Abstract
We report physiological, anatomical and molecular differences in two economically important grapevine (Vitis vinifera L.) cultivars cv. Grenache (near-isohydric) and Chardonnay (anisohydric) in their response to water-stress induced cavitation. The aim of the study was to compare organ vulnerability (petiole and stem) to cavitation by measuring ultrasonic acoustic emissions (UAE) and percent loss of conductance of potted grapevines subject to the onset of water-stress. Leaf (ψL) and stem water potential (ψS), stomatal conductance (gs), transpiration (E), petiole hydraulics (KPet), and xylem diameter were also measured. Chardonnay displayed hydraulic segmentation based on UAE, with cavitation occurring at a less negative ψL in the petiole than in the stem. Vulnerability segmentation was not observed in Grenache, with both petioles and stems equally vulnerable to cavitation. Leaf water potential that induced 50% of maximum UAE was significantly different between petioles and stems in Chardonnay (ψ50Petiole = -1.14 and ψ50Stem = -2.24 MPa) but not in Grenache (ψ50Petiole = -0.73 and ψ50Stem = -0.78 MPa). Grenache stems appeared more susceptible to water-stress induced cavitation than Chardonnay stems. Grenache displayed (on average) a higher KPet likely due to the presence of larger xylem vessels. A close relationship between petiole hydraulic properties and vine water status was observed in Chardonnay but not in Grenache. Transcriptional analysis of aquaporins in the petioles and leaves (VvPIP1;1, VvPIP2;1, VvPIP2;2 VvPIP2;3, VvTIP1;1, and VvTIP2;1) showed differential regulation diurnally and in response to water-stress. VvPIP2;1 showed strong diurnal regulation in the petioles and leaves of both cultivars with expression highest predawn. Expression of VvPIP2;1 and VvPIP2;2 responded to ψL and ψS in both cultivars indicating the expression of these two genes are closely linked to vine water status. Expression of several aquaporin genes correlated with gas exchange measurements, however, these genes differed between cultivars. In summary, the data shows two contrasting responses in petiole hydraulics and aquaporin expression between the near-isohydric cultivar, Grenache and anisohydric cultivar, Chardonnay.
Keywords: aquaporin, cavitation, water-stress, isohydric, anisohydric, petiole, hydraulic conductivity, xylem embolism
Introduction
Grapevines respond to water deficit with a variety of physiological and molecular mechanisms including modifications to the liquid pathways of water movement through the root and shoot, and vapor movement through stomata (Lovisolo et al., 2010). Under conditions of water-stress, grapevines are susceptible to xylem cavitation and embolism (Tyree and Sperry, 1989) resulting in reduced hydraulic conductivity of the xylem pathway. Cultivar and species differences have been observed in Vitis sp. in their physiological responses to drought stress (Schultz, 2003; Soar et al., 2006; Alsina et al., 2007; Vandeleur et al., 2009; Gerzon et al., 2015) indicating that variation in drought tolerance is a genetically controlled trait that can extend to cultivar differences.
Vulnerability to xylem cavitation is dependent on the hydraulic architecture of plants, a feature that varies between and within many plant species (Tyree et al., 1994; Schultz, 2003; Alsina et al., 2007). This variation is influenced by the segmented structure of dicotyledonous plants that permits hydraulic segmentation of different plant organs. In Juglans regia L. (Tyree et al., 1993), Acer saccharinum L. (Tsuda and Tyree, 1997), and Vitis sp. (Lovisolo et al., 2008a; Charrier et al., 2016; Hochberg et al., 2016), petioles have been shown to be vulnerable to cavitation under drought conditions, encouraging leaf shedding and stem preservation against further water-stress. Leaf shedding is also known to occur in grapevines under extreme episodes of drought (Keller, 2005).
The ability of plants to repair cavitated and embolized vessels is necessary to maintain the hydraulic pathway following drought (Zwieniecki and Holbrook, 2009), but how this occurs is not fully understood and refilling under negative pressure is still widely debated (Cochard et al., 2013, 2015). Holbrook et al. (2001) demonstrated using nuclear magnetic resonance (NMR) imaging that upon re-watering following drought treatment, grapevines were able to refill embolized vessels but only under non-transpiring conditions. Brodersen et al. (2010) subsequently demonstrated refilling of embolized vessels in V. vinifera (cv. Chardonnay) using high-resolution X-ray computed tomography. Flow rates from surrounding cells were quantified and successful refilling was demonstrated in stems that were under tension, but the repairing conduit were considered to be hydraulically isolated from the bulk of the xylem. It has been proposed that aquaporin’s located in the surrounding xylem parenchyma cells may contribute to water movement required for embolism recovery (Holbrook and Zwieniecki, 1999; Tyree et al., 1999; Lovisolo et al., 2010). More recently, models have been proposed describing how refilling of xylem conduits may occur through aquaporin facilitated movement of water via the phloem and living xylem parenchyma cells (Holbrook and Zwieniecki, 1999; Nardini et al., 2011; Brodersen and McElrone, 2013; Secchi et al., 2016).
In walnut (J. regia), increased expression of two aquaporin genes, JrPIP2;1 and JrPIP2;2 in parenchyma cells associated with xylem vessels, was found to correlate with refilling after winter embolism (Sakr et al., 2003). Kaldenhoff et al. (2008) reported that reduced expression of NtPIP2 aquaporin (using RNAi) in tobacco shoots delayed embolism repair compared to the wildtype control and to tobacco plants with reduced expression of NtPIP1 aquaporin’s. Secchi and Zwieniecki (2010) correlated up-regulation of the PtPIP1 subfamily in Populus trichocarpa (Torr. and Gray) stems with xylem embolism and proposed a continuous embolism/refilling cycle under normal conditions. In V. vinifera cv. Grenache, VvPIP2;1 has been shown to be expressed in petiole tissue and in vessel associated cells (VACs) (Chitarra et al., 2014).
Stomatal closure is thought to minimize water-stress induced cavitation in grapevine (Lovisolo and Schubert, 1998). However, grapevine cultivars are known to vary in their degree of stomatal closure in response to water-stress. Cultivars, such as Grenache, are considered to be near-isohydric, since midday water potential does not decrease substantially under water-stress due to stomatal closure (Schultz, 2003; Vandeleur et al., 2009). In contrast, cultivars such as Shiraz (Syrah) and Chardonnay are more anisohydric with stomata being less sensitive to declining water potential (Schultz, 2003; Soar et al., 2006; Rogiers et al., 2009; Vandeleur et al., 2009). The classification of isohydry and anisohydry for species and cultivars is dependent on many factors including water potential regulation, stomatal behavior, and hydraulic transport under drought conditions (Martínez-Vilalta and Garcia-Forner, 2016). A recent meta-analysis examined factors influencing stomatal conductance in grapevine in response to water availability proposing that there is a continuum of stomatal responses that are dependent upon the scion – rootstock combination and the interaction with different soil types (Lavoie-Lamoureux et al., 2017).
Xylem embolism formation and refilling has been studied extensively in grapevines due to the presence of large xylem vessels that provide a good model for both physiological and molecular studies (Holbrook et al., 2001; Brodersen et al., 2010; Choat et al., 2010). With the sequencing of the grapevine genome (Jaillon et al., 2007; Velasco et al., 2007), and identification of aquaporin families and sub-families (Fouquet et al., 2008; Shelden et al., 2009), grapevine provides an excellent model of a woody perennial fruit tree for studying gene responses to water-stress.
Many studies have investigated the role of aquaporins in root xylem hydraulic function (Lovisolo et al., 2008b; Vandeleur et al., 2009; Perrone et al., 2012); however, there are only a limited number of studies in the shoots (Pou et al., 2013; Chitarra et al., 2014). In this study, we compare the cavitation vulnerability and hydraulic properties of two economically important grapevine cultivars, Vitis vinifera L. cv. Grenache (isohydric) and Chardonnay (anisohydric) in response to water-stress (Schultz, 2003; Vandeleur et al., 2009). The objectives of this study were firstly, to compare organ vulnerability to cavitation by measuring xylem cavitation in the petiole and stem, petiole hydraulic conductivity (Kpetiole) and xylem anatomical differences between the two cultivars in response to moderate water-stress; secondly, to determine if aquaporin expression was altered in the petioles and leaves both diurnally and in response to water-stress induced cavitation. In order to do this, we measured the transcript abundance of six aquaporin genes, VvPIP1;1, VvPIP2;1, VvPIP2;2, VvPIP2;3, VvTIP1;1, and VvTIP2;1. Vitis PIP2 and TIP genes have previously been functionally characterized as water conducting channels in Xenopus oocytes (Shelden et al., 2009; Vandeleur et al., 2009), however, VvPIP1;1 only shows water conducting capacity when co-expressed with VvPIP2 genes (Vandeleur et al., 2009).
Materials and Methods
Plant Material
Vitis vinifera L. Grenache (clone SA38) and Chardonnay (clone I10V1) 1-year-old rootlings (own roots) (Glen Avon Nursery, Langhorne Creek, SA, Australia) were planted in 4.7 L pots containing a modified University of California (UC) soil mix [61.5% (v/v) sand, 38.5% (v/v) peat moss, 0.50 g L-1 calcium hydroxide, 0.90 g L-1 calcium carbonate, 100 g per 100 L-1 Nitrophoska (12:5:14, N:P:K plus trace elements; IncitecPivot, Melbourne, VIC, Australia) at pH 6.8] and fertilized with 0.08 g L-1 (soil) per month of Osmocote Standard (Scotts Australia Pty Ltd., Baulkham Hills, NSW, Australia) as described previously (Shelden, 2008; Shelden et al., 2009). Plants were grown in controlled temperature glasshouses maintained at 25°C day/20°C night with extended light period provided by 1000 watt mercury halide lamps (14 h day/10 h night). Plants were watered to field capacity every 2 days and spur pruned to have two shoots. Pot-grown vines were subjected to a drying cycle to impose water deficit. Plants were watered to field capacity in the evening prior to starting all experiments, then water was withheld for the remainder of the experiment.
Drought Experiment 1 – Measurement of Acoustic Emissions
Ultrasonic acoustic emissions (UAE) have been used to measure drought-induced cavitation in woody plants including grapevine (Milburn and Johnson, 1966; Tyree and Dixon, 1983; Tyree et al., 1984; Ikeda and Ohtsu, 1992; Kikuta et al., 1997; Kikuta et al., 2003; Johnson et al., 2009). A cavitation event occurs in the xylem vessels due to increased tension as a result of drought, resulting in a rapid relaxation of energy that produces an acoustic emission of energy (AE). Ultrasonic acoustic emissions were measured using an acoustic monitoring system (Model 4615 DSM, Physical Acoustic Corporation, Princeton Junction, NJ, United States) with I151 sensors. Signals were amplified in the range of 150–400 kHz. At least five individual plants grown in the glasshouse were monitored over the growing season for each variety. Sensors were positioned on the basal portion of the plant between nodes two and six on fully mature leaves. One sensor was clamped to the middle of an internode and one to a petiole. A thin layer of silicon grease was put onto the transducer to allow better acoustic contact with the plant. The plant was watered to field capacity at the start of the experiment and UAE’s recorded continuously over the drought period until wilting point was reached. As Chardonnay reached wilting point before Grenache, measurements were taken over a longer period for Grenache. The cumulative UAEs (cUAE) were determined and plotted against time of water-stress. In addition, cUAEs were normalized relative to the maximum number of cavitated vessels observed at extreme dehydration in each organ (petiole and stem) and plotted against the mean leaf water potential (ψL). A sigmoidal dose-response curve (variable) slope was fitted to the data using GraphPad Prism® Version 4.0.
The cavitation threshold value (ψ10) is the leaf water potential determined at the point at which cavitation is triggered and is taken as 10% of the maximum UAEs (Salleo et al., 1996; Nardini et al., 2001). Both ψ10 and ψ50 were determined for the stem and petiole of at least three plants of each cultivar. Significance between means were determined with an unpaired t-test using GraphPad Prism®.
Leaf Water Potential
Leaf water potential (ψL) measurements were made using the PSYPROTM data logger with L-51(A)-SF leaf psychrometer sensors (Wescor, Inc., Logan, UT, United States). Psychrometers were calibrated with NaCl solutions as described by Campbell and McInnes (1999). Leaf psychrometers were positioned on four basal leaves surrounding the acoustic sensors. Prior to attachment, the leaf cuticle was removed from the abaxial side of the leaf with 1200 grit sandpaper as described by Campbell and McInnes (1999). The scan sequence program was set as follows: cooling current time 15 s, measurement period seconds 20 s, delay seconds after cooling 5.2 s, and read average 6 s. For each psychrometer a reading was taken every 15 min over the duration of the experiment. Where indicated, leaf water potentials were also measured with a pressure chamber (PMS Instruments, Albany, OR, United States). A calibration performed of leaf psychrometers versus pressure chamber yielded a linear relationship (Supplementary Figure S1). The use of psychrometers was preferential as it was non-destructive and allowed continuous in situ monitoring of ψL over the experiment duration.
Xylem Anatomy
Transverse sections of fresh petioles were stained with toluidine blue O and examined to determine the vessel diameter of mature xylem vessels in Chardonnay and Grenache vines. Hand sections were made using a single razor blade in the middle of the petiole. Sections were stained with 0.05% v/v toluidine blue O for 1 min, rinsed with distilled water and mounted onto slides. Sections were viewed under a light microscope (Zeiss Axiophot Pol Photomicroscope, Oberkochen, Germany). Images were captured using a Nikon DXM1200F digital Video (Coherent Life Sciences) and Nikon ACT-1 software. The mean xylem vessel diameter was determined for each variety by sampling petioles from four vines and measuring the diameter of xylem vessels per petiole segment. The weighted hydraulic diameter (dhyd) was calculated as:
where r is the radius of the vessel, as described by Sperry et al. (1994).
Drought Experiment 2
Plants were grown in controlled temperature glasshouses as described above and watered to field capacity the evening prior to beginning observations. Four individual plants were harvested at the following time points: 6:00, 11:00, 16:00, and 21:00 h on each sampling day (well-watered and water-stressed) (16 plants in total for each cultivar). The same plants were sampled for each time point under well-watered and water-stressed conditions. The 6:00 h time point was harvested in the dark and is referred to in the text as predawn ψL, and 11:00 h as midday ψL.
One leaf from each plant was taken to measure ψL at each time point with the Scholander pressure chamber (PMS Instruments, Albany, OR, United States). Stem water potential (ψs) was determined by covering a leaf on each plant with a plastic bag wrapped in aluminum foil for 1 h prior to measurement with the Scholander pressure chamber (Begg and Turner, 1970). One petiole with leaf attached was harvested for measurement of hydraulic conductance and Percent Loss of Conductance (PLC). One leaf per plant was used to measure transpiration and stomatal conductance (gS) with a LI-6400 Portable Photosynthesis System (LI-COR, Lincoln, NE, United States) at 11:00and 16:00 h on the days of sampling. Over the course of the experiment, midday stomatal conductance (gS) was measured on all plants using a porometer (Delta T AP4; Delta-T Devices Ltd., Cambridge, United Kingdom).
On the first sampling day (well-watered), two petioles and corresponding leaves were sampled from nodal positions three to five at the basal portion of the stem at all time points for aquaporin expression analysis. Petioles were cut parallel to the shoot axis with a sharp razor blade and the petiole detached from the base of the leaf. Tissues were wrapped separately in aluminum foil and immediately snap frozen in liquid nitrogen. Water was withheld from plants for the next 3 days at which time the same plants were sampled again (water-stressed, when midday leaf water potentials were predicted to be approximately -1.5 MPa). The same plants were sampled for each time point under well-watered (WW) and water-stress conditions (WS).
Petiole Hydraulic Conductivity and Percent Loss Conductance (PLC)
The petiole specific hydraulic conductivity (Kh, m4 s-1 MPa-1) and PLC was measured using the XYL’EM Embolism Meter apparatus (Bronkhorst, France). The XYL’EM apparatus was equipped with a pressure transducer and two flow meters (Liquiflow, Instrutec; 5 and 50 g h-1). Degassed glass distilled water (Labglass Cascade, Graintech, Australia) was used to fill the captive air tank. Petioles were cut parallel to the node submerged under MilliQ water and immediately attached to luer tubes that were connected to the XYL’EM apparatus. The leaf blade was removed from the petiole after connection to the luer tube. To obtain Kinit, petioles were perfused with degassed distilled water at 4 kPa. Petioles were then flushed at 0.15 MPa for 1 min and Kfinal determined. The water flow (F; m3 s-1) entering the petiole was measured when exposed to a positive pressure (P; MPa) of 0.15 MPa and recorded when flow rate was stable (usually between 1 min). PLC was computed:
On occasions, Kfinal values were lower than Kinitial computing a negative PLC value. This can result from blockage of the xylem vessels during measurement (Cochard et al., 2013). Where this occurred, these values were recorded as zero PLC in the analysis.
The petiole specific hydraulic conductivity, KPet_h, was computed as:
Where L is the length of the petiole segment (m).
Leaf area (LA; m2) was measured with a leaf area meter (AM200, ADC Bioscientific Ltd., Herts, England). Leaf specific conductivity (KPet_LA) was determined by:
The mean KPet_h was calculated from averaging KPet_h values from all time points of well-watered vines.
Total and Poly(A)+ RNA Isolation cDNA Synthesis
Total RNA was isolated from petiole tissue as described previously (Shelden et al., 2009) and treated with RNase free DNase 1 (Ambion, Melbourne, VIC, Australia). First strand cDNA synthesis from normalized total RNA was synthesized using the iScriptTM cDNA synthesis kit (Bio-rad, Hercules, CA, United States) from 1 μg of total RNA. Leaf total RNA was extracted using the Spectrum Plant Total RNA Kit (Sigma) and cDNA synthesized using the Superscript III First Strand Kit (Invitrogen). Quantity and purity of total RNA was determined with a Nanodrop Spectrophotometer ND-1000 (Biolab Ltd., Australia).
Quantitative Real-Time Polymerase Chain Reaction (QRT-PCR)
Gene specific primers (Sigma–Aldrich, Castle Hill, NSW, Australia) were designed with Primer31 (accessed July 2006) to previously described aquaporin cDNAs, VvPIP1;1, VvPIP2;1, VvPIP2;2, VvPIP2;3, VvTIP1;1, and VvTIP2;1 (Shelden et al., 2009), in regions with highest sequence divergence (Supplementary Table S1). Primers were designed to amplify amplicons between 110 and 230 bp in length with Tm between 58 and 65°C, and GC content not higher than 55%.
A 2 × mix of KAPA SYBR®FAST qPCR (KAPA Biosystems) was used for all real-time PCR reactions. The reaction mix contained 2 × KAPA Master Mix, 10 μM of each primer and an amount of cDNA template equivalent to 15 ng of total RNA. Twenty microliter reactions were used and each reaction was performed in duplicate. Thermocycling was conducted in a QuantStudio 12K Flex Real-Time PCR system (Life Technologies): 95°C for 3 min, 40 cycles consisting of 95°C for 1 s, 55°C for 20 s, and 72°C for 10 s. Prior to melt curve analysis a final denaturation step at 95°C for 30 s was performed. Melt curve analysis was performed between 57 and 97°C at 0.5°C increments for 30 s. To ensure single-product amplification, melt curve analysis was performed by heating the PCR products for 40 cycles starting at 52°C and increasing by 0.5°C per cycle with continuous fluorescence detection.
Amplification efficiencies varied between 95 and 100%. To confirm correct amplicon, PCR products were sequenced using Dye Terminator 3 (Applied Biosystems, Foster, CA, United States) and analyzed by the Institute of Medical and Veterinary Sciences (Adelaide, SA, Australia). Data were normalized with VveELFγ, VvACT, and VvUbq that have been shown to be constitutively expressed in grapevine (Deluc et al., 2006, 2008). Standard curves were generated for each gene using gene specific primers and 10-fold serial dilutions of purified PCR amplified gene specific products. The correlation coefficient and PCR amplification efficiency (E) was determined for each primer set. Relative changes in gene expression were determined using the Pfaffl method (Pfaffl, 2001). The values were calculated relative to each reference gene and then the geometric mean was determined (Vandesompele et al., 2002). Outliers were removed using the ROUT algorithm (Motulsky and Brown, 2006). The Log2 ratio was calculated for diurnal expression as 11:00/6:00 h, 16:00/6:00 h, and 21:00/6:00 h and for water-stress treatment as WS/WW. Data are presented as the mean normalized expression of four biological representatives (petioles and leaves taken from individual plants) ± SE, each with two technical replicates.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism®(GraphPad Software Inc, San Diego, CA, United States). One-way ANOVA and two-way ANOVA were performed to test differences between experimental groups. Two tailed unpaired t-tests were used to compare mean values. The correlation matrix was performed using RStudio software (Version 1.0.1532). Hydraulic conductivity and QPCR data were log normal transformed prior to performing correlation in RStudio.
Results
Drought Experiment 1: Leaf Water Status and Cavitation Vulnerability
Leaf water potential measurements were made continuously with leaf psychrometers over the course of the drought experiments on both Chardonnay and Grenache vines. A linear regression of ψL measured with the pressure chamber and psychrometers showed a good correlation with no significant difference observed (Supplementary Figure S1). The pressure chamber tended to measure lower values for ψL particularly at higher water potentials than psychrometers as has been reported in other species (Martinez et al., 2011). Psychrometers recorded water potential every 15 min, thus oscillations were observed throughout the day (Supplementary Figure S2) indicating the continuously changing water status of the leaf most likely due to localized changes in water availability and stomatal conductance (During and Loveys, 1996). The mean predawn ψL of well-watered Chardonnay vines was -0.26 MPa and in Grenache -0.29 MPa (Table 1). In water-stressed Grenache vines the predawn and midday ψL were significantly higher (-0.7 and -1.05 MPa, respectively) than Chardonnay vines (-1.17 and -1.44 MPa) in response to water-stress.
Table 1.
Drought experiment 1 predawn (ψL_Predawn, MPa) and midday leaf water potential (ψL_Midday, MPa) for Chardonnay and Grenache vines used for cavitation analysis under well-watered (WW) and water-stressed (WS) conditions.
| Cultivar | WW ψL_Predawn | WW ψL_Midday | WS ψL_Predawn | WS ψL_Midday |
|---|---|---|---|---|
| Chardonnay | -0.26 ± 0.06a | -0.31 ± 0.01b | -1.17 ± 0.01c | -1.44 ± 0.01d |
| Grenache | -0.29 ± 0.10a | -0.34 ± 0.11b | -0.70 ± 0.09e | -1.05 ± 0.06f |
Plants were grown in control temperature glasshouses, watered to field capacity the evening prior to WW measurements being recorded and water withheld until wilting point. WS is reported after 96 h of water withheld. Leaf water potential was recorded continuously with psychrometers on the same plant as ultrasonic acoustic emissions (UAEs) were recorded. Mean values of three independent experiments ± SEM (n = 3) are shown. Superscript letters show significance between both cultivars and time of day (two-way ANOVA, Bonferroni post-test, p > 0.05).
Cavitation was measured in the internodes and the middle of petioles of both Chardonnay and Grenache vines, by the detection of UAEs using specialized microphones (Supplementary Figure S2). Vulnerability curves were generated by plotting the cUAEs against the leaf water potential, for three independent drying experiments for both Chardonnay and Grenache (Figure 1). The number of emissions per day increased over time as the water-stress became more severe. In well-watered Chardonnay vines, cavitation was only detected in the petiole and not in the stem internode (Figure 1A). As the water-stress increased (ψL values ∼-1.5 MPa), UAEs were detected in both the stem and petiole (Figure 1A). In Grenache, UAEs were detected simultaneously in both the petioles and stems of well-watered vines (Figure 1B). From the vulnerability curves, it is possible to determine the ψCAV threshold value, taken as 10% of the maximum cUAEs, for the point at which cavitation is triggered (Salleo et al., 1996; Nardini et al., 2001). In Grenache vines, the petiole and stem had very similar ψ10 values of -0.13 and -0.19 MPa, respectively (Table 2). In Chardonnay vines, the leaf water potential (ψ10) at which cavitation was triggered in the petioles and stems was significantly different (p < 0.05), with threshold ψ10= -0.53 and -1.81 MPa for the petioles and stems, respectively. There was no significant difference between cultivars in threshold ψ10 for petioles, however, the stem ψ10 was significantly different. Leaf water potential that induced 50% loss of conductance was significantly different between petioles and stems in Chardonnay (ψ50Petiole = -1.14 and ψ50Stem= -2.24 MPa) but not in Grenache (ψ50Petiole = -0.73 and ψ50Stem= -0.78 MPa). There was no significant difference between cultivars in ψ50Petiole, however, ψ50Stem was significantly more negative in Chardonnay than Grenache.
FIGURE 1.
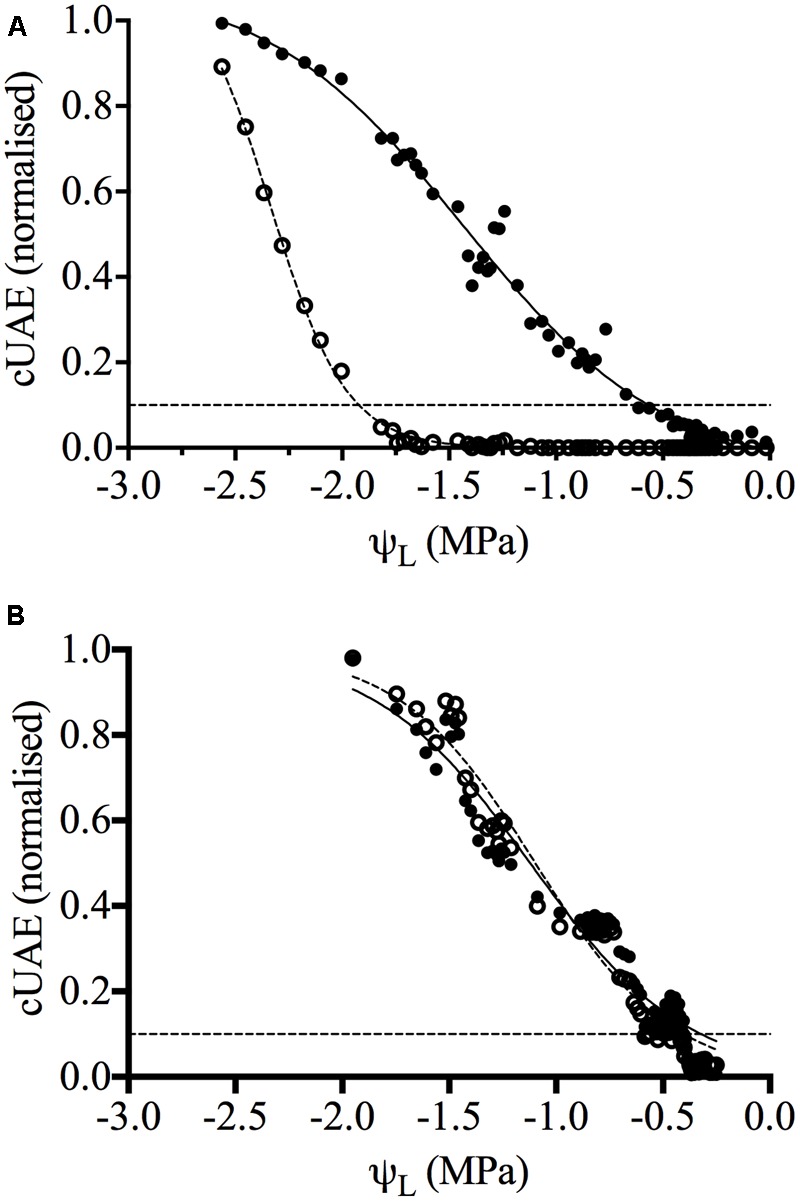
Vulnerability curves for Chardonnay (A) and Grenache (B) grapevines. Data shown is the normalized cumulative ultrasonic acoustic emissions (cUAE) plotted against mean leaf water potential for both the petiole (filled circles, bold line) and stem (open circles, dotted line) (n = 1). Curves were generated by fitting a sigmoidal dose-response curve (variable slope) to the data using Graphpad Prism software. The hillslope (r2 in brackets) for Chardonnay petiole and stem is –0.9 (0.99) and –2.4 (0.99), respectively, and for Grenache petiole and stem –1.2 (0.94) and –1.4 (0.97), respectively. The dotted black line represents 10% of the maximum cUAEs.
Table 2.
Leaf water potential for threshold cavitation (ψ10) and 50% loss of conductance (ψ50) assayed by acoustic emissions (AEs) in the petioles and stems of Chardonnay and Grenache potted vines.
| Cultivar | ψ10Petiole (MPa) | ψ10Stem (MPa) | ψ50Petiole (MPa) | ψ50Stem (MPa) |
|---|---|---|---|---|
| Chardonnay | -0.53 ± 0.04a | -1.81 ± 0.23b | -1.13 ± 0.13a | -2.24 ± 0.23b |
| Grenache | -0.13 ± 0.10a,c | -0.19 ± 0.11c | -0.73 ± 0.2a | -0.78 ± 0.18a |
Ψ10 is determined as the leaf water potential for which 10% of the maximum AE. Ψ50 is the leaf water potential that induces 50% loss of hydraulic conductance. Data is the mean ± SEM of at least three independent experiments. Superscript letters indicate significance between cultivar and organ (t-test, p < 0.05).
Drought Experiment 2: Leaf Water Status, Hydraulic Conductivity, and Xylem Anatomy
In Drought Experiment 2, leaf and stem water potential displayed a diurnal pattern for both varieties (Table 3). In Chardonnay, a significant difference was observed between well-watered and water-stress at each time point except for predawn (6:00 h) after 72 h of withholding water. In Grenache, there was no significant difference between well-watered and water-stressed plants except at 21:00 h. No significant differences in ψL were observed between cultivars under well-watered and water-stressed conditions (Table 3). The stem water potential was significantly different between well-watered and water-stressed conditions for both Chardonnay and Grenache at each time point (Table 3). A significant difference in ψS was observed at 16:00 h between water-stressed Chardonnay (-1.38 MPa) and Grenache (-1.18 MPa) with Grenache maintaining a less negative ψs. Both E and gS were significantly decreased in water-stressed Chardonnay and Grenache compared to well-watered vines at 16:00 h (Figures 2A,B). Midday stomatal conductance (gS) measurements showed Chardonnay had a significantly higher gS than Grenache on D2 after water was withheld (Figure 2C). In both Chardonnay and Grenache, midday gS decreased significantly as the vines became more water-stressed after 3 days of water withheld. Both stem and leaf water potential were strongly positively correlated with E and gS in Chardonnay, however, only stem water potential correlated in Grenache.
Table 3.
Drought Experiment 2 leaf water potential (ψL, MPa) and stem water potential (ψS, MPa) for Chardonnay and Grenache WW and WS vines.
| Chardonnay |
Grenache |
|||
|---|---|---|---|---|
| ψL (MPa) |
ψL (MPa) |
|||
| Time | WW | WS | WW | WS |
| 6:00 | -0.2 ± 0.03a | -0.5 ± 0.05a | -0.3 ± 0.02a | -0.5 ± 0.06a |
| 11:00 | -0.7 ± 0.03c | -1.0 ± 0.16d | -0.9 ± 0.04b,c | -1.0 ± 0.06b,e |
| 16:00 | -1.0 ± 0.08e | -1.5 ± 0.04f | -1.1 ± 0.18b | -1.3 ± 0.07b,e |
| 21:00 | -0.3 ± 0.02a | -1.0 ± 0.02 d | -0.6 ± 0.04a,c | -1.2 ± 0.04e |
|
ΨS (MPa) |
ΨS (MPa) |
|||
| Time | WW | WS | WW | WS |
| 6:00 | – | – | – | – |
| 11:00 | -0.5 ± 0.03a | -0.8 ± 0.03b | -0.6 ± 0.02a | -0.9 ± 0.06b |
| 16:00 | -0.6 ± 0.07a | -1.4 ± 0.05c | -0.8 ± 0.07b | -1.2 ± 0.06c |
| 21:00 | -0.4 ± 0.03a | -1.2 ± 0.07c | -0.5 ± 0.0a | -1.1 ± 0.02c |
Plants were grown in control temperature glasshouse and measurements conducted at the time of tissue sampling for QPCR analysis. Plants were watered to field capacity the evening prior to WW measurements, and water withheld for 72 h for WS measurements. Leaf water potential and stem water potential measurements were taken with the pressure chamber. Mean ± SEM (n = 4). Superscript letters indicate significance differences (two-way ANOVA, Bonferroni post-test, p < 0.05).
FIGURE 2.
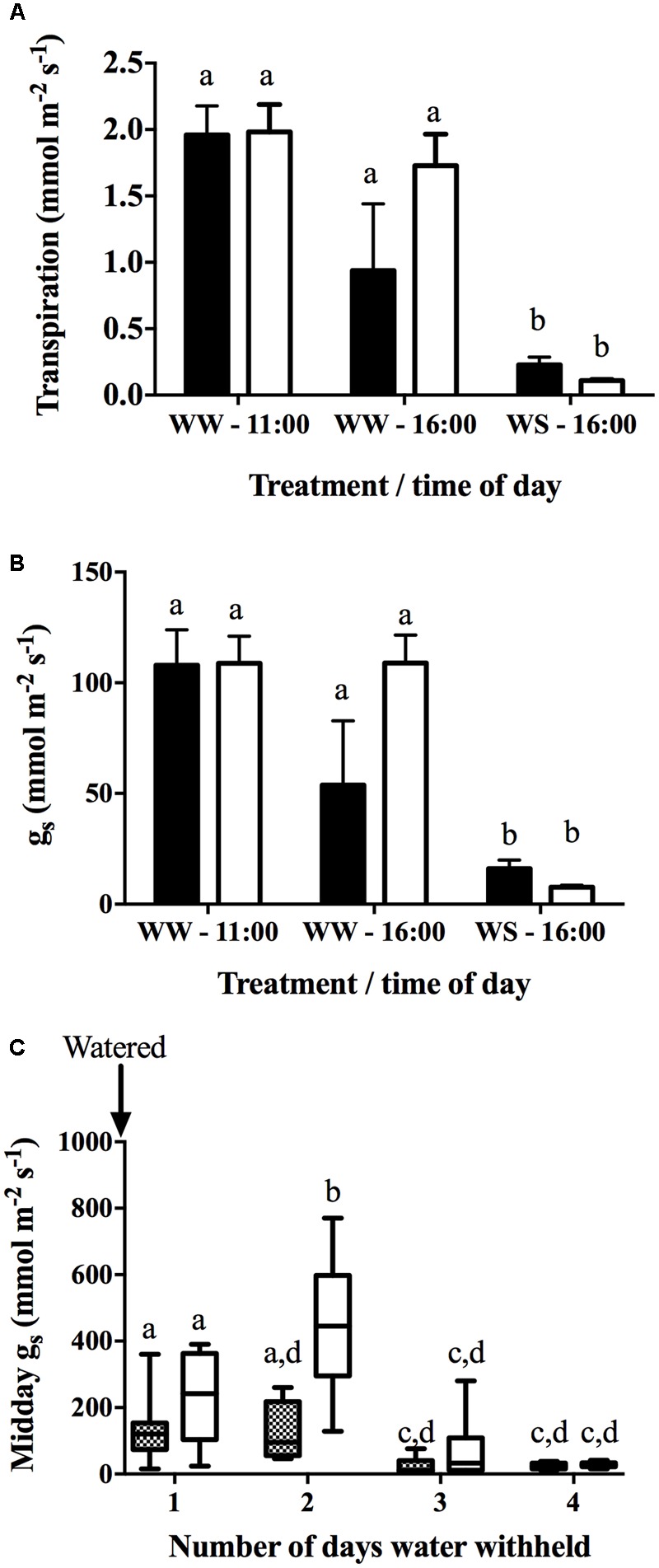
Stomatal conductance (gS) (A) and transpiration (E) (B) were measured at 11:00 and 16:00 h on well-watered (WW) (D1) plants and at 16:00 h of water-stressed (WS) (D4) plants with the Licor (n = 4). Plants were watered to field capacity the evening prior to the first day of sampling (D1 – WW) and water was withheld for 4 days (D4 – WS). Data shows the mean ± SEM. Midday leaf stomatal conductance (gS) (C) measured on Grenache and Chardonnay plants using the porometer over the duration of water withholding experiment. Data shows the mean ± min/max value (n = 9–16). Significant differences between cultivars and time points are indicated by different letters (p < 0.05).
Hydraulic conductivity measurements were conducted on excised petioles from Chardonnay and Grenache vines grown in a temperature-controlled glasshouse. In response to well-watered and water-stressed conditions, no significant diurnal changes in petiole hydraulic conductivity (KPet_h and KPet_LA) were evident in either cultivar (Two-way ANOVA, p > 0.05) (Figures 3A,B). Water-stress significantly decreased petiole KPet_h and KPet_LA for Chardonnay at 11:00 h. There were no significant differences in KPet_h or KPet_LA for well-watered and water-stressed Grenache vines. KPet_LA showed a significantly linear decline with decreasing ψL in Chardonnay but not in Grenache (Figure 3C). Both KPet_h and KPet_LA were higher in Grenache petioles than Chardonnay, although this difference was not significant. The mean KPet_h was 3.7 × 10-10 m4 MPa-1 s-1 and 5.2 × 10-10 m4 MPa-1 s-1 in Chardonnay and Grenache, respectively. PLC significantly increased under water-stress in Grenache petioles with maximum PLC 35% at 16:00 h when both ψL and ψS were most negative (Supplementary Figure S3). In Chardonnay, maximum PLC was 23%, however, there was no significant difference at any time point in response to water-stress. When PLC was plotted against stem water potential no difference in slope was observed between cultivars (Figure 4). When ψS > -0.80 MPa, the fitted slope was not significantly different from zero, however, ψS < -0.80 MPa, PLC increased with a slope of 14.1 and 7.5% PLC per MPa for Grenache and Chardonnay, respectively, however, these were not significantly different.
FIGURE 3.
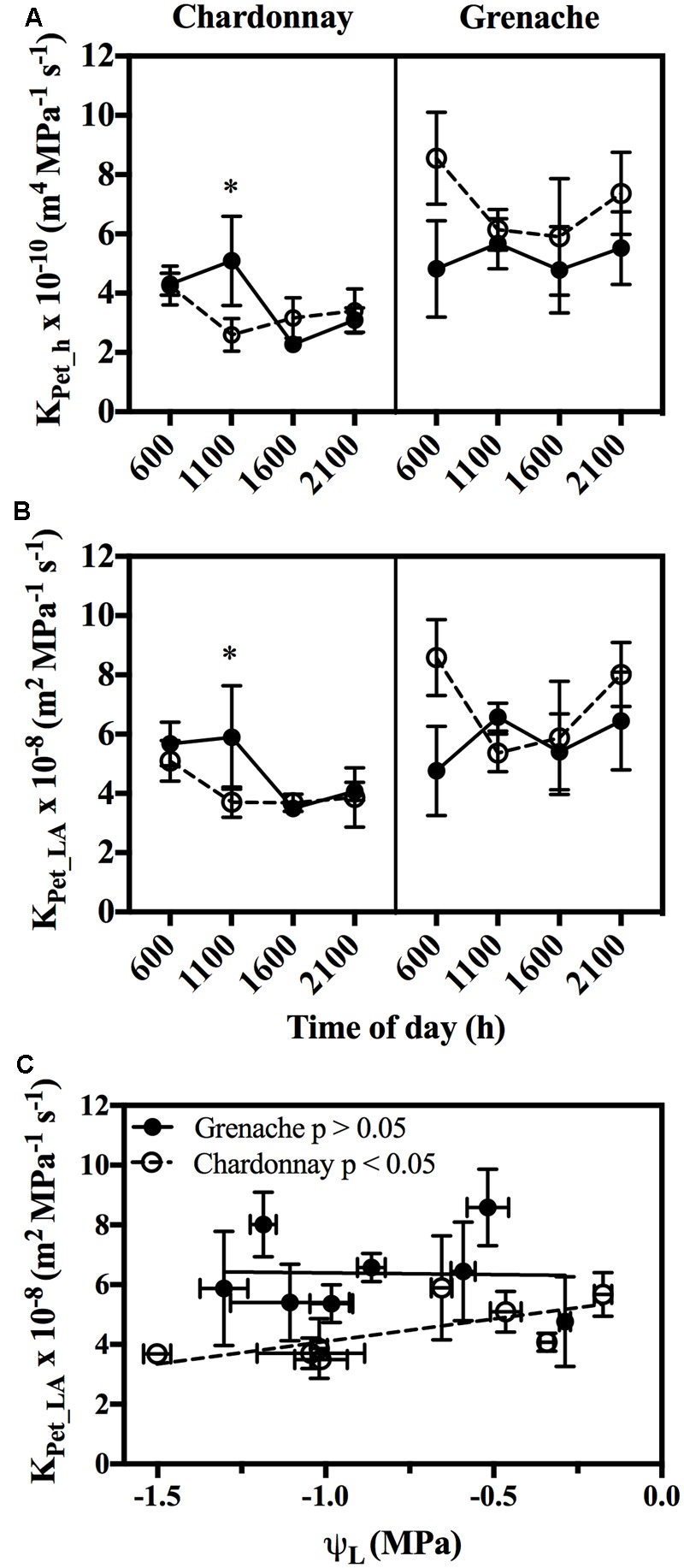
Mean petiole specific hydraulic conductivity (KPet_h) (A) and mean leaf area specific conductivity (KPet-LA) (B) over a diurnal period in WW (filled circles, bold line) and WS (open circles, dotted line) Chardonnay and Grenache vines. The relationship between KPet-LA and leaf water potential (ψL) for Chardonnay and Grenache was determined by fitting a linear regression (significant p < 0.05) (C). Data shows the mean ± SEM (n = 4). Significant differences between WW and WS are indicated by ∗ (p < 0.05).
FIGURE 4.
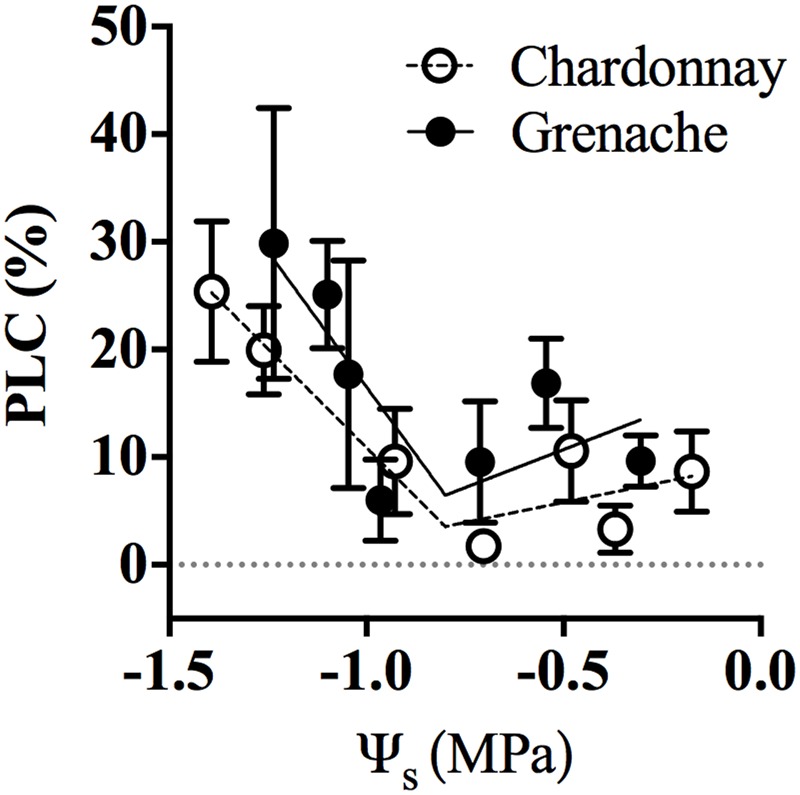
Percent loss of conductance (PLC) in response to stem water potential (ψS) for Chardonnay (open circles) and Grenache petioles (filled circles). Well-watered and water-stressed data were combined for each cultivar and ψS ranked. Data was segmented in to approximately equal steps in ψS and means ± SEM calculated for each category (n = 3–9 for each point). There were 32 separate observations for Chardonnay and 31 for Grenache. For each cultivar data was fitted with a segmental linear regression; the break point for both = –0.9 MPa. The first slope is not significantly different from zero and the intercept is also not significantly different from zero (i.e., zero PLC above –0.8 MPa). Below –0.8 MPa the PLC increases with a slope of 14.1 and 7.5% PLC per MPa for Grenache and Chardonnay, respectively, however, these are not significantly different.
Xylem vessel diameters were measured on mature petioles of Chardonnay and Grenache. The frequency of petioles with xylem diameter between 20 and 29 μm was similar for both Chardonnay and Grenache; however, xylem vessel diameters greater than 30 μm were frequently measured in Grenache petioles (Figure 5). In Grenache petioles, the average xylem vessel diameter was significantly larger than Chardonnay petioles, as were both the minimum and maximum xylem diameters (Table 4). Chardonnay had a weighted hydraulic diameter (dhyd) of 32.1 μm and Grenache had a dhyd of 44.1 μm calculated according to Sperry et al. (1994). Thus, based on dhyd, Chardonnay petioles would have less capacity to conduct water compared to Grenache.
FIGURE 5.
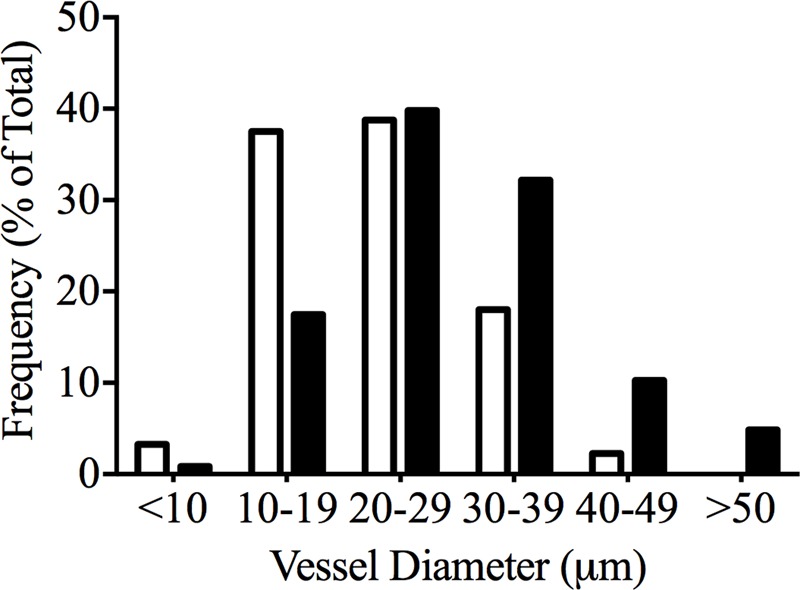
Relative frequency of different xylem vessel diameters in mature Chardonnay (black bars) and Grenache (white bars) petioles calculated from transverse sections of four individual petioles.
Table 4.
Minimum, maximum and mean xylem vessel diameter (μm) for mature Chardonnay and Grenache petioles for plants grown in a growth chamber.
| Min. vessel diameter (μm) | Mean vessel diameter (μm) | Max. vessel diameter (μm) | |
|---|---|---|---|
| Chardonnay | 5.0 | 20.7 ± 0.7a | 55 |
| Grenache | 7.5 | 30.5 ± 3.5b | 65 |
Vessel diameters (μm) were obtained from transverse sections of petioles harvested from four individual plants. Data for vessel diameter is the mean ± SEM (n = 4 petioles). Superscript letters indicate significance between cultivars (p < 0.05).
Diurnal Regulation of AQP Expression under Well-Watered Conditions
To investigate if aquaporin gene expression in the petioles and leaves correlated with physiological parameters we monitored the expression of six genes VvPIP1;1, VvPIP2;1, VvPIP2;2, VvPIP2;3, VvTIP1;1, and VvTIP2;1 using QPCR over a diurnal cycle of well-watered and water-stressed Chardonnay and Grenache vines. Transcripts of all these genes have previously been detected in other vegetative tissues in grapevine (stem, tendrils, leaves, and roots) and thus are not specific to the petiole and leaves (Shelden, 2008; Vandeleur et al., 2009). Petioles and leaves were sampled from four independent plants used for physiological measurements, but due to the extremely low expression of some aquaporin genes (undetectable by QPCR), some data points represent less biological representatives. A major problem linked to studying gene expression in conductive tissues (i.e., petioles) is the low transcript abundance of mRNA (Sakr et al., 2003).
To analyze the diurnal/circadian response of aquaporin gene expression under well-watered conditions, the expression data at each time point was compared to the expression levels predawn (6:00 h) (Table 5). Several aquaporin genes examined showed evidence of diurnal regulation of gene expression. The expression of VvPIP2;1 negatively correlated with time in the petioles and leaves of both cultivars (Figures 6A,B). VvPIP1;1petiole and VvPIP2;3petiole negatively correlated with time in Grenache only (Figure 6B). The diurnal expression pattern of VvPIP2;1 was similar for both cultivars and tissues examined (petioles and leaves) with expression highest predawn, subsequently decreasing over the day with expression levels lowest at 21:00 h (Supplementary Figures S4–S7). Expression of VvPIP2;1 was significantly decreased at all time points in Chardonnay petioles and at 16:00 h and 21:00 h in all other samples compared with predawn levels (6:00 h). The mean normalized expression of VvPIP2;1 was significantly higher in Grenache petioles than Chardonnay petioles (Supplementary Figures S4, S5).
Table 5.
Diurnal expression of aquaporin genes in Chardonnay and Grenache petioles and leaves.
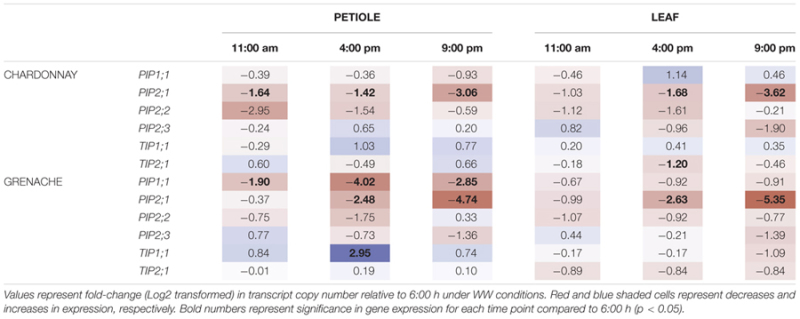 |
FIGURE 6.
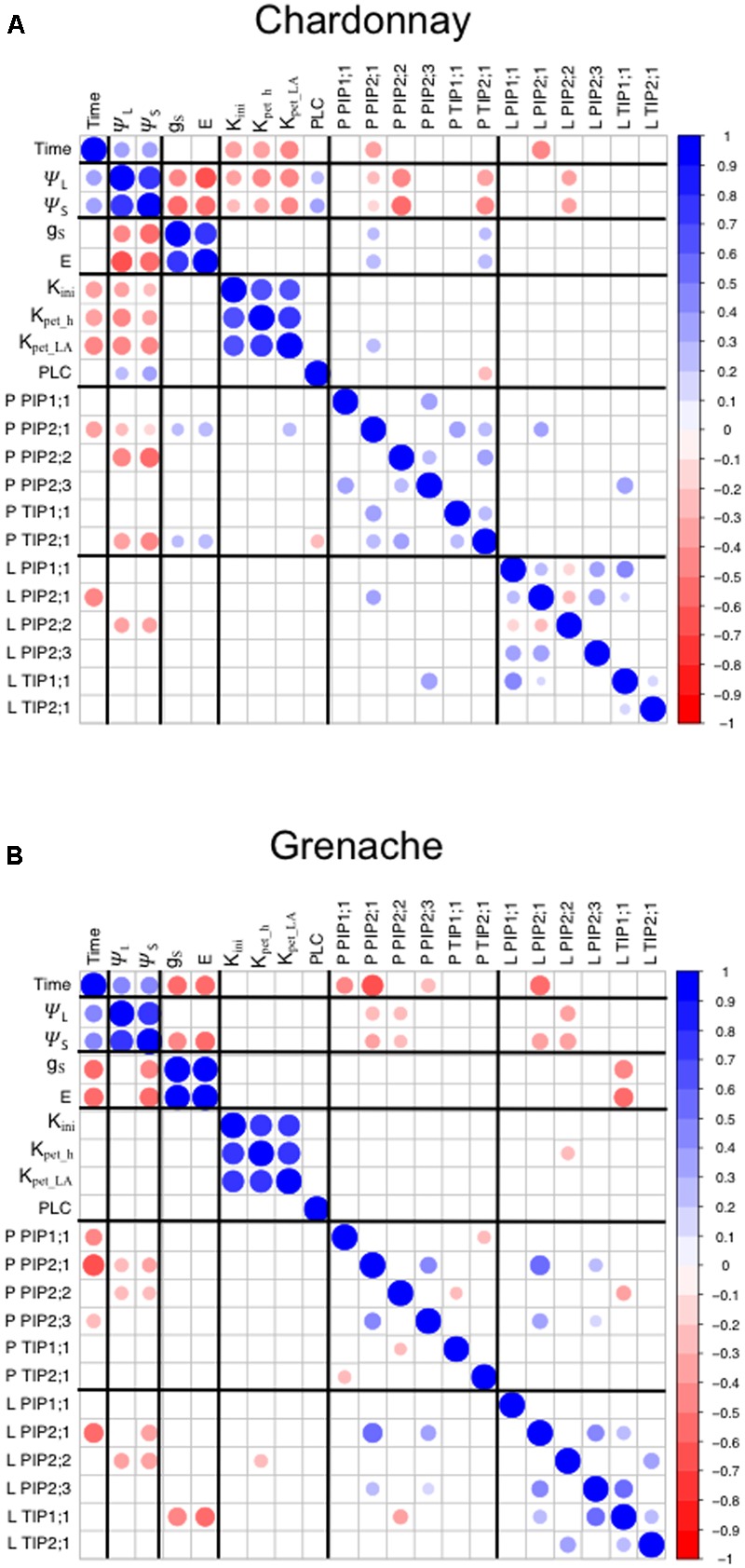
Correlation matrix of physiological parameters and aquaporin gene expression in Chardonnay (A) Grenache (B). All data was obtained from Drought Experiment 2. Leaf and stem water potentials were assumed to be the same for 6:00 h (predawn). Only significant Pearson correlation coefficients are shown. P-petiole and L-leaf for aquaporin gene expression.
Expression of VvPIP2;2 in Chardonnay and Grenache was highest predawn and decreased throughout the day, however, the response was not significant. In contrast to VvPIP2;1, expression levels of VvPIP2;2 increased at 21:00 h to values similar to predawn levels. There were no significant changes in expression of VvPIP2;3 in the petioles and leaves for either cultivar compared with 6:00 h (Table 5).
No significant diurnal response was observed in the petioles or leaves of either cultivar for VvTIP2;1 (Supplementary Figures S4–S7) although a significant decrease in expression was observed at 16:00 h in Chardonnay leaves. Expression of VvTIP1;1 significantly increased in Grenache petioles at 16:00 h compared to predawn levels but no other changes were observed. No diurnal response for VvTIP1;1 was observed in Chardonnay petioles or leaves of either cultivar.
Transcriptional Regulation of Aquaporins in Response to Water-Stress
To determine the transcriptional response of aquaporin’s to a moderate water-stress, petioles and leaves were harvested from Chardonnay and Grenache when midday ψL (11:00 h) was approximately -1.0 MPa. Chardonnay and Grenache differed in their response to water-stress, with expression of aquaporin’s in Chardonnay petioles tending to be more down-regulated than in Grenache petioles compared to well-watered expression (Table 6).
Table 6.
Aquaporin gene expression in response to water-stress treatment.
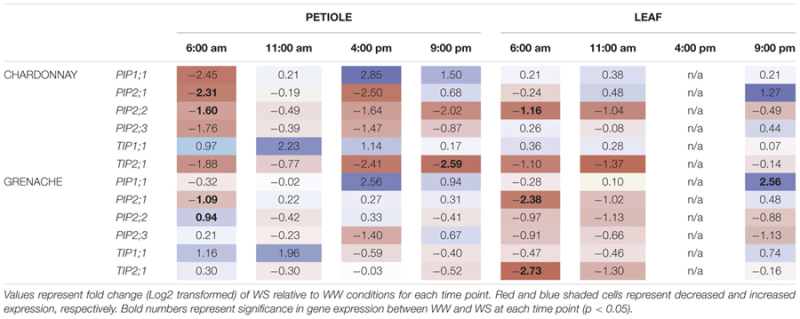 |
In response to water-stress, there was a significant down-regulation of VvPIP2;1 at 6:00 h (predawn) in both Chardonnay and Grenache petioles and in the leaves of Grenache (Table 6). Mean normalized expression was highest predawn for both cultivars and tissues under water-stress (Supplementary Figures S4–S7). Both VvPIP2;1 and VvPIP2;2 were strongly down-regulated in Chardonnay petioles but not Grenache petioles in response to water-stress. In Chardonnay, VvPIP2;2 was significantly down-regulated at 6:00 h in both petioles and leaves in response to WS, however, was upregulated at 6:00 h in Grenache petioles.
VvTIP2;1 was down-regulated in both Chardonnay and Grenache in response to water-stress, however, this was only significant at 6:00 h in Grenache leaves and 21:00 h in Chardonnay petioles. The significant down-regulation of VvPIP2;1 and VvTIP2;1 compared to well-watered plants also correlated with significant decreases in leaf water potential (Figure 7).
FIGURE 7.
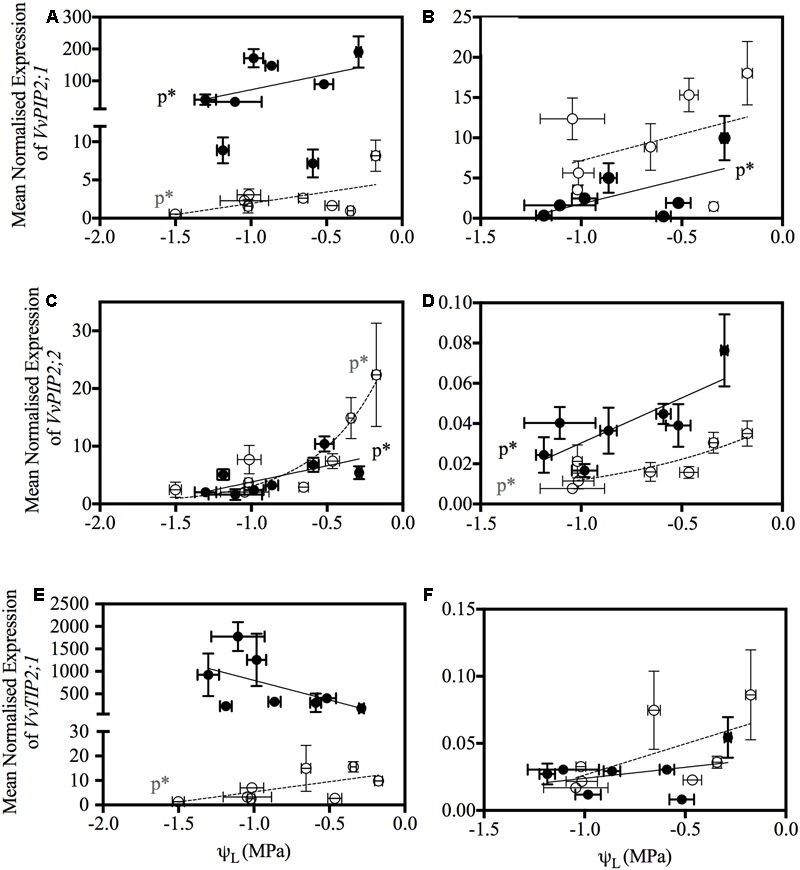
Relationship between aquaporin gene expression and leaf water potential (ψL) in Chardonnay (open gray circles, dotted line) and Grenache (filled black circles, bold line) petioles and leaves. Expression of VvPIP2;1 in petioles (A) and leaves (B), VvPIP2;2 in petioles (C) and leaves (D) and VvTIP2;2 in petioles (E) and leaves (F). Only gene expression data that had a significant Pearson correlation with ψL are shown. Significance is indicated by p∗.
Correlation of Grapevine Physiology with Aquaporin Gene Expression
A correlation analysis of the physiological data (time, ψL, ψs, gS, E, Kinit, KPet_h, KPet_LA, and PLC) with aquaporin gene expression revealed some significant results (Figure 6). In Chardonnay ψL and ψS positively correlated with time and negatively correlated with gS, E and hydraulic conductivity measurements (Kinit, KPet_h, and KPet_LA) (Figure 6A). ψL and ψS both positively correlated with PLC. VvTIP2;1petiole negatively correlated with PLC in Chardonnay. In Chardonnay, ψL and ψS were negatively correlated with aquaporin gene expression of VvPIP2;1petiole, VvPIP2;2petiole, VvTIP2;1petiole, and VvPIP2;2leaf (Figures 6A, 7). Expression of VvPIP2;1petiole and VvTIP2;1petiole positively correlated with gS and E. Hydraulic conductivity measurements (Kinit, KPet_h, and KPet_LA) correlated with time in Chardonnay but not Grenache.
In Grenache, ψL and ψS negatively correlated with aquaporin expression of VvPIP2;1petiole, VvPIP2;2petiole, and VvPIP2;2leaf (Figure 7). ψS negatively correlated with both gS and E and VvPIP2;1leaf. gS, E, and K parameters did not correlate with ψL in Grenache (Figure 6B). Expression of VvPIP2;1leaf positively correlated with the expression of VvPIP2;1petiole, VvPIP2;3petiole, VvPIP2;3leaf, and VvTIP1;1leaf (Figure 6B). gS and E correlated with time in Grenache but not Chardonnay.
Discussion
Hydraulic Properties Differ between Grapevine Cultivars
Chardonnay vines displayed hydraulic segmentation, with petioles being more susceptible to cavitation than stems (Figure 1A and Table 2). Previous studies on grapevines have shown the roots to be more vulnerable to cavitation than the shoots and this seems to be common in anisohydric species in order to protect the stem from catastrophic cavitation during drought (Lovisolo and Schubert, 2006; Lovisolo et al., 2008a). Hydraulic segmentation has been reported in woody species including Juglans regia L. (Tyree et al., 1993), Acer saccharum (Tsuda and Tyree, 1997) with highest cavitation vulnerability for these species in the petioles. Grapevines also display vulnerability segmentation with a number of studies showing stems are more resistant to water-stress induced embolism than petioles (Alsina et al., 2007; Choat et al., 2010; Zufferey et al., 2011; Charrier et al., 2016). Hydraulic vulnerability segmentation in grapevine has been demonstrated in both cv. Syrah and Cabernet Sauvignon using X-ray microcomputed tomography (Charrier et al., 2016; Hochberg et al., 2016). Higher vulnerability in petioles compared to shoots has been proposed to be a form of hydraulic segmentation leading to leaf shedding in response to drought (Tyree et al., 1993). In this study Chardonnay vines maintained midday leaf water potential close to the cavitation threshold (-1.8 MPa for stems) as has been reported for other anisohydric species (McDowell et al., 2008). This is in good agreement with other studies; NMR imaging showed dehydrated Chardonnay vines only suffered significant stem embolism ψL < -2.0 MPa, and when ψL was above -1.5 MPa the majority of vessels remained filled (Choat et al., 2010). In Cabernet Sauvignon vines imaged with X-ray microcomputed tomography, embolized vessels increased when ψL< -1.5 MPa and ψ50Stem= -1.73 and ψ50Petiole= -0.98 MPa. Grenache was more susceptible to water-stress induced cavitation as indicated by the higher ψ10 at which cavitation begins to occur, however, showed no evidence of hydraulic segmentation between the stems and petioles (Figure 1B and Table 2). In this study, under moderate water-stress, Grenache did not show any evidence of developing run-away cavitation as was observed for Chardonnay stems (Supplementary Figure S2). Grenache stems have previously been reported to be more susceptible to the formation of xylem embolism than both Syrah and Chardonnay (Schultz, 2003; Alsina et al., 2007).
It has been proposed that early cavitation events may act as a hydraulic signal for stomatal closure (Salleo et al., 2000) and involves chemical signals such as ABA that may promote embolism repair (Lovisolo et al., 2008a). The early onset of xylem embolism in Grenache stems and petioles may be responsible for triggering midday stomatal closure thus contributing to their near-isohydric behavior (Figure 2 and Table 1). Stomata also respond to transient changes in leaf water potential (Meinzer et al., 2001) that can occur as a result of cavitation, and this may influence the occurrence of stomatal patchiness (During and Loveys, 1996).
Grenache exhibited higher petiole specific hydraulic conductivity than Chardonnay and this is most likely due to the higher frequency of larger xylem vessel diameters (Figure 5) (Scholander et al., 1955; Essau, 1965; Lovisolo and Schubert, 1998). The relative measured KPet-h between cultivars (Chardonnay/Grenache = 0.71) was consistent with the relative dhyd (Chardonnay/Grenache = 0.72) and suggests that Grenache are adapted to supply a greater leaf surface area than Chardonnay. This difference was also observed in the rachis xylem comparing Grenache and Shiraz (Scharwies and Tyerman, 2017).
A close relationship between petiole hydraulic properties and vine water status (ψL and ψS) was observed in Chardonnay vines, but not in Grenache vines (Figure 3C). The decline in petiole hydraulic conductivity with increasing water-stress, may be reflective of a decline in the permeability of the water conducting pathway either through the xylem vessels or by way of the cell to cell pathway (via aquaporins). Many studies have examined the contribution of aquaporins in the cell-to-cell pathway of water movement in roots and there is substantial evidence that increases in root hydraulic conductance are correlated with aquaporin expression (Javot and Maurel, 2002; Tyerman et al., 2002; Vandeleur et al., 2009; Chaumont and Tyerman, 2014). Aquaporin’s have also been shown to contribute to leaf hydraulic conductivity in several species and alter in response to specific environmental cues (Prado and Maurel, 2013). Aquaporin’s are highly expressed in xylem parenchyma cells in several species including Arabidopsis (Prado et al., 2013), walnut (Sakr et al., 2003), and maize (Hachez et al., 2008). Expression in xylem parenchyma cells is crucial for radial water movement from xylem vessels and for the refilling of embolized xylem vessels (Secchi and Zwieniecki, 2016). VvPIP2;1 expression in the petiole of Chardonnay correlated with vine water status, gas exchange and KPet_LA and therefore may play a significant role in regulating petiole and leaf hydraulics in anisohydric cultivars. VvPIP2;1 also decreased under water deficit in field grown vines (Dayer et al., 2017). In soybean leaves, differential expression of some aquaporin genes correlated with a midday decrease in KPet_LA indicative of a potential role in regulating diurnal fluctuations in leaf water status (Locke and Ort, 2015).
Aquaporin Genes Are Diurnally Expressed in Grapevine Petioles and Leaves
Both PIP and TIP aquaporins (mRNA and protein) have been shown to be diurnally regulated, with expression generally higher in the day correlating with transpiration. Diurnal and/or circadian changes in aquaporin expression have been observed in maize and Arabidopsis roots (Lopez et al., 2003; Takase et al., 2011; Caldeira et al., 2014) and in the leaves of Nicotiana excelsior (Yamada et al., 1997), Samanea saman (Moshelion et al., 2002), and maize (Hachez et al., 2008). A diurnal mRNA expression pattern was evident in the petioles and leaves for both Chardonnay and Grenache, however, the response was cultivar, isoform and tissue dependent.
The most predominant diurnal expression pattern was for VvPIP2;1. VvPIP2;1 expression in both the leaves and petioles of Chardonnay and Grenache was strongly diurnally regulated under well-watered conditions with expression highest predawn and decreasing over the course of the day (Table 5 and Supplementary Figures S3–S6). A diurnal pattern was still evident even under water-stress conditions, however, in Grenache leaves and petioles expression was decreased compared to well-watered conditions and the peak in expression was at 11:00 h. This may reflect the near-isohydric nature of Grenache, where the transpirational demand tends to be highest in the morning. A strong diurnal regulation of PIP aquaporin’s has previously been reported in the leaves of Fragaria vesca (strawberry) under both well-watered conditions and drought conditions (Šurbanovski et al., 2013). The expression of VvPIP2;1 correlated with ψL in both Chardonnay and Grenache indicating that expression of this aquaporin isoform responds to leaf water status (Figure 7). The high expression predawn in both Chardonnay and Grenache petioles may be consistent with a role in night-time refilling of xylem vessels under well-watered conditions.
VvPIP2;2, also demonstrated an apparent diurnal regulation with expression highest at predawn and decreasing during the day in both Chardonnay and Grenache leaves and petioles, with night-time expression increasing to values close to predawn expression in all samples except Grenache leaves. VvPIP2;2 expression in the leaves and petioles was negatively correlated with plant water status in both Chardonnay and Grenache, indicating that the expression of this gene is finely tuned to both diurnal fluctuations in plant water status and in response to water-stress (Figure 7).
In contrast to the VvPIP2 genes, expression of VvTIP1;1 increased during the late afternoon in the petioles of Grenache when ψL was most negative (Table 3). This is consistent for a role of TIPs maintaining plant water status during high transpiration by facilitating water movement from the vacuoles. In Grenache leaves, VvTIP1;1 expression was negatively correlated with stomatal conductance and transpiration (Figure 6). Diurnal changes in TIP expression have previously been reported in the guard cells of sunflower and are thought to be involved in closing of stomata (Sarda et al., 1997). In Chardonnay petioles, VvTIP2;1 correlated with both leaf and stem water status, thus the transcriptionally regulation of VvTIPs is different in anisohydric/near-isohydric cultivars.
Aquaporin Gene Expression in Response to Water-Stress
The differences in aquaporin gene expression in response to water-stress in Chardonnay and Grenache is a molecular reflection of the different physiological properties of these two cultivars under water-stress. Changes in aquaporin gene expression in plants has been previously reported in response to abiotic stresses including drought (Yamaguchi-Shinozaki et al., 1992; Yamada et al., 1995; Mariaux et al., 1998; Barrieu et al., 1999; Sarda et al., 1999; Secchi et al., 2007, 2016; Vandeleur et al., 2009; Pou et al., 2013), salt stress (Suga et al., 2002), light (Cochard et al., 2007), cold stress (Li et al., 2000), and diurnal fluctuations (Lopez et al., 2003). The response of aquaporins to water-stress is species and isoform dependent, and in grapevine, also cultivar dependent. Previous studies have demonstrated that grapevine cultivars can either exhibit isohydric or anisohydric responses and this may be linked to aquaporin expression (Vandeleur et al., 2009).
Expression of VvPIP2;1 and VvPIP2;2 in Chardonnay petioles, showed a strong, rapid down-regulation in response to water-stress (decrease in ψL) compared with Grenache petioles (Table 6 and Figures 7A,B). Refilling of embolized vessels has been observed in Chardonnay stems under non-transpiring conditions (Brodersen et al., 2010); however, the strong down-regulation of aquaporins under water-stress in this study indicates refilling is unlikely in Chardonnay, at least under transpiring conditions. VvPIP2;1 has previously been shown to be expressed in VACs of embolized and recovering petioles in Grenache supporting the hypothesis that aquaporins play a major role in xylem refilling (Chitarra et al., 2014). Interestingly, a number of PIP and TIP genes showed increased expression predawn (VvPIP2;2 was significant) in response to water-stress in Grenache petioles perhaps indicating a potential role in night-time refilling in this cultivar (Secchi et al., 2007). Night-time refilling in Grenache may prevent the onset of run-away cavitation as water-stress increases in severity. VvPIP2;1 and VvPIP2;2 share high homology with the walnut aquaporins, JrPIP2;1 and JrPIP2;2, both postulated to be involved in embolism refilling and expressed in the xylem parenchyma cells of walnut (Sakr et al., 2003), thus these may be a good candidates for modulating refilling in grapevine petioles. In V. labrusca L. cv. Concord (fox grape), embolized vessels formed in the stem while the plant was actively transpiring and under considerable water-stress (Holbrook et al., 2001). Refilling of these vessels occurred only when combined with an increase in leaf water potential and a cessation of sap flow. Other authors have proposed that refilling of embolized vessels can occur when transpiration rates are high (McCully et al., 1998; McCully, 1999). Refilling of grapevine vessels has been shown in Chardonnay at a moderate water-stress under non-transpiring conditions and is believed to be dependent on water movement from the living xylem parenchyma cells into the xylem vessels (Brodersen et al., 2010). Given the physiological and molecular differences observed between Chardonnay and Grenache it is possible that different refilling strategies exist between these cultivars.
In leaves, the aquaporin expression profile differed to petioles, with Grenache exhibiting greater down-regulation of aquaporin genes in response to water-stress than Chardonnay. In Chardonnay leaves, the expression of aquaporin genes was isoform specific, with VvPIP2;2 and VvTIP2;1 decreasing expression relative to well-watered (similar to Grenache) yet other genes only showed small insignificant increases/decreases in expression. Interestingly, VvPIP1;1 that is thought to be involved in regulation of other aquaporins, was upregulated in petioles and leaves in response to water-stress in both cultivars. This is most likely a reflection of the different tissue types, with petioles primarily behaving as water conduits to the photosynthetic machinery in the leaves. Increased aquaporin expression could be linked to photosynthesis (Hachez et al., 2008) and/or embolism repair (Holbrook et al., 2001). The regulation of aquaporin gene expression in Grenache leaves appears to be related to the near-isohydric behavior and conservative water use strategy, whereas the response of aquaporin’s in Chardonnay leaves is less dramatic, and may reflect the anisohydric behavior of this cultivar.
The overexpression of SoTIP2;2 in tomato has been suggested to be involved in regulating anisohydric behavior in response to drought stress by maintaining vacuolar water permeability and thus osmotic buffering in response to abiotic stress conditions (Sade et al., 2009). Overexpression of VvPIP2;4N in the anisohydric cultivar, Brachetto, resulted in greater leaf capacitance compared to wildtype, indicating that aquaporins’ may be involved in the control of hydraulic capacitance in grapevine (Vitali et al., 2016). VvTIP2;1 expression in Chardonnay petioles correlates with both gas exchange and water status and thus may help regulate vacuolar water permeability in response to water-stress conditions in anisohydric grapevine cultivars, perhaps through control of hydraulic capacitance. A link between aquaporins’ and hydraulic capacitance needs to be further evaluated in iso/anisohydric cultivars.
Conclusion
In summary, the data show that there were two contrasting responses in petiole hydraulics and aquaporin expression between the near-isohydric cultivar Grenache and anisohydric cultivar Chardonnay. We have shown that Grenache (near-isohydric variety) was more susceptible to the onset of xylem embolism in both the petioles and stems than Chardonnay (anisohydric), most likely linked to larger xylem vessels. It is apparent that Grenache employs a molecular and physiological strategy to conserve cellular water balance. To further understand the role of aquaporin’s in isohydric/anisohydric response to water-stress, further investigation into both the transcriptional and post-translational regulation of aquaporin’s in specific cell types (i.e., xylem parenchyma cells) needs to be investigated. The different water use strategy of these two cultivars, Chardonnay and Grenache needs to be accounted for in irrigation management. Furthermore, the petiole and leaf signature of expressed aquaporin’s may be used in combination with other petiole assessments in screening different genotypes for differences in isohydric/anisohydric behavior.
Author Contributions
MS, BK, and ST conceived and designed the experiments. MS, RV, and ST conducted the experiments and analyzed the data. MS and ST wrote the manuscript and all authors contributed to editing the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Some experiments described in this manuscript were conducted during MS’s Ph.D. candidature at the University of Adelaide and thus some of the data has been described previously (Shelden, 2008). The Ph.D. thesis represents the only medium this research has appeared in and this publication is in line with the author’s university policy. We thank Wendy Sullivan for expert technical assistance and Kara Levin for help with RNA extractions and QPCR.
Funding. This research was supported by the Grape and Wine Research and Development Corporation and the Australian Research Council (ARC) Centre of Excellence in Plant Energy Biology (CE140100008). The ARC and University of Adelaide have supported this research by funding Dr. MS (DE140100575).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2017.01893/full#supplementary-material
References
- Alsina M. M., De Herralde F., Aranda X., Save R., Biel C. (2007). Water relations and vulnerability to embolism are not related: experiments with eight grapevine cultivars. Vitis 46 1–6. [Google Scholar]
- Barrieu F., Marty-Mazars D., Thomas D., Chaumont F., Charbonnier M., Marty F. (1999). Desiccation and osmotic stress increase the abundance of mRNA of the tonoplast aquaporin BOBtip26-1 in cauliflower cells. Planta 209 77–86. 10.1007/s004250050608 [DOI] [PubMed] [Google Scholar]
- Begg J. E., Turner N. C. (1970). Water potential gradients in field tobacco. Plant Physiol. 46 343–346. 10.1104/pp.46.2.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen C. R., McElrone A. J. (2013). Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Front. Plant Sci. 4:108. 10.3389/fpls.2013.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen C. R., McElrone A. J., Choat B., Matthews M. A., Shackel K. A. (2010). The dynamics of embolism repair in xylem: In vivo visualizations using high-resolution computed tomography. Plant Physiol. 154 1088–1095. 10.1104/pp.110.162396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira C. F., Jeanguenin L., Chaumont F., Tardieu F. (2014). Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nat. Commun. 5:5365. 10.1038/ncomms6365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. S., McInnes K. J. (1999). Response of in situ leaf psychrometer to cuticle removal by abrasion. Agron. J. 91 859–862. 10.2134/agronj1999.915859x [DOI] [Google Scholar]
- Charrier G., Torres-Ruiz J. M., Badel E., Burlett R., Choat B., Cochard H., et al. (2016). Evidence for hydraulic vulnerability segmentation and lack of xylem refilling under tension. Plant Physiol. 172 1657–1668. 10.1104/pp.16.01079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F., Tyerman S. D. (2014). Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol. 164 1600–1618. 10.1104/pp.113.233791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitarra W., Balestrini R., Vitali M., Pagliarani C., Perrone I., Schubert A., et al. (2014). Gene expression in vessel-associated cells upon xylem embolism repair in Vitis vinifera L. Petioles. Planta 239 887–899. 10.1007/s00425-013-2017-7 [DOI] [PubMed] [Google Scholar]
- Choat B., Drayton W. M., Brodersen C., Matthews M. A., Shackel K. A., Wada H., et al. (2010). Measurement of vulnerability to water stress-induced cavitation in grapevine: a comparison of four techniques applied to a long-vesseled species. Plant Cell Environ. 33 1502–1512. 10.1111/j.1365-3040.2010.02160.x [DOI] [PubMed] [Google Scholar]
- Cochard H., Badel E., Herbette S., Delzon S., Choat B., Jansen S. (2013). Methods for measuring plant vulnerability to cavitation: a critical review. J. Exp. Bot. 64 4779–4791. 10.1093/jxb/ert193 [DOI] [PubMed] [Google Scholar]
- Cochard H., Delzon S., Badel E. (2015). X-ray microtomography (micro-CT): a reference technology for high-resolution quantification of xylem embolism in trees. Plant Cell Environ. 38 201–206. 10.1111/pce.12391 [DOI] [PubMed] [Google Scholar]
- Cochard H., Venisse J. S., Barigah T. S., Brunel N., Herbette S., Guilliot A., et al. (2007). Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol. 143 122–133. 10.1104/pp.106.090092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer S., Peña J. P., Gindro K., Torregrosa L., Voinesco F., Martínez L., et al. (2017). Changes in leaf stomatal conductance, petiole hydraulics and vessel morphology in grapevine Vitis vinifera cv. Chasselas) under different light and irrigation regimes. Funct. Plant Biol. 44 679–693. 10.1071/FP16041 [DOI] [PubMed] [Google Scholar]
- Deluc L., Barrieu F., Marchive C., Lauvergeat V., Decendit A., Richard T., et al. (2006). Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 140 499–511. 10.1104/pp.105.067231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc L., Bogs J., Walker A. R., Ferrier T., Decendit A., Merillon J. M., et al. (2008). The transcription factor vvmyb5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 147 2041–2053. 10.1104/pp.108.118919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- During H., Loveys B. R. (1996). Stomatal patchiness of field-grown sultana leaves: diurnal changes and light effects. Vitis 35 7–10. [Google Scholar]
- Essau K. (1965). Plant Anatomy. New York, NY: Wiley. [Google Scholar]
- Fouquet R., Leon C., Ollat N., Barrieu F. (2008). Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep. 27 1541–1550. 10.1007/s00299-008-0566-1 [DOI] [PubMed] [Google Scholar]
- Gerzon E., Biton I., Yaniv Y., Zemach H., Netzer Y., Schwartz A., et al. (2015). Grapevine anatomy as a possible determinant of isohydric or anisohydric behavior. Am. J. Enol. Vitic. 66 340–347. 10.5344/ajev.2015.14090 [DOI] [Google Scholar]
- Hachez C., Heinen R. B., Draye X., Chaumont F. (2008). The expression pattern of plasma membrane aquaporins in maize leaf highlights their role in hydraulic regulation. Plant Mol. Biol. 68 337–353. 10.1007/s11103-008-9373-x [DOI] [PubMed] [Google Scholar]
- Hochberg U., Albuquerque C., Rachmilevitch S., Cochard H., David-Schwartz R., Brodersen C. R., et al. (2016). Grapevine petioles are more sensitive to drought induced embolism than stems: evidence from In vivo MRI and microcomputed tomography observations of hydraulic vulnerability segmentation. Plant Cell Environ. 39 1886–1894. 10.1111/pce.12688 [DOI] [PubMed] [Google Scholar]
- Holbrook N. M., Ahrens E. T., Burns M. J., Zwieniecki M. A. (2001). In vivo observation of cavitation and embolism repair using magnetic resonance imaging. Plant Physiol. 126 27–31. 10.1104/pp.126.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. M., Zwieniecki M. A. (1999). Embolism repair and xylem tension: do we need a miracle? Plant Physiol. 120 7–10. 10.1104/pp.120.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Ohtsu M. (1992). Detection of xylem cavitation in field-grown pine trees using the acoustic-emission technique. Ecol. Res. 7 391–395. 10.1007/BF02347106 [DOI] [Google Scholar]
- Jaillon O., Aury J. M., Noel B., Policriti A., Clepet C., Casagrande A., et al. (2007). The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449 463–U465. 10.1038/nature06148 [DOI] [PubMed] [Google Scholar]
- Javot H., Maurel C. V. (2002). The role of aquaporins in root water uptake. Ann. Bot. 90 301–313. 10.1093/aob/mcf199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. M., Meinzer F. C., Woodruff D. R., Mcculloh K. A. (2009). Leaf xylem embolism, detected acoustically and by cryo-SEM, corresponds to decreases in leaf hydraulic conductance in four evergreen species. Plant Cell Environ. 32 828–836. 10.1111/j.1365-3040.2009.01961.x [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R., Ribas-Carbo M., Flexas J., Lovisolo C., Heckwolf M., Uehlein N. (2008). Aquaporins and plant water balance. Plant Cell Environ. 31 658–666. 10.1111/j.1365-3040.2008.01792.x [DOI] [PubMed] [Google Scholar]
- Keller M. (2005). Deficit irrigation and vine mineral nutrition. Am. J. Enol. Vitic. 56 267–283. [Google Scholar]
- Kikuta S. B., Hietz P., Richter H. (2003). Vulnerability curves from conifer sapwood sections exposed over solutions with known water potentials. J. Exp. Bot. 54 2149–2155. 10.1093/jxb/erg216 [DOI] [PubMed] [Google Scholar]
- Kikuta S. B., Logullo M. A., Nardini A., Richter H., Salleo S. (1997). Ultrasound acoustic emissions from dehydrating leaves of deciduous and evergreen trees. Plant Cell Environ. 20 1381–1390. 10.1046/j.1365-3040.1997.d01-34.x [DOI] [Google Scholar]
- Lavoie-Lamoureux A., Sacco D., Risse P. A., Lovisolo C. (2017). Factors influencing stomatal conductance in response to water availability in grapevine: a meta-analysis. Physiol. Plant 159 468–482. 10.1111/ppl.12530 [DOI] [PubMed] [Google Scholar]
- Li L. G., Li S. F., Tao Y., Kitagawa Y. (2000). Molecular cloning of a novel water channel from rice: its products expression in xenopus oocytes and involvement in chilling tolerance. Plant Sci. 154 43–51. 10.1016/S0168-9452(99)00269-1 [DOI] [PubMed] [Google Scholar]
- Locke A. M., Ort D. R. (2015). Diurnal depression in leaf hydraulic conductance at ambient and elevated [co2] reveals anisohydric water management in field-grown soybean and possible involvement of aquaporins. Environ. Exp. Bot. 116 39–46. 10.1016/j.envexpbot.2015.03.006 [DOI] [Google Scholar]
- Lopez M., Bousser A. S., Sissoeff I., Gaspar M., Lachaise B., Hoarau J., et al. (2003). Diurnal regulation of water transport and aquaporin gene expression in maize roots: contribution of PIP2 proteins. Plant Cell Physiol. 44 1384–1395. 10.1093/pcp/pcg168 [DOI] [PubMed] [Google Scholar]
- Lovisolo C., Perrone I., Carra A., Ferrandino A., Flexas J., Medrano H., et al. (2010). Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update. Funct. Plant Biol. 37 98–116. 10.1071/fp09191 [DOI] [Google Scholar]
- Lovisolo C., Perrone I., Hartung W., Schubert A. (2008a). An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytol. 180 642–651. 10.1111/j.1469-8137.2008.02592.x [DOI] [PubMed] [Google Scholar]
- Lovisolo C., Schubert A. (1998). Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. J. Exp. Bot. 49 693–700. [Google Scholar]
- Lovisolo C., Schubert A. (2006). Mercury hinders recovery of shoot hydraulic conductivity during grapevine rehydration: evidence from a whole-plant approach. New Phytol. 172 469–478. 10.1111/j.1469-8137.2006.01852.x [DOI] [PubMed] [Google Scholar]
- Lovisolo C., Tramontini S., Flexas J., Schubert A. (2008b). Mercurial inhibition of root hydraulic conductance in Vitis spp. Rootstocks under water stress. Environ. Exp. Bot. 63 178–182. 10.1016/j.envexpbot.2007.11.005 [DOI] [Google Scholar]
- Mariaux J. B., Bockel C., Salamini F., Bartels D. (1998). Desiccation- and abscisic acid-responsive genes encoding major intrinsic proteins (MIPs) from the resurrection plant Craterostigma plantagineum. Plant Mol. Biol. 38 1089–1099. 10.1023/A:1006013130681 [DOI] [PubMed] [Google Scholar]
- Martinez E. M., Cancela J. J., Cuesta T. S., Neira X. X. (2011). Review. Use of psychrometers in field measurements of plant material: accuracy and handling difficulties. Spanish J. Agric. Res. 9 313–328. 10.5424/sjar/20110901-295-10 [DOI] [Google Scholar]
- Martínez-Vilalta J., Garcia-Forner N. (2016). Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant Cell Environ. 40 962–976. 10.1111/pce.12846 [DOI] [PubMed] [Google Scholar]
- McCully M. E. (1999). Root xylem embolisms and refilling. Relation to water potentials of soil, roots, and leaves, and osmotic potentials of root xylem sap. Plant Physiol. 119 1001–1008. 10.1104/pp.119.3.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully M. E., Huang C. X., Ling L. E. C. (1998). Daily embolism and refilling of xylem vessels in the roots of field-grown maize. New Phytol. 138 327–342. 10.1046/j.1469-8137.1998.00101.x [DOI] [PubMed] [Google Scholar]
- McDowell N., Pockman W. T., Allen C. D., Breshears D. D., Cobb N., Kolb T., et al. (2008). Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 178 719–739. 10.1111/j.1469-8137.2008.02436.x [DOI] [PubMed] [Google Scholar]
- Meinzer F. C., Clearwater M. J., Goldstein G. (2001). Water transport in trees: current perspectives, new insights and some controversies. Environ. Exp. Bot. 45 239–262. 10.1016/S0098-8472(01)00074-0 [DOI] [PubMed] [Google Scholar]
- Milburn J. A., Johnson R. P. C. (1966). The conduction of sap. Ii. Detection of vibrations produced by sap cavitation in Ricinus xylem. Planta 66:43052. 10.1007/BF00380209 [DOI] [PubMed] [Google Scholar]
- Moshelion M., Becker D., Biela A., Uehlein N., Hedrich R., Otto B., et al. (2002). Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell 14 727–739. 10.1105/tpc.010351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Brown R. E. (2006). Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformat. 7:123. 10.1186/1471-2105-7-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A., Salleo S., Jansen S. (2011). More than just a vulnerable pipeline: xylem physiology in the light of ion-mediated regulation of plant water transport. J. Exp. Bot. 62 4701–4718. 10.1093/jxb/err208 [DOI] [PubMed] [Google Scholar]
- Nardini A., Tyree M. T., Salleo S. (2001). Xylem cavitation in the leaf of prunus laurocerasus and its impact on leaf hydraulics. Plant Physiol. 125 1700–1709. 10.1104/pp.125.4.1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone I., Gambino G., Chitarra W., Vitali M., Pagliarani C., Riccomagno N., et al. (2012). The grapevine root-specific aquaporin VvPIP2;4N controls root hydraulic conductance and leaf gas exchange under well-watered conditions but not under water stress. Plant Physiol. 160 965–977. 10.1104/pp.112.203455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou A., Medrano H., Flexas J., Tyerman S. D. (2013). A putative role for tip and pip aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ. 36 828–843. 10.1111/pce.12019 [DOI] [PubMed] [Google Scholar]
- Prado K., Boursiac Y., Tournaire-Roux C., Monneuse J. M., Postaire O., Da Ines O., et al. (2013). Regulation of Arabidopsis leaf hydraulics involves light-dependent phosphorylation of aquaporins in veins. Plant Cell 25 1029–1039. 10.1105/tpc.112.108456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado K., Maurel C. (2013). Regulation of leaf hydraulics: from molecular to whole plant levels. Front. Plant Sci. 4:255. 10.3389/fpls.2013.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogiers S. Y., Greer D. H., Hutton R. J., Landsberg J. J. (2009). Does night-time transpiration contribute to anisohydric behaviour in a Vitis vinifera cultivar? J. Exp. Bot. 60 3751–3763. 10.1093/jxb/erp217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade N., Vinocur B. J., Diber A., Shatil A., Ronen G., Nissan H., et al. (2009). Improving plant stress tolerance and yield production: is the tonoplast aquaporin SLTIP2;2 a key to isohydric to anisohydric conversion? New Phytol. 181 651–661. 10.1111/j.1469-8137.2008.02689.x [DOI] [PubMed] [Google Scholar]
- Sakr S., Alves G., Morillon R. L., Maurel K., Decourteix M., Guilliot A., et al. (2003). Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiol. 133 630–641. 10.1104/pp.103.027797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S., Logullo M. A., Depaoli D., Zippo M. (1996). Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytol. 132 47–56. 10.1111/j.1469-8137.1996.tb04507.x [DOI] [PubMed] [Google Scholar]
- Salleo S., Nardini A., Pitt F., Lo Gullo M. A. (2000). Xylem cavitation and hydraulic control of stomatal conductance in laurel (Laurus nobilis L.). Plant Cell Environ. 23 71–79. 10.1046/j.1365-3040.2000.00516.x [DOI] [Google Scholar]
- Sarda X., Tousch D., Ferrare K., Cellier F., Alcon C., Dupuis J. M., et al. (1999). Characterization of closely related delta-tip genes encoding aquaporins which are differentially expressed in sunflower roots upon water deprivation through exposure to air. Plant Mol. Biol. 40 179–191. 10.1023/A:1026488605778 [DOI] [PubMed] [Google Scholar]
- Sarda X., Tousch D., Ferrare K., Legrand E., Dupuis J. M., Casse-Delbart F., et al. (1997). Two tip-like genes encoding aquaporins are expressed in sunflower guard cells. Plant J. 12 1103–1111. 10.1046/j.1365-313X.1997.12051103.x [DOI] [PubMed] [Google Scholar]
- Scharwies J. D., Tyerman S. D. (2017). Comparison of isohydric and anisohydric Vitis vinifera L. cultivars reveals a fine balance between hydraulic resistances, driving forces and transpiration in ripening berries. Funct. Plant Biol. 44 324–338. 10.1071/FP16010 [DOI] [PubMed] [Google Scholar]
- Scholander P. F., Lowe W. E., Kanwisher J. W. (1955). The rise of sap in tall grapevines. Plant Physiol. 30 94–104. 10.1104/pp.30.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz H. R. (2003). Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. Cultivars during drought. Plant Cell Environ. 26 1393–1405. 10.1046/j.1365-3040.2003.01064.x [DOI] [Google Scholar]
- Secchi F., Lovisolo C., Schubert A. (2007). Expression of OePIP2.1 aquaporin gene and water relations of Olea europaea twigs during drought stress and recovery. Ann. Appl. Biol. 150 163–167. 10.1111/j.1744-7348.2007.00118.x [DOI] [Google Scholar]
- Secchi F., Pagliarani C., Zwieniecki M. A. (2016). The functional role of xylem parenchyma cells and aquaporins during recovery from severe water stress. Plant Cell Environ. 40 858–871. 10.1111/pce.12831 [DOI] [PubMed] [Google Scholar]
- Secchi F., Zwieniecki M. A. (2010). Patterns of pip gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the pip1 aquaporin subfamily as moderators of refilling process. Plant Cell Environ. 33 1285–1297. 10.1111/j.1365-3040.2010.02147.x [DOI] [PubMed] [Google Scholar]
- Secchi F., Zwieniecki M. A. (2016). Accumulation of sugars in the xylem apoplast observed under water stress conditions is controlled by xylem ph. Plant Cell Environ. 39 2350–2360. 10.1111/pce.12767 [DOI] [PubMed] [Google Scholar]
- Shelden M. C. (2008). A Comparison of Water Stress-Induced Xylem Embolism in Two Grapevine Cultivars, Chardonnay and Grenache, and the Role of Aquaporins. Ph.D. thesis, University of Adelaide; Adelaide, SA. [Google Scholar]
- Shelden M. C., Howitt S. M., Kaiser B. N., Tyerman S. D. (2009). Identification and functional characterisation of aquaporins in the grapevine, Vitis vinifera. Funct. Plant Biol. 36 1065–1078. 10.1071/fp09117 [DOI] [PubMed] [Google Scholar]
- Soar C. J., Speirs J., Maffei S. M., Penrose A. B., Mccarthy M. G., Loveys B. R. (2006). Grape vine varieties shiraz and grenache differ in their stomatal response to VPD: Apparent links with ABA physiology and gene expression in leaf tissue. Austr. J. Grape Wine Res. 12 2–12. 10.1111/j.1755-0238.2006.tb00038.x [DOI] [Google Scholar]
- Sperry J. S., Nichols K. L., Sullivan J. E. M., Eastlack S. E. (1994). Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern utah and interior alaska. Ecology 75 1736–1752. 10.2307/1939633 [DOI] [Google Scholar]
- Suga S., Komatsu S., Maeshima M. (2002). Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiol. 43 1229–1237. 10.1093/pcp/pcf148 [DOI] [PubMed] [Google Scholar]
- Šurbanovski N., Sargent D. J., Else M. A., Simpson D. W., Zhang H., Grant O. M. (2013). Expression of Fragaria vesca PIP aquaporins in response to drought stress: PIP down-regulation correlates with the decline in substrate moisture content. PLOS ONE 8:e74945. 10.1371/journal.pone.0074945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase T., Ishikawa H., Murakami H., Kikuchi J., Sato-Nara K., Suzuki H. (2011). The circadian clock modulates water dynamics and aquaporin expression in Arabidopsis roots. Plant Cell Physiol. 52 373–383. 10.1093/pcp/pcq198 [DOI] [PubMed] [Google Scholar]
- Tsuda M., Tyree M. T. (1997). Whole-plant hydraulic resistance and vulnerability segmentation in Acer Saccharinum. Tree Physiol. 17 351–357. 10.1093/treephys/17.6.351 [DOI] [PubMed] [Google Scholar]
- Tyerman S. D., Niemietz C. M., Bramley H. (2002). Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ. 25 173–194. 10.1046/j.0016-8025.2001.00791.x [DOI] [PubMed] [Google Scholar]
- Tyree M. T., Cochard H., Cruiziat P., Sinclair B., Ameglio T. (1993). Drought-induced leaf shedding in walnut - evidence for vulnerability segmentation. Plant Cell Environ. 16 879–882. 10.1111/j.1365-3040.1993.tb00511.x [DOI] [Google Scholar]
- Tyree M. T., Davis S. D., Cochard H. (1994). Biophysical perspectives of xylem evolution - is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction. Iawa J. 15 335–360. 10.1163/22941932-90001369 [DOI] [Google Scholar]
- Tyree M. T., Dixon M. A. (1983). Cavitation events in Thuja occidentalis L - utrasonic acoustic emissions from the sapwood can be measured. Plant Physiol. 72 1094–1099. 10.1104/pp.72.4.1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T., Dixon M. A., Tyree E. L., Johnson R. (1984). Ultrasonic acoustic emissions from the sapwood of cedar and hemlock - an examination of 3 hypotheses regarding cavitations. Plant Physiol. 75 988–992. 10.1104/pp.75.4.988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T., Salleo S., Nardini A., Lo Gullo M. A., Mosca R. (1999). Refilling of embolized vessels in young stems of laurel. Do we need a new paradigm? Plant Physiol. 120 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T., Sperry J. S. (1989). Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40 19–38. 10.1146/annurev.pp.40.060189.000315 [DOI] [Google Scholar]
- Vandeleur R. K., Mayo G., Shelden M. C., Gilliham M., Kaiser B. N., Tyerman S. D. (2009). The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 149 445–460. 10.1104/pp.108.128645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:Research0034. 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco R., Zharkikh A., Troggio M., Cartwright D., Cestaro A., Pruss D., et al. (2007). A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLOS ONE 2:e1326. 10.1371/journal.pone.0001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali M., Cochard H., Gambino G., Ponomarenko A., Perrone I., Lovisolo C. (2016). VvPIP2;4N aquaporin involvement in controlling leaf hydraulic capacitance and resistance in grapevine. Physiol. Plant. 158 284–296. 10.1111/ppl.12463 [DOI] [PubMed] [Google Scholar]
- Yamada S., Katsuhara M., Kelly W. B., Michalowski C. B., Bohnert H. J. (1995). A family of transcripts encoding water channel proteins - tissue-specific expression in the common ice plant. Plant Cell 7 1129–1142. 10.1105/tpc.7.8.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Komori T., Myers P. N., Kuwata S., Kubo T., Imaseki H. (1997). Expression of plasma membrane water channel genes under water stress in Nicotiana excelsior. Plant Cell Physiol. 38 1226–1231. 10.1093/oxfordjournals.pcp.a029109 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Koizumi M., Urao S., Shinozaki K. (1992). Molecular cloning and characterisation of 9 cDNAs for genes that are responsive to dessication in Arabidopsis thaliana: sequence analysis of one cDNA clone that encodes a putative transmembrane channel protein. Cell Physiol. 33 217–224. 10.1093/oxfordjournals.pcp.a078243 [DOI] [Google Scholar]
- Zufferey V., Cochard H., Ameglio T., Spring J. L., Viret O. (2011). Diurnal cycles of embolism formation and repair in petioles of grapevine (Vitis vinifera cv. Chasselas). J. Exp. Bot. 62 3885–3894. 10.1093/jxb/err081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki M. A., Holbrook N. M. (2009). Confronting maxwell’s demon: biophysics of xylem embolism repair. Trends Plant Sci. 14 530–534. 10.1016/j.tplants.2009.07.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


