Abstract
Purpose of review
An important challenge in neurology is identifying the neural mechanisms underlying behavioral deficits after brain injury. Here, we review recent advances in understanding the effects of focal brain lesions on brain networks and behavior.
Recent findings
Neuroimaging studies indicate that the human brain is organized in large-scale resting state networks (RSNs) defined via functional connectivity, that is the temporal correlation of spontaneous activity between different areas. Prior studies showed that focal brain lesion induced behaviorally relevant changes of functional connectivity beyond the site of damage. Recent work indicates that across domains, functional connectivity changes largely conform to two patterns: a reduction in interhemispheric functional connectivity and an increase in intrahemispheric functional connectivity between networks that are normally anticorrelated, for example dorsal attention and default networks. Abnormal functional connectivity can exhibit a high degree of behavioral specificity such that deficits in a given behavioral domain are selectively related to functional connectivity of the corresponding RSN, but some functional connectivity changes allow prediction across domains. Finally, as behavioral recovery proceeds, the prestroke pattern of functional connectivity is restored.
Summary
Investigating changes in RSNs may shed light on the neural mechanisms underlying brain dysfunction after stroke. Therefore, resting state functional connectivity may represent an important tool for clinical diagnosis, tracking recovery and rehabilitation.
Keywords: recovery, resting state functional connectivity, resting state network, stroke
INTRODUCTION
A fundamental challenge in neurology is to understand the effects of focal lesions on brain activity and subsequent behavioral impairments. Traditionally, anatomical damage of brain modules was thought to affect corresponding cognitive functions, leading to behavioral impairments. However, since the seminal paper of von Monakow on diaschisis [1], it has been shown that stroke also induces changes in function and physiology of brain regions distant from the site of anatomical damage (see a recent review by Carrera and Tononi [2]). A promising way to understand the widespread functional changes induced by stroke is to study the functional organization of brain networks. Measurements of the temporal correlation of blood-oxygenated-level-dependent (BOLD) signals between different brain regions in the absence of a task [3,4], that is resting-state functional connectivity, can be used to identify such networks, called resting state networks (RSNs). RSNs are associated with specific functions (e.g. vision, attention, motor) and their topography recapitulates that of networks activated during tasks [5–10]. Notably, a growing number of studies indicates that stroke induces changes of functional connectivity within and between RSNs [11–13] and that these changes correlate with behavioral impairment at acute [11,14,15] and chronic [11,16▪] phases (see Varsou et al. [17], for a review). Here, we review the latest reports showing behaviorally relevant stroke-induced changes of functional connectivity at acute and chronic phases after stroke and after rehabilitation.
BEHAVIORALLY RELEVANT CHANGES OF RESTING STATE FUNCTIONAL CONNECTIVITY AFTER STROKE AT ACUTE STAGE
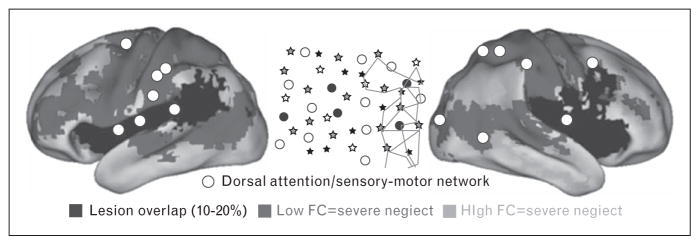
In 2007, He et al. [11] demonstrated that the severity of spatial neglect, that is a deficit in processing stimuli on the body and in the space contralateral to the lesion, was correlated with the degree of reduction of the interhemispheric functional connectivity of regions of the so-called ‘dorsal attention network’ (DAN), which are involved in the control of visuo-spatial attention [18]. Similar results were obtained in another small sample study in 2010 [14]. Baldassarre et al. reexamined the relationship between neglect and functional connectivity across several RSNs in a large and heterogeneous (i.e. not only neglect) group of first ever stroke patients [15]. They observed that the severity of spatial and nonspatial deficits in neglect patients was associated with two correlated patterns of abnormal functional connectivity involving dorsal attention/sensory motor networks: a bilateral reduction of interhemispheric functional connectivity within these networks; an increase of functional connectivity between these networks and fronto-parietal/default mode networks in the right hemisphere, which normally are anticorrelated (Fig. 1). Therefore, neglect was related to the functional connectivity within and between (i.e. interaction) multiple RSNs. Siegel et al. [19▪] subsequently showed that these two patterns of behaviorally relevant functional connectivity are observed across many behavioral domains such as attention, language, motor function, verbal memory, and spatial memory.
FIGURE 1.
Patterns of resting state functional connectivity associated to spatial neglect at acute stage. The white circles indicate the regions of interest (ROIs) belonging to dorsal attention/sensory motor networks, whose functional connectivity is associated to the degree of the rightward spatial biases in attention typical of neglect patients (schematically illustrated in central inset, in a large sample of stroke patients (n = 84). Spatial neglect is associated with two abnormal patterns of functional connectivity derived from the white ROIs: reduced interhemispheric functional connectivity with regions of dorsal attention/sensory motor networks in the opposite hemisphere (blue voxels); increased functional connectivity (reduced segregation) with regions of default mode/fronto-parietal networks (orange voxels). Note that dorsal attention/sensory motor and default mode/fronto-parietal networks are typically anticorrelated in healthy individuals, hence, the increased functional connectivity should be read as reduction of ‘segregation’ between two sets of networks. Black color indicates voxels damaged in 10–20% of patients. Reproduced with permission [15].
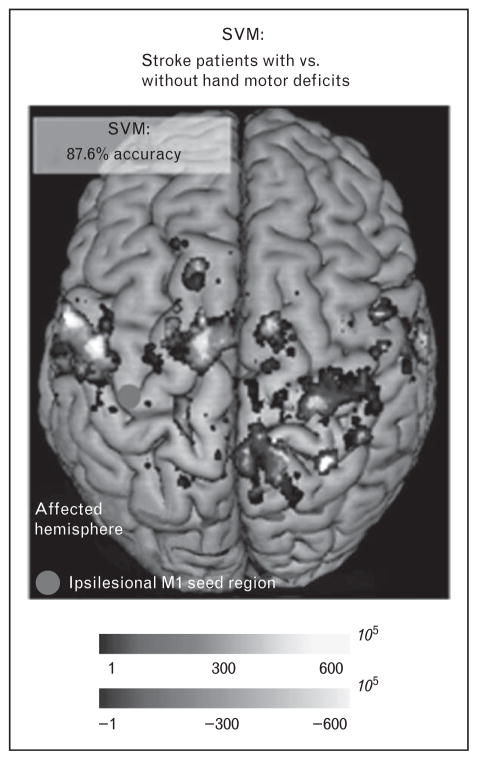
A large number of studies have examined the relationship between behavioral deficits and changes in functional connectivity in the sensory motor domain. In 2010, a study in humans [14] and one in rodents [20] showed that motor deficits at the acute stage after stroke were correlated with the reduction of interhemispheric functional connectivity of the motor network. Similar observations have been recently reported in other studies [21–25]. In an interesting study, Rehme et al. [26▪▪] used a multivariate fMRI approach to classify stroke patients with and without motor impairments. The authors studied 20 stroke patients, half with and half without deficits of hand function and a group of 10 healthy controls. Using functional connectivity between the ipsilesional primary motor cortex (M1) and the rest of the brain as the input features to a support vector machine, the authors classified patients with hand deficits compared with controls and patients without deficits with high accuracy (82.6–87.6%; Fig. 2). The classification was driven by a decrease of interhemispheric functional connectivity of M1 and an increase of functional connectivity between M1 and premotor areas.
FIGURE 2.
Multivariate SVM classification distinguishes patients with versus without motor deficits. Resting-state functional connectivity of ipsilesional M1 provided 87.6% mean accuracy for the classification of stroke patients into hand motor impairment groups. Blue: areas of voxelwise resting-state connectivity, which contribute to the classification of stroke patients without hand motor deficit (i.e. stroke patients with hand motor impairment were characterized by reduced connectivity with these areas). Red: areas of voxelwise resting-state connectivity, which contribute to the classification of stroke patients with hand motor impairment. The scaling of the weights reflects the relative contribution of voxelwise resting-state connectivity with ipsilesional M1 to group classification. The green dot indicates the seed voxel coordinate in ipsilesional M1. SVM, Support Vector Machine. Reproduced with permission [26▪▪].
Strokes often produce a cluster of correlated behavioral deficits [27]. For instance, patients with right hemisphere damage may exhibit unilateral spatial neglect, a general slowing of reaction time, spatial memory deficits, and motor impairments of the left side of the body (arm/limb) [27]. Accordingly, disentangling behavioral-specific patterns of functional connectivity might guide clinical and theoretical understanding. Baldassarre et al. [25] investigated this issue by studying the relationship between attention and motor deficits and interhemispheric functional connectivity of the dorsal attention and motor networks in a large group of right hemisphere damaged patients. A partial correlation technique that orthogonalized behavioral deficits and network functional connectivity was used to isolate the (relative) unique correlation between each deficit and network. For example, when the correlation between the attention deficit and the DAN was computed, both the motor deficit and motor network functional connectivity were regressed out. Using this procedure, a double dissociation was found that linked deficits of a given domain, for example attention, selectively and preferentially to the reduction of the interhemispheric functional connectivity of the corresponding network, for example the DAN. Therefore, this study provided evidence for the behavioral specificity of functional connectivity even for two related domains such as attention and motor, although only under circumstances in which correlated influences were removed. Conversely, Siegel et al. [19▪] showed that some changes in functional connectivity predict behavioral deficits across a broad range of domains rather than being highly specific.
Although the above studies show the behavioral significance of resting state functional connectivity, they do not address the relative importance of functional connectivity and lesion topography for predicting behavioral deficits. Siegel et al. used machine-learning models to compare how well functional connectivity and lesion topography predicted behavioral deficits in each of six domains (attention, visual memory, verbal memory, language, motor, visual) in a large cohort of stroke patients (n = 132) [19▪]. Although sensory motor functions (visual and motor domains) were better predicted by lesion topography, associative functions (visual memory and verbal memory) were better predicted by functional connectivity. Attention and language deficits were well predicted by both.
Beyond correlation, a number of other measures based on the BOLD signal have been used to classify abnormalities after stroke. Graph theory, the mathematics of network organization, is one promising tool for understanding poststroke changes. In this framework, regions of interest (ROIs) in the brain are called ‘nodes’, whereas the pairwise relationships between nodes, indexed by functional connectivity, are defined as ‘ties’. An assemblage of nodes and ties represents a graph or network. In a recent study, Zhu et al. [28] found that, as compared to healthy individuals, patients showed higher nodal centrality as indexed by nodal degree (i.e., number of nodes exhibiting correlation with a given node) and nodal efficiency (i.e., how good is the communication between a node’s neighbors when the node is removed) in several regions of the default mode network. Critically, the altered nodal centrality of the middle frontal gyrus was associated with poor cognitive efficiency as indexed by a low mini-mental state examination score. The amplitude of low-frequency fluctuations (ALFFs), a measure of the BOLD signal within a region, also has been used to investigate the consequences of stroke. This metric, usually obtained by measuring the total power within a specific frequency band (i.e. 0.01–0.1 Hz), is an index of the strength of low frequency oscillations. Previous studies showed that ALFFs reflect physiological signals (3) and exhibit behavioral relevance in clinical [29] and healthy [30] populations. In the language domain, Yang et al. [31] reported that aphasic patients, compared to healthy individuals, showed increased ALFFs in contralesional mesial temporal cortex (hippocampus/parahippocampus). Changes in ALFFs were negatively correlated with the construction score in the Aphasia Battery of Chinese, i.e. low ALFFs were associated with poor language production.
Overall, these studies strongly argue in favor of a network-wide account of brain architecture in which structural damage produces abnormal interactions between distant areas leading to behavioral impairments of cognitive functions in a selective manner.
RESTING STATE FUNCTIONAL CONNECTIVITY AT CHRONIC STAGE
Although the studies described above have investigated the effects of stroke on functional connectivity at the acute phase, three recent reports examined the patterns of abnormal functional connectivity at the chronic stage, for example ~3–4 months after stroke onset. Tang et al. [32] reported that as compared to healthy individuals, chronic patients with subcortical stroke exhibited reduced homologous interhemispheric functional connectivity of the precentral gyrus, postcentral gyrus, inferior frontal gyrus, middle temporal gyrus, thalamus, cerebellum anterior lobe, and cerebellum posterior lobe (CPL). Notably, the interhemispheric functional connectivity of the CPL was correlated with the Fugl–Meyer score of hand (FMA-H) and illness duration such that patients with low functional connectivity exhibited poor FMA-H and longer duration of impairment. These findings suggest that the functional connectivity of the CPL may represent a good indicator of motor functions at the chronic stage.
Almeida et al. [33] showed reduced functional connectivity between motor and executive control and visuospatial networks in patients with motor deficits vs. healthy controls, indicating that chronically, poor motor outcome is associated with abnormal functional connections across multiple networks.
Finally, Liu et al. [34] found higher interhemispheric functional connectivity of M1 in chronic stroke patients with well recovered motor functions as compared to healthy individuals. This increased functional connectivity was negatively correlated with the degree of damage of the cortical spinal tract and M1–M1 anatomical fibers, suggesting a compensatory mechanism of functional connectivity restoration when motor functions have recovered.
CHANGES OF RESTING STATE FUNCTIONAL CONNECTIVITY AND RECOVERY
Previous work in humans [11] and rodents [20] indicated that behavioral recovery proceeds in parallel with the restoration of normal resting state functional connectivity. More recent studies have primarily focused on recovery in the domains of attention and language function. We briefly consider each domain below.
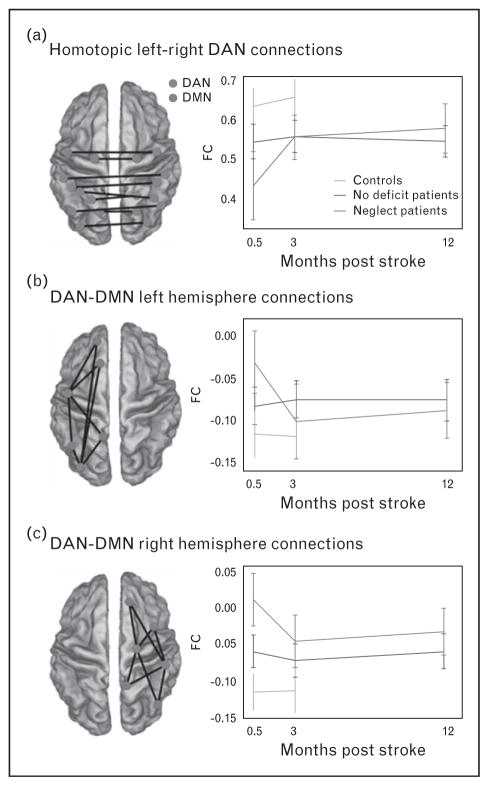
For patients with acute neglect, recovery appears dependent on the normalization of connectivity in attention and motor areas. Ramsey et al. [16▪] investigated the mechanisms underlying neglect recovery by studying longitudinally the resting state functional connectivity of a large cohort of stroke patients. Beginning with a-priori knowledge of disruptions associated with acute neglect, they correlated longitudinal changes (at 0.5, 3, and 12 months) of spatial and nonspatial deficits with changes in the functional connectivity of dorsal attention/sensory motor networks [16▪] (Fig. 3). A whole-brain data-driven analysis was also conducted to identify patterns of functional connectivity behavior correlations related to neglect recovery. Both analyses indicated that recovery of neglect, mostly complete by 3 months, was associated with restoration of interhemispheric functional connectivity across attention, sensory, and motor networks as well as with the return of normal anticorrelation functional connectivity patterns in the right hemisphere.
FIGURE 3.
Recovery of spatial neglect is associated to restoration of the interhemispheric functional connectivity of DAN and anticorrelation between DAN and default mode network (DMN). Interhemispheric dorsal attention network (DAN) (a), left hemisphere DAN to DMN (b) and right hemisphere DAN to DMN (c) functional connectivity over time for the deficit (red) and no deficit (blue) patients and controls (green). The brain figures show a subset of the connections that were averaged in the graphs (blue dots represent DAN ROIs and red dots DMN ROIs). Reproduced with permission [16▪].
In the language domain, two recent reports investigated the changes of functional connectivity at the chronic phase after stroke onset. In a small sample study (n = 4), Sebastian et al. [35] showed that recovery of naming deficits at 5–7 months after stroke in three out of four patients was mirrored by increased functional connectivity within the language network, whereas a patient with no recovery exhibited decreased functional connectivity. Although based on few cases, the study suggests that restoration of functional connectivity of language network may track the recovery of language production. Nair et al. [36] reported that, acutely, patients without documented clinical deficits of language as compared to healthy controls exhibited decreased functional connectivity of the language network, which then recovered at the chronic stage. Moreover, longitudinal changes in functional connectivity between left putamen and right superior parietal cortex were positively correlated with improved verbal fluency scores. These findings indicate that even in patients without a clear diagnosis of aphasia, both language network and functions can be impaired and recover in parallel.
The reports reviewed above indicate that longitudinal changes in behavioral performance are parallel to the restoration of the patterns of resting state functional connectivity, suggesting that RSNs may be used to track recovery after focal brain injury.
NEW INSIGHT FOR REHABILITATION?
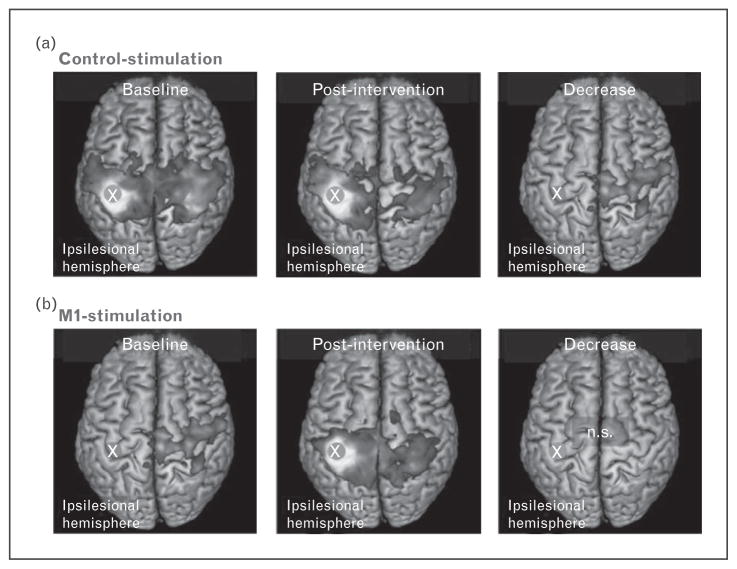
Although the studies described above have investigated the relationship between resting state functional connectivity and (relatively) spontaneous recovery, several investigations have focused on functional connectivity as related to rehabilitation after behavioral and brain stimulation treatment. In a longitudinal study, Volz et al. [37▪▪] investigated the effects of intermittent theta-burst stimulation (iTBS) over ipsilesional primary motor cortex on motor function and resting state functional connectivity in a group of acute stroke patients prior to physiotherapy treatment. Patients with hand motor deficits were pseudo-randomized into two matched groups in which iTBS was delivered for 5 days either over ipsilesional primary motor cortex (M1-stimulation group) or parieto-occipital vertex (control-stimulation group). Whole brain resting state functional connectivity of ipsilesional M1 and motor functions as indexed by grip strength and the Jebsen–Taylor Hand Function Test, were measured at two time points: one day before the first iTBS session (baseline) and one day after the last iTBS session (posttreatment). Although both groups recovered over timepoints, the M1-stimulation group exhibited a significantly better outcome than the control-stimulation group. Notably, although the control-stimulation group showed a significant reduction of interhemispheric functional connectivity of ipsilesional M1 with several motor areas, the M1-stimulation group did not exhibit such decrease of motor network functional connectivity (Fig. 4) between pretreatment and posttreatment time points. Furthermore, changes in functional connectivity were correlated with improvement of motor functions. These findings indicate that iTBS mitigates the disruption of motor network as well as facilitates a good behavioral outcome.
FIGURE 4.
iTBS treatment increases inter-hemispheric functional connectivity of motor network after stroke. Interhemispheric resting-state connectivity of ipsilesional M1 decreased in the early subacute phase in control-stimulated patients (a). Interestingly, no significant decrease was evident in the M1-stimulation group (b). Reproduced with permission [37▪▪].
Another stimulation tool for improving behavioral performance after stroke is transcranial direct current stimulation (tDCS). Two recent studies investigated the effects of tDCS on motor and language functions and on resting state functional connectivity after stroke. In a pilot study on a small sample of stroke patients (n = 5), Chen and Schlaug [38] employed tDCS for 10 sessions within 2 weeks, with the anode and cathode electrodes placed over the ipsilesional and contralesional motor cortex, respectively. The treatment induced significant behavioral amelioration of motor functions as well as increased functional connectivity of ipsilesional motor cortex with contralateral premotor cortex and bilateral precuneus. Similarly, in the language domain, Marangolo et al. [39] investigated the effects of tDCS on language recovery and resting state functional connectivity in a small group (n = 9) of aphasic patients with articulatory deficits. Patients underwent two 15-day sessions of language therapy and real or sham tDCS treatment with a 14-day intersession interval between the real and the sham condition. As compared to sham, tDCS stimulation induced a significant improvement in syllable and word production. Resting state functional connectivity was indexed by eigenvector centrality, a graph-based metric that quantitatively identifies network profiles across the whole brain [40]. A high eigenvector centrality score is assigned to nodes exhibiting several connections with nodes that in turn are highly connected with many other nodes. Accordingly, this topological measure captures the influence of a given node across multiple networks. Between-session analyses revealed that several regions of motor, default, and control network exhibited increased eigenvector centrality after tDCS treatment as compared to sham tDCS. Interestingly, these changes were correlated with the improvement of syllables repetition accuracy after treatment.
Taken together, these studies indicate that treatment leading to recovery of behavioral functions is correlated with the restoration of patterns of resting state functional connectivity of multiple RSNs, suggesting that intrinsic brain activity may represent a biomarker for tracking rehabilitation.
CONCLUSION
The findings reviewed here have important clinical and theoretical implications. First, functional connectivity measurements of resting state activity can predict behavioral deficits after stroke. Second, changes in resting state functional connectivity may be used to track behavioral recovery longitudinally. Finally, functional connectivity may serve as a marker to better understand effects of rehabilitation paradigms. Future studies should aim at understanding the degree to which specific patterns of behavioral deficits are selectively related to patterns of abnormal functional connectivity. Understanding this relationship will facilitate the development of targeted/individualized treatments in clinical practice, because the functional connectivity score of a patient can be used in parallel with anatomical images to formulate an early diagnosis after stroke. Finally, from a theoretical point of view, these studies suggest that interindividual variability in complex behavior is partially accounted for by individual differences in patterns of spontaneous activity.
KEY POINTS.
The human brain is organized in large-scale resting state networks (RSNs).
Stroke induces behaviorally relevant changes of resting state functional connectivity within and across RSNs.
Longitudinal changes of resting state functional connectivity reflect spontaneous recovery and rehabilitation outcome.
Resting state functional connectivity can be used to predict behavioral deficits, track recovery and monitor the rehabilitation.
Acknowledgments
Financial support and sponsorship
This work was supported by the following grants: Grant Fellowship 2016 from Fondazione Umberto Veronesi, Italy; Grant ‘Giovani Ricercatori – Ricerca Finalizzata 2013’ code GR-2013-02358806 from Ministry of Health Italy; Grant from the National Institute of Neurological Disorders and Stroke RO1 NS095741-01; and, funds from the Rehabilitation Institute of St. Louis.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.von Monakow C. Lokalisation der Hirnfunktionen [Localization of brain functions] Journal fur Psychologie and Neurologie. 1911;17:185–200. [Google Scholar]
- 2.Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137(Pt 9):2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- 3.Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 4.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 5.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doucet G, Naveau M, Petit L, et al. Brain activity at rest: a multiscale hierarchical functional organization. J Neurophysiol. 2011;105:2753–2763. doi: 10.1152/jn.00895.2010. [DOI] [PubMed] [Google Scholar]
- 8.Hacker CD, Laumann TO, Szrama NP, et al. Resting-state network estimation in individual subjects. NeuroImage. 2013;82:616–633. doi: 10.1016/j.neuroimage.2013.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole MW, Bassett DS, Power JD, et al. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon EM, Laumann TO, Adeyemo B, et al. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He BJ, Snyder AZ, Vincent JL, et al. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Ovadia-Caro S, Villringer K, Fiebach J, et al. Longitudinal effects of lesions on functional networks after stroke. J Cereb Blood Flow Metab. 2013;33:1279–1285. doi: 10.1038/jcbfm.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Qin W, Zhang J, et al. Altered functional organization within and between resting-state networks in chronic subcortical infarction. J Cereb Blood Flow Metab. 2014;34:597–605. doi: 10.1038/jcbfm.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter AR, Astafiev SV, Lang CE, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldassarre A, Ramsey L, Hacker CL, et al. Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain. 2014;137(Pt 12):3267–3283. doi: 10.1093/brain/awu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Ramsey LE, Siegel JS, Baldassarre A, et al. Normalization of network connectivity in hemispatial neglect recovery. Ann Neurol. 2016;80:127–141. doi: 10.1002/ana.24690. The study indicates that recovery of spatial neglect is associated with the restoration of two large-scale patterns of resting state functional connectivity across multiple RSNs: a reduction in inter-hemispheric functional connectivity and an increase in intrahemispheric functional connectivity between networks that are normally anticorrelated such as dorsal attention and default networks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varsou O, Macleod MJ, Schwarzbauer C. Functional connectivity magnetic resonance imaging in stroke: an evidence-based clinical review. Int J Stroke. 2013;9:191–198. doi: 10.1111/ijs.12033. [DOI] [PubMed] [Google Scholar]
- 18.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 19▪.Siegel JS, Ramsey LE, Snyder AZ, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci USA. 2016;113:E4367–E4376. doi: 10.1073/pnas.1521083113. Using a large stroke cohort and a machine-learning approach, the study shows that visual memory and verbal memory deficits are better predicted by functional connectivity than by lesion location and visual and motor deficits are better predicted by lesion location than functional connectivity. Attention and language deficits were well predicted by both. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Meer MP, van der Marel K, Wang K, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JL, Schlaug G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front Neurol. 2013;4:178. doi: 10.3389/fneur.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Meer MP, Otte WM, van der Marel K, et al. Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. J Neurosci. 2012;32:4495–4507. doi: 10.1523/JNEUROSCI.3662-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer AQ, Kraft AW, Wright PW, et al. Optical imaging of disrupted functional connectivity following ischemic stroke in mice. NeuroImage. 2014;99:388–401. doi: 10.1016/j.neuroimage.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014;13:206–216. doi: 10.1016/S1474-4422(13)70264-3. [DOI] [PubMed] [Google Scholar]
- 25.Baldassarre A, Ramsey L, Rengachary J, et al. Dissociated functional connectivity profiles for motor and attention deficits in acute right-hemisphere stroke. Brain. 2016;139(Pt 7):2024–2038. doi: 10.1093/brain/aww107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪▪.Rehme AK, Volz LJ, Feis DL, et al. Identifying neuroimaging markers of motor disability in acute stroke by machine learning techniques. Cereb Cortex. 2015;25:3046–3056. doi: 10.1093/cercor/bhu100. This study shows that multivarite fMRI approach is able to identify patients with versus without motor deficits based on the pattern of resting state functional connectivity at acute stage after stroke. These findings may have important clinical implications for diagnosis of motor impairment and other neurological disorders after focal brain injury. [DOI] [PubMed] [Google Scholar]
- 27.Corbetta M, Ramsey L, Callejas A, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85:927–941. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y, Bai L, Liang P, et al. Disrupted brain connectivity networks in acute ischemic stroke patients. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9525-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Mennes M, Zuo XN, Kelly C, et al. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. NeuroImage. 2011;54:2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Li J, Li Y, et al. Altered intrinsic regional activity and interregional functional connectivity in poststroke aphasia. Sci Rep. 2016;6:24803. doi: 10.1038/srep24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang C, Zhao Z, Chen C, et al. Decreased functional connectivity of homotopic brain regions in chronic stroke patients: a resting state fMRI study. PLoS One. 2016;11:e0152875. doi: 10.1371/journal.pone.0152875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida SR, Vicentini J, Bonilha L, et al. Brain connectivity and functional recovery in patients with ischemic stroke. J Neuroimaging. 2016 doi: 10.1111/jon.12362. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Qin W, Zhang J, et al. Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke. 2015;46:1045–1051. doi: 10.1161/STROKEAHA.114.007044. [DOI] [PubMed] [Google Scholar]
- 35.Sebastian R, Long C, Purcell JJ, et al. Imaging network level language recovery after left PCA stroke. Restor Neurol Neurosci. 2016;34:473–489. doi: 10.3233/RNN-150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair VA, Young BM, La C, et al. Functional connectivity changes in the language network during stroke recovery. Ann Clin Transl Neurol. 2015;2:185–195. doi: 10.1002/acn3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪▪.Volz LJ, Rehme AK, Michely J, et al. Shaping early reorganization of neural networks promotes motor function after stroke. Cereb Cortex. 2016;26:2882–2894. doi: 10.1093/cercor/bhw034. This report describes how iTBS over ipsilesional primary motor cortex induces behavioral improvement as well as restoration of typical resting state functional connectivity of motor network. This study provides relevant insight in understanding the neural mechanisms of recovery after treatment and it exhibits important clinical implication for the rehabilitation of motor functions after stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JL, Schlaug G. Increased resting state connectivity between ipsilesional motor cortex and contralesional premotor cortex after transcranial direct current stimulation with physical therapy. Sci Rep. 2016;6:23271. doi: 10.1038/srep23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marangolo P, Fiori V, Sabatini U, et al. Bilateral transcranial direct current stimulation language treatment enhances functional connectivity in the left hemisphere: preliminary data from aphasia. J Cognit Neurosci. 2016;28:724–738. doi: 10.1162/jocn_a_00927. [DOI] [PubMed] [Google Scholar]
- 40.Lohmann G, Margulies DS, Horstmann A, et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS One. 2010;5:e10232. doi: 10.1371/journal.pone.0010232. [DOI] [PMC free article] [PubMed] [Google Scholar]