Abstract
The posterior parietal cortex (PPC) is traditionally associated with attention, perceptual decision making and sensorimotor transformations, but more recent human neuroimaging studies support an additional role in episodic memory retrieval. In this Opinion article, we present a functional–anatomical model of the involvement of the PPC in memory retrieval. Parietal regions involved in perceptual attention and episodic memory are largely segregated and often show a push–pull relationship, potentially mediated by prefrontal regions. Moreover, different PPC regions carry out specific functions during retrieval — for example, representing retrieved information, recoding this information based on task demands, or accumulating evidence for memory decisions.
The posterior parietal cortex (PPC) is traditionally associated with visuospatial and sensorimotor functions on the basis of neurophysiological studies in monkeys1,2, and neuroimaging3,4 and lesion studies5,6 in humans. The PPC contains multisensory maps in which objects in the environment are represented according to their location with respect to the body and their behavioural relevance. The PPC also contains motor plans for looking or reaching towards interesting objects7–9. Thus, the PPC is perfectly suited for orienting attention to important information in the environment on the basis of internal goals and the sensory distinctiveness and adaptive value of stimuli4, and for sampling sensory information that leads eventually to decisions and actions10.
However, the contribution of the PPC to human behaviour is not limited to these functions and may include memory retrieval. In the past decade, several hypotheses have been proposed to explain the surprisingly consistent response of the PPC during the retrieval of episodic memories (reviewed in REFS 11–15). Retrieval-related activity is usually stronger in the left hemisphere16 and generalizes over cue modality17, stimulus material18 and reward contingencies19. At the same time, the parietal response is sensitive to key memory manipulations, such as those involving the strength20 and depth17 of encoding, and to the distinction between recollection (that is, the retrieval of contextual details) and familiarity (that is, the mere sense of ‘oldness’)13,21. PPC activity during episodic retrieval is particularly intriguing, as patients with PPC lesions do not show clear amnesic symptoms (reviewed in REFS 22,23). This paradox may reflect the insensitivity of standard tests of memory performance to subtle memory deficits24 or the correlative nature of neuroimaging data, as ancillary processes might recruit the PPC during memory retrieval.
The simplest episodic memory task (for example, deciding whether a picture or word is new or seen before) requires the interplay of several cognitive processes, including perceptual analysis of the probe stimulus, guided retrieval of relevant memory representations and accumulation of evidence for a decision that may feed into a motor plan. Most hypotheses about the role of the PPC in memory retrieval have considered one or two of such processes, including attention12,25, event representation13,26, decision making27 or subjective experience24. However, later studies28,29,45 have shown that several parietal subregions are characterized by distinctive functional properties during memory retrieval that indicate the implausibility of ‘single-process’ accounts (reviewed in REF. 15).
In this Opinion article, we provide a novel functional–anatomical model of memory-related activity in the human PPC (BOX 1) that builds on functional neuroimaging studies, focusing on the topography and the temporal profile of blood-oxygen-level-dependent (BOLD) activity during memory retrieval. These studies highlight multiple processes that dynamically interact during memory tasks and are localized to different parietal subregions. We believe that this signal-based approach considerably advances our understanding of the involvement of the PPC in episodic memory retrieval.
Box 1. A model of parietal involvement in episodic memory retrieval.
The figure presents a parcellation scheme of the left posterior parietal cortex (PPC) (see the figure, left panel) and a functional–anatomical model (see the figure, right panel) of the relationship between PPC regions that are involved in memory retrieval and other cortical regions that are associated with memory formation and consolidation, movement execution, perceptual attention and task-level control. The arrows indicate the direction of information flow between areas. Top-down (TD) biases and bottom-up (BU) biases are also indicated. Although retrieval-related activity is more consistently observed in the left PPC, a similar scheme might also apply to the right hemisphere.
In this model, PPC regions that are associated with perceptual attention (depicted in green in the figure) and episodic memory retrieval (in purple or red) are largely segregated31, although they can co-activate when retrieved information is used for a perceptual task41. Regions that are involved in perceptual attention — the posterior intraparietal sulcus (pIPS) and superior parietal lobule (SPL) — form part of the dorsal attention network (DAN) and provide TD modulation to sensory regions. Regions that are involved in memory retrieval — the angular gyrus (AG), which is part of the default mode network (DMN; purple), and the lateral IPS (latIPS; red outlined in black) and postcentral sulcus (PoCS; red), which form part of the frontoparietal control network (FPCN; red) — co-activate with medial temporal lobe (MTL) regions that are involved in memory formation and consolidation. PPC regions for perceptual attention and memory retrieval show a push–pull relationship31, owing to either mutual suppression (indicated by the double arrows between DAN and DMN regions) or modulation by a cingulo-opercular network (CON)61, which includes the dorsal anterior cingulate cortex (dACC)– presupplementary motor area (preSMA) and the anterior insula (aINS)–frontal operculum (fO). The influence of the CON on the PPC may reflect either task control or sustained attention or arousal.
Within the memory component of the PPC, the AG and the latIPS serve different functions29. The AG shows strong connectivity with the DMN, including the MTL, and is involved in the representation of retrieved events. Regions in the latIPS and PoCS show connectivity with the FPCN and participate in transforming and manipulating the retrieved information according to the task at hand. Further evidence suggests that the latIPS acts as an accumulator of mnemonic information98, receiving signals from the AG, and possibly directly from the MTL, for familiarity judgements or for recoding retrieved information to answer questions about past events. Unlike perceptual accumulators, the putative mnemonic accumulator is not coupled to motor intention, thus requiring an additional step before action preparation and, rather than serving as a general purpose mechanism for memory-based decisions, only tracks evidence for old responses.
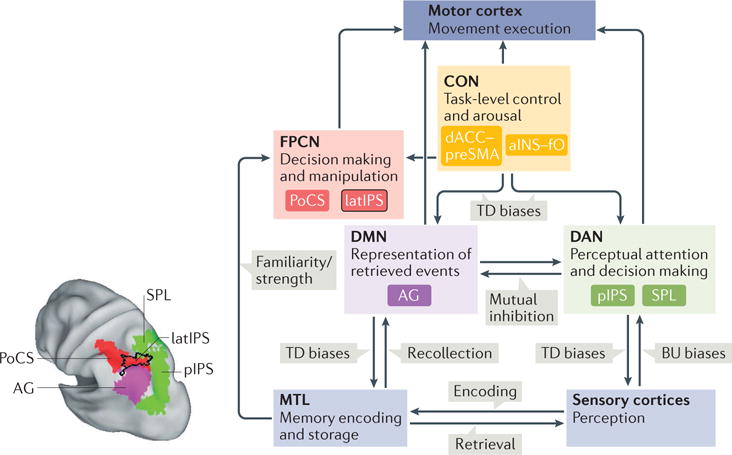
The left panel of the figure is republished with permission of Society for Neuroscience, from Memory accumulation mechanisms in human cortex are independent of motor intentions, Sestieri, C. et al., J. Neurosci. 34, 6993–7006 (2014); permission conveyed through Copyright Clearance Center, Inc.
Attention and episodic retrieval
Topographic segregation
Given that the PPC has long been associated with visuospatial and sensorimotor functions, a fundamental question surrounding its role during memory tasks concerns the degree of overlap between activity related to memory retrieval and that related to perceptual attention. As the only comparison to date involved a meta-analysis of the memory retrieval and perceptual attention literatures30, several years ago we performed a direct contrast in the same group of participants31.
Classical item-recognition paradigms tend to squeeze perceptual, mnemonic, decision and motor processes within a short time period that is below the temporal resolution of functional MRI (fMRI). Thus, activity related to one process, such as attending to the probe stimulus, cannot be temporally distinguished from activity related to another process, such as memory retrieval. Therefore, we designed tasks in which memory or perceptual search extended over a sustained period, such that these processes could be more easily separated29,31. We found that parietal regions showing positive responses to perceptual and memory search were adjacent but non-overlapping31 (FIG. 1a). Memory search-related activity was observed on the lateral aspect of the PPC, including the angular gyrus (AG), the lateral bank of the intraparietal sulcus (latIPS) and the postcentral sulcus (PoCS), and medially along the precuneus, the posterior cingulate cortex and the retrosplenial cortex. Many of these regions overlap with the default mode network (DMN) — a set of brain regions that show highly correlated activity at rest and consistent deactivation during externally oriented tasks32,33. By contrast, perceptual search-related activations were observed along the medial bank of the posterior IPS (pIPS) and the ventral IPS (vIPS), and in regions of the superior parietal lobule (SPL), which together form part of the dorsal attention network (DAN) — a set of brain regions that are putatively involved in the orienting and reorienting of attention based on internal goals or expectations4,34. This anatomical segregation was evident in both hemispheres and in individual subjects.
Figure 1. Perceptual attention and episodic memory retrieval in the PPC: segregation and competition.
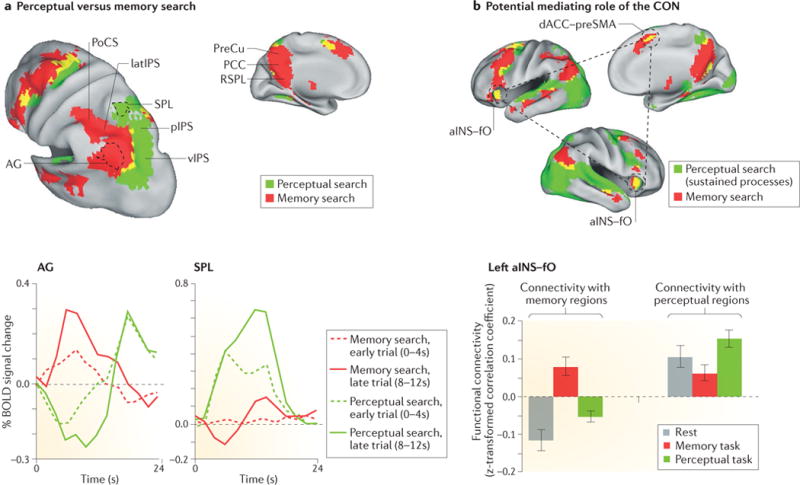
a | The upper panels show a conjunction map of blood-oxygen-level-dependent (BOLD) activity that was observed when participants searched in episodic memory to judge the accuracy of statements about a previously watched television show (memory search) or searched for specific visual targets in movie clips (perceptual search). A minimal overlap of the areas that are activated during these two tasks (depicted in yellow) is observed in the posterior parietal cortex (PPC). The time courses of BOLD activity from the angular gyrus (AG; bottom left panel), which was more active during memory search, and the superior parietal lobule (SPL; bottom right panel), which was more active during perceptual search, indicate a push–pull mechanism between sustained activation for the preferred task and sustained deactivation for the non-preferred task that scales with search duration (early trials, in which participants responded between 0 and 4 seconds after the cue, are depicted using dashed lines; late trials, in which participants responded between 8 and 12 seconds after the cue, are depicted using solid lines). Similar results were observed in the right hemisphere. b | The upper panels show a conjunction map of sustained activity during memory (memory search; red) and perceptual tasks (average of search and audiovisual stimulation; green). The cingulo–opercular network (CON62; in the figure, indicated by the black dashed circles) includes the bilateral anterior insula (aINS)–frontal operculum (fO) and the dorsal anterior cingulate cortex (dACC)–presupplementary motor area (preSMA) and showed activity in both tasks (yellow). The coupling of CON regions (bottom panel) with perceptual regions (left plot), including the bilateral posterior intraparietal sulcus (pIPS) and frontal eye fields, or with memory regions (right plot), including the bilateral AG and posterior cingulate cortex (PCC) and precuneus (PreCu), was higher during the preferred task. Error bars represent standard error of the mean. This flexible pattern of connectivity is consistent with the idea that the CON mediates the dynamic competition between PPC regions. latIPS, lateral IPS; PoCS, postcentral sulcus; RSPL, retrosplenial cortex; vIPS, ventral IPS. Part a is republished with permission of Society for Neuroscience, from Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex, Sestieri, C., Shulman, G. L. & Corbetta, M., J. Neurosci. 30, 8445–8456 (2010); permission conveyed through Copyright Clearance Center, Inc. Part b is reprinted from REF. 61 by permission of the MIT Press.
Causal support for a segregation of parietal regions that are involved in memory (albeit semantic) retrieval and perceptual attention comes from a recent study35 that combined electroencephalography (EEG) with the inhibitory effect of repetitive transcranial magnetic stimulation36,37 (rTMS) during task anticipation. We found that both performance and anticipatory desynchronization of oscillations in the alpha band were affected by stimulation of the pIPS only during the perceptual attention task and of the AG only during the semantic retrieval task, demonstrating a double dissociation between PPC regions and task demands.
Another study by Cabeza et al.38 reported a greater overlap in PPC activation between a memory-search and a perceptual-search task, especially in the IPS. According to the authors, the overlap reflects the involvement of the same area in orienting attention to memory and perceptual stimuli, as suggested by the Attention to Memory (AtoM) model12,39. This model proposes that dorsal PPC regions maintain retrieval goals, whereas ventral PPC regions monitor retrieval output, paralleling a model of perceptual attention orienting that links dorsal parietal regions to top-down attention and ventral parietal regions to bottom-up attention4. However, the overlap observed by Cabeza et al. in their study might reflect the constant presence of visual stimulation in both search tasks, and thus shared perceptual attention to the target (probe) stimulus. Free recall paradigms could help to resolve this issue, as they offer a way to study memory-orienting activity in the absence of perceptual stimulation. A recent study by Kragel and Polyn indicates the absence of sustained activity in the dorsal PPC during long periods of free recall40, but more studies on this issue are needed.
Importantly, overlap between perceptual attention and episodic memory tasks is expected whenever the contrast between two memory conditions reflects a difference in the amount or duration of perceptual attention to the probe stimulus, or when information retrieved from memory is used to complete a perceptual or motor task, as in a study by Summerfield and colleagues41 (for similar results, see also REF. 42). In this study, the use of long-term memory to orient visuospatial attention evoked activity in both dorsal (pIPS) and ventral (AG) PPC regions. Therefore, when retrieved memory information is used to perform a perceptual task, visuospatial responses in the dorsal PPC co-occur with retrieval-related responses in the ventral PPC.
Attention to memories
The results of our study31 and the aforementioned meta-analysis30 argue against a strict anatomical and functional correspondence between attention to episodic memory and perceptual attention. The available literature also suggests a similar dissociation for reorienting responses in the two domains30 (but see REF. 38), although this issue is still debated43,44. Importantly, these results do not preclude the functional distinctions that were drawn by Cabeza and colleagues12 and their mapping to relatively more dorsal and ventral parietal regions. The AtoM model explains several findings in the memory literature by associating difficult retrieval conditions that are characterized by weak memories and low-confidence responses with the need for top-down attention and, conversely, associating vivid, strong, detailed retrieval conditions with bottom-up attentional capture by memory contents12,39. Nonetheless, we emphasize the difficulty of isolating signals that are related to the orienting of attention to memories in traditional item-recognition paradigms, as researchers also inevitably measure other intervening processes, including perceptual attention to the probe stimulus. Furthermore, some of the memory effects reported in the SPL, including the preference for old versus new items and for low-versus high-confidence judgements, can be explained by alternative factors13,15, such as general task relevance13 or time-on-task effects45, rather than the orienting of attention to memories.
In addition, there are several open questions about the analogy between mechanisms for orienting and reorienting attention to memory and the environment. In the dorsal parietal cortex, multivoxel patterns distinguish between attention to different perceptual features (for example, red versus green46) or dimensions (for example, location versus colour47,48), indicating that parietal signals depend on the specific aspect of the stimulus that is attended. Moreover, dorsal parietal signals exert a causal influence over the activity of the visual cortex49,50. However, it is not known whether multivoxel patterns in the dorsal parietal cortex also depend on the specific content of what is being retrieved from memory; it is also not clear whether the dorsal PPC exerts causal influence over medial temporal regions. Similarly, dorsal and ventral cortical attention systems work in concert during reorienting of perceptual attention, with co-activation of both systems34, but it is not clear if dorsal and ventral parietal regions also work together to reorient retrieval from episodic memory. Co-activation of dorsal and ventral parietal regions was not observed in a study that specifically investigated the attention- reorienting response to memories51, although the available literature on this issue is limited25,52.
Dynamic competition
The segregation of mechanisms for perceptual and episodic memory search is further supported by analyses of activity time series in our perceptual- search and memory-search tasks31. Regions activated during one task were deactivated during the other task, and the amplitude of this negative covariation was proportional to search duration and related to behavioural performance in both tasks. Although this pattern of ‘push–pull’ (REFS 53,54) was observed using a cued recollection instead of a typical item- recognition paradigm, it nonetheless suggests a dynamic competition between perceptual and memory systems, which might serve to suppress task-irrelevant regions and reduce crosstalk between potentially competing representations. Alternatively, BOLD-signal deactivation might indicate more-selective, task-relevant responses (‘filter’), although this hypothesis is less parsimonious, as it posits that regions would need to reverse their functional role according to task demands.
Other lines of evidence support a competitive interaction between perceptual and memory systems. Sustained deactivation in the DAN, coupled with sustained activity in ventral regions of the DMN, was observed during long (75 s) periods of free recall from a studied list of words40. In addition, an item-recognition study55 in which participants were presented with multiple pictures and had to identify the previously studied one showed that trials that required more perceptual attention to suppress false recognition evoked greater activation in the dorsal PPC but lower activation in those ventral PPC regions that further distinguished between true and false memories. Furthermore, behavioural studies have shown that the division of attention between memory retrieval and a concurrent visual continuous reaction-time task produces a memory deficit, especially in free recall56. Finally, we have shown that transient inhibition of the AG using rTMS significantly decreased item-recognition accuracy compared with SPL stimulation, with sham stimulation producing an intermediate level of performance57. One interpretation of this pattern of results is that a subtle negative effect of AG stimulation on performance was enhanced by contrasting it with a subtle positive effect of SPL stimulation, in line with a competitive interaction between these regions.
In summary, human PPC regions that are involved in perceptual attention and episodic memory are not only anatomically segregated but also seem to be organized in a dynamic competition.
Prefrontal control of PPC competition
A mechanism of direct inhibition between different PPC regions might also coexist with the presence of a higher-order system that modulates PPC activity, similar to the way in which top-down influences on associative regions and occipital cortex can enhance or suppress the processing of sensory information4,58–60. By analogy, we asked whether the push–pull pattern in PPC subregions might depend on top-down signals from other cortical regions61.
Because such a top-down function could only be performed by regions that are active during both perceptual and memory search, we first identified ‘domain-general’ regions (FIG. 1b; top panel) that showed a sustained response during both tasks of our previous study31. The most consistent overlap across subjects coincided with the so-called cinguloopercular network (CON), a set of regions that have been associated with maintenance of a task set (that is, the configuration of cognitive processes that is actively maintained for task performance)62. These regions also flexibly changed their interactions with perceptual and memory PPC regions, as indexed by functional connectivity, depending on task demands (FIG. 1b; bottom panel) — potentially consistent with a role mediating the push–pull in the PPC63. Although some authors have proposed the CON to be part of a larger frontoparietal control network (FPCN) that is involved in the flexible, adaptive control of behaviour64,65, we observed a functional distinction, both at rest and during task execution, between the CON and putative nodes of the FPCN61. This result fits with the hypothesis that the two networks are involved in different aspects of cognitive control66.
These observations are consistent with the hypothesis that the CON partly mediates a push–pull relationship in the PPC, but they are only suggestive. Functional connectivity measures are bidirectional and do not imply causation, which is better supported by measure of effective connectivity67. Furthermore, our results are compatible with an alternative hypothesis whereby CON activity reflects the general availability of cognitive resources68,69.
Representing retrieved information
Episodic retrieval often occurs in the service of a particular behavioural goal. For example, in order to determine which desk drawer to open, we may need to retrieve an episode in which we put away the object we want to find. Our model proposes a distinction between retrieving and representing the episode, and selecting specific information from that representation relevant to the task at hand. We suggest that this distinction corresponds anatomically to two different regions in the PPC: the AG and the latIPS.
An influential set of hypotheses maintains that PPC regions represent retrieved information11, either by holding an actual representation13 or by linking distributed memory traces26. According to Vilberg and Rugg13, this function implies the generation and maintenance of an integrated representation of information retrieved from episodic memory, meaning that the PPC may act as an episodic buffer70. According to the CoBRA (cortical binding of relational activity) hypothesis26, with the passage of time from the event, the parietal lobe substitutes for hippocampal functions during retrieval by binding episodic features that are stored in different cortical regions into a coherent representation.
There is substantial support for the view that activity in the AG reflects retrieved information, including processes supporting the online maintenance of this information. The AG is activated when memory retrieval involves recollection of specific details of an event13, and AG responses are stronger as more episodic information is retrieved71. Perhaps the strongest evidence that the AG represents what is being remembered comes from a study that used multivoxel pattern classification to decode the content of retrieved information from the BOLD signal during recall of word–picture associations72. In this study, activity in a region of the AG that was associated with subjective vivid remembering could be used to distinguish not only the reactivated category (face versus scene) but also the individual event (associated face versus non-associated face) cued by words. Interestingly, the vividness of recollection is reported to decrease in patients with parietal lesions73,74.
The representational role of the AG may generalize to other forms of memory. Meta-analyses have demonstrated that the AG is the region most reliably activated by general semantic memory tasks75 and shows considerable overlap with the region showing recollection-specific effects76. Given this pattern of activity across several semantic tasks, Binder and Desai77 have proposed that the AG has a unique role in the representation of event concepts, implying a stronger (neurobiological) link between episodic and semantic memory than previously assumed on the basis of theoretical distinctions78. The AG might also have a similar representational role in the imagination of hypothetical or future events79.
The AG versus the lateral IPS
The above-described results for the AG are consistent with either the binding (CoBRA) or the buffer hypothesis. However, the way in which the time course of AG activity depends on the memory task may reveal more clues. One study80 tracked the time course of activity as participants maintained the retrieved information in working memory for a variable interval, as they waited to answer one of three possible questions. A region of the AG showed sustained activity over the entire delay period, whereas the response in the medial temporal lobe (MTL) was transient, being associated with picture presentation but not with delay activity.
However, when retrieved information is continuously recoded in accord with task demands, rather than simply maintained, the activity in the AG, as well as in the MTL, is only transient. We showed this by exploiting the large variability in decision times in our memory-search paradigm29 to track how parietal activity unfolded over time (FIG. 2). Activity in both the AG and the MTL (particularly the posterior parahippocampus) scaled in amplitude with retrieval duration, yet remained transient, peaking approximately at the same time across trials, even in trials with long decision times (>8 s). This result is consistent with a role for the AG in the initial representation of retrieved information. By contrast, activity in the latIPS as well as in the PoCS exhibited sustained activation until a final decision was made, followed by transient activity in the motor cortex. The time course of the BOLD response in the latIPS fits with a region that is involved in manipulating retrieved information according to task dictates.
Figure 2. Different roles for the AG and latIPS during episodic memory retrieval.
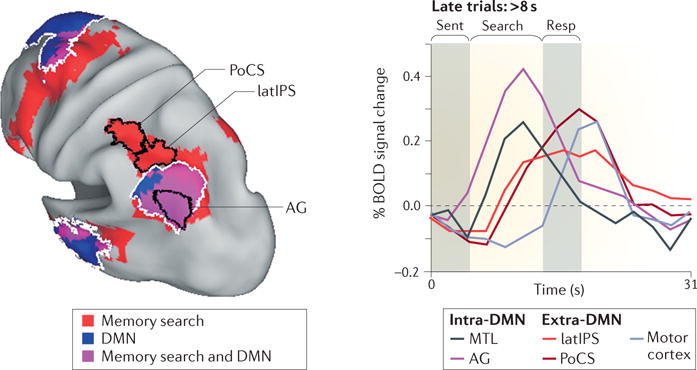
The brain image (left panel) shows a conjunction map illustrating the superimposition of memory search-related activity and the default mode network (DMN), independently defined through resting-state functional connectivity analysis. Regions of overlap include the angular gyrus (AG) but not the lateral intraparietal sulcus (latIPS) or the postcentral sulcus (PoCS). The line graph on the right panel shows the functional responses of the regions that are illustrated in the left panel during sentence reading (‘Sent’), memory search and response (‘Resp’). When retrieved information is continuously recoded as part of the decision process during long memory-search trials (lasting longer than 8 s), intra-DMN regions of the medial temporal lobe (MTL) and of the AG show more-transient activity than do extra-DMN regions of the latIPS and of the PoCS. A transient blood-oxygen-level-dependent (BOLD) response is last observed in motor cortex (light grey). Republished with permission of Society for Neuroscience, from Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses, Sestieri, C., Corbetta, M., Romani, G. L. & Shulman, G. L., J. Neurosci. 31, 4407–4420 (2011); permission conveyed through Copyright Clearance Center, Inc.
The functional dissociation between the AG and the latIPS is supported by results from other lines of research. Wheeler and Buckner21 were probably the first to point to a functional distinction between the AG and the latIPS on the basis of their profile of BOLD activity during familiarity versus recollection judgements. In addition, the two regions cluster with different resting-state networks. The AG, along with the posterior cingulate cortex and the MTL, is part of the DMN29 (see also REF. 81). Evidence for a general involvement of the DMN in episodic memory retrieval can be traced back to the work of Andreasen et al.82 (see also REF. 83), but a more recent study emphasized the specific contribution of a subnetwork including the AG and the MTL in constructing a mental scene based on memory84. By contrast, more-dorsal and more-anterior regions of the latIPS and PoCS are considered part of the FPCN64,85.
Supporting evidence for a functional dissociation between the AG and the latIPS comes also from non-human primate research, although interspecies comparisons are complicated by the considerable relative expansion of the inferior parietal lobule (IPL) in humans86 and by the difficulty of designing comparable retrieval tasks across species87. Anatomical studies have shown that the MTL is selectively connected with the IPL both in monkeys88,89 and in humans90. The MTL may thus have a key mediating role in providing access to stored information91,92 in both species. In addition, an fMRI study in awake monkeys demonstrated a similar functional dissociation between regions of the IPL (area PG/PGOp) and of the IPS (area PEa/DIP) on the basis of the pattern of evoked activity during a serial probe-recognition task and the pattern of anatomical and functional connectivity93. In particular, the monkey IPL region might correspond to the human AG, given its anatomical position, the presence of a primacy effect (which is thought to reflect long-term memory retrieval) and the pattern of anatomical and functional connectivity with the MTL. By contrast, the more dorsal region of the monkey IPS, which showed a recency effect (associated with working-memory functions) and strong anatomical and functional connectivity with prefrontal regions, might be homologous to the human latIPS.
In summary, the current evidence supports a division of labour between the MTL, which is involved early in the retrieval processes, the AG, which represents details of retrieved information, and the latIPS and PoCS, which participate in transforming and manipulating the retrieved information according to the task at hand.
Decision making and motor intention
Accumulating memory-decision evidence
The sustained response of the latIPS and PoCS regions in our memory-search experiment29 is also compatible with decisional aspects of the retrieval task. Wagner et al. first proposed that parietal regions might serve as a ‘mnemonic accumulator’ of evidence for memory decisions11 (see also REF. 27), given the well-known association between parietal activity and perceptual decisions that had been established in electrophysiological studies in non-human primates94. Specifically, the ramp-like activity that is observed in the lateral intraparietal area (LIP; a sensorimotor area) of monkeys during simple perceptual decisions10 matches the mechanism of evidence accumulation that is proposed by mathematical models of decision making, such as the drift-diffusion model95. According to this model, decisions result from the continuous accumulation of noisy information until one of two response thresholds is reached. Interestingly, an early formulation of the drift-diffusion model described retrieval as a process of evidence accumulation about the relatedness between probes and items in the memory set96.
The involvement of parietal regions in the accumulation of mnemonic evidence is consistent with studies showing that regions near or at the IPS track perceived oldness, regardless of whether the response is correct or incorrect20,97. Moreover, as evidence for a decision accumulates over time, a region that is involved in decision making is expected to show sustained activity until a response is made, as observed for latIPS and PoCS regions in our memory-search study29.
We investigated the relationship between parietal activity and the putative mnemonic accumulator by manipulating the amount of evidence for old and new responses and by inserting a temporal delay before response execution98 (BOX 2). Notably, the BOLD signal in the latIPS increased in a graded manner with the amount of evidence for an old decision but not for a new decision, consistent with an asymmetric accumulator (that is, an accumulator only for old responses). Thus, the latIPS may be involved in a relatively late stage of the retrieval process in which decisions about oldness are made.
Box 2. Evidence accumulation during memory-based decisions.
An open question about the mnemonic accumulator hypothesis concerns the way blood- oxygen-level-dependent (BOLD) activity tracks the manipulation of decision evidence15. In theory, a neural accumulator would show a steeper accumulation process when more evidence is available (that is, for easier decisions)115. However, because the BOLD signal has a temporally lower resolution than the neural signal, the BOLD response does not reflect the instantaneous neural activity but rather the integral of the neuronal activity over time. Thus, if subjects respond as soon as a decision is reached (for example, in a reaction-time paradigm), more evidence is typically associated with lower BOLD activity116,117, as easier decisions are also faster. In this context, it is difficult to isolate accumulator regions, as their predicted BOLD pattern is similar to that of regions showing mere time-on-task effects. Instead, if subjects hold responses until a ‘go’ signal (for example, in a delay paradigm), the relationship between amount of decision evidence and BOLD activity should be reversed. Specifically, under the assumption that accumulator regions maintain the level of activity that is reached during the decision process until the response, easier decisions are now associated with higher BOLD activity, as the accumulation process reaches the bound more rapidly and the integral of neural activity is larger.
In addition, it is not clear whether the hypothetical accumulator tracks the amount of evidence favouring both ‘old’ and ‘new’ responses (symmetric) or just shows a preference for older items (asymmetric). Whereas symmetric accumulation is common in perceptual decision making10,94,103, the original drift-diffusion model of episodic retrieval96 proposed that the judgement of a stimulus as ‘new’ is driven by the absence of a memory signal rather than by the presence of a novelty signal. In this model, stimuli can be judged to be old when enough evidence towards relatedness (that is, the number of matching features between probes and items in the memory set) is accumulated, whereas stimuli are judged to be new when these comparisons terminate in non-matches. Therefore, old–new decisions might be supported by an asymmetric accumulator that tracks perceived memory strength (which is greater for older memories than for newer ones).
We recently manipulated the amount of evidence for old and new decisions in a picture-recognition paradigm with delayed responses98. Evidence towards old and new responses was varied by manipulating the frequency of image presentation at encoding and the similarity between new and old images at retrieval, respectively. Participants had to delay their memory judgements, which were indicated by either an eye movement or a hand movement. Consistent with a mechanism for the asymmetric accumulation of evidence towards old decisions, the peak of decision-related activity in the left lateral intraparietal sulcus scaled parametrically with the amount of perceived memory strength.
A definitive test of the mnemonic accumulation hypothesis will require techniques that can track evidence accumulation with higher temporal resolution than fMRI. Several studies conducted with EEG in the 1990s identified a parietal effect that starts around 400–500 ms after the stimulus and is strongly associated with recollection (reviewed in REF. 99). However, the spatial resolution of the EEG does not allow the contribution of different PPC regions to be distinguished, and techniques with better spatial resolution, such as magnetoencephalography, have not yet provided a robust functional parcellation of the PPC (reviewed in REF. 100; see also REF. 101). Recently, an electrocorticography study102 reported greater sustained high-frequency gamma power, a proxy signal for multiunit activity, for old versus new correct decisions in the IPS; this increase in gamma power decayed only 200 ms before the motor response, consistent with an accumulator hypothesis.
Memory decisions and motor intentions
Neurophysiological studies in monkeys have demonstrated a close relationship between signals that are related to evidence accumulation in area LIP and motor planning, suggesting that decisions are inseparable from the actions that are used to report them94. Human studies have also shown that perceptual decision-related signals can be observed in different parietal effector-preferring regions depending on the specific sensorimotor associations that are required for the task103–105. For example, during difficult face–place discriminations103, activity in regions of the PPC that preferred saccades106,107 and pointing108,109 was modulated by the amount of decision evidence (that is, amount of noise in the test stimulus) cueing their preferred effector (FIG. 3a). Similarly, parietal activity during memory retrieval might in part reflect the preparation of motor responses15. This issue was first explored in a study that required participants to manually respond only to old or new items in different sessions17. The independence of PPC activity from response contingency that was observed in this study seems to be inconsistent with a motor preparation account, although participants might still have prepared a response without executing it15.
Figure 3. Distinction between perceptual and memory-based decision making.

a | Evidence accumulation and motor intention are coupled in perceptual decisions. The top panel shows between-subject overlap of the individual anterior parietal reach region (aPRR) and posterior intraparietal sulcus (pIPS), which exhibited a preference for pointing and saccadic movements, respectively, in a localizer experiment. Both an aPRR and a posterior PRR were identified, although the two regions showed a very similar response profile. Only the left hemisphere is displayed in the figure, but the two hemispheres showed similar results. Decision-related activity in both of these regions (aPPR, in the example) was modulated by the amount of evidence (manipulated by changing the amount of noise in the stimulus) towards the preferred effector (bottom panel). b | Evidence accumulation and motor intention are independent in memory-based decisions. A localizer experiment identified an effector-preferring PRR and pIPS (top left panel). Decision-related activity in these regions (PPR, in the example) was not modulated by the amount of evidence for memory-based decisions associated with their preferred effector (bottom left panel). Anatomical location of the lateral IPS (latIPS) region showed activity compatible with a putative mnemonic accumulator during memory based- decisions (top right panel). Activity in the latIPS was not modulated by the amount of evidence for a particular action (bottom right panel). Specifically, a constant preference for older items was observed regardless of whether old responses were associated with eye or hand movements, supporting the independence between signals coding for memory strength and motor intentions. S.C., signal change. Part a is adapted with permission from REF. 103, Macmillan Publishers Limited. Part b is republished with permission of Society for Neuroscience, from Memory accumulation mechanisms in human cortex are independent of motor intentions, Sestieri, C. et al., J. Neurosci. 34, 6993–7006 (2014); permission conveyed through Copyright Clearance Center, Inc.
This account also makes the key prediction that memory-related activity should occur in sensorimotor PPC regions that code for the particular effector that is used to report the decision. We tested this prediction by examining whether signals in effector-preferring regions of the PPC reflected the accumulation of evidence during memory decisions as observers reported old judgements through eye movements and new judgements through hand movements, or vice versa98. Importantly, decision-related activity in effector-preferring regions of the PPC was independent of the amount of evidence associated with a particular action (FIG. 3b) and therefore did not reflect the strength of action–intention processes. Moreover, the graded preference for older items in the latIPS was consistently observed regardless of whether old decisions were associated with eye- or hand-movement responses, supporting the independence between evidence accumulation and motor intention.
We therefore conclude that it is unlikely that PPC activation during memory retrieval reflects action intention, and that key variables for memory and perceptual decisions are encoded in sensorimotor areas to very different extents.
Memory versus perceptual decisions
Two main features of the signals that are observed in the human PPC during memory decisions do not fit a simple analogy with the pattern of firing rate that is observed in the monkey LIP during perceptual decisions, suggesting that perceptual and memory-based decisions rely on different mechanisms.
First, unlike perceptual decisions in monkeys10,94 and humans103–105, effector- preferring PPC regions do not seem to represent the amount of evidence favouring a particular action during memory-based decisions98. The independence between signals for memory decision and motor intention might reflect the relevance of the decision in guiding actions. As hand and eye movements are routinely made to objects on the basis of their perceptual characteristics, it is plausible that evidence signals from different perceptual systems feed continuously into intentional mechanisms. However, memory-based decisions may be less likely to be routinely coupled to effectors. Although some information that we retrieve from memory is highly relevant to motor planning (such as whether an object is a source of nutrients), much of the information we retrieve (for example, “what movie did I see last night?”) is not.
Second, whereas perceptual accumulation signals in the LIP are thought to generalize across different perceptual discriminations (although most evidence comes from variants of the same task — that is, the random dot motion paradigm), it is not clear which types of memory-based decisions involve a parietal accumulator. Accounts of decision- related parietal activations during episodic retrieval have largely focused on old–new judgements11. However, naturalistic uses of episodic memory often involve retrieval of additional information associated with an object or event. The invariant preference of the latIPS for old items across different paradigms suggests that this proposed accumulator might just reflect evidence for oldness. Parietal regions, including the right latIPS, have been shown to track the overall memory strength of a pair of old stimuli (that is, the frequency of repetition at encoding of both stimuli) but not the amount of evidence supporting the actual memory decision (judging which of the two stimuli was presented more frequently)110, suggesting that the response of these regions may be inflexible. The absence of generalization over different memory paradigms does not fit the traditional view of a general decision mechanism.
In summary, the activity profile of the latIPS is compatible with an asymmetric accumulator of evidence for old decisions, but, unlike for perceptual decisions, the accumulation mechanism is uncoupled from motor intentions, and its generalization to other memory judgements seems to be limited.
Conclusions
In this Opinion article, we have discussed experimental findings on the relationship between memory retrieval and other processes that are localized in the human PPC, with particular emphasis on neuroimaging studies that track the temporal evolution of activity during a memory task. These studies suggest a functional–anatomical parcellation of the human PPC that is consistent with other schemes that are based on resting-state functional connectivity85,111 and task-evoked activity15,28,45. All these schemes agree on the presence of at least three sets of PPC regions: dorsal regions of the DAN (the pIPS and SPL) that are related to visuospatial attention and eye movements; regions of the FPCN (the latIPS) that track the sense of familiarity or perceived memory strength; and regions of the DMN (the AG) that show recollection-specific effects. This model can improve the analysis of neuropsychological deficits that are caused by focal lesions or neurosurgical ablations of the human PPC. It may also be helpful to target these cortical regions for stimulation (for example, TMS) in experiments aimed at modulating activity during normal cognition or in correcting activity in patients with brain injuries for rehabilitation.
We also suggest several outstanding issues for future research. The nature of the push– pull mechanism between ventral and dorsal PPC regions is still not clear, and more research is needed to unravel its underlying physiological mechanisms and its relevance for behaviour. For example, do dorsal and ventral PPC regions directly interact or does this happen through a third party? We have suggested the putative role of the CON in orchestrating the push– pull dynamics in the PPC; this should be assessed with analyses of causation. Also, the apparent difference between the brain mechanisms for memory and perceptual decisions deserves further investigation. Notably, different neuroimaging techniques and decision paradigms should be used in the memory domain to reach the same level of understanding that we have for perceptual decisions. Last, a joint examination of memory-related activity in lateral versus medial PPC regions, and of their relationship with other regions that are involved in memory retrieval (including the MTL112, prefrontal cortex113 and basal ganglia114), will lead to a more comprehensive model of PPC involvement in the retrieval of information from long-term memory.
Acknowledgments
This work was supported by the J. S. McDonnell Foundation and by US National Institutes of Health (grant RO1 NS095741) to M.C.
Footnotes
Competing interests statement
The authors declare no competing interests.
Contributor Information
Carlo Sestieri, Department of Neuroscience, Imaging and Clinical Sciences, Institute of Advanced, Biomedical Technologies, University of Chieti, 66100 Chieti, Italy.
Gordon L. Shulman, Department of Neurology, Washington University School of Medicine
Maurizio Corbetta, Department of Neuroscience, University of Padua, 35122 Padua, Italy; at the Department of Neurology, Radiology, Neuroscience, Biomedical Engineering, Washington University School of Medicine, St. Louis, Missouri 63110, USA.
References
- 1.Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- 2.Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- 3.Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- 4.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 5.Vallar G, Perani D, editors. The Anatomy of Spatial Neglect in Humans. Elsevier Science Publishers; 1987. [Google Scholar]
- 6.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Phil Trans R Soc Lond B. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- 8.Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 11.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy DA. Towards an understanding of parietal mnemonic processes: some conceptual guideposts. Front Integr Neurosci. 2012;6:41. doi: 10.3389/fnint.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uncapher MR, Gordon AM, Wagner AD. In: The Cognitive Neurosciences. Mangun GR, Gazzaniga MS, editors. MIT Press; 2014. pp. 567–576. [Google Scholar]
- 16.Guerin SA, Miller MB. Lateralization of the parietal old/new effect: an event-related fMRI study comparing recognition memory for words and faces. Neuroimage. 2009;44:232–242. doi: 10.1016/j.neuroimage.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duarte A, Henson RN, Graham KS. Stimulus content and the neural correlates of source memory. Brain Res. 2011;1373:110–123. doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Huettel SA, Raposo A, Adcock RA, Dobbins IG. Functional significance of striatal responses during episodic decisions: recovery or goal attainment? J Neurosci. 2010;30:4767–4775. doi: 10.1523/JNEUROSCI.3077-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Schoo LA, et al. The posterior parietal paradox: why do functional magnetic resonance imaging and lesion studies on episodic memory produce conflicting results? J Neuropsychol. 2011;5:15–38. doi: 10.1348/174866410X504059. [DOI] [PubMed] [Google Scholar]
- 23.Berryhill ME. Insights from neuropsychology: pinpointing the role of the posterior parietal cortex in episodic and working memory. Front Integr Neurosci. 2012;6:31. doi: 10.3389/fnint.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb Cortex. 2010;20:479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor AR, Han S, Dobbins IG. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J Neurosci. 2010;30:2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011;11:277–291. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- 27.Donaldson DI, Wheeler ME, Petersen SE. Remember the source: dissociating frontal and parietal contributions to episodic memory. J Cogn Neurosci. 2010;22:377–391. doi: 10.1162/jocn.2009.21242. [DOI] [PubMed] [Google Scholar]
- 28.Nelson SM, et al. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31:4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn Mem. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sestieri C, Shulman GL, Corbetta M. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J Neurosci. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 33.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capotosto P, et al. Task and regions specific top-down modulation of alpha rhythms in parietal cortex. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw278. http://dx.doi.org/10.1093/cercor/bhw278. [DOI] [PMC free article] [PubMed]
- 36.Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capotosto P, Babiloni C, Romani GL, Corbetta M. Differential contribution of right and left parietal cortex to the control of spatial attention: a simultaneous EEG–rTMS study. Cereb Cortex. 2011;22:446–454. doi: 10.1093/cercor/bhr127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabeza R, et al. Overlapping parietal activity in memory and perception: evidence for the attention to memory model. J Cogn Neurosci. 2011;23:3209–3217. doi: 10.1162/jocn_a_00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Kragel JE, Polyn SM. Functional interactions between large-scale networks during memory search. Cereb Cortex. 2015;25:667–679. doi: 10.1093/cercor/bht258. [DOI] [PubMed] [Google Scholar]
- 41.Summerfield JJ, Lepsien J, Gitelman DR, Mesulam MM, Nobre AC. Orienting attention based on long-term memory experience. Neuron. 2006;49:905–916. doi: 10.1016/j.neuron.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Rosen ML, Stern CE, Michalka SW, Devaney KJ, Somers DC. Cognitive control network contributions to memory-guided visual attention. Cereb Cortex. 2016;26:2059–2073. doi: 10.1093/cercor/bhv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012;16:338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson SM, McDermott KB, Petersen SE. In favor of a ‘fractionation’ view of ventral parietal cortex: comment on Cabeza et al. Trends Cogn Sci. 2012;16:399–400. doi: 10.1016/j.tics.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Hutchinson JB, et al. Functional heterogeneity in posterior parietal cortex across attention and episodic memory retrieval. Cereb Cortex. 2014;24:49–66. doi: 10.1093/cercor/bhs278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Hospadaruk L, Zhu DC, Gardner JL. Feature-specific attentional priority signals in human cortex. J Neurosci. 2011;31:4484–4495. doi: 10.1523/JNEUROSCI.5745-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci. 2010;30:14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu T, Hou Y. A hierarchy of attentional priority signals in human frontoparietal cortex. J Neurosci. 2013;33:16606–16616. doi: 10.1523/JNEUROSCI.1780-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruff CC, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 50.Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28:10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciaramelli E, Grady C, Levine B, Ween J, Moscovitch M. Top-down and bottom-up attention to memory are dissociated in posterior parietal cortex: neuroimaging and neuropsychological evidence. J Neurosci. 2010;30:4943–4956. doi: 10.1523/JNEUROSCI.1209-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaeger A, Konkel A, Dobbins IG. Unexpected novelty and familiarity orienting responses in lateral parietal cortex during recognition judgment. Neuropsychologia. 2013;51:1061–1076. doi: 10.1016/j.neuropsychologia.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hester RL, et al. Predicting success: patterns of cortical activation and deactivation prior to response inhibition. J Cogn Neurosci. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- 54.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 55.Guerin SA, Robbins CA, Gilmore AW, Schacter DL. Interactions between visual attention and episodic retrieval: dissociable contributions of parietal regions during gist-based false recognition. Neuron. 2012;75:1122–1134. doi: 10.1016/j.neuron.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Craik FI, Govoni R, Naveh-Benjamin M, Anderson ND. The effects of divided attention on encoding and retrieval processes in human memory. J Exp Psychol Gen. 1996;125:159–180. doi: 10.1037//0096-3445.125.2.159. [DOI] [PubMed] [Google Scholar]
- 57.Sestieri C, Capotosto P, Tosoni A, Luca Romani G, Corbetta M. Interference with episodic memory retrieval following transcranial stimulation of the inferior but not the superior parietal lobule. Neuropsychologia. 2013;51:900–906. doi: 10.1016/j.neuropsychologia.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 58.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 59.Higo T, Mars RB, Boorman ED, Buch ER, Rushworth MF. Distributed and causal influence of frontal operculum in task control. Proc Natl Acad Sci USA. 2011;108:4230–4235. doi: 10.1073/pnas.1013361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat Neurosci. 2011;14:830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sestieri C, Corbetta M, Spadone S, Romani GL, Shulman GL. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J Cogn Neurosci. 2014;26:551–568. doi: 10.1162/jocn_a_00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dosenbach NU, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadaghiani S, D’Esposito M. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cereb Cortex. 2015;25:2763–2773. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coste CP, Kleinschmidt A. Cingulo-opercular network activity maintains alertness. Neuroimage. 2016;128:264–272. doi: 10.1016/j.neuroimage.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 70.Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 71.Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuhl BA, Chun MM. Successful remembering elicits event-specific activity patterns in lateral parietal cortex. J Neurosci. 2014;34:8051–8060. doi: 10.1523/JNEUROSCI.4328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J Neurosci. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davidson PS, et al. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim H. Default network activation during episodic and semantic memory retrieval: a selective meta-analytic comparison. Neuropsychologia. 2016;80:35–46. doi: 10.1016/j.neuropsychologia.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tulving E. Elements of Episodic Memory. Oxford Univ Press; 1983. [Google Scholar]
- 79.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 80.Vilberg KL, Rugg MD. The neural correlates of recollection: transient versus sustained fMRI effects. J Neurosci. 2012;32:15679–15687. doi: 10.1523/JNEUROSCI.3065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vincent JL, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 82.Andreasen NC, et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 83.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 84.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 87.Templer VL, Hampton RR. Episodic memory in nonhuman animals. Curr Biol. 2013;23:R801–R806. doi: 10.1016/j.cub.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 90.Uddin LQ, et al. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyashita Y. Inferior temporal cortex: where visual perception meets memory. Annu Rev Neurosci. 1993;16:245–263. doi: 10.1146/annurev.ne.16.030193.001333. [DOI] [PubMed] [Google Scholar]
- 92.Higuchi S, Miyashita Y. Formation of mnemonic neuronal responses to visual paired associates in inferotemporal cortex is impaired by perirhinal and entorhinal lesions. Proc Natl Acad Sci USA. 1996;93:739–743. doi: 10.1073/pnas.93.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miyamoto K, et al. Functional differentiation of memory retrieval network in macaque posterior parietal cortex. Neuron. 2013;77:787–799. doi: 10.1016/j.neuron.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 94.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 95.Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ratcliff R. A theory of memory retrieval. Psychol Rev. 1978;85:59–108. [Google Scholar]
- 97.Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sestieri C, et al. Memory accumulation mechanisms in human cortex are independent of motor intentions. J Neurosci. 2014;34:6993–7006. doi: 10.1523/JNEUROSCI.3911-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 100.Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev. 2010;34:1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seibert TM, Gimbel SI, Hagler DJ, Jr, Brewer JB. Parietal activity in episodic retrieval measured by fMRI and MEG. Neuroimage. 2011;55:788–793. doi: 10.1016/j.neuroimage.2010.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gonzalez A, et al. Electrocorticography reveals the temporal dynamics of posterior parietal cortical activity during recognition memory decisions. Proc Natl Acad Sci USA. 2015;112:11066–11071. doi: 10.1073/pnas.1510749112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tosoni A, Galati G, Romani GL, Corbetta M. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nat Neurosci. 2008;11:1446–1453. doi: 10.1038/nn.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Donner TH, Siegel M, Fries P, Engel AK. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr Biol. 2009;19:1581–1585. doi: 10.1016/j.cub.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 105.Gould IC, Nobre AC, Wyart V, Rushworth MF. Effects of decision variables and intraparietal stimulation on sensorimotor oscillatory activity in the human brain. J Neurosci. 2012;32:13805–13818. doi: 10.1523/JNEUROSCI.2200-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- 107.Patel GH, et al. Topographic organization of macaque area LIP. Proc Natl Acad Sci USA. 2010;107:4728–4733. doi: 10.1073/pnas.0908092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galletti C, Fattori P, Kutz DF, Gamberini M. Brain location and visual topography of cortical area V6A in the macaque monkey. Eur J Neurosci. 1999;11:575–582. doi: 10.1046/j.1460-9568.1999.00467.x. [DOI] [PubMed] [Google Scholar]
- 109.Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a ‘parietal reach region’ in the human brain. Exp Brain Res. 2003;153:140–145. doi: 10.1007/s00221-003-1587-1. [DOI] [PubMed] [Google Scholar]
- 110.Guerin SA, Miller MB. Parietal cortex tracks the amount of information retrieved even when it is not the basis of a memory decision. Neuroimage. 2011;55:801–807. doi: 10.1016/j.neuroimage.2010.11.066. [DOI] [PubMed] [Google Scholar]
- 111.Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 114.Scimeca JM, Badre D. Striatal contributions to declarative memory retrieval. Neuron. 2012;75:380–392. doi: 10.1016/j.neuron.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004;27:161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 116.Ho TC, Brown S, Serences JT. Domain general mechanisms of perceptual decision making in human cortex. J Neurosci. 2009;29:8675–8687. doi: 10.1523/JNEUROSCI.5984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kayser AS, Buchsbaum BR, Erickson DT, D’Esposito M. The functional anatomy of a perceptual decision in the human brain. J Neurophysiol. 2010;103:1179–1194. doi: 10.1152/jn.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


