Abstract
Objective
To investigate the modulated effects of HRF on cyclooxygenase isoform expression and its activity, using the human umbilical vein endothelial cell (HUVEC) model induced by interleukin-1 beta (IL-1β).
Methods
Cells were treated with indomethacin (positive control), HRF, and its components at various concentrations prior to treatment with IL-1β at 24 h. Cell viability was determined by MTT assay. Moreover, the anti-inflammatory effects of HRF and its components through mRNA and protein expression were established using real-time quantitative PCR and Western blot, respectively. COX activity was identified via exogenous and endogenous PGE2 productions using the EIA.
Result
There was no cytotoxicity in HUVECs treated with HRF. None of the experimental conditions used in the study affected the expression of COX-1, but COX-2 protein expression was inhibited at concentrations under 10 µg/mL. Despite the significantly increased levels of exogenous PGE2, HRF had no effect on COX-2 mRNA expression. However, the production of PGE2 was lower at a concentration of 100 µg/mL HRF than at a concentration below 10 µg/mL. Interestingly, each component of HRF revealed different effects of the Ha-Rak formula.
Conclusion
Our preliminary findings suggest that HRF and its components provide diverse modulation of COX-2 and PGE2 at the in vitro level.
1. Introduction
Fever, an excessively high body temperature, is a defensive mechanism of humans and is found as a clinical sign of inflammation [1]. Numerous endogenous and exogenous factors trigger a febrile response and lead to the release of various inflammatory mediators from immune and nonimmune cells, such as leukocytes, macrophages, and endothelial cells [2, 3]. These cells play an essential role in generating several proinflammatory cytokines, namely, interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factors (TNF), and interferons (IFNs), which act as inflammatory inducers in a febrile response [4–6].
IL-1, a proinflammatory cytokine, is a one of the endogenous pyrogens (EPs) implicated in fever induction. EPs are able to circulate in the blood vessels and activate prostaglandin E2 (PGE2) synthesis in the brain via cAMP and other neurotransmitter activation, resulting in an elevation of the thermostatic set point and, in turn, an increase in heat loss and temperature [4, 7]. Moreover, IL-1 is also involved in the synthesis of prostaglandins (PGs) and lipid eicosanoids through cyclooxygenase enzyme (COX) activity. COX is a key enzyme that present two isoforms (COX-1 and COX-2), both of which act as rate-limiting enzymes in PG biosynthesis by metabolizing arachidonic acid. COX-1 is commonly expressed in various cells and tissues and facilitates housekeeping functions, whereas COX-2 is induced by proinflammatory cytokines, growth factors, infections, and other harmful stimuli. Additionally, COX-2 is one of the inflammatory markers, and it is associated with fever-related diseases [8–10]. In clinical practice, nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin, indomethacin, diclofenac, and ibuprofen, are usually used to block COX activities and attenuate inflammatory responses, including fever [11, 12]. However, some toxicological effects stemming from the prolonged use of NSAIDs can cause side effects, such as hepatotoxicity, gastrointestinal irritation, renal impairment, and allergic reactions [13, 14].
Thai herbal Ha-Rak formula (HRF), also known as Bencha-Loga-Wichienis, is a polyherbal formula consisting of the roots of five medical plants: Capparis micracantha DC. (CM), Clerodendrum petasites S. Moore (CP), Harrisonia perforate Merr. (HP), Ficus racemosa L. (FR), and Tiliacora triandra Diels (TT). It has been traditionally used as an antipyretic and anti-inflammatory drug for fever treatment, and it is included in the National List of Essential Medicines of Thailand. Previous in vitro and in vivo studies have indicated that HRF shows diverse pharmacological effects, including antioxidant, [15] anti-inflammatory [16], antipyretic and antinociceptive properties [17]. Additionally, certain components of HRF, namely, the extracts from FR and CP, have been shown to exert anti-inflammatory and antipyretic effects [18–20]. Nevertheless, the modulated effects of HRF on COX activity and prostaglandin synthesis are still imprecise and need clarification. We therefore investigated the regulation of HRF and its components on COX inhibition, using IL-1β induced in the human umbilical vein endothelial cell (HUVEC) model.
2. Materials and Methods
2.1. Reagents
All powders of HRF and its components were prepared by the Manufacturing Unit of Herbal Medicines and Products Ayurved Siriraj, Center of Applied Thai Traditional Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University. The human endothelial-SFM basal growth medium with L-glutamine was obtained from Gibco (Gibco, USA). The human COX-1 and COX-2 monoclonal antibodies and standards were purchased from Cayman Chemical (Ann Arbor, MI, USA). The ethanol reagent was purchased from Scharlau (Scharlau, Spain). The fetal bovine serum (FBS), penicillin, streptomycin, indomethacin, and other chemical reagents were purchased from Sigma-Aldrich (MO, USA).
2.2. Preparation of HRF and Its Component Extracts
The herbal powders were extracted with an 80% ethanol solution at a ratio of 1 : 10 (w/v). All of the HRF and its component extractions were evaporated under 40°C at a pressure within 110–180 mbar (Buchi, Switzerland) and kept in a minus 80°C freezer prior to lyophilization. The freeze-dried extracts were stored in the dark in a controlled temperature and humidity environment.
2.3. Human Umbilical Vein Endothelial Cell Isolation and Treatment
HUVECs were derived from umbilical cords obtained from normal, pregnant women, as previously described [21]. The isolated HUVECs were cultured in T-75 flasks with human endothelial-SFM basal growth medium and with L-glutamine (Gibco, USA) supplemented with fetal bovine serum 10% (FBS), penicillin (100 U/mL), and streptomycin (100 mg/mL), at 37°C in 5% CO2 in an incubator. When the cells were over 80% confluent, the HUVECs were treated with indomethacin (100 µg/mL), HRF, and its component extracts at various concentrations prior to treatment with IL-1β (1 ηg/mL); they were subsequently incubated at 37°C in 5% CO2 for 24 h.
2.4. Cell Viability Assay
MTT assay, as previously described [22], was conducted to determine the cytotoxicity of the test compounds. Briefly, HUVECs (3 × 104 cells/well) were seeded on a 96-well plate and pretreated with HRF and the component extracts (0.00001–100 µg/mL) for 24 h; after that, 200 µL of MTT (200 µg/mL) was added to each well and incubated for 1 h. To dissolve formazan, 100 µL of DMSO solution was added to each well and measured using a spectrophotometer (SpectraMAX M5, Molecular Devices, CA) at an absorbance of 595 nm.
2.5. Real-Time Quantitative PCR Analysis (qRT-PCR)
The total RNA from each treatment was extracted with an Illustra RNA spin Mini RNA isolation kit (GE Healthcare, UK). All of the primer sequences are described in Table 1. The conditions are 95°C for 10 min and 95°C for 15 min, followed by 40 cycles of amplification at 60°C for 40 min, and, subsequently, at 72°C for 40 min. Analysis of the data was performed with the cycle threshold (Ct) method (ΔΔCt), normalized with the GAPDH gene used as a housekeeping gene and internal control.
Table 1.
List of primer sequences.
| Primer name | GenBank | Sense primer (5′ → 3′) | Anti-sense primer (5′ → 3′) |
|---|---|---|---|
| COX-1 | NM_001271368.1 | GACCCGCCTCATCCTCATAG-3 | CCACCGA TCTTGAAGGAGTCA |
| COX-2 | NM_006662.2 | CAAAAGCTGGGAAGCCTTCT | CCATCCTTGAAAAGGCGCAG |
| GAPDH | NM_001289746.1 | GACCACTTTGTCAAGCTCATTTCC | TGAGGGTCTCTCTCTTCCTCTTGT |
COX-1: cyclooxygenase-1; COX-2: cyclooxygenase-2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
2.6. Western Blot Analysis
HUVECs were treated with indomethacin, HRF, and the extracts (1, 10, and 100 µg/mL) prior to IL-1β (1 ηg/mL) stimulation for 24 h. The COX determination, including a Bradford protein assay, was performed as previously described [21]. Briefly, all samples were loaded into SDS-PAGE, underwent electrophoresis, and were transferred to nitrocellulose blotting membranes (Bio-Rad, Germany). After blocking with a solution of 5% skim milk for 1.5 h at room temperature, the membranes were incubated overnight with a specific monoclonal COX-1 or COX-2 antibody at 4°C and an anti-mouse IgG of COX-1 or anti-COX-2 (Sigma-Aldrich, USA, dilution 1/10000) for 1.5 h, respectively. β-Actin (Sigma-Aldrich, USA, dilution 1/5000) was used as an internal control in the experiment. The COX protein bands were visualized using VersaDoc™ Imaging Systems (Bio-Rad, Germany).
2.7. Determination of COX Activity
After treating the cells with the test compounds, the supernatant of each sample was collected after 24 h to measure the endogenous level of PGE2. To determine the exogenous PGE2 production, the medium from the HUVEC culture was discarded. The cells were washed with a phosphate-buffered saline (PBS) solution (138 mM NaCl; 2.7 mM KCl, 8 mM Na2HPO4; and 1.46 mM KH2PO4), incubated with a medium containing arachidonic acid (10 µM) for 10 min. The level of PGE2 was measured by using an enzyme immunoassay kit (GE Healthcare, UK).
2.8. Statistical Analysis
Data were presented as mean ± standard error of the mean (SEM). All experiments were performed in triplicate, and their results were analyzed by one-way analysis of variance (ANOVA), followed by Dunnett's post hoc test using GraphPad Prism version 5 for Windows (GraphPad Software Inc., San Diego, CA, USA). The statistically significantly value was set at p < 0.05.
3. Results
3.1. Cell Viability Assessments
The HUVECs were treated with HRF and its components at the increasing concentrations of 0.0001, 0.001, 0.01, 0.1, 1, 10, and 100 µg/mL (data not shown). At 100 µg/mL, the cell viabilities were higher than 90%, except for the cells treated with Ficus racemosa L. (Table 2). This result suggests that no obvious cytotoxicity was observed in the HUVECs incubated with HRF at up to 100 µg/mL.
Table 2.
Cell viability of 100 µg/mL of HRF and its components against HUVECs.
| Herbal | Cell viability (%) |
|---|---|
| Thai herbal Ha-Rak formula | 94 ± 5 |
| Harrisonia perforate Merr. | 120 ± 4 |
| Capparis micracantha DC. | 125 ± 3 |
| Clerodendrum petasites S. Moore | 99 ± 2 |
| Ficus racemosa L. | 58 ± 4 |
| Tiliacora triandra Diels | 99 ± 4 |
The data represent mean ± SEM of triplicate wells from at least 3 separate experiments performed on different days.
3.2. Inhibitory Effects of HRF and Its Components on COX mRNA Expression
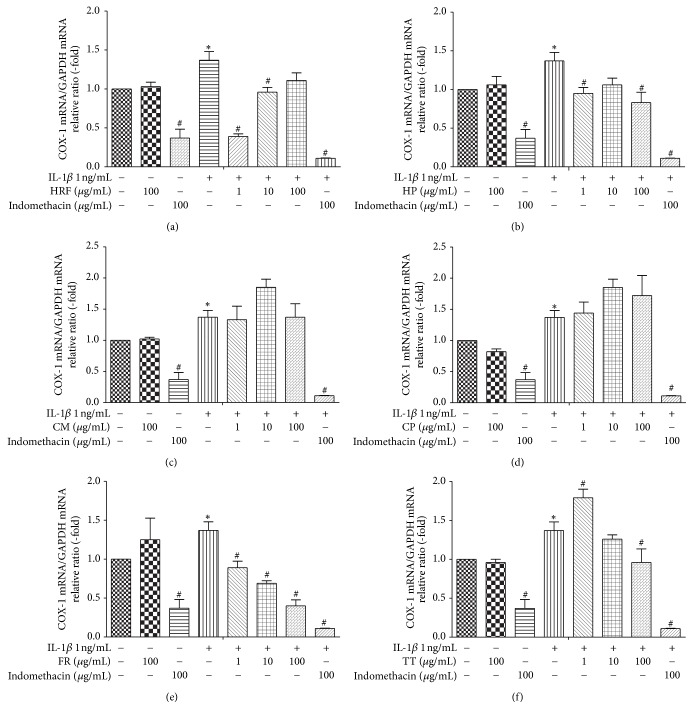
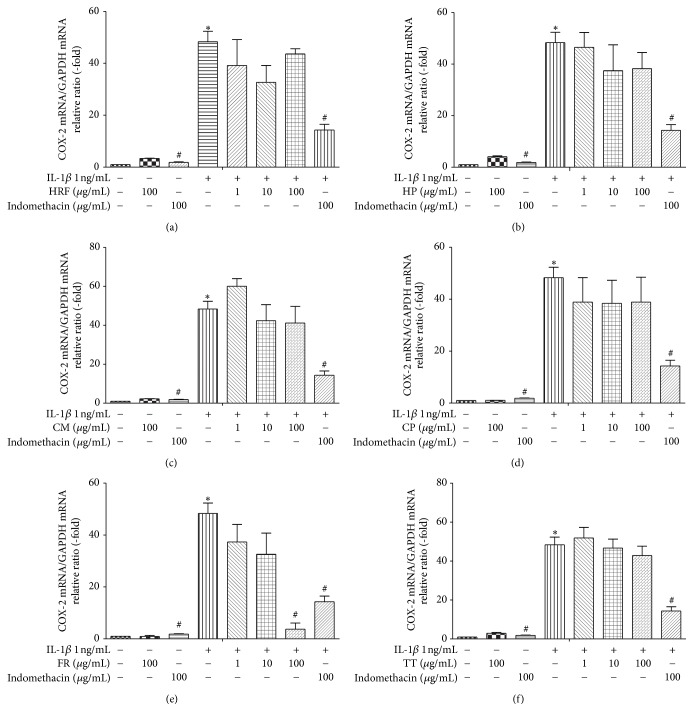
The results demonstrated that IL-1β (1 ηg/mL) significantly increased the COX1 and COX-2 mRNA expressions, relative to the untreated group of HUVECs (p < 0.05). Treatment with or without indomethacin (100 μg/mL) in the IL-1β-induced HUVECs significantly attenuated the mRNA expressions of COX-1 and COX-2 (p < 0.05; Figures 1 and 2).
Figure 1.
The effects of HRF (a) and its components: Harrisonia perforata Merr. (HP), Capparis micracantha DC. (CM), Clerodendrum petasites S. Moore (CP), Ficus racemosa L. (FR), and Tiliacora triandra Diels (TT) (b–f) (1, 10, and 100 μg/mL) on COX-1 mRNA expression in HUVECs treated with IL-1β 1 ng/mL for 24 h. Control: nonaddition. ∗p < 0.05, versus control group; #p < 0.05, versus IL-1β only.
Figure 2.
The effects of HRF (a) and its components: Harrisonia perforata Merr. (HP), Capparis micracantha DC. (CM), Clerodendrum petasites S. Moore (CP), Ficus racemosa L. (FR), and Tiliacora triandra Diels (TT) (b–f) (1, 10, and 100 μg/mL) on COX-2 mRNA expression in HUVECs treated with IL-1β 1 ng/mL for 24 h measured by qRT-PCR. Control: nonaddition. ∗p < 0.05, versus control group; #p < 0.05, versus IL-1β only.
Furthermore, treatment with the HRF (1, 10 μg/mL), TT (100 μg/mL), and FR (1, 10, and 100 μg/mL) extracts prior to the IL-1β challenge suppressed the COX-1 mRNA expression, with the FR extracts showing an inhibitory effect on COX-1 in a dose-dependent manner (Figures 1(a), 1(b), 1(e), and 1(f)). In addition, the highest dose of the FR extract (100 μg/mL) provided the greatest inhibition of COX-2 mRNA expression induced by IL-1β stimulation (Figure 2(e)). However, the other treatment compounds (HRF, HP, CM, TT, and CP extracts) showed a tendency to decrease the COX-2 mRNA levels in HUVECs induced by IL-1β (Figures 2(a), 2(b), 2(c), and 2(d)).
3.3. HRF and Its Components Attenuated COX Protein Expression
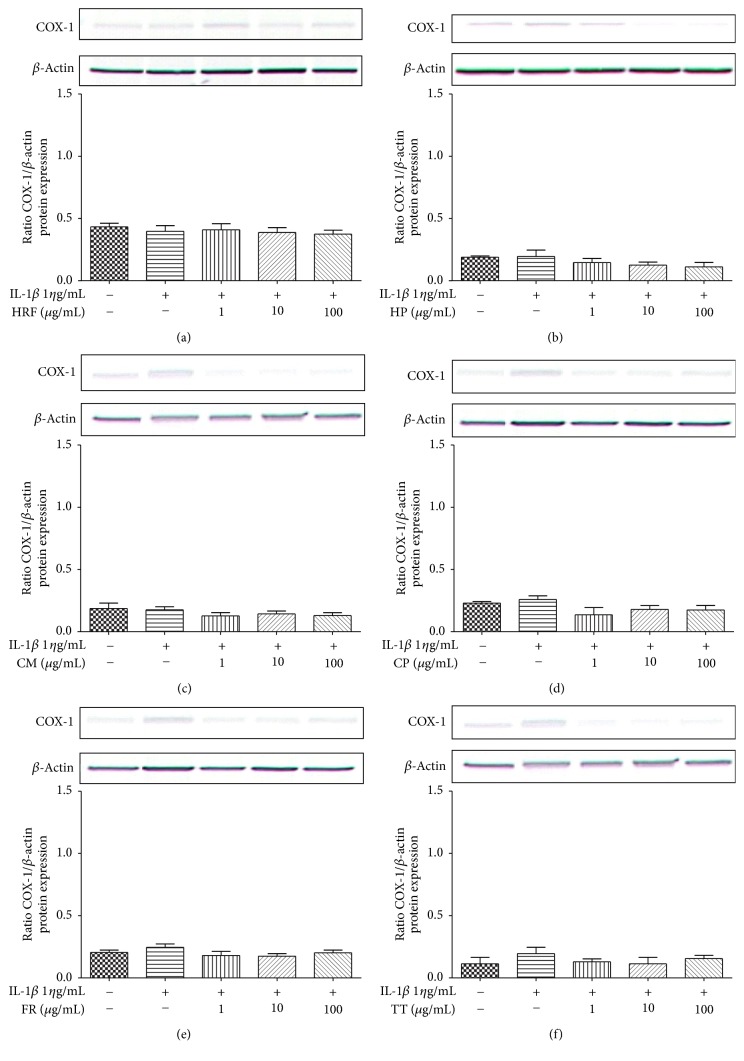
No statistically significant differences were observed in the COX-1 protein expression treated with HRF and its components prior to the IL-1β challenge (Figure 3).
Figure 3.
The effects of HRF (a) and its components: Harrisonia perforata Merr. (HP), Capparis micracantha DC. (CM), Clerodendrum petasites S. Moore (CP), Ficus racemosa L. (FR), and Tiliacora triandra Diels (TT) (b–f) on COX-1 protein expression in IL-1β-treated HUVECs for 24 h. COX protein was detected by Western blot. Control: nonaddition.
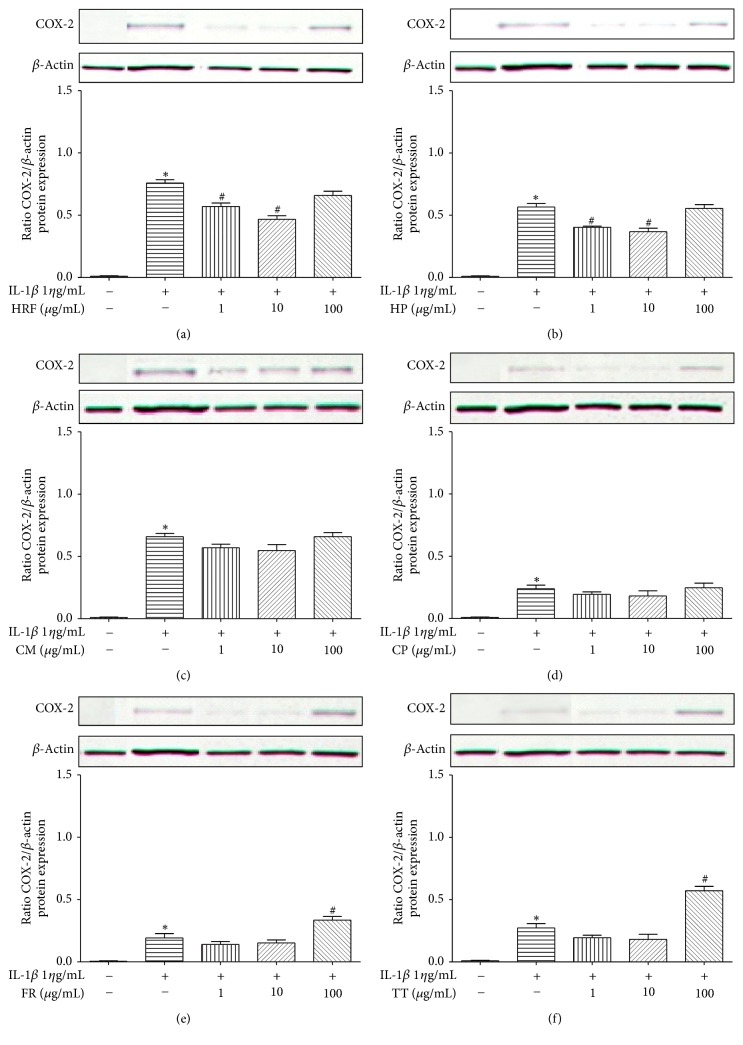
IL-1β (1 ηg/mL) noticeably induced COX-2 expression (p < 0.05) compared to the control groups (Figure 4). HRF (1 and 10 μg/mL) and HP (1 and 10 μg/mL) significantly decreased levels of COX-2 protein induced by IL-1β. However, the highest dose of HRF (100 μg/mL) and HP (100 μg/mL) extracts remarkably affected COX-2 protein inhibition (Figures 4(a) and 4(b)).
Figure 4.
The effects of HRF (a) and its components: Harrisonia perforata Merr. (HP), Capparis micracantha DC. (CM), Clerodendrum petasites S. Moore (CP), Ficus racemosa L. (FR), and Tiliacora triandra Diels (TT) (b–f) on COX-2 protein expression in IL-1β-treated HUVECs for 24 h. COX protein was detected by Western blot. Control: nonaddition. ∗p < 0.05, versus control group; #p < 0.05, versus IL-1β only.
3.4. Effects of HRF and Its Components on COX Activity through Endogenous and Exogenous PGE2 Production
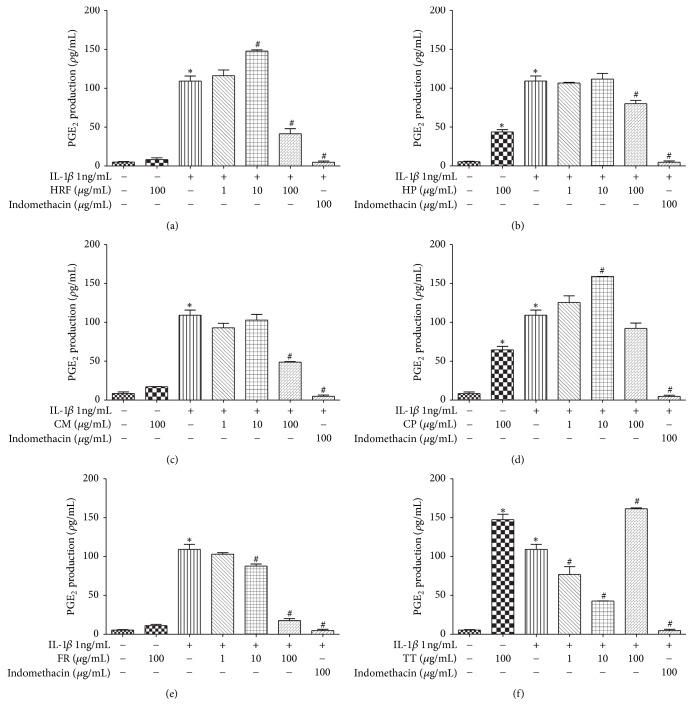
HRF (100 µg/mL), HP (100 µg/mL), CM (100 µg/mL), FR (10 and 100 µg/mL), and TT (1 and 10 µg/mL) extracts significantly restrained endogenous PGE2 production (Figures 5(a)–5(c), 5(e), and 5(f)). Meanwhile, HRF (10 µg/mL) and CP (10 µg/mL) noticeably increased PGE2 accumulation (Figures 5(a) and 5(d)) in IL-1β induced HUVECs (p < 0.05).
Figure 5.
The effects of HRF (a) and its components: Harrisonia perforata Merr. (HP), Capparis micracantha DC. (CM), Clerodendrum petasites S. Moore (CP), Ficus racemosa L. (FR) and Tiliacora triandra Diels (TT) (b–f) on COX activity in the presence of endogenous AA in IL-1β-treated HUVECs. The data represent mean ± SEM of three experiments. Control: nonaddition. ∗p < 0.05, versus control group; #p < 0.05, versus IL-1β only.
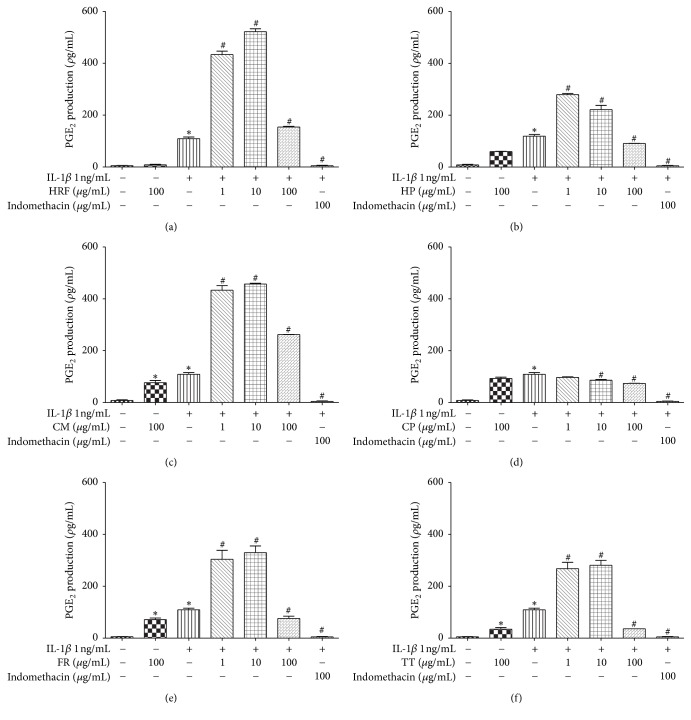
As for exogenous PGE2 generation, the results indicated that all concentrations of the test compounds, including HRF and its components, significantly enhanced COX activity, whereas CP (10 and 100 µg/mL) significantly restrained exogenous PGE2 production (Figure 6).
Figure 6.
The effects of HRF (a) and its components: Harrisonia perforata Merr. (HP), Capparis micracantha DC. (CM), Clerodendrum petasites S. Moore (CP), Ficus racemosa L. (FR), and Tiliacora triandra Diels (TT) (b–f) on COX activity in the presence of exogenous AA in IL-1β-treated HUVECs. The data represent mean ± SEM of three experiments. Control: nonaddition. ∗p < 0.05, versus control group; #p < 0.05, versus IL-1β only.
4. Discussion
We have presented the modulatory effects of HRF and its components by focusing on the COX-PGE2 pathway related to the febrile response in the HUVEC model. Several scientific reports have indicated that many cytokines and other mediators, including IL-1β, play a critical role in fever induction through increased levels of PGE2 in the hypothalamic thermoregulatory center [23]. Moreover, the COX-PGE2 pathway is also responsible for inflammatory response development [24]. Cyclooxygenase enzymes (COXs), comprising two isoforms (COX-1 and COX-2), are the key enzymes in prostaglandin generation [25]. The induction of COX-2 by several proinflammatory cytokines represents an important mechanism controlling the overall production of prostanoids and the evolution of the inflammatory response [26]. Our results also confirm a previous report that IL-1β can activate COX-2 mRNA and protein expressions and can consequently generate PGE2 production in the HUVEC model [27]. In this study, we also observed the effect of IL-1β on endogenous and exogenous PGE2 releases through COX-2 expression in HUVECs. We found that IL-1β could mediate endogenous and exogenous PGE2 production through COX-2 metabolites [26]. Thus, the anti-inflammatory effect is probably due to IL-1β's ability to inhibit the COX-2 enzyme [28]. After the endogenous PGE2 production was measured, exogenous AA was used to treat the cells. The amount of PGE2 measured in this media reflects the level of PGE2 synthesis by the exogenous AA and also the COX activity [29]. Interestingly, HRF significantly enhanced exogenous AA, leading to the upregulation of COX-2 expression induced by IL-1β (Figure 6(a)). These results suggest that the use of exogenous AA as substrates will help to evaluate the COX enzyme directly [30].
In this study, HRF and its components did inhibit the COX-2 protein expression in HUVECs (Figure 4), but the amount of COX-1 protein expression was not affected. The predicted modulatory effects of HRF present in the protein clearly suggest its potential of being an inhibitor of COX-2. Our findings demonstrate that the formula and some components can modulate COX isoforms, as found in previous reports; for instance, Clerodendrum petasites S. Moore showed an inhibition of COX-2-mediated. PGE2 production in vitro [31] and a bioassay-guided fractionation of the ethanol extract of Ficus racemosa L. showed potent inhibitory activity against both COX-1 and 5-LOX in vitro [19].
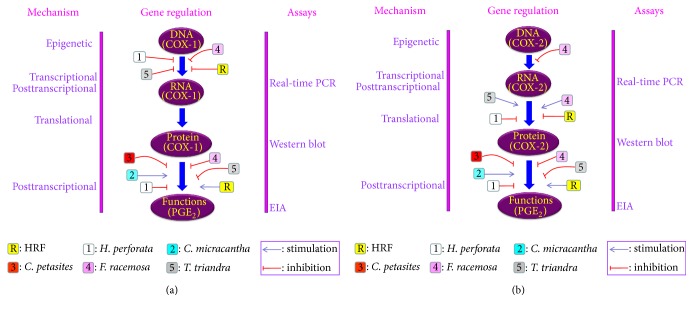
Our findings provide preliminary evidence that HRF and its components can modulate the COX enzymes directly involved with PGE2 production in IL-1β-induced in HUVECs (Figure 7).
Figure 7.
Summary of the effects of HRF and its components on COX-1 (a) and COX-2 (b) gene regulation pathway in HUVECs.
However, further studies to elucidate HRF's pharmacological effects and other signaling pathways involving its anti-inflammatory and antipyretic potentials should be investigated to support its traditional usage and clinical application and to develop HRF as a novel antipyretic agent in the future.
Acknowledgments
This research was supported by the Thailand Research Fund (Grant no. DBG5380040) and the Chalermphrakiat Grant, Faculty of Medicine, Siriraj Hospital, Mahidol University.
Ethical Approval
The protocol of this study was approved by the Ethics Committee on Research Involving Human Subjects, Faculty of Medicine, Mahidol University (EC no. Si412/2011).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Harden L. M., Kent S., Pittman Q. J., Roth J. Fever and sickness behavior: friend or foe? Brain, Behavior, and Immunity. 2015;50:322–333. doi: 10.1016/j.bbi.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Evans S. S., Repasky E. A., Fisher D. T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nature Reviews Immunology. 2015;15(6):335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao C., Matsumura K., Yamagata K., Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1β: A possible site of prostaglandin synthesis responsible for fever. Brain Research. 1996;733(2):263–272. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- 4.Netea M. G., Kullberg B. J., Van der Meer J. W. M. Circulating cytokines as mediators of fever. Clinical Infectious Diseases. 2000;31(5):S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello C. A., Cannon J. G., Wolff S. M. New concepts on the pathogenesis of fever. Reviews of Infectious Diseases. 1988;10(1):168–189. doi: 10.1093/clinids/10.1.168. [DOI] [PubMed] [Google Scholar]
- 6.Shah A., Unger E., Bain M. D., et al. Cytokine and adhesion molecule expression in primary human endothelial cells stimulated with fever-range hyperthermia. International Journal of Hyperthermia. 2002;18(6):534–551. doi: 10.1080/02656730210157843. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello C. A. Infection,fever, and exogenous and endogenous pyrogens: some concepts have changed. Journal of Endotoxin Research. 2004;10(4):201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 8.Vane J. R., Bakhle Y. S., Botting R. M. Cyclooxygenases 1 and 2. Annual Review of Pharmacology and Toxicology. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Ricciotti E., Fitzgerald G. A. Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell J. A., Akarasereenont P., Thiemermann C., Flower R. J., Vane J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proceedings of the National Acadamy of Sciences of the United States of America. 1993;90(24):11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons D. L., Wagner D., Westover K. Nonsteroidal anti-inflammatory drugs, acetaminophen, cyclooxygenase 2, and fever. Clinical Infectious Diseases. 2000;31:S211–S218. doi: 10.1086/317517. [DOI] [PubMed] [Google Scholar]
- 13.Bjorkman D. Nonsteroidal anti-inflammatory drug-associated toxicity of the liver, lower gastrointestinal tract, and esophagus. American Journal of Medicine. 1998;105(5 A):17S–21S. doi: 10.1016/S0002-9343(98)00276-9. [DOI] [PubMed] [Google Scholar]
- 14.Asero R. Single NSAID hypersensitivity is associated with atopic status. European Annals of Allergy and Clinical Immunology. 2015;47(2):48–53. [PubMed] [Google Scholar]
- 15.Pluemsamran T., Tripatara P., Phadungrakwittaya R., Akarasereenont P., Laohapand T., Panich U. Redox mechanisms of AVS022, an oriental polyherbal formula, and its component herbs in protection against induction of matrix metalloproteinase-1 in UVA-irradiated keratinocyte HaCaT cells. Evidence-Based Complementary and Alternative Medicine. 2013;2013:10. doi: 10.1155/2013/739473.739473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juckmeta T., Itharat A. Anti-inflammatory and antioxidant activities of thai traditional remedy called “ya-ha-rak”. Journal of Health Research. 2012;26(4) [Google Scholar]
- 17.Konsue A., Sattayasai J., Puapairoj P., Picheansoonthon C. Antipyretic effects of Bencha-Loga-Wichien herbal drug in rats. Thai Journal of Pharmacology. 2008;29(1):79–82. [Google Scholar]
- 18.Panthong A., Kanjanapothi D., Taesotikul T., Wongcome T., Reutrakul V. Anti-inflammatory and antipyretic properties of Clerodendrum petasites S. Moore. Journal of Ethnopharmacology. 2003;85(1):151–156. doi: 10.1016/S0378-8741(02)00368-9. [DOI] [PubMed] [Google Scholar]
- 19.Li R. W., Leach D. N., Myers S. P., Lin G. D., Leach G. J., Waterman P. C. A new anti-inflammatory glucoside from ficus racemosa L. Planta Medica. 2004;70(5):421–426. doi: 10.1055/s-2004-818969. [DOI] [PubMed] [Google Scholar]
- 20.Rao R. B., Anupama K., Swaroop K. R. L. A., Murugesan T., Pal M., Mandal S. C. Evaluation of anti-pyretic potential of Ficus racemosa bark. Phytomedicine. 2002;9(8):731–733. doi: 10.1078/094471102321621340. [DOI] [PubMed] [Google Scholar]
- 21.Akarasereenont P., Techatraisak K., Chotewuttakorn S., Thaworn A. The expression of cyclooxygenase-2 in human umbilical vein endothelial cell culture from preeclampsia. Journal of the Medical Association of Thailand. 1999;82(2):167–171. [PubMed] [Google Scholar]
- 22.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Dinarello C. A., Gatti S., Bartfai T. Fever: Links with an ancient receptor. Current Biology. 1999;9(4):R147–R150. doi: 10.1016/S0960-9822(99)80085-2. [DOI] [PubMed] [Google Scholar]
- 24.Seibert K., Zhang Y., Leahy K., et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proceedings of the National Acadamy of Sciences of the United States of America. 1994;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons D. L., Botting R. M., Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacological Reviews. 2004;56(3):387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 26.Bonazzi A., Bolla M., Buccellati C., et al. Effect of endogenous and exogenous prostaglandin E2 on interleukin-1β-induced cyclooxygenase-2 expression in human airway smooth-muscle cells. American Journal of Respiratory and Critical Care Medicine. 2000;162(6):2272–2277. doi: 10.1164/ajrccm.162.6.2003127. [DOI] [PubMed] [Google Scholar]
- 27.Akarasereenont P., Techatrisak K., Chotewuttakorn S., Thaworn A. The induction of cyclooxygenase-2 in IL-1β-treated endothelial cells is inhibited by prostaglandin E2 through cAMP. Mediators of Inflammation. 1999;8(6):287–294. doi: 10.1080/09629359990298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turini M. E., DuBois R. N. Cyclooxygenase-2: a therapeutic target. Annual Review of Medicine. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 29.Plasen D. The roles of cyclooxygenase and nitric oxide synthase on acute effects of lipid component activated endothelial cells. 2001.
- 30.Zarghi A., Arfaei S. Selective COX-2 inhibitors: a review of their structure-activity relationships. Iranian Journal of Pharmaceutical Research. 2011;10(4):655–683. [PMC free article] [PubMed] [Google Scholar]
- 31.Deep P., Ansari M. T., Singh A. K., Raghav P. Pharmacological Potentials of Ficus racemosa - a Review. Journal of Pharmaceutical Sciences Review and Research. 2013;22(1):29–34. [Google Scholar]