Abstract
Nanofibrous matrices hold great promise in skin wound repair partially due to their capability of recapturing the essential attributes of native extracellular matrix (ECM). With regard to limited studies on the effect of nanofibrous matrices on keratinocytes, the present study was aimed to understand how the topographical feature of nanofibrous matrices regulates keratinocyte motility by culturing keratinocytes on polycaprolactone (PCL)/collagen nanofibrous matrices (rough surface with fiber diameters of 331±112 nm) or the matrices coated with a thin layer of collagen gel to form a secondary ultrafine fibrous network (smooth surface with ultrafine fiber diameters of 55±26 nm). It was found that the PCL/collagen nanofibrous matrices alone did not stimulate cell migration, while collagen gel coating could significantly increase cell motility. Further studies demonstrated that the ultrafine fibrous network of collagen gel coating significantly activated integrin β1, Rac1 and Cdc42, facilitated the deposition of laminin-332 (formerly called laminin-5), and promoted the expression of active matrix metalloproteinases (MMPs) (i.e., MMP-2 and 9). Neutralization of integrin β1 activity abrogated the gel coating-induced keratinocyte migration. These findings provide important evidence on the role of topographical features of nanofibrous matrices in regulating the phenotypic alteration of keratinocytes and suggest the possible utility of collagen-containing nanofibrous matrices for skin regeneration especially in re-epithelialization.
Keywords: nanofibers, human keratinocytes, topography, reepithelialization, integrin β1, Rac1, Cdc42, laminin-332 (laminin-5)
1. Introduction
In skin wound repair, rapid restoration of the epithelial barrier function is crucial to minimize potential threats from the environment such as bacterial invasion and dehydration [1]. Extensive efforts have been made to regenerate epidermis by transplanting cultured epithelial cell sheets [2–5], grafting meshed split skin [6, 7], using tissue-engineered skin grafts with an epidermal layer [8–10], or stimulating the re-epithelialization from wound edge or intact hair follicles [1, 11]. In either approach, complete re-epithelialization cannot be achieved until further migration of keratinocytes from the graft and wound edge to bridge the open wound surface.
In recognition of the importance of keratinocyte migration in wound re-epithelialization, it is highly desirable to create a microenvironment with the ability to promptly recruit autologous keratinocytes from the intact wound edge and promote their migration for faster re-epithelialization. Electrospun nanofibrous matrices, featuring high surface-to-volume ratio, high porosity and good interconnectivity, have great potential to mimic the skin ECM in both morphology and composition [12,13]. It has been reported that nanofibrous matrices support the adhesion and spreading of normal human keratinocytes [14]. However, the most critical behavior of keratinocyte in wound healing, i.e., migration on electrospun nanofibrous matrices has never been studied.
The activation of keratinocyte migration can initiate soon after injury, while its migration rate significantly relies on the wound bed and underlying ECM [15–17] and involves the synergistic coordination among several cellular events including cell attachment/detachment, cytoskeletal reorganization, ECM degradation and redistribution of membrane integrins [16,18, 19]. It has been found that ECM molecules like collagen type I and type IV, laminin, fibronectin and vitronectin can regulate keratinocyte migration by interacting with various integrins on the cell surface [16,17, 20–22]. In this regard, a plausible strategy for creating skin grafts is to incorporate relevant ECM molecules in the scaffolds, for example, either directly incorporated into electrospun nanofibers or coated onto the fiber surface post-electrospinning [23], to promote keratinocyte proliferation and migration. Furthermore, transplantation of such grafts to the wound bed can also formulate a stimulatory microenvironment to recruit autologous keratinocytes for an accelerated re-epithelialization. Upon injury, keratinocytes of the epidermis are activated and migrate onto dermis, which is rich with type I collagen fibrils [16, 17, 24], suggesting the stimulatory effect of type I collagen on keratinocyte migration. Previous results showed that type I collagen-containing electrospun nanofibers promote the adhesion and spreading of human epidermal keratinocytes and support the formation of epidermal layers [25]. However, electrospun collagen fibers normally have diameters ranging from 100 to 1200 nm [25, 26], much larger than collagen fibrils in dermis (~50 nm in diameter), which would result in different surface roughness and subsequently induce differential keratinocyte migratory response. In this regard, it becomes necessary to determine whether electrospun nanofibers are optimal for wound repair, especially for rapid re-epithelialization, and if not, what kind of modification should be made to further improve them.
In this study, collagen-containing nanofibrous matrices with two different topographical features, i.e., electrospun polycaprolactone (PCL)/collagen nanofibrous matrices with an average fiber diameter of 331±112 nm and electrospun PCL/collagen nanofibrous matrices coated with a thin layer of type I collagen gel to form a secondary ultrafine fibrous network (average diameter of 55±26nm) bridging the electrospun fibers, were fabricated. The proliferation, morphology and migration of human epidermal keratinocytes on both nanofibrous matrices were side-by-side studied.
2. Materials and Methods
2.1. Materials
1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) was obtained from Oakwood products Inc (West Columbia, SC). Poly(epsilon-caprolactone) (PCL, Mw=80,000) was purchased from Sigma-Aldrich (St. Louis, MO) and type I collagen (Coll) was obtained from Elastin Products Inc. (Owensville, MO). All other reagents and solutions were obtained from Invitrogen (Carlsbad, CA) except as indicated.
2.2. Nanofibrous matrix preparation, modification and characterization
Nanofibrous matrices of collagen or PCL/Coll were fabricated using the electrospinning technique following the same procedures as previously described [27]. Briefly, 8% (w/v) pure collagen or 3:1 PCL/Coll (w/w) blend solutions were prepared by dissolving PCL and collagen in HFIP. Then, the solution was loaded into a 5-mL syringe with a 20-gauge blunt-tip stainless steel needle and electrospun at 15 kV using a customized electrospinning apparatus. The polymer solution was dispensed using a syringe pump (Kdscientific, Holliston, MA) at 10 μL/min. Nanofibrous matrices collected onto round glass cover slips (Ø=12mm) under sterile conditions were used for the studies unless indicated. Then, PCL/Coll nanofibrous matrices were placed in 24-well culture plates.
To modify the electrospun nanofibrous matrices with collagen gel, fresh collagen solution was prepared by dissolving type I collagen in 0.01N HCl and stored at 4°C for further use. To modify the PCL/Coll nanofibrous matrices, prepared collagen solution was diluted in PBS at a ratio of 4:1 and then neutralized by adding 0.1N NaOH on ice. The neutralized collagen solution with a final concentration of 3 mg/mL was added onto the PCL/Coll nanofibrous matrix surface and then immediately removed as much as possible. After incubation for 1 h at 37°C for a complete gelation, the matrices were washed with PBS and then immediately used for cell culture or for characterization as described below. To better compare the effect of substrate surface on keratinocyte behavior, two additional groups were also included: collagen adsorbed PCL/Coll nanofibrous matrices and electrospun collagen nanofibrous matrices. For the former, a neutralized collagen solution of 50 μg/mL was added onto PCL/Coll nanofibrous matrix surface and incubated for 2 hours at room temperature for adsorption. After adsorption, the meshes were washed with PBS and used for cell culture or further characterization.
Scanning electron microscopy (SEM) and atomic force microscopy (AFM) were used to characterize these nanofibrous matrices. For SEM examination, the dehydrated samples were sputter-coated with gold and then examined with a LEO 982 FEG SEM. To determine the diameter of nanofibers, images of five randomly selected areas were captured and analyzed by analysis software (NIS-elements BR 3.10, Nikon, Japan). The surface roughness of different nanofibrous matrices was determined by using Aglient NanoR AFM in a “dynamic force mode”, that is, a “tapping mode.” Three randomly selected areas of the surface with the size of 10×10 μm (x direction) were scanned,, y respectively. Close contact mounted cantilevers (Pacific Nanotechnology, Santa Clara, CA) were used. To describe the topography and roughness of the substrates, the roughness parameter for the surface, Rms, the root-mean-square height of the surface, was calculated by NanoRule.
2.3. Cell and cell culture
Human keratinocytes immortalized by expressing the catalytic subunit of telomerase were a gift from Dr. James Rheinwald (NIH Harvard Skin Disease Research Center). Cells were plated in serum-free keratinocyte medium supplemented with recombinant EGF (2.5 μg/500 mL), bovine pituitary extract (25 mg/500 mL), 0.3 mM CaCl2, 100 μg/mL streptomycin and 100 IU/mL of penicillin (Sigma, St Louis, MO) and cultured at 37°C in a humidified 5% CO2 atmosphere. Medium was replaced every 48 h until they reached 50–60% confluence.
Keratinocytes were seeded onto various nanofibrous matrices or glass cover slips at a density of 5×103 cells/cm2 and allowed to attach for 60 min prior to the addition of fully supplemented medium. The cells were further cultured for up to 7 days. The effect of nanofibrous matrices on cell behaviors was determined throughout the 1-week culture period.
2.4. Cell migration assay
In vitro CytoSelect™ 24-Well Wound Healing Assay Kit purchased from Cell Biolabs Inc. (San Diego, CA) was used to evaluate the migratory capacity of keratinocytes. Briefly, 2×105 keratinocytes suspended in the culture media were seeded onto various matrix surfaces with inserts in place. After culturing for 12 h, the insert was removed to generate a consistent 0.9 mm wound gap among the cells. Cells were allowed to migrate into the wound gap for 24 and 48 h. After staining the cells with methylene blue, images of the wound gap were taken to analyze the migration distance of keratinocytes. 10 representative points along the “wound” of each sample were used to evaluate average migration (n=4).
To determine the involvement of integrin β1 in regulating keratinocyte migration, anti-integrinβ1 antibody (Millipore, Clone P4C10, 1:200) was added to the culture right after the removal of insert to neutralize the integrin β1 function.
2.5. Cell proliferation
Cell proliferation on various substrates was measured using CyQUANT® Cell Proliferation Assay Kit (Molecular Probes, Inc., Eugene, OR) following the protocol suggested by the manufacturer. Briefly, A standard curve over cell number was first established (y=0.007x-2.977, R2=0.995). Samples were then harvested on day 1, 3 and 7 (n=4 per group for each time point). After removal of medium and washing with PBS, the samples were frozen and stored at −80°C. After collecting all the time-point samples, the samples were lysed in CyQUANT® cell-lysis buffer for 1 h at room temperature and then 200 μL of CyQUANT® GR dye/cell-lysis buffer was added to each sample and incubated for 2–5 minutes in the dark at room temperature. The fluorescence of cell lysates was measured using the multi-mode BioTek microplate reader at ~480 nm excitation and ~520 nm emission. Cell numbers were calculated based on the standard curve.
2.6. Immunofluorescent microscopy
Immunofluorescent staining of cells was performed following the procedures previously described [28]. Briefly, cultured cells were fixed in 4% paraformaldehyde for 10 minutes and then permeabilized with 0.2% Triton X-100 in PBS. Antibodies used in the study were as follows: phalloidin-TRITC (Sigma, 1:500), anti-vinculin-FITC (Sigma, 1:200), antibody against the active conformation of β1 integrin (Millipore, clone HUTS-4, 1:300) and anti-laminin-332 (also known as anti-laminin-5, Millipore, clone P3H9-2, 1:200). For those primary antibodies without fluorescent labels, the cells were further incubated with goat anti-mouse IgG-FITC conjugate secondary antibody (Caltag, 1:400). Cell nuclei were stained with DAPI (Sigma, 1:1000). The staining was examined under a Nikon Eclipse 80i epifluorescent microscope. The fluorescence intensity of the lysate of cells stained for active integrin β1 was also quantified by using a multi-mode microplate reader (Synergy™ HT, BioTek Instruments, Inc., Winooski, VT).
2.7. Determination of polarized keratinocytes
Keratinocytes were seeded on various matrices at a density of 2×104 cells/cm2 and cultured for 24 h and then fixed with 4% formaldehyde. After staining the cells with TRITC-phalloidin and DAPI, images were taken from three randomly selected fields containing approximately 200 cells for each culture well. To score a cell as polarized, the cell needed to possess two defined morphological features, including (1) elongation and (2) the presence of long cytoplasmic extensions (lamellipodia and filopodia). Cells that did not show these two features but spread were counted as nonpolarized. Cells that were not spread were not counted.
2.8. Zymography
The expression of MMP-2 and MMP-9 in keratinocytes was analyzed by zymography as described previously [29, 30]. Briefly, supernatants of each culture were collected on day 1, 3 and 7 and stored at −80°C for further analysis. The protein concentration of each sample was determined by bicinchoninic acid assay (BCA protein assay kit; Pierce, Rockford, IL). Equal amounts of proteins (5μg) were loaded on 10% sodium dodecyl sulfate (SDS)–polyacrylamide gels (Sigma) containing 1% gelatin (Sigma) and then separated by electrophoresis on ice (at 125 V for 120 min). The proteins were renatured in the renaturing buffer (Biorad, Hercules, CA) at room temperature for 1h, allowing gelatinases to cleave the gelatin substrate copolymerized in the gel. The gel was then incubated with developing buffer (Biorad) at 37°C overnight. Afterwards, the gel was stained with 0.5% Coomassie blue for 30 min, and then destained with distilled water for appropriate color contrast. Clear bands were indicative of the location of proteolytic activity. The identities of the MMPs were based on their molecular weights.
2.9. Measurement of the activity of integrin β1 by flow cytometry
Analysis of cell surface expression of active integrin β1 by flow cytometry was performed as described previously [31]. 1×106 fibroblasts seeded on various matrices were trypsinized and washed with FACS buffer (5% BSA, 0.1% sodium azide in PBS). Cells were resuspended and incubated with 2.5 μg HUTS-4 or isotype-matched IgG (IgG2b) negative control (Millipore) for 1h at 4°C. After washing with the FACS buffer, the cells were labeled with FITC-conjugated secondary antibody for 1h at 4°C. Cells were then washed and analyzed with the Cell Lab Quanta™ SC MPL flow cytometer using Quanta analysis program (Beckman Coulter, USA). Approximately 5,000 cells were analyzed in each experiment, and the results shown were representative of three independent experiments.
2.10. Measurement of Rac1 and Cdc42 activation by affinity pull-down assay
The activation of Rac1 and Cdc42 was measured based on the specific interaction of activated Rac1 and Cdc42 with their effectors PAK1 p21-binding domain (PBD) and N-WASP GTPase binding domain (GBD), respectively [32]. Briefly, PBD and GBD cDNAs in the pGEX-2T plasmid were expressed in Escherichia coli, and bacteria were lysed by sonication. The PBD-GST and GBD-GST fusion proteins were isolated with glutathione-agarose beads (GE Healthcare Life Sciences). Cell lysates containing 1 mg of proteins were incubated with 15 μg of the bead-bound GST fusion proteins at 4°C for 60 min. Affinity-purified proteins were separated by 12% SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and immunoblotted using Rac1 and Cdc42 antibodies. Bound primary antibody was detected with anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody and chemiluminescence detection reagents. The bands were assessed by densitometry (Image J), and the amount of PBD-bound Rac1 and GBD-bound Cdc42 was compared with that of total cellular Rac1 and Cdc42 determined by direct immunoblot analysis.
2.11. Statistical Analysis
Each experiment was repeated at least 3 times on different time points and data were expressed as the mean ±SD. All the cytotoxicity and cell attachment measurements were collected in triplicate for each group. Unpaired student t-test was used to evaluate the significance between experimental groups. A value of p<0.05 was considered statistically significant.
3. Results
Characterization of nanofibrous matrices
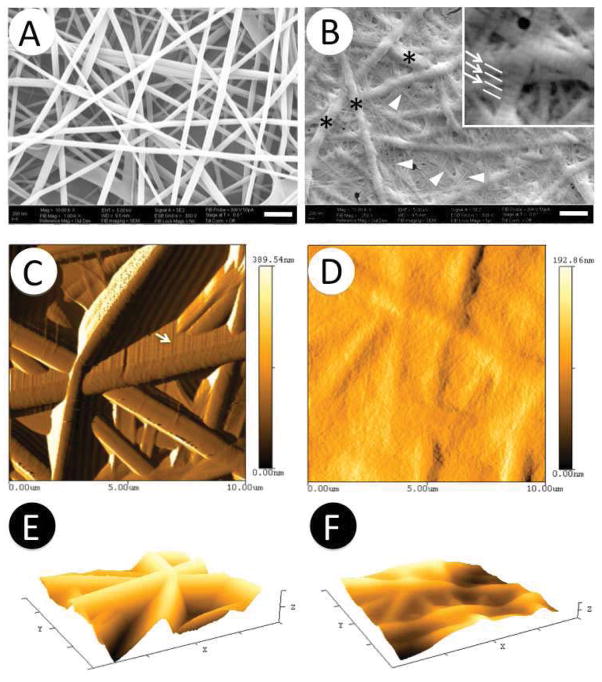
Using the established electrospinning condition as described in Materials and Methods, electrospun PCL/Coll nanofibers collected on the glass coverslips had a random arrangement with an average diameter of 331±112 nm (Figure 1A and Table 1). Distinctively, the coating of PCL/Coll electrospun nanofibrous matrices with 3mg/mL collagen gel yielded a secondary fiber network (arrowhead in Figure 1B and Table 1) with an average diameter of 55±26 nm. These ultrafine fibers of collagen gel evenly distributed among the interstitial space of PCL/Coll nanofibers (asterisks in Figure 1B) in a random fashion to bridge the PCL/Coll nanofibers and formed a smoother surface. Close examination of these ultrafine fibers revealed the characteristic D-banding patterns with a typical length in the range of 64 to 67 nm (as shown by arrows in Figure 1B inset), similar to those of native collagen fibrils. Measurement of collagen gel-coated PCL/Coll fibrous matrices by AFM further confirmed that ultrafine fibers of collagen gel indeed filled the interstitial space of PCL/Coll nanofibrous matrices and led to a rather smooth surface (Figure 1D). In order to better characterize the roughness of these matrices, roughness parameters (Rrms, root mean square roughness) were determined by using Nanorule software supplied with AFM. As shown in Table 1, Figure 1E and 1F, Rrms of PCL/Coll nanofibrous matrices alone was 596±15 nm, while it decreased to 269±7 nm after coating with collagen gel. The actual difference on the surface roughness between collagen gel-coated and noncoated PCL/Coll nanofibrous matrices could be even more pronounced without artifacts that appeared in the images of PCL/Coll nanofibrous matrices (arrows in Figure 1C).
Figure 1.
Characterization of PCL/collagen nanofibrous matrix alone (A, C, E) and with collagen gel coating (B, D, F). A–B) SEM micrographs; C–D) Two-dimensional AFM micrographs; E–F) Three-dimensional AFM micrographs. Arrowheads: collagen gel ultrafine fibers; Asterisks: Electrospun PCL/collagen nanofibers, Arrows in B: D- banding patterns of collagen gel ultrafine fibers. Scale: 1μm
Table 1.
Characterization of PCL/collagen nanofibrous matrices alone or with collagen gel coating.
| Nanofiber | Gel coating | |
|---|---|---|
| Composition | PCL/collagen nanofibers | PCL/collagen nanofibers coated with 3mg/mL collagen gel |
| Average fiber diameter (nm) | 331±112* | 55±26* |
| Roughness Rrms (nm) | 596±15* | 269±7* |
Statistically significant, p<0.001
Accelerated keratinocyte migration on collagen gel-coated nanofibrous matrices
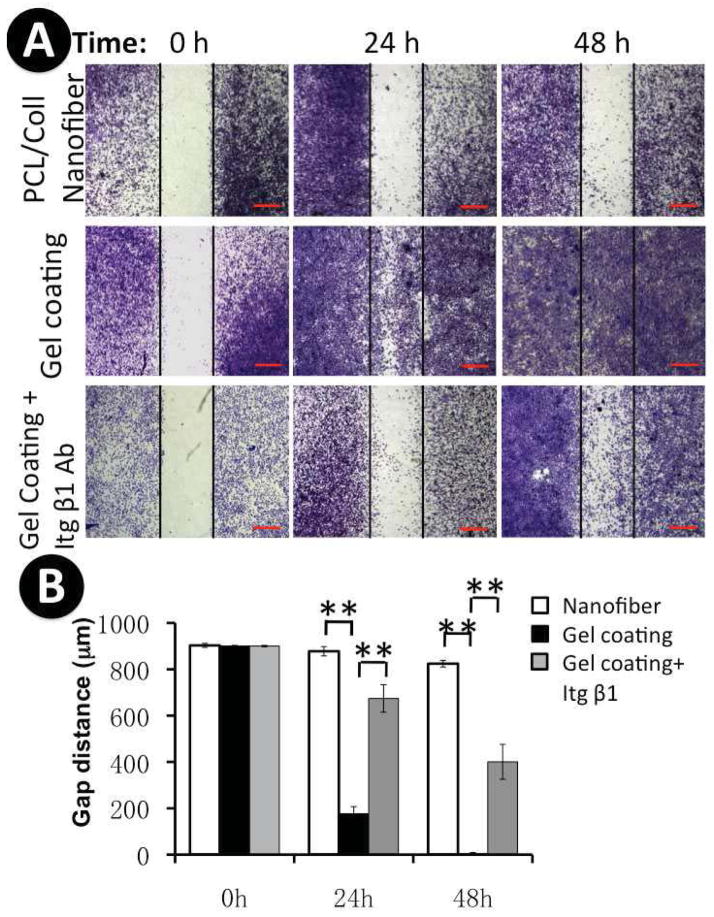
In order to evaluate the effect of various nanofibrous matrices on keratinocyte migration, an in vitro wound-healing assay was performed by culturing the cells on various matrices with a “wound gap” created in the middle (Figure 2). After 24 and 48 hours, staining of the culture with methylene blue revealed that keratinocyte migratory behavior was greatly correlated with their underlying matrices. As shown in Figure 2A and 2B, keratinocytes barely migrated into the “wound gap” on PCL/Coll nanofibrous matrices even after 48-hour culture. In contrast, keratinocyte migration was significantly enhanced on the surface of collagen gel-coated PCL/Coll nanofibrous matrices, in which the “wound gap” was completely closed by 48 h (Figure 2A and 2B). In order to further elaborate whether the accelerated motility of keratinocytes on collagen gel coated nanofibers was due to chemical variation or the topographical changes, wound healing assay was also similarly done on electrospun collagen nanofibrous matrices with an average diameter of 363± 87 nm, PCL/Coll nanofibrous matrices with adsorbed nonfibrous collagen and glass cover slips (see supplement Fig. 1). Interestingly, neither pure collagen nanofibrous matrices nor collagen-adsorbed PCL/Coll nanofibrous matrices promoted keratinocyte migration. However, high motility of keratinocytes was observed on glass coverslips, similar to that on the collagen gel-coated nanofibrous matrices.
Figure 2.
Keratinocyte migration on various nanofibrous matrices. A) Cells were seeded on PCL/collagen nanofibers without or with collagen gel coating with an insert in the middle. After 12 h, the insert was removed to generate a 900μm “wound gap”. Cells were allowed to migrate into the wound gap, and visualized after 24 and 48 h using methylene blue staining. In order to determine the involvement of integrin β1 in regulating keratinocyte migration, anti-integrin β1 antibody was added to the culture to neutralize the integrin β1 function. Scale bar: 500μm; B) Quantification of the “wound gap” distance between the front lines of the majority of migrating keratinocytes (n=4). ** Statistically significant, p < 0.001.
Enhanced keratinocyte proliferation on collagen gel-coated nanofibrous matrices
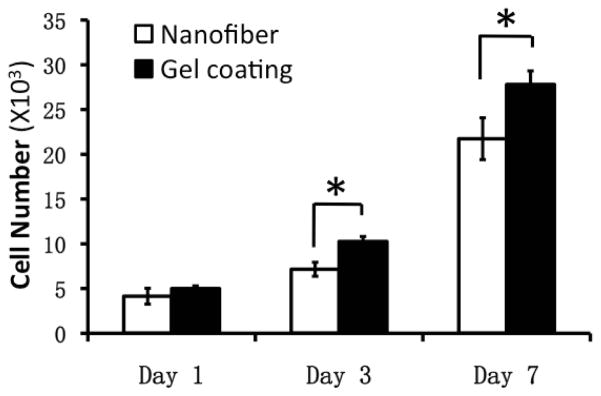
Substrates that favor continuous cell proliferation are considered advantageous especially for re-epithelialization. Surface chemistry of substrates has been well recognized for its crucial contribution to the cell/substrate interactions [25, 33–35]. However, it remains unclear whether surface topography of nanofibrous matrices also influences keratinocyte adhesion and proliferation. In this study, the proliferation of keratinocytes on both matrices was determined by DNA assay for up to 7 days. Continuous cell proliferation was observed on both matrices and keratinocytes proliferated slightly faster on collagen gel-coated matrices compared to the noncoated matrices on day 3 and 7 (Figure 3).
Figure 3.
Keratinocyte proliferation plated on PCL/collagen nanofibrous matrices alone or with collagen gel coating for up to 7 days (n=4) * Statistically significant, p < 0.05
Promotion of keratinocyte polarization by collagen gel-coated nanofibrous matrices
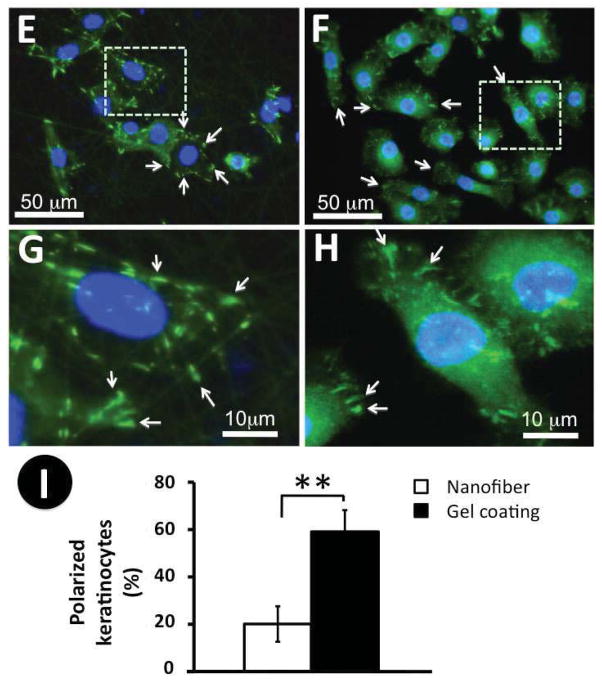
Keratinocytes change their morphology dramatically as they go from stationary to migratory [17, 36]. To determine the cell morphology on nanofibrous matrices with or without collagen gel coating, keratinocytes cultured on these matrices for 24 hours were immunostained for the cytoskeleton protein, F-actin, and the focal adhesion protein, vinculin. F-actin staining showed that keratinocytes exhibited a typical cobblestone-like morphology on the noncoated nanofibrous matrices as characterized by the lack of lamellipodia/filopodia and the existence of prominent cortical F-actin after 2-day culture (Figure 4A and 4C). Interestingly, most keratinocytes on the collagen gel-coated matrices became elongated with at least one lamellipodium/filopodium presented (Figure 4B and 4D, arrowhead), consistent with the increased propensity for the motile behavior. Some keratinocytes had more than one lamellipodium/filopodium accompanied by a more elongated and spindle-like morphology (Figure 4D). The presence of more than one lamellipodium/filopodium is often related to their frequent switches of the motion direction [37]. Staining of vinculin indicated that various matrices indeed affected the spatial distribution of focal adhesion plaques on the cell membranes. On the PCL/Coll nanofibrous matrices, vinculin clusters mainly located on the circumferential region of the cell membrane along the nanofibers (arrows in Figure 4E and 4G). On the collagen gel-coated nanofibrous matrices, vinculin clusters in keratinocytes were scattered in lamellipodia (arrows in Figure 4F and 4H) and stained vinculin clusters were slightly smaller than those on the PCL/Coll nanofibrous matrices (Figure 4G vs. 4H). To better quantify the morphology difference of keratinocytes on various matrices, polarized keratinocytes from each group were counted. It was found that more than 60% of keratinocytes on collagen gel-coated nanofibrous matrices remained polarized while only about 20% of cells polarized on PCL/Coll nanofibrous matrices (Figure 4I).
Figure 4.
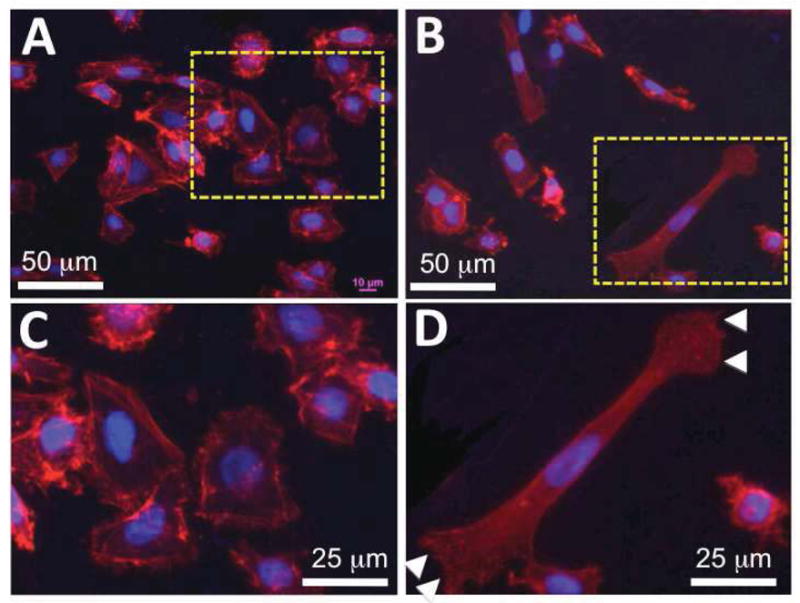
Morphology of keratinocytes on PCL/collagen nanofibrous matrices alone or with collagen gel coating. A–H) Immunofluorescent staining of intracellular cytoskeleton protein of F-actin (Red) and nuclei with DAPI (purple/blue) (A, C: PCL/collagen nanofiber alone; B, D: with collagen gel coating), and focal adhesion proteins of vinculin (Green) (E, G: PCL/collagen nanofiber alone; F, H: with collagen gel coating). Arrowhead: keratinocyte lamellipodium; arrow: spatial distribution of vinculin. I) Percentage of polarized keratinocytes (n=3) ** Statistically significant, p < 0.001
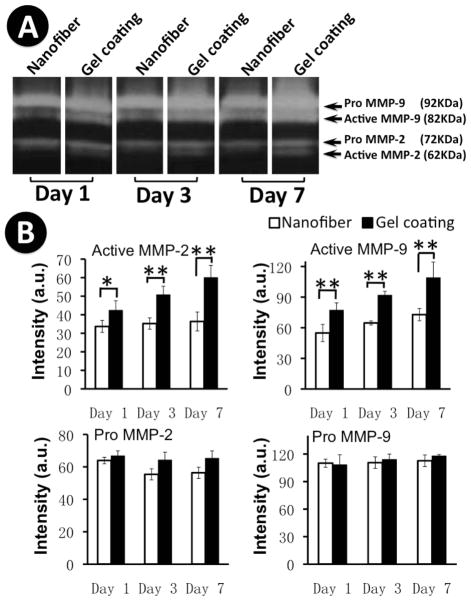
Upregulation of MMP-2 and MMP-9 activity in keratinocytes by collagen gel-coated nanofibrous matrices
Cell motility requires the release of cells from adhesion sites, which is regulated, at least in part, by proteolysis of ECM molecules with MMPs [38]. Thus, the activities of MMP-2 and -9 released to the supernatants of the culture were determined by gelatin zymography for up to 7 days. As shown in Figure 5A, the active form of MMP-2 (i.e., 62 KDa) was detected at all three time points (day 1, 3 and 7) from the culture on collagen gel-coated nanofibrous matrices and reached its maximal activity on day 7. In contrast, the supernatant from the culture on PCL/Coll nanofibrous matrices alone did not show detectable active MMP-2 at all the investigated time points. With regard to MMP-9 activity, the active form, i.e., 82KDa was detected in all supernatant samples from both matrices and its activity increased steadily over the extended culture (Figure 5A). However, a notably higher level of active MMP-9 was detected in the cultures on collagen gel-coated matrices (Figure 5A). The inactive zymogen of either pro-MMP-2 or pro-MMP-9 remained constant in the supernatants for all the cultures during the entire investigated times and showed no difference between collagen gel-coated and noncoated nanofibrous matrices. Semi-quantitative analysis of the optical density of the bands from three separate experiments confirmed the consistency of the observations (Figure 5B). These results suggest that collagen gel coating indeed induces the activation of both MMP-2 and MMP-9.
Figure 5.
Gelatin zymograms showing gelatinase activities of keratinocytes on PCL/collagen nanofibrous matrices alone or with collagen gel coating for up to 7 days. A) Representative zymogram image; B) Quantification of MMP-2 and MMP-9 expression (n=3). * Statistically significant, p<0.05; ** Statistically significant, p<0.001
Increase of laminin-332 (formerly termed laminin-5) deposition by collagen gel-coated nanofibrous matrices
Laminin-332, a crucial ECM component secreted by keratinocytes is responsible for the repair of the damaged basement membrane in skin wounds. It is deposited over collagen fibrils and required for keratinocyte anchorage to and migration on the provisional basement membrane [36, 39]. To determine whether collagen gel-coated matrices may favor the deposition of endogenous laminin-332, immunostaining for laminin-332 was performed on the samples cultured overnight. Compared to the limited deposition of laminin-332 on non-coated matrices, a substantial amount of laminin-322 deposited around and beyond the keratinocytes (strong fluorescence was seen far from nuclei) on the collagen gel-coated matrices (Figure 6). Growth factors, cytokines and lysophospholipids have been shown to stimulate laminin-332 synthesis in keratinocytes [40]. Our new results demonstrate that collagen gel-coated scaffolds also enhance laminin-332 deposition, which may facilitate keratinocyte migration.
Figure 6.
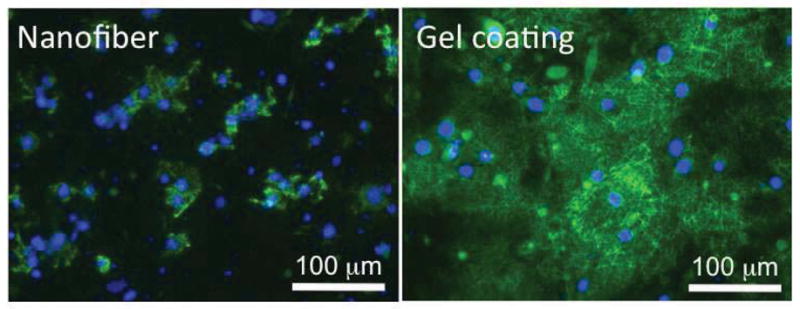
Deposition of laminin-332 (laminin-5) by keratinocytes on PCL/collagen nanofibrous matrices alone or with collagen gel coating. Scale bar: 100μm.
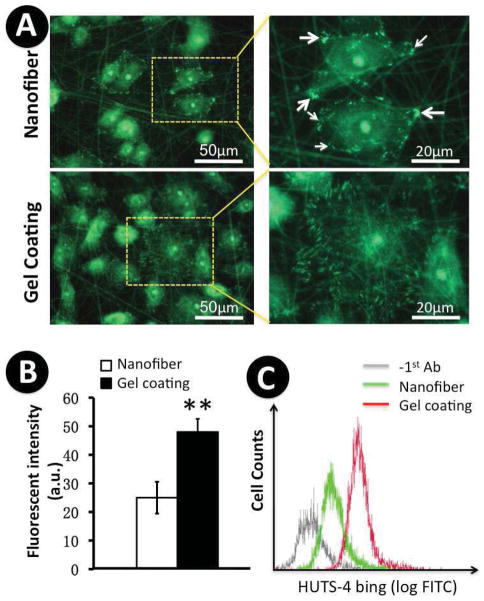
Activation of integrin β1 in keratinocytes by collagen gel-coated nanofibrous matrices
Integrin β1 is one of the major ECM receptors present in keratinocytes. It is activated through “outside-in” and “inside-out” signaling, and mediates keratinocyte adhesion and migration during the re-epithelialization [15, 17, 41–43]. To examine how various nanofibrous matrices regulate integrin β1 activation, immunofluorescent staining was performed on the cultured keratinocytes to detect the active conformation of integrin β1 using a monoclonal antibody, HUST-4. A large amount of positive staining, i.e., active integrin β1, appeared on the peripheral region of those keratinocytes cultured on collagen gel-coated nanofibrous matrices (Figure 7A). In contrast, only a limited number of active integrin β1 plaques were found in the keratinocytes cultured on PCL/Coll nanofibrous matrices (Figure 7A arrows). Noticeably, the size and distribution of active integrin β1 on keratinocyte membranes varied and depended on underlying matrix. On collagen gel-coated matrices, elongated active integrin β1 evenly distributed on the circumferential region of cell membrane, while round dots along the fiber orientation were observed on PCL/Coll nanofibrous matrices. Notably, some intracellular staining was also observed, which required further studies in the future. Interestingly, the expression and arrangement of active integrin β1 in those keratinocytes cultured on glass coverslip surfaces was similar to that on the collagen gel-coated matrices (data not shown). Quantification of the active integrin β1 by measuring the total fluorescence intensity of the lysate of stained cells via multi-mode microplate reader showed about two times the active integrin β1 on the collagen gel-coated matrices in comparison to that on the non-coated ones (P<0.01) (Figure 7B). To further ascertain that collagen gel-coated matrices indeed affect the activity of integrin β1, we performed flow cytometry analysis. As shown in Figure 7C, the level of HUTS-4 binding (FITC staining) was found much higher on collagen gel-coated matrices than that on the non-coated ones, consistent with the immunostaining results.
Figure 7.
Expression of active β1 integrins in keratinocytes on PCL/collagen nanofibrous matrices alone or with collagen gel coating. A) Immunofluorescent staining of active integrin β1. B) Quantification of the lysates of cells fluorescently stained for active β1 integrins with a fluorometer (n=3). ** Statistically significant, p < 0.001. C) HUTS-4 binding determined via flow cytometry (n=3).
Role of integrin β1 in keratinocyte migration on nanofibrous matrices
To determine the regulatory involvement of integrin β1 in keratinocyte motility on collagen gel-coated matrices, a wound-healing assay was performed on the keratinocytes with integrin β1 activity neutralized by the neutralizing antibody. Apparently, antibody neutralization significantly inhibited keratinocyte migration on the collagen gel-coated matrices. Even after 48 h, the “wound gap” remained unclosed (Figure 2A). This result was consistently observed at various seeding densities. This result together with those of Figure 7 indicated that integrin β1, whose activation was stimulated by collagen gel coating, played a key role in regulating keratinocyte migration on collagen gel-coated matrices.
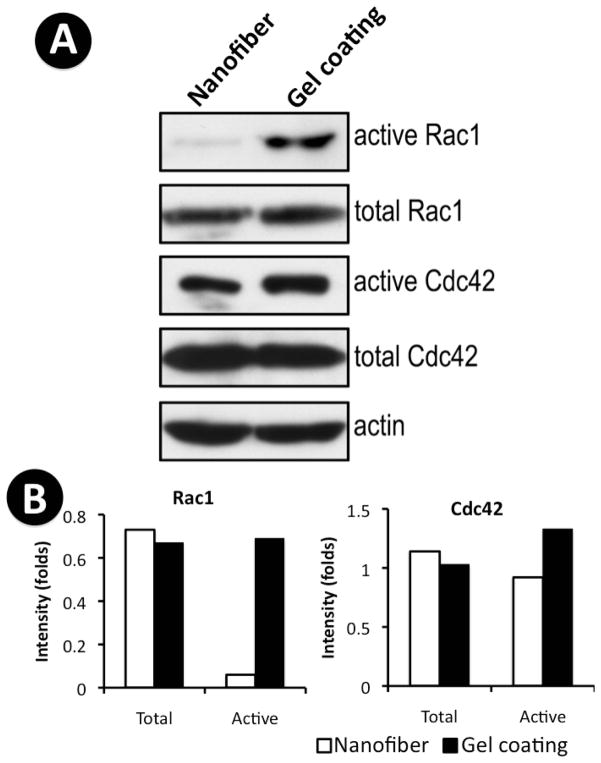
Activation of Rac1 and Cdc42 in keratinocytes enhanced by collagen gel-coated nanofibrous matrices
Rac1 and Cdc42 of the Rho GTPase family are key downstream signal-transduction molecules of growth factor receptors and integrins. Their activation induces the formation of lamellipodia and filopodia, thereby mediating integrin-dependent cell motility [44]. Therefore, the activation of Rac1 and Cdc42 in cultured keratinocytes on both matrices was studied by affinity pull-down assay using PBD- and GBD-GST fusion proteins. It was found that the activation of Rac1 is markedly increased in keratinocytes cultured on collagen gel-coated matrices, while that of Cdc42 is slightly increased (Figure 8). In contrast, there is no significant change of total Rac1 and Cdc42 on both collagen gel-coated and non-coated matrices (Figure 8). Thus, our results suggest that the collagen gel-coated matrices stimulate keratinocyte motility likely by activating integrin β1 and its downstream effectors Rac1 and Cdc42.
Figure 8.
Activation of Rac1 and Cdc42 in keratinocytes enhanced by collagen gel-coated PCL/collagen nanofibers matrices. A) Activation of Cdc42 and Rac1 in keratinocytes cultured on PCL/collagen nanofibrous matrices alone or with collagen gel coating were determined by affinity pull-down assay. The total Cdc42 and Rac1 were measured by western blot analysis. Actin was used as a loading control. (B) Protein bands were quantified by densitometry and plotted.
Discussion
With regard to limited studies on the effect of nanofibrous matrices on keratinocytes, the present study was aimed to determine what kind of topographical features of nanofibrous matrices favor keratinocyte migration and what the associated underneath mechanism is. Cultures of human keratinocytes on electrospun PCL/Coll nanofibrous matrices with or without type I collagen gel coating showed that collagen gel coating, which bridged the interstitial space of electrospun PCL/Coll nanofibers with ultrafine fibers, favored both cell proliferation and migration, important to re-epithelialization. Further studies showed that collagen gel coating increased active MMP-2 and -9 levels (consistent with the increased MMP levels at early wound healing stages for cell migration [16, 45–47]), promoted endogenous laminin-332 deposition, activated integrin β1 and its downstream signaling molecules, Rac1 and Cdc42. This finding not only elaborates the possible mechanism for enhanced keratinocyte motility on collagen-gel coated PCL/Coll nanofibrous matrices, but also indicates the similarity for keratinocytes on collagen gel-coated nanofibers and wound provisional matrix. Thus, electrospun nanofibrous matrices coated with collagen gel may also be used as an in vitro model for provisional matrix to study the cell-matrix interactions relevant to wound healing process.
Culturing keratinocytes on various collagen-containing nanofibrous matrices showed that only collagen gel-coated PCL/Coll nanofibrous matrices elevated keratinocyte motility, while neither pure electrospun collagen nanofibrous matrices nor electrospun PCL/Coll nanofibrous matrices adsorbed with nonfibrous collagen showed such stimulatory effect (Supplement Figure 1), indicating the surface topography of matrices indeed play an essential role in regulating the migratory activity of keratinocytes. Different from the large pores (~2 μm) in PCL/Coll electrospun nanofiber matrices, collagen gel coating on the surface of PCL/Coll nanofibers led to the formation of a secondary fibril network composed of ultrafine fibers (55± 26 nm in average diameter) with interstitial pores less than 200 nm. This ultrafine fibril network turned the surface of PCL/Coll nanofibrous matrices into a relatively smooth one (Figure 1B and 1D). Keratinocytes are relatively smaller with short lamellipodia in comparison to dermal fibroblasts. On PCL/Coll electrospun nanofibrous matrices, the interfiber distance may be too large for keratinocytes to form sufficient cell/matrix contacts to migrate; as a result, fewer focal adhesion plaques would be developed. On the other hand, collagen gel-coated matrices provide a relatively smooth surface for keratinocytes to adhere, which may allow the formation of more contact points. Indeed, immunostaining for vinculin did show fewer focal adhesion points formed on keratinocytes cultured on non-coated matrices and their distribution pattern closely correlated with the spatial arrangement of electrospun fibers (Figure 4E and 4G). In contrast, more fluorescently stained vinculin was observed in the keratinocytes cultured on collagen gel-coated matrices (Figure 4F) and its distribution followed a radial pattern for individual cells (Figure 4H).
As a membrane cytoskeletal protein, vinculin links intracellular actin filaments to the integrin components of focal adhesion complexes [48], thus its distribution would greatly affect cell morphology. Staining for F-actin revealed distinct cell morphology between the coated and non-coated matrices. Keratinocytes on the collagen gel-coated matrices showed more polarization and the formation of lamellipodia (Figure 4A vs. 4B, and Figure 4I), indicating the possible presence of more migrating keratinocytes[36]. We also found that gel-coated matrices significantly increased the active forms of MMP-2 and -9 (Figure 5), which are known for their participation in epithelial cell migration by cleaving ECM molecules in concert with other MMPs like MMP-1[16, 45]. MMP-2 was reported to localize in the cell/ECM contactlike structures of migrating keratinocytes [47] and modify ECM molecules to induce cell migration. It has also been reported that MMP-2 colocalizes with laminin-332 by binding to its γ2 chain [49] and subsequently cleaves the γ2 subunit at residue 587, exposing a putative cryptic promigratory site on laminin-332 that triggers cell motility [50]. In this study, a substantial amount of laminin-332 was indeed detected in the culture on gel-coated PCL/Coll nanofibrous matrices while little was seen on non-coated matrices (Figure 6).
Deposition of laminin-332 onto gel-coated PCL/Coll nanofibrous matrices would switch the adhesion and signaling of keratinocytes from collagen-dependent to laminin-332 dependent, mediated by different integrins [51]. Keratinocytes then use these freshly deposited laminin-332 in locomotion. Since integrin β1 is a known receptor for this ligand, it is reasonable to assume that ECM-induced integrin β1 expressions can differentially regulate keratinocyte motility. It was found that keratinocytes cultured on collagen gel-coated matrices showed a significant increase of active integrin β1 and the neutralization of its activity using blocking antibody partially abolished the facilitated migration (Figures 2 and 7), consistent with prior mouse genetic studies indicating the participation of β1 integrins in epidermal growth and repair [41]. A Possible explanation to β1 integrin-induced cell motility is that β1 integrin triggers a more motile phenotype via activating small GTPases Rac1 mediated by FAK/Cas/Crk signaling pathway and Cdc42. It is known that Rac1 and Cdc42 control cell migration through increasing the formation of motile actin-based structures including lamellipodia and helping establish cellular polarity in response to ECM [52–54]. We indeed observed higher activation of Rac1 and Cdc42 in keratinocyte on collagen gel-coated matrices (Figure 8). Clearly, β1 integrins act as a critical mediator for outside-in signal transduction for keratinocytes specifically on the collagen gel-coated matrices.
Our studies highlighted the important role of topographical feature of nanofibrous matrices in regulating keratinocytes for their re-epithelialization function conveyed by integrin β1 signal transduction, which is mediated via Rac1 and Cdc42. Clearly, the findings would provide an insightful understanding of the regulatory mechanisms of nanofiber-modulated phenotypic expression of human keratinocytes for appropriate wound repair, but also offer an interesting paradigm to study the potential contributions of various ECM-like fibrous matrices to in vivo wound healing processes.
Conclusion
In this study, we explored keratinocyte motility and other behaviors related to wound healing on various nanofibrous matrices. Apparently, collagen-containing nanofibers with diameters of 331±112 nm do not support human keratinocyte migration. On the other hand, modification of such nanofibrous matrices with a secondary ultrafine collagen fibrous network with the fiber diameter down to 55± 26 nm can significantly stimulate keratinocyte migration. Our further studies have shown that such a secondary fibrous network stimulates the adhesion, proliferation and migration of keratinocytes by regulating the activation and distribution of integrin β1 and the downstream effectors of Rac1 and Cdc42, facilitating the deposition of laminin-332, and promoting the expression of active MMPs (MMP-2 and 9). Clearly, nanofiber-induced distinct keratinocyte responses are closely regulated by the topography of the nanofibrous matrices, which might be as important as the chemistry of the matrices.
Supplementary Material
Acknowledgments
This investigation was sponsored by the Grant Number 1R21 AR056416 from NIAMS. Dr. Xiaoling Fu was supported by the Innovation & Entrepreneurship Doctoral Fellowship from the Stevens Institute of Technology.
Footnotes
Author Disclosure Statement
No competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raja, Sivamani K, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–68. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- 2.Billingham RE, Reynolds J. Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspensions. Br J Plast Surg. 1952;5:25–36. doi: 10.1016/s0007-1226(52)80004-9. [DOI] [PubMed] [Google Scholar]
- 3.Hefton JM, Finkelstein JL, Madden MR, Shires GT. Grafting of burn patients with allografts of cultured epidermalcells. Lancet. 1983;322:428–30. doi: 10.1016/s0140-6736(83)90392-6. [DOI] [PubMed] [Google Scholar]
- 4.Hefton JM, Caldwell D, Biozes DG, Balin AK, Carter DM. Grafting of skin ulcers with cultured autologous epidermal cells. J Am Acad Dermatol. 1986;14(3):399–405. doi: 10.1016/s0190-9622(86)70048-0. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor N, Mulliken J, Banks-Schlegel S, Kehinde O, Green H. Grafting of burns with cultured epithelium prepared fromautologous epidermal cells. Lancet. 1981;317:75–8. [PubMed] [Google Scholar]
- 6.Kreis RW, Mackie DP, Hermans RP, Vloemans AR. Expansion techniques for skin grafts: comparison between mesh and Meek island (sandwich-) grafts. Burns. 1994;20(Supplement 1):S39–S42. doi: 10.1016/0305-4179(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 7.Billingham RE, Medawar PB. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385–402. [Google Scholar]
- 8.Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin: Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305–13. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 9.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874–80. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 10.Eaglstein WH, Falanga V. Tissue engineering and the development of Apligraf®, a human skin equivalent. Clin Ther. 1997;19:894–905. doi: 10.1016/s0149-2918(97)80043-4. [DOI] [PubMed] [Google Scholar]
- 11.Nanney LB, Paulsen S, Davidson MK, Cardwell NL, Whitsitt JS, Davidson JM. Boosting epidermal growth factor receptor expression by gene gun transfection stimulates epidermal growth in vivo. Wound Repair Regen. 2000;8(2):117–27. doi: 10.1046/j.1524-475x.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 12.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 13.Bhattarai SR, Bhattarai N, Yi HK, Hwang PH, Cha DI, Kim HY. Novel biodegradable electrospun membrane: scaffold for tissue engineering. Biomaterials. 2004;25:2595–602. doi: 10.1016/j.biomaterials.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Electrospinning of collagen nanofibers Effects on the behavior of normal human keratinocytes and early stage wound healing. Biomaterials. 2006;27:1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25:1289–1297. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 15.Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992;101:1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 17.O’Toole EA. Extracellular matrix and keratinocyte migration. Clin Exp Dermatol. 2001;26:525–30. doi: 10.1046/j.1365-2230.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 18.Odland G, Ross G. Human wound repair I. epidermal regeneration. J Cell Biol. 1968;39:135–51. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 20.Guo M, Toda K, Grinnell F. Activation of human keratinocyte migration on type I collagen and fibronectin. J Cell Sci. 1990;96:197–205. doi: 10.1242/jcs.96.2.197. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Wu J, Spong S, Sheppard D. The integrin alphavbeta6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci. 1998;111:7. doi: 10.1242/jcs.111.15.2189. [DOI] [PubMed] [Google Scholar]
- 22.Nasca MR, O’Toole EA, Palicharla P, West DP, Woodley DT. Thalidomide increases human keratinocyte migration and proliferation. J Invest Dermatol. 1999;113:720–4. doi: 10.1046/j.1523-1747.1999.00744.x. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49:5603–21. [Google Scholar]
- 24.Patel GK, Wilson CH, Harding KG, Finlay AY, Bowden PE. Numerous Keratinocyte Subtypes Involved in Wound Re-Epithelialization. J Invest Dermatol. 2005;126:497–502. doi: 10.1038/sj.jid.5700101. [DOI] [PubMed] [Google Scholar]
- 25.Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–61. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan J, Kotaki M, Zhang Y, Mo X, Ramakrishna S, Ramakrishna S. Recent advances in polymer nanofibers. J Nanosci Nanotechnol. 2004;4:52–65. [PubMed] [Google Scholar]
- 27.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583–9. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 28.Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, et al. α6β4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of α3β1 integrin. J Cell Sci. 2003;116:3543–56. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- 29.Kleiner DE, Stetlerstevenson WG. Quantitative zymography: Detection of picogram quantities of gelatinases. Anal Biochem. 1994;218:325–9. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- 30.Galis Z, Sukhova G, Libby P. Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995;9:974–80. doi: 10.1096/fasebj.9.10.7615167. [DOI] [PubMed] [Google Scholar]
- 31.Oinuma L, Katach H, Negishi M. Semaphorin 4D/plexin-B1 –mediated R-Ras GAP activity inhibits cell migration by regulating β1 integrin activity. J Cell Biol. 2006;173:601–613. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X, Liu J, Qi Y, Brakebusch C, Chrostek-Grashoff A, Edgar D, et al. Rac1 is essential for basement membrane-dependent epiblast survival. Mol Cell Biol. 2010;30:3569–3581. doi: 10.1128/MCB.01366-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25:1289–97. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 34.Min BM, You Y, Kim JM, Lee SJ, Park WH. Formation of nanostructured poly(lactic-co-glycolic acid)/chitin matrix and its cellular response to normal human keratinocytes and fibroblasts. Carbohydr Polym. 2004;57:285–92. [Google Scholar]
- 35.Noh HK, Lee SW, Kim JM, Oh JE, Kim KH, Chung CP, et al. Electrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials. 2006;27:3934–44. doi: 10.1016/j.biomaterials.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Frank DE, Carter WG. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci. 2004;117:13. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- 37.Sehgal BU, DeBiase PJ, Matzno S, Chew TL, Claiborne JN, Hopkinson SB, et al. Integrin beta 4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006;281:35487–98. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamenkovic I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 39.Zhang K, Kramer RH. Laminin 5 Deposition Promotes Keratinocyte Motility. Exp Cell Res. 1996;227:309–22. doi: 10.1006/excr.1996.0280. [DOI] [PubMed] [Google Scholar]
- 40.Amano S, Akutsu N, Ogura Y, Nishiyama T. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. Br J Dermatol. 2004;151:961–70. doi: 10.1111/j.1365-2133.2004.06175.x. [DOI] [PubMed] [Google Scholar]
- 41.Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fässler R, et al. A crucial role of β1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129:2303–15. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- 42.Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–26. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 44.Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390(6660):632–6. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 45.Madlener M, Parks WC, Werner S. Matrix Metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242:201–10. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- 46.Salo T, Mäkelä M, Kylmäniemi M, Autio-Harmainen H, Larjava H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab Invest. 1994;70:176–82. [PubMed] [Google Scholar]
- 47.Mäkelä M, Larjava H, Pirilä E, Maisi P, Salo T, Sorsa T, et al. Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp Cell Res. 1999;251:67–78. doi: 10.1006/excr.1999.4564. [DOI] [PubMed] [Google Scholar]
- 48.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–33. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 49.Wahlgren J, Väänänen A, Teronen O, Sorsa T, Pirilä E, Hietanen J, et al. Laminin-5 gamma 2 chain is colocalized with gelatinase-A (MMP-2) and collagenase-3 (MMP-13) in odontogenic keratocysts. J Oral Pathol Med. 2003;32:100–7. doi: 10.1034/j.1600-0714.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 50.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen BP, Gil SG, Carter WG. Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J Biol Chem. 2000;275:31896–907. doi: 10.1074/jbc.M006379200. [DOI] [PubMed] [Google Scholar]
- 52.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nature Reviews Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz MA, Shattil SJ. Signaling networks linking integrins and Rho family GTPases. Trends Biochem Sci. 2000;25(8):388–391. doi: 10.1016/s0968-0004(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 54.Choma DP, Pumiglia K, DiPersio CM. Integrin α3β1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci. 2004;117:3947–3959. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.