INTRODUCTION
Neuroblastoma accounts for 7% of all malignant tumors in children and 10% of pediatric cancer deaths1, 2. Approximately 50% of patients present with high-risk disease,1 and their multimodal treatment includes induction with multi-agent chemotherapy, surgical resection, consolidation with myeloablative chemotherapy and autologous hematopoietic cell transplant (AHCT), radiation therapy (RT), and treatment of minimal residual disease (MRD) with isotretinoin, the anti-GD2 monoclonal antibody (mAb) dinutuximab, granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin 2 (IL-2)3. The addition of an immunotherapy MRD treatment phase was a significant advancement in the treatment of high-risk neuroblastoma. However, despite treatment with this intensive multimodal regimen, the 2-year event-free survival (EFS) for children with high-risk neuroblastoma who complete therapy remains low at 66 ± 5%,3 and new therapeutic strategies are needed. Given the improved outcomes with using dinutuximab in MRD therapy, new uses for anti-GD2 antibodies are being evaluated including for the treatment of relapsed patients4–8.
The anti-GD2 mAb dinutuximab has several limitations. First, the administration of dinutuximab causes significant neuropathic pain. GD2, a surface disialoganglioside present on neuroblastoma cells, is also expressed on peripheral nerves. The pain that occurs with dinutuximab is felt to be a result of complement activation eliciting local neuronal activity, an on-target off-tumor effect of an anti-GD2 mAb9. Second, dinutuximab is a chimeric human-mouse antibody which may result in the development of human anti-mouse antibodies (HAMA). In comparison, hu14.18K322A is a humanized anti-GD2 mAb that is designed with a single point mutation (K332A) that reduces complement activation, the intent of which was to cause less complemented-mediated pain4. This decreased pain of hu14.18K322A compared to dinutuximab has been demonstrated in a rat model of pain,9 as well as in a retrospective comparison of narcotic usage in patients getting either dinutuximab or hu14.18K322A (at dosages more than twice that of dinutuximab)10. Additionally, as hu14.18K322A is approximately 98% human-derived it is theoretically less immunogenic4.
In an effort to further improve outcomes for pediatric patients with high-risk neuroblastoma, we have developed a phase 2 trial for pediatric patients with newly diagnosed high-risk neuroblastoma which is evaluating the incorporation of hu14.18K322A and cytokines (GM-CSF and IL-2) throughout all treatment phases (NCT01857934)11, 12. In the consolidation phase of this trial, patients receive a busulfan/melphalan (Bu/Mel) conditioning regimen, AHCT, and experimental immunotherapy with hu14.18K322A, GM-CSF, and IL-2, with or without haploidentical NK infusions. Because the mechanism of action of hu14.18K322A is primarily through antibody-dependent cell-mediated cytotoxicity (ADCC) rather than complement activation4, 13, 14, we sought to optimize strategies to enhance ADCC. Natural killer cells (NKs) are major effector cells in ADCC, but their effect can be mitigated by the presence of other hematopoietic cell types15. Therefore, in an effort to avoid potential inhibition by other cells types while also maximizing hu14.18K322A efficacy, we are adoptively transferring haploidentical NKs to patients with suitable parental donors during consolidation therapy, a time of maximal endogenous hematopoietic cell depletion. Additionally, the adoptive transfer of haploidentical NK cells may mediate a graft-versus-tumor cytotoxic effect.
Here we describe our initial experience with using this experimental consolidation therapy regimen in newly diagnosed pediatric high-risk neuroblastoma patients. We also describe the feasibility of procuring autologous hematopoietic progenitor cells (HPCs) in the setting of induction therapy with multi-agent chemotherapy, hu14.18K322A and cytokines. We focus on the feasibility and tolerability of this experimental AHCT regimen. The initial results of the induction phase of this regimen have been reported elsewhere11, 12.
METHODS
Patient selection
This prospective phase 2 trial for pediatric patients with newly diagnosed high-risk neuroblastoma (NCT01857934) was approved by our institutional review board. The trial opened in May 2013 and enrollment continues. We defined high-risk neuroblastoma using participant age, the International Neuroblastoma Staging System (INSS) disease stage, MYC-N status, and other biologic features in a schema identical to that used by the Children’s Oncology Group (COG)16. Parents aged 18 years or older were eligible for evaluation as haploidentical NK donors. Written informed consent was obtained from all participants (both patients and donors) in accordance with institutional guidelines. All patients were treated at St. Jude Children’s Research Hospital (Memphis, TN).
Treatment plan
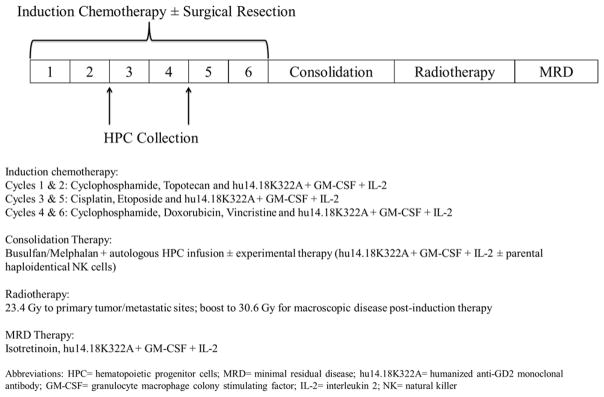
Figure I summarizes the overall treatment schema of this protocol. Patients underwent 6 cycles of induction therapy, with each cycle combining multi-agent chemotherapy, hu14.18K322A, and cytokines (GM-CSF and IL-2). The chemotherapy backbone used is identical to the COG studies ANBL02P1 (NCT00070200)17 and ANBL0532 (NCT00567567)18. HPCs were collected after induction cycle 2 or 4. Primary tumors were resected or debulked during induction. Following induction, patients underwent disease evaluation, as well as evaluation for concurrent medical problems as per our pre-AHCT standard operating procedures. The results of the end of induction disease response did not factor into eligibility to proceed with consolidation therapy. Consolidation therapy included AHCT with a Bu/Mel conditioning regimen and for consenting patients, experimental therapy with hu14.18K322A, cytokines and, when available, parental haploidentical NKs. After recovering from AHCT, patients receive RT to the primary tumor bed, followed by MRD therapy with hu14.18K322A, cytokines, and isotretinoin on a schedule identical to that used in the COG study ANBL00323.
Figure I.
Phase 2 trial treatment schema for pediatric patients with newly diagnosed high-risk neuroblastoma, including consolidation therapy with a busulfan/melphalan conditioning regimen, autologous hematopoietic cell transplant, and immunotherapy with hu14.18K322A (an anti-GD2 monoclonal antibody), GM-CSF, and IL-2, with or without adoptive transfer of parental haploidentical NK cells.
The AHCT conditioning regimen included busulfan (0.8 mg/kg/dose for patients > 12 kg or 1.1 mg/kg/dose for patients ≤ 12 kg, given intravenously every 6h over days −6 to −3, with dose adjustments based on therapeutic drug monitoring [goal steady-state concentration of 700–800ng/mL]), followed by melphalan (70 mg/m2/dose for patients > 10 kg or 2.3 mg/kg/dose for patients ≤ 10 kg daily, given intravenously on days −2 to −1). Autologous HPC infusion was given the day after completing chemotherapy (day 0). On days 2 to 5, consenting patients began experimental therapy with hu14.18K322A. Dose escalation of hu14.18K322A (25 mg/m2/dose or 40 mg/m2/dose) was based on the frequency of protocol defined dose-limiting toxicities. IL-2 was given subcutaneously (106 units/m2/dose) beginning on day 3, and continued every other day for a total of 4 doses. Participants with a consenting and suitable parental donor received a single infusion of haploidentical NKs between days 2 and 5 (depending on donor availability). Daily GM-CSF administration (250μg/m2/dose) began on day 10 and continued until the absolute neutrophil count (ANC) exceeded 2000/mm3 for 2 consecutive days. After haploidentical NK infusion, recipient blood samples were obtained at 2 points for NK chimerism testing (approximately 10 days and 21 days post-NK infusion). Chimerism testing was conducted on sorted NKs from recipient blood samples, and the percentages of donor and recipient DNA from NKs were determined using variable nucleotide tandem repeats19.
Autologous HPC collection and processing
Initial evaluation for HPC collection occurred after induction cycle 2; if collection was not possible at that time, patients were re-evaluated after induction cycle 4. The preferred collection method was peripheral blood leukapheresis. For HPC mobilization, patients were given granulocyte colony-stimulating factor (G-CSF) at 5μg/kg/day, in addition to the GM-CSF (250μg/m2/dose) that was given as part of the induction therapy regimen. Collection began when the absolute peripheral CD34+ count was at least 20/μL, with the intent of collecting a minimum of 2 × 106 CD34+ cells/kg for AHCT. Peripheral leukapheresis was performed over 1 to 2 days. If an adequate HPC graft could not be collected after 2 leukapheresis cycles, bone marrow was harvested. The unpurged HPC product was cryopreserved in accordance with institutional standard operating procedures.
Haploidentical NK collection and processing
Potential parental NK donors were screened in accordance with FDA guidance 21 CFR 1271. KIR genotyping and phenotyping of parental and recipient NK cells was performed. KIR mismatch was a factor in donor selection, but not a requirement to be a donor. Donors were evaluated by an independent physician to ensure they could safely undergo peripheral blood apheresis. To obtain the NK (CD3−CD56+) product, donors underwent a non-mobilized, peripheral blood apheresis (2 total blood volumes [TBV]). The blood products were then processed using microbead labeling via a CliniMACS system (Miltenyi Biotec, Auburn, CA) in accordance with the manufacturer’s instructions. The therapeutic cells underwent CD3 depletion followed by CD56 positive selection20. NK cell dosing was not protocol defined, and patients received the entirety of the purified NK product. Purified NKs were administered to patients within 36h of collection, without cryopreservation.
Supportive care during AHCT
Patients received levetiracetam for seizure prophylaxis during busulfan conditioning. Veno-occlusive disease (VOD) prophylaxis consisted of continuous infusion of low-dose heparin (or enoxaparin if the patient was receiving it as treatment for a known thrombus), with or without oral ursodiol. The prophylactic regimen used was based on the institutional standard operating procedure at the time. Pain management was personalized based on a patient’s previous medication requirements with hu14.18K322A treatment during induction therapy, and included opioid infusions via patient controlled analgesia pumps. Infection prophylaxis included fluconazole or micafungin (antifungal), acyclovir (antiviral) and trimethoprim-sulfamethoxazole or pentamidine (for Pneumocystis jiroveci pneumonia [PJP] coverage). During the neutropenic phase after autologous HPC infusion, antibacterial prophylaxis included levofloxacin or cefepime with or without vancomycin.
Toxicity monitoring
Patients were monitored for adverse events from the start of the AHCT conditioning regimen until they began RT. Protocol defined unacceptable toxicities, if determined to be attributable to the experimental immunotherapy regimen, included the following: toxicity requiring the use of vasopressors (grade 4 cytokine release syndrome, grade 3 or 4 hypotension), grade 4 respiratory failure, failure of ANC recovery >500/mm3 by day 35, and grade 3 or 4 neurotoxicity with MRI evidence of new CNS thrombi, infarction or bleeding. Hepatic VOD was defined according to the Baltimore criteria (bilirubin ≥ 2 mg/dL with at least 2 of the following: hepatomegaly, ascites, or weight gain ≥ 5% from baseline) or the modified Seattle criteria (any 2 of the following: painful hepatomegaly with right-upper-quadrant pain, fluid retention with weight gain ≥ 2% from baseline, or bilirubin ≥ 2 mg/dL)21. Patients were considered to have VOD if they met either the Baltimore or Seattle criteria. VOD with multi-organ dysfunction (MOD) included renal (creatinine ≥ 3 times baseline or need for dialysis) or pulmonary (requiring supplemental oxygen for > 24 h or need for mechanical ventilation) involvement. Other adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Date of neutrophil engraftment was defined as the first day on which the ANC was at least 500/mm3 for 3 consecutive days. Date of platelet engraftment was defined as the first day on which the platelet count was at least 20 × 103/mm3, without transfusion in the preceding 7 days.
Statistical methods
Descriptive statistics were summarized as frequency for categorical variables, and mean and standard derivation for continuous variables. The difference in frequencies were tested using the Fisher exact test. The difference of means were tested using the Wilcoxon rank-sum test. All analyses were done using StatXact 11 and SAS 9.4. All tests were 2-sided and statistical significance level was set at 0.05.
RESULTS
Patient characteristics
At the time of this analysis, 34 patients had enrolled on this trial from July 2013 to February 2016. Of these, 2 patients did not proceed with consolidation therapy with AHCT due to parental decision to be taken off study prior to entering this phase of therapy. An additional patient had recurrent respiratory viral illnesses that precluded them from undergoing AHCT and 1 patient underwent AHCT without the experimental therapy due to parental preference. Here we report on the 30 patients who completed consolidation therapy with AHCT and experimental immunotherapy. Table I presents the clinical features of these patients at initial diagnosis. The median age at diagnosis was 32.8 months (range, 6.1 – 182.5 months); 25 patients had INSS stage 4 disease and 15 patients had MYC-N amplification. No patient developed progressive disease during induction chemotherapy12. Twenty-two patients had an eligible parental NK donor, 21 of which received NKs. The clinical status of 1 patient with an eligible NK donor precluded them from receiving this experimental therapy. For the 8 patients with no eligible NK donor, 11 parents were available for evaluation and found to be ineligible: 5 were seropositive for a relevant communicable disease, 5 had recently traveled to an area of high infection risk, and 1 had recently obtained a tattoo.
Table I.
Characteristics at initial diagnosis of high-risk pediatric neuroblastoma patients who have completed consolidation therapy with AHCT and hu14.18K322A/GM-CSF/IL-2, with and without haploidentical NK infusion (n=30)
| Characteristic | Number (%) |
|---|---|
|
| |
| Age (median 32.8 months) | |
| < 12 months | 2 (6.7) |
| 12 – < 18 months | 1 (3.3) |
| ≥ 18 months | 27 (90) |
|
| |
| Sex | |
| Male | 19 (63.3) |
| Female | 11 (36.6) |
|
| |
| Race | |
| Caucasian | 17 (56.7) |
| African American | 9 (30) |
| Other | 4 (13.3) |
|
| |
| Primary Tumor Site | |
| Adrenal | 18 (60) |
| Abdomen/Pelvis | 3 (10) |
| Other | 9 (30) |
|
| |
| INSS Stage | |
| Stage 2B | 1 (3.3) |
| Stage 3 | 4 (13.3) |
| Stage 4 | 25 (83.4) |
|
| |
| MYC-N | |
| Amplified | 15 (50) |
| Not Amplified | 15 (50) |
Abbreviations: AHCT = autologous hematopoietic cell transplant; hu14.18K322A = humanized anti-GD2 monoclonal antibody; GM-CSF = granulocyte-macrophage colony stimulating factor; IL-2 = interleukin 2; NK = natural killer cell; INSS=International Neuroblastoma Staging System
Feasibility of autologous HPC collection
Adequate products for AHCT were obtained from all 30 patients who underwent HPC collection. The median dose of autologous CD34+ cells collected was 15.9 × 106 cells/kg (range, 2.8 –46.88 × 106 cells/kg). Twenty-eight patients underwent initial HPC collection after induction cycle 2, with 27 requiring a single peripheral leukapheresis and 1 (who weighed < 10 kg) a bone-marrow harvest. The latter patient underwent an additional leukapheresis after induction cycle 4 because the initial bone-marrow harvest was inadequate. Two patients underwent initial HPC collection after induction cycle 4 because they had active infections after cycle 2; the first required a single peripheral leukapheresis and the second a bone-marrow harvest after unsatisfactory leukapheresis.
AHCT and experimental therapy characteristics
The median time from enrollment on study to starting conditioning chemotherapy for AHCT was 163.5 days (range, 136 – 203 days). Patients received a median dose of 8.33 × 106 cells/kg (range, 2.5 – 46.88 × 106 cells/kg) of autologous CD34+ cells and a median dose of 1.9 × 106 cells/kg (range, 0.5 – 8.7 × 106 cells/kg) of autologous CD3+ cells. Sixteen patients received 25 mg/m2/dose, daily × 4 days, of hu14.18K322A and 14 received 40 mg/m2/dose, daily × 4 days. No protocol defined dose-limiting toxicities occurred at either dose level. For VOD prophylaxis, 12 patients received heparin, 17 received heparin with ursodiol, and 1 received enoxaparin with ursodiol. Patients were transferred back to their primary service a median of 34.5 days (range, 27 – 106 days) post-HPC infusion, and began RT a median of 48 days (range, 28 – 131 days) after beginning consolidation therapy. These times were similar between patients who did or did not receive NKs (to primary service: median 35 days [NKs; range 27 – 106] vs. 34 days [no NKs; 27 – 49], P=0.56; to start of RT: median 48 days [NKs; range 34 – 131] vs. 44 days [no NKs; range 35 – 69], P=0.36).
Twenty-one patients received haploidentical NKs at a median of 4 days (range, 3 – 5 days) post-HPC infusion. The infused NK products contained a median of 27.6 × 106 cells/kg CD56+ cells (range, 4.1 – 113.979 × 106 cells/kg) and 0.01 × 106 cells/kg CD3+ cells (range, 0.003 – 0.104 × 106 cells/kg). All 21 patients had at least 1 peripheral blood sample collected and sorted for NK chimerism testing. Twenty patients had a sample collected at the first time point (median 11 days post-HPC infusion; range 8 – 14), 15 of which had sufficient NK cells for testing. Of these, 14 samples had detectable donor DNA (median 3.5% donor DNA [range, 0% – 68%]. Eighteen patients had a sample collected at the second time point (median 25 days post-HPC infusion; range 22 – 50), all of which were adequate for testing. Of these NK cell samples, 6 contained detectable donor DNA (median donor DNA 1% [range, 0% – 35%]).
Toxicity
All 30 patients who received AHCT with experimental immunotherapy achieved neutrophil engraftment prior to day 30 post-HPC infusion (median, 13 days; range, 10 – 28 days) and platelet engraftment of at least 20 × 103/mm3 by day 102 (median, 36.5 days; range, 0 – 102 days). The median time to neutrophil engraftment (13 days [NKs; range 10 – 28 days] vs. 15 days [no NKs; range 11 – 22 days]; P= 0.07) and platelet engraftment (52 days [NKs; range 13 – 102 days] vs. 35 days [no NKs; range 13 – 79 days]; P =0.7) was similar between those that did or did not receive NKs. One patient that received NK cells was slow to engraft after the initial HPC infusion, and received an autologous HPC boost at day 24 after the initial infusion. This patient ultimately engrafted neutrophils by day 28 and platelets by day 102 after the initial HPC infusion.
Table II reports grade 3 or 4 adverse events of the 30 patients who received AHCT with experimental immunotherapy. No grade 5 events occurred. One patient developed a grade 3 infusion reaction while receiving hu14.18K322A; this was medically managed and did not prohibit subsequent doses. One patient developed multi-organ system dysfunction with an upper gastrointestinal bleed, coagulopathy, pneumatosis, small-bowel obstruction, grade 4 encephalopathy, and grade 4 respiratory failure requiring repeated intubation and mechanical ventilation; this patient did not have VOD. On lung biopsy, this patient had pathologic findings consistent with pulmonary injury due to chemotherapy toxicity, considered to be related to the Bu/Mel conditioning regimen. One patient developed respiratory failure requiring intubation in the setting of an aspiration pneumonia; this patient subsequently developed VOD, but had no signs of VOD at the time of intubation. Toxicities were similar for patients that did or did not receive NKs. No patients developed acute graft-versus-host disease.
Table II.
Toxicities of patients with high-risk neuroblastoma treated with AHCT and hu14.18K322A/GM-CSF/IL-2, with and without haploidentical NK infusion*
| Toxicity† | All patients (n=30) | NK cell recipients (n=21) | Non-NK cell recipients (n=9) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Grade 3 [total #] | Grade 4 [total #] | Grade 3/4 (%) | Grade 3/4 in NK recipients [total #] | Grade 3/4 in non-NK recipients [total #] | P-value** | |
|
| ||||||
| Infectious | ||||||
| Fever with neutropenia | 20 | 0 | 66.7 | 15 | 5 | 0.43 |
| Fever without neutropenia | 3 | 0 | 10 | 3 | 0 | 0.54 |
| Bacteremia | 1 | 0 | 3.3 | 1 | 0 | 1 |
| Enterocolitis | 16 | 0 | 53.3 | 13 | 3 | 0.24 |
| Upper respiratory infection | 8 | 1 | 30 | 5 | 4 | 0.39 |
| Cholangitis/cholecystitis | 3 | 0 | 10 | 2 | 1 | 1 |
| Urinary tract infection | 4 | 0 | 13.3 | 2 | 2 | 0.56 |
| Peritonitis | 1 | 0 | 3.3 | 1 | 0 | 1 |
|
| ||||||
| Digestive | ||||||
| Mucositis | 17 | 0 | 56.7 | 13 | 4 | 0.21 |
| Anorexia | 6 | 0 | 20 | 4 | 2 | 0.36 |
| Nausea/vomiting | 6 | 0 | 20 | 3 | 3 | 0.33 |
|
| ||||||
| Hepatic | ||||||
| Elevated AST | 4 | 2 | 20 | 5 | 1 | 0.64 |
| Elevated ALT | 1 | 3 | 13.3 | 3 | 1 | 1 |
| Elevated bilirubin | 0 | 3 | 10 | 3 | 0 | 0.54 |
| Elevated GGT | 0 | 1 | 3.3 | 1 | 0 | 1 |
|
| ||||||
| Electrolyte Abnormalities | ||||||
| Hypokalemia | 11 | 4 | 50 | 10 | 5 | 1 |
| Hyperkalemia | 1 | 1 | 6.7 | 2 | 0 | 1 |
| Hypocalcemia | 1 | 2 | 10 | 3 | 0 | 0.54 |
| Hypophosphatemia | 5 | 0 | 16.7 | 5 | 0 | 0.29 |
| Hyponatremia | 2 | 0 | 6.7 | 2 | 0 | 1 |
|
| ||||||
| Other | ||||||
| Infusion reaction | 1 | 0 | 3.3 | 1 | 0 | 1 |
| Encephalopathy | 0 | 1 | 3.3 | 1 | 0 | 1 |
| GI bleed | 1 | 0 | 3.3 | 1 | 0 | 1 |
| Respiratory | 0 | 2 | 6.7 | 2 | 0 | 1 |
National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0
Data represent grade 3 or 4 toxicities occurring from the start of the AHCT conditioning regimen until the start of radiation therapy; a toxicity was counted once per patient at its maximal grading; no grade 5 events occurred.
P -value comparing total # grade 3/4 toxicities between NK and non-NK recipients
Abbreviations: AHCT = autologous hematopoietic cell transplant; hu14.18K322A = humanized anti-GD2 monoclonal antibody; GM-CSF = granulocyte-macrophage colony stimulating factor; IL-2 = interleukin 2; NK = natural killer cell; AST = aspartate aminotransferase; ALT = alanine aminotransferase; GGT = gamma-glutamyl transferase
Eight patients developed VOD, with a median onset of 21 days post-HPC infusion (range, 16 – 25) (Table III). Five patients had hepatic VOD only, 4 of which received defibrotide treatment. Three patients had VOD with MOD, all of which were treated with defibrotide. One manifested as pulmonary edema requiring supplemental oxygen, and 2 had acute renal injury. No patients with VOD required dialysis or mechanical ventilation. The median duration of VOD was 15.5 days (range, 9 – 24), and all 8 patients experienced complete resolution. The incidence of VOD was not statistically significant between those patients that did or did not receive NKs.
Table III.
Veno-occlusive disease (VOD) in patients with high-risk neuroblastoma treated with AHCT with Busulfan/Melphalan, and hu14.18K322A/GM-CSF/IL-2, with and without haploidentical NK infusion.
| All patients (n=30) | NK cell recipients (n=21) | Non-NK cell recipients (n=9) | |||
|---|---|---|---|---|---|
|
| |||||
| Total # of cases | Incidence (%) | Total # of cases | Total # of cases | P-value* | |
|
| |||||
| VOD | 8 | 26.7 | 6 | 2 | 1 |
| Hepatic-only VOD** | 5 | 16.7 | 4 | 1 | 1 |
| VOD with MOD# | 3 | 10 | 2 | 1 | 1 |
P-value comparing VOD between NK and non-NK recipients
Hepatic VOD was defined according to the Baltimore criteria (bilirubin ≥ 2 mg/dL with at least 2 of the following: hepatomegaly, ascites, or weight gain ≥ 5% from baseline) or the modified Seattle criteria (any 2 of the following: painful hepatomegaly with right-upper-quadrant pain, fluid retention with weight gain ≥ 2% from baseline, or bilirubin ≥ 2 mg/dL)
VOD with multi-organ dysfunction (MOD) included renal (creatinine ≥ 3 times baseline or need for dialysis) or pulmonary (requiring supplemental oxygen for > 24 h or need for mechanical ventilation) involvement
Abbreviations: AHCT = autologous hematopoietic cell transplant; hu14.18K322A = humanized anti-GD2 monoclonal antibody; GM-CSF = granulocyte-macrophage colony stimulating factor; IL-2 = interleukin 2; NK = natural killer cell; VOD = veno-occlusive disease; MOD = multi-organ dysfunction
DISCUSSION
The incorporation of the anti-GD2 mAb dinutuximab into upfront MRD treatment for high-risk neuroblastoma represented a significant therapeutic advance3 and is now considered the standard of care. New uses for anti-GD2 antibodies are being evaluated, including for the treatment of relapsed patients4–8. Additionally, there are several ongoing studies testing the feasibility and tolerability of adding NK cells (including ex-vivo expanded autologous NKs [NCT02573896] or allogeneic NKs [NCT02650648]) to treatment with an anti-GD2 mAb. In this phase II study, we are incorporating hu14.18K322A4, GM-CSF and IL-2 into the treatment of newly diagnosed patients throughout several phases of therapy, including during consolidation with AHCT in conjunction with the adoptive transfer of haploidentical NKs (NCT01857934). This regimen seeks to further intensify the systemic therapy given during the consolidation phase of multi-modal high-risk neuroblastoma treatment, prior to patients beginning RT for local control. We hypothesized that the adoptive transfer of haploidentical NKs at a time of maximal endogenous hematopoietic cell depletion would enhance hu14.18K322A efficacy by improving ADCC through increased engagement with the anti-GD2 antibody13, 14. Additionally, donor NK cell infusion into a leukopenic environment should facilitate in vivo NK expansion22–26.
In this report, we found that following induction therapy with multi-agent chemotherapy (identical to COG ANBL02P117 and ANBL053218), hu14.18K322A and cytokines, consolidation with AHCT using a Bu/Mel conditioning regimen in conjunction with our experimental immunotherapy (hu14.18K322A, low-dose IL-2, GM-CSF, with or without haploidentical NK cells), was both feasible and tolerable. We procured adequate autologous HPC products for AHCT from all 30 patients, with most patients requiring a single peripheral leukapheresis. All patients had adequate hematologic recovery after AHCT. In these 30 patients, 8 developed VOD, 3 of which had mild MOD. The incidence of other toxicities (Table II) were similar to those previously reported for AHCT using a Bu/Mel conditioning regimens27–33. We did not appreciate any difference in toxicities between those patients that did and did not receive NKs. We cannot yet report on the late toxicities with this consolidation regimen, as our median post-AHCT follow-up for this initial cohort is only 636.5 days (range, 258 – 1224).
The results of our study should be interpreted in the context of similar trials and institutional results using a Bu/Mel AHCT consolidation regimen for high-risk pediatric neuroblastoma patients. In the HR-NBL1 trial by SIOPEN, pediatric patients with high-risk neuroblastoma were randomized to receive AHCT using either a Bu/Mel or CEM conditioning regimen. Of the 296 patients that were randomized to receive the Bu/Mel AHCT regimen, the average age at start of therapy was 2.7 years old, with 87% having INSS stage 4 disease, 44% of which had MYCN amplification. Prior to AHCT, patients underwent induction therapy with rapid COJEC (8 courses in total), and if inadequate metastatic disease response at that time, went on to receive 2 courses of topotecan, vincristine and doxorubicin prior to proceeding to AHCT. The Bu/Mel regimen consisted of either oral or IV busulfan, at doses that ranged from 0.8–1.2 mg/kg per dose for 16 doses (dose based on patient weight), followed by a single 140 mg/m2 dose of Melphalan IV31. For the 267 patients that received the Bu/Mel regimen, there was a median ANC recovery of 12 days, and a median platelet > 20 × 103/mm3 recovery of 20 days34. The Children’s Hospital of Philadelphia (CHOP) group retrospectively reviewed their experience using a Bu/Mel consolidation AHCT regimen. Twenty-one patients were analyzed, with an average age of 4.2 years old at the time of AHCT. Patients were considered to have high-risk disease based on INSS stage, age, MYCN status, ploidy and histology at diagnosis. Most patients (95%) received induction chemotherapy using a COG-type induction per ANBL0532 (6 cycles of chemotherapy, with combinations of cyclophosphamide/topotecan, cisplatin/etoposide and cyclophosphamide/doxorubicin/ vincristine)18. Our trial uses an identical induction chemotherapy regimen, with the addition of hu14.18K322A, GMCSF and IL-2. Their Bu/Mel regimen consisted of IV busulfan (16 doses with dosing based on levels) and melphalan (doses not reported). Patients had a median ANC recovery of 13 days and platelets > 20 × 103/mm3 of 20 days33. Nationwide Children’s Hospital has also reported on a small cohort (n=6) of high-risk neuroblastoma patients who underwent consolidation with a Bu/Mel AHCT regimen after receiving COG-type induction chemotherapy (6 cycles of chemotherapy). The average age of these patients was 4.3 years old, and high-risk disease was defined using the COG guidelines Busulfan was given IV, 1 mg/kg/dose for 16 doses over 4 days, without PK dosing guidance, followed by a single dose of Melphalan at 140 mg/m2. In this cohort, patients had ANC recovery by 10 – 11 days, and platelets > 20 × 103/mm3 by day 2529. The ANC and platelet engraftment times seen in these reports are similar to what we report for our study, where patients had adequate hematologic recovery after consolidation therapy with AHCT and experimental immunotherapy, with a median time to neutrophil and platelet engraftment of 13 and 36.5 days, respectively.
Pediatric patients with neuroblastoma are at a higher risk than patients with other solid tumors for developing VOD during AHCT35. The risk of VOD is influenced by the agents used during conditioning chemotherapy, with busulfan being of particular concern36–38. It is important to interpret VOD in the context of the criteria, prophylactic regimen and grading systems used, as these factors are not uniform across institutions. The criteria and grading system we used were based on that used in the Defibrotide Treatment IND protocol (NCT 00628498) that was available at that time39, and our VOD prophylaxis was heparin/enoxaparin with or without ursodiol. In our study, 8 patients (n =30, 26.7%) developed VOD, 7 of which then received treatment with defibrotide. Three patients had mild MOD, 1 with pulmonary insufficiency and 2 with acute renal injury (Table III). No patients developed VOD with severe MOD. This incidence of VOD is similar to previously reported data from several groups using a Bu/Mel based AHCT consolidation regimen. This includes SIOPEN (60 of 267 patients [22%]; diagnosis using the Bearman criteria40; ursodiol as VOD prophylaxis)31, CHOP (5 of 21 patients [23.8%]; diagnosis using modified Settle or Baltimore criteria; no VOD prophylaxis and treatment of VOD with defibrotide)33, and Nationwide (1 of 6 patients [16.7%]; diagnosis criteria not reported; no VOD prophylaxis)29.
Our institution has extensive experience with the use of adoptively transferred haploidentical NKs as a component of therapeutic regimens for hematologic malignancy, both in conjunction with chemotherapy and with allogeneic HCT41–44. In these other studies, the haploidentical NK cell products were processed in the same manner and the entirety of the purified NK cell product was administered without protocol defined NK cell dosing (doses administered ranged from 1.65 – 103 × 106 cells/kg CD56+ cells)42, 44. Consistent with our experience in these other trials, the use of haploidentical NKs in this experimental consolidation regimen was feasible and well tolerated. Of the 30 patients who consented to therapy with hu14.18K322A and cytokines in conjunction with AHCT, 21 (70%) received haploidentical NK infusions. No patients developed identifiable NK infusion-related complications. Although results must be interpreted cautiously due to small numbers, it is encouraging that engraftment of both neutrophils and platelets does not appear to be negatively impacted by the adoptive transfer of haploidentical NKs, and that other toxicities were comparable between those patients that did and did not receive NKs. The most common reason for NK donor ineligibility was recent travel to an area where high-risk communicable diseases are endemic, reflecting our international patient population.
Ideally, donor NKs would remain in the recipient while hu14.18K322A is present. In a previous study evaluating the use of hu14.18K322A in a refractory/relapsed neuroblastoma pediatric cohort, after 4 consecutive daily doses of hu14.18K322A the median T1/2α and T1/2β of hu14.18K322A were 1.7 and 21.1 days, respectively4. In this study, 15 patients had evaluable NK samples at a median of 11 days post-HPC infusion, and donor derived DNA was detected in 14 of these samples. However, at a median of 25 days post-HPC infusion, 18 patients had evaluable NK samples, of which donor DNA was detectable in only 6 samples. This transient haploidentical NK engraftment is consistent with prior studies at our institution, including in pediatric AML patients that received haploidentical NK cells (median dose 29 × 106 cells/kg) in conjunction with chemotherapy, and were found to have a median transient NK engraftment of 10 days, with a median peak NK chimerism of 7% donor43. It is unknown whether the addition of haploidentical NKs will improve outcomes or what threshold of haploidentical NK engraftment may be necessary to augment ADCC. This is an area that will require further evaluation, possibly in the context of a randomized trial. Additionally, the role of donor/recipient KIR mismatch on patient outcomes is not known. This will be evaluated in this study once more patients with a longer length of follow-up are available for analysis.
In conclusion, in newly diagnosed pediatric patients with high-risk neuroblastoma who have received intensive multi-modal induction therapy (including immunotherapy with an anti-GD2 mAb), the use of a consolidation therapy regimen that includes AHCT with a Bu/Mel conditioning regimen and immunotherapy with hu14.18K322A, GM-CSF, and IL-2, with or without haploidentical NK infusions, is feasible and tolerable. The long-term effects of this treatment regimen, as well as the resultant event-free and overall survival in this difficult-to-treat population continue to be explored. Since we saw no identifiable additional toxicity related to the incorporation of hu14.18K322A, cytokines and haploidentical NK cells in the immediate post-AHCT period, we believe that this approach could be incorporated into other neuroblastoma AHCT regimens, including tandem transplant. Future directions to determine the value of this experimental AHCT regimen could include randomization of patients to receive a myeloablative AHCT, with or without anti-GD2 mAb and cytokines, with or without the adoptive transfer of haploidentical NKs.
Acknowledgments
Funding sources: This work was supported by the St. Jude Children’s Research Hospital Comprehensive Cancer Center Support Grant (2 P30 CA021765), American Lebanese Syrian Associated Charities, Cookies for Kids’ Cancer and Cure Childhood Cancer Foundation
We wish to acknowledge the St. Jude Children’s Research Hospital Comprehensive Cancer Center, American Lebanese Syrian Associated Charities, Cookies for Kids’ Cancer and Cure Childhood Cancer Foundation for their support of this work. We thank the St. Jude Children’s Research Hospital GMP facility for manufacturing the hu14.18K322A antibody. We also thank senior scientific editor Keith Laycock, PhD for his critical review of this manuscript.
Footnotes
Financial Disclosure statement: The authors have no financial disclosures.
Conflict of Interest statement: The authors have no conflicts of interest to disclose.
References
- 1.Park JR, Bagatell R, London WB, et al. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatric blood & cancer. 2013;60:985–993. doi: 10.1002/pbc.24433. [DOI] [PubMed] [Google Scholar]
- 2.Grupp SA, Asgharzadeh S, Yanik GA. Neuroblastoma: issues in transplantation. Biology of blood and marrow transplantation. 2012;18:S92–100. doi: 10.1016/j.bbmt.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. The New England journal of medicine. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navid F, Sondel PM, Barfield R, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. Journal of clinical oncology. 2014;32:1445–1452. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kushner BH, Ostrovnaya I, Cheung IY, et al. Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with Anti-G immunotherapy and isotretinoin: a prospective Phase II study. Oncoimmunology. 2015;4:e1016704. doi: 10.1080/2162402X.2015.1016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shusterman S, London WB, Hank JA, et al. A feasibility and phase II study of the hu14.18-IL2 immunocytokine in combination with GM-CSF and isotretinoin in patients with recurrent or refractory neuroblastoma: A Children’s Oncology Group study. Journal of Clinical Oncology. 2015;33 <Go to ISI>://000358036900021. [Google Scholar]
- 7.Federico S, Leung W, Pappo AS, et al. Humanized Anti-GD2 Antibody (hu14.18K322A) Given with Chemotherapy +/− Parental Natural Killer (NK) Ceclls in Children with Recurrent or Refractory Neuroblastoma. Pediatric blood & cancer. 2014;61:S123–S123. <Go to ISI>://000343932100067. [Google Scholar]
- 8.Mody RNA, Van Ryn C, Yu AL, et al. Phase II randomized trial of irinotecan/temozolomide (I/T) with temsirolimus (TEM) or dinutuximab plus granulocyte colony stimuating factor (DIN/GMCSF) in children with refractory or relapsed neuroblastoma: A report from the Children’s Oncology Group (COG) Journal of clinical oncology. 2016;34(suppl) [Google Scholar]
- 9.Sorkin LS, Otto M, Baldwin WM, 3rd, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149:135–142. doi: 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anghelescu DL, Goldberg JL, Faughnan LG, et al. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatric blood & cancer. 2015;62:224–228. doi: 10.1002/pbc.25280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wayne Lee Furman SMF, McCarville Mary B, Davidoff Andrew M, et al. Improved clinical responses with the concomitant use of an anti-GD2 monoclonal antibody and chemotherapy in newly diagnosed children with high-risk (HR) neuroblastoma (NB): Preliminary results of a phase II study. Journal of Clinical Oncology. 2016:34. [Google Scholar]
- 12.Wayne Lee Furman BLS, Federico Sara Michele, McCarville Mary B, et al. Early response rates and Curie scores at end of induction: An update from a phase II study of an anti-GD2 monoclonal antibody (mAb) with chemotherapy (CT) in newly diagnosed patients (pts) with high-risk (HR) neuroblastoma (NB). ASCO Annual Meeting; 2017. [Google Scholar]
- 13.Barker E, Mueller BM, Handgretinger R, Herter M, Yu AL, Reisfeld RA. Effect of a Chimeric Anti-Ganglioside GD2 Antibody on Cell-mediated Lysis of Human Neuroblastoma Cells. Cancer Res. 1991;51:144–149. http://cancerres.aacrjournals.org/content/51/1/144.abstract. [PubMed] [Google Scholar]
- 14.Raffaghello L, Marimpietri D, Pagnan G, et al. Anti-GD2 monoclonal antibody immunotherapy: a promising strategy in the prevention of neuroblastoma relapse. Cancer Lett. 2003;197:205–209. doi: 10.1016/s0304-3835(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 15.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Current opinion in pediatrics. 2005;17:7–13. doi: 10.1097/01.mop.0000150631.60571.89. http://www.ncbi.nlm.nih.gov/pubmed/15659956. [DOI] [PubMed] [Google Scholar]
- 17.Park JR, Scott JR, Stewart CF, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children’s Oncology Group study. Journal of clinical oncology. 2011;29:4351–4357. doi: 10.1200/JCO.2010.34.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JRKS, London WB, Naranjo A, Lerner Cohn S, Hogarty M, et al. A phase III randomized clinical trial (RTC) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-rish neuroblastoma (HR-NB): A Children’s Oncology Group (COG) study. Journal of clinical oncology. 2016;34(suppl) [Google Scholar]
- 19.Schichman SA, Suess P, Vertino AM, Gray PS. Comparison of short tandem repeat and variable number tandem repeat genetic markers for quantitative determination of allogeneic bone marrow transplant engraftment. Bone marrow transplantation. 2002;29:243–248. doi: 10.1038/sj.bmt.1703360. [DOI] [PubMed] [Google Scholar]
- 20.Iyengar R, Handgretinger R, Babarin-Dorner A, et al. Purification of human natural killer cells using a clinical-scale immunomagnetic method. Cytotherapy. 2003;5:479–484. doi: 10.1080/14653240310003558. [DOI] [PubMed] [Google Scholar]
- 21.Carreras E. Veno-occlusive disease of the liver after hemopoietic cell transplantation. European journal of haematology. 2000;64:281–291. doi: 10.1034/j.1600-0609.2000.9r200.x. http://www.ncbi.nlm.nih.gov/pubmed/10863974. [DOI] [PubMed] [Google Scholar]
- 22.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. European journal of immunology. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 23.Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer immunology, immunotherapy : CII. 2008;57:1579–1587. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nature reviews. Immunology. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 25.Nair MP, Schwartz SA. Suppression of human natural and antibody-dependent cytotoxicity by soluble factors from unstimulated normal lymphocytes. J Immunol. 1982;129:2511–2518. http://www.ncbi.nlm.nih.gov/pubmed/6183335. [PubMed] [Google Scholar]
- 26.Beum PV, Lindorfer MA, Taylor RP. Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J Immunol. 2008;181:2916–2924. doi: 10.4049/jimmunol.181.4.2916. http://www.ncbi.nlm.nih.gov/pubmed/18684983. [DOI] [PubMed] [Google Scholar]
- 27.Proust-Houdemont S, Pasqualini C, Blanchard P, et al. Busulfan-melphalan in high-risk neuroblastoma: the 30-year experience of a single institution. Bone marrow transplantation. 2016 doi: 10.1038/bmt.2016.75. [DOI] [PubMed]
- 28.Elborai Y, Hafez H, Moussa EA, et al. Comparison of toxicity following different conditioning regimens (busulfan/melphalan and carboplatin/etoposide/melphalan) for advanced stage neuroblastoma: Experience of two transplant centers. Pediatric transplantation. 2016;20:284–289. doi: 10.1111/petr.12638. [DOI] [PubMed] [Google Scholar]
- 29.Soni S, Pai V, Gross TG, Ranalli M. Busulfan and melphalan as consolidation therapy with autologous peripheral blood stem cell transplantation following Children’s Oncology Group (COG) induction platform for high-risk neuroblastoma: early results from a single institution. Pediatric transplantation. 2014;18:217–220. doi: 10.1111/petr.12202. [DOI] [PubMed] [Google Scholar]
- 30.Molina B, Alonso L, Gonzalez-Vicent M, et al. High-dose busulfan and melphalan as conditioning regimen for autologous peripheral blood progenitor cell transplantation in high-risk neuroblastoma patients. Pediatr Hematol Oncol. 2011;28:115–123. doi: 10.3109/08880018.2010.537434. [DOI] [PubMed] [Google Scholar]
- 31.Ladenstein R, Potschger U, Pearson AD, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. The lancet oncology. 2017;18:500–514. doi: 10.1016/S1470-2045(17)30070-0. [DOI] [PubMed] [Google Scholar]
- 32.Yalcin B, Kremer LC, van Dalen EC. High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma. The Cochrane database of systematic reviews. 2015:CD006301. doi: 10.1002/14651858.CD006301.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai AV, Heneghan MB, Li Y, et al. Toxicities of busulfan/melphalan versus carboplatin/etoposide/melphalan for high-dose chemotherapy with stem cell rescue for high-risk neuroblastoma. Bone marrow transplantation. 2016;51:1204–1210. doi: 10.1038/bmt.2016.84. [DOI] [PubMed] [Google Scholar]
- 34.Morgenstern DAS, Potschger U, Wheeler K, Pasqualini C, Valteau-Couanet D, Garaventa A, Papadakis V, Yaniv I, Ladenstein R. Engraftment following busulfan/melphalan (BuMel) high-dose chemotherapy for high-risk neuroblastoma. A report from the HR-NBL-1/SIOPEN trial. Advances in Neuroblastoma Research Congress. 2016 [Google Scholar]
- 35.Horn B, Reiss U, Matthay K, McMillan A, Cowan M. Veno-occlusive disease of the liver in children with solid tumors undergoing autologous hematopoietic progenitor cell transplantation: a high incidence in patients with neuroblastoma. Bone marrow transplantation. 2002;29:409–415. doi: 10.1038/sj.bmt.1703393. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Yoo KH, Sung KW, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone marrow transplantation. 2010;45:1287–1293. doi: 10.1038/bmt.2009.349. [DOI] [PubMed] [Google Scholar]
- 37.Cheuk DK, Wang P, Lee TL, et al. Risk factors and mortality predictors of hepatic veno-occlusive disease after pediatric hematopoietic stem cell transplantation. Bone marrow transplantation. 2007;40:935–944. doi: 10.1038/sj.bmt.1705835. [DOI] [PubMed] [Google Scholar]
- 38.Valteau-Couanet D, Benhamou E, Vassal G, et al. Consolidation with a busulfan-containing regimen followed by stem cell transplantation in infants with poor prognosis stage 4 neuroblastoma. Bone marrow transplantation. 2000;25:937–942. doi: 10.1038/sj.bmt.1702376. [DOI] [PubMed] [Google Scholar]
- 39.Richardson PG, Smith AR, Triplett BM, et al. Defibrotide for Patients with Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome: Interim Results from a Treatment IND Study. Biology of blood and marrow transplantation. 2017;23:997–1004. doi: 10.1016/j.bbmt.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–3020. http://www.ncbi.nlm.nih.gov/pubmed/7756636. [PubMed] [Google Scholar]
- 41.Leung W. Infusions of allogeneic natural killer cells as cancer therapy. Clinical cancer research. 2014;20:3390–3400. doi: 10.1158/1078-0432.CCR-13-1766. [DOI] [PubMed] [Google Scholar]
- 42.Rubnitz JE, Inaba H, Kang G, et al. Natural killer cell therapy in children with relapsed leukemia. Pediatric blood & cancer. 2015;62:1468–1472. doi: 10.1002/pbc.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. Journal of clinical oncology. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Triplett BM, Shook DR, Eldridge P, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone marrow transplantation. 2015;50:1012. doi: 10.1038/bmt.2015.139. [DOI] [PubMed] [Google Scholar]