Abstract
Neutralizing antibodies (NAb) interfering with glycoprotein complex-mediated virus entry into host cells are thought to contribute to the protection against herpesvirus infection. However, using herpesvirus glycoprotein complexes as vaccine antigens can be complicated by the necessity of expressing multiple subunits simultaneously to allow efficient complex assembly and formation of conformational NAb epitopes. By using a novel bacterial artificial chromosome (BAC) clone of the clinically deployable Modified Vaccinia Ankara (MVA) vector and exploiting ribosomal skipping mediated by 2A peptides, MVA vectors were generated that expressed self-processing subunits of the human cytomegalovirus (HCMV) pentamer complex (PC) composed of gH, gL, UL128, UL130, and UL131A. These MVA vectors expressed 2A-linked HCMV PC subunits that were efficiently cleaved and transported to the cell surface as protein complexes forming conformational neutralizing epitopes. In addition, vaccination of mice by only two immunizations with these MVA vectors resulted in potent HCMV NAb responses that remained stable over a period of at least six months. This method of eliciting NAb by 2A-linked, self-processing HCMV PC subunits could contribute to develop a HCMV vaccine candidate and may serve as a template to facilitate the development of subunit vaccine strategies against other herpesviruses.
Keywords: Herpesvirus, glycoprotein, vaccine, neutralizing antibody, artificial chromosome, 2A peptide
Introduction
Herpesviruses include a number of ubiquitous and highly-adapted human pathogens that can cause severe illnesses in individuals with impaired immune system such as transplant recipients or AIDS patients or in congenitally infected fetuses (Arvin et al., 2007). While licensed vaccines are available for varicella-zoster virus (VZV), vaccines candidates for other human herpesviruses including herpes-simplex viruses (HSV), human cytomegalovirus (HCMV), Epstein-Barr virus (EBV), and Kaposi sarcoma-associated herpesvirus (KSHV) remain elusive (Arvin et al., 2007; Stratton et al., 2000). Considerable efforts to develop herpesvirus vaccines have been made using approaches based on selected humoral or cellular immunodominant antigens as safer and more cost-effective alternatives to live-attenuated, inactivated, or replication-defective herpesvirus vaccines. These subunit vaccine approaches using plasmids, viral vectors, purified protein, or virus-like particles showed promising results in different animal models and clinical trials (Cohen, 2015; Dasgupta et al., 2009; Johnston et al., 2016; Pass et al., 2009; Schleiss, 2008; Schleiss, 2016).
Eliciting neutralizing antibodies (NAb) that interfere with glycoprotein complex-mediated virus entry into host cells is thought to be important for a vaccine formulation to prevent or control herpesvirus infection (Nelson et al., 2017; Plotkin, 2013). While the essential and highly-conserved herpesvirus envelope glycoprotein complexes composed of gB and gH/gL are principal targets for NAb, additional glycoprotein complexes or accessory glycoproteins associating with gH/gL can represent critical immune targets that contribute to the stimulation of herpesvirus neutralizing activity (Heldwein, 2016; Macagno et al., 2010; Sathiyamoorthy et al., 2017; Vanarsdall and Johnson, 2012). Over the past years it has been discovered for HCMV that NAb blocking infection of many biologically relevant host cells recognize in majority three accessory glycoproteins called UL128, UL130, and UL131A that form a pentamer complex (PC) with gH/gL (Fouts et al., 2012; Macagno et al., 2010; Wang and Shenk, 2005). While this complex is dispensable for HCMV infection of fibroblasts (FB), it is required for efficient infection of epithelial cells (EC), endothelial cells, and other cells thought to be important for HCMV dissemination and transmission (Hahn et al., 2004; Sinzger et al., 2008; Vanarsdall and Johnson, 2012; Wang and Shenk, 2005). In contrast to NAb interfering with the essential entry function of gB and gH/gL complexes, NAb interfering with PC-mediated entry are unable to block FB infection, though they are substantially more potent than NAb targeting gB or gH/gL epitopes in preventing infection of non-FB cell types such as EC (Chiuppesi et al., 2015; Macagno et al., 2010). These results suggest that the PC could be an important vaccine component to prevent or control HCMV infection.
Since NAb targeting the HCMV PC recognize mainly quaternary conformational epitopes that are formed effectively only upon assembly of UL128/130/131A with gH/gL, vaccine-mediated induction of HCMV NAb is promoted by simultaneous expression of all five PC subunits (Chandramouli et al., 2017; Chiuppesi et al., 2017a; Chiuppesi et al., 2015; Chiuppesi et al., 2017b; Ciferri et al., 2015a; Ciferri et al., 2015b; Macagno et al., 2010; Wussow et al., 2014; Wussow et al., 2013). Ribosomal skipping mediated by 2A peptides of picornaviruses is a widely used mechanism to express multiple proteins via a single transcript because of the relative small size of the 2A peptides (18–22 amino acids) and the potential stoichiometric expression of the co-expressed proteins (de Felipe, 2004; Kim et al., 2011; Szymczak et al., 2004). By using a novel bacterial artificial chromosome (BAC) clone of the well-characterized and clinically-deployable Modified Vaccinia Ankara (MVA) vector (Cottingham et al., 2008; Gilbert, 2013; Verheust et al., 2012), the use of 2A-mediated ribosomal skipping was exploited to stimulate NAb by self-processing subunits of the HCMV PC. The results indicate that recombinant MVA vectors either expressing 2A-linked polyproteins composed of all five PC subunits or co-expressing 2A-linked polyproteins composed of UL128/130/131A and gH/gL stimulate robust HCMV NAb responses in mice. This method of eliciting HCMV NAb by 2A-linked polycistronic expression constructs could contribute to develop a HCMV vaccine candidate and may serve as template to facilitate the development of vaccine approaches based on multi-subunit glycoprotein complexes of other herpesvirus.
Materials and Methods
Cells and viruses
Baby hamster kidney (BHK-21) cells, ARPE-19, MRC-5 (American Type Culture Collection [ATCC]) and chicken embryo fibroblasts (CEF; Charles River) were maintained by standard procedures. MVA was propagated in BHK-21 and CEF cells as described previously (Wussow et al., 2014). HCMV and MVA virus stocks were prepared following virus propagation in ARPE-19 or BHK-21 cells, respectively, and titrated as described (Chiuppesi et al., 2017a; Chiuppesi et al., 2015; Wussow et al., 2014). HCMV TB40/E expressing GFP was derived from TB40/Ewt-GFP BAC DNA (O’Connor and Murphy, 2012). MVA 1974/NIH clone 1 were kindly provided by Bernard Moss (NIAID) (Mayr and Malicki, 1966). The construction of MVA-PC has been described previously (Wussow et al., 2014).
Plasmids
Transfer plasmids for generating a novel MVA BAC, termed MVABAC-TK (Figure 1), or MVABAC-TK-derived recombinants with 2A-linked HCMV PC subunits were constructed by standard molecular biology cloning techniques. For generating MVABAC-TK, a transfer vector was generated in which pBeloBAC11 sequences (New England Biolabs) and a GFP expression cassette with vaccinia P11 late promoter were flanked by DNA sequences homologues to the MVA Thymidine kinase (TK) gene locus (Figure 1). MVA TK homology flanks were derived by PCR from MVA 1974/NIH clone 1 and corresponded to base pairs 69313–70000 and 70001–70703 (MVA Acambis, Accession Nr. AY603355.1). The GFP marker with P11 promoter was derived by PCR from plasmid pLW73 (Wyatt et al., 2009). A unique AvrII restriction site was introduced between the ends of the TK homology flanks to allow linearization of the entire transfer construct (Figure 1). Transfer constructs for inserting P2A-linked HCMV PC subunits into MVABAC-TK by En passant mutagenesis were generated by cloning codon-optimized and P2A-linked UL128/UL130/UL131A or gH/gL subunit subset gene sequences between the vaccinia modified H5 early/late promoter (mH5) and vaccinia transcription termination signal (TTTTAT) of pGEM-T-mH5 (Wussow et al., 2013). A kanamycin expression cassettes with adjacent I-SceI homing endonuclease restriction sites and flanking 50bp gene duplication was subsequently introduced into the pGEM-T-mH5-cloned HCMV PC subunit subset genes sequences (Tischer et al., 2010; Wussow et al., 2013). All HCMV PC subunit gene sequences were based on HCMV strain TB40/E (TB40/E-BAC; Accession Nr. EF999921). Codon-optimized and P2A-linked PC gene sequences of HCMV TB40/E were synthesized by Genescript. Optimization of the HCMV TB40/E gene sequences for Vaccinia codon usage was performed using the Codon Optimization Tool from Integrated DNA Technologies. Runs of more than three nucleotides of the same type in a row within the codon-optimized P2A-linked HCMV gene sequences were silently mutated to enhance the stability of HCMV genes within MVA (Wyatt et al., 2009). Gene internal Kanamycin/I-SceI cassettes flanked by 50 bp gene duplications were derived by PCR from plasmid pEPkan-S2 using primers that provided sequences for the 50 bp gene duplications. Detailed sequence maps generated by Vector NTI (Invitrogen) for all plasmids are available upon request. All cloned transfer constructs were confirmed by sequencing.
Figure 1. Construction of MVA expressing self-processing HCMV PC subunits.
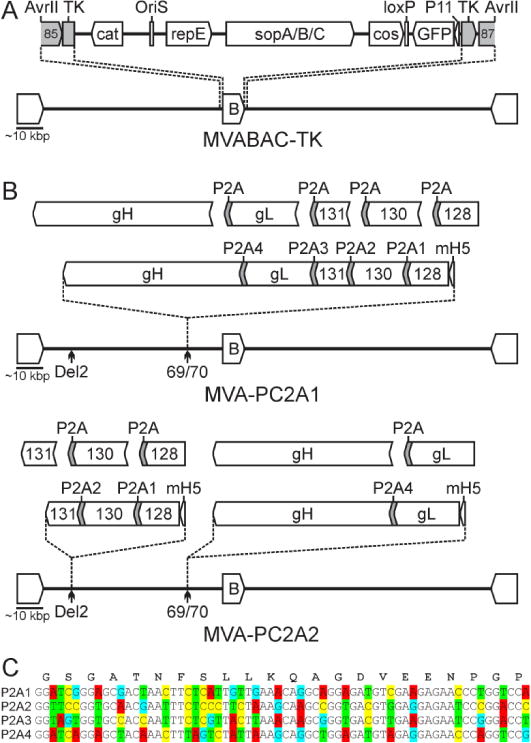
A) MVABAC-TK construction. pBeloBAC11 vector sequences (B = cat, OriS, repE, sopA/B/C, cos, loxP site) and a GFP expression cassette (GFP, Vaccinia P11 promoter) were inserted into the Thymidine kinase (TK) gene MVA utilizing ~700bp homologous sequences (gray filled elements). 85 and 87 = MVA ORFs 85 and 87 (Accession Nr. U94848). B) Construction of MVA-PC2A1 and MVAPC2A2. HCMV PC subunits (gH, gL, UL128, UL130, UL131A) linked by different P2A sequences (P2A1-P2A4) were inserted into MVABAC-TK either all together into the MVA intergenic region 69/70 (IGR69/70) to generate MVA-PC2A1, or separately as UL128/130/131A and gH/gL subunit subsets into the MVA deletion 2 (Del2) site and IGR69/70 to generate MVA-PC2A2. B = BAC vector; mH5 = modified H5 promoter. C) P2A sequences (P2A1-P2A4) with different codon usage were used to link the HCMV PC subunits within the MVA constructs as indicated in B. Lower 4 lines indicate DNA sequences with mutated nucleotides (marked in colors) that were used to encode the different P2A peptides between the HCMV subunits. Upper line shows the amino acid sequences of the P2A peptide.
BAC construction
MVABAC-TK was generated by a procedure similar to that described previously by Domi and Moss (Domi and Moss, 2002). Briefly, 70–90% confluent CEF cells were infected with MVA 1974/NIH clone 1 at 0.01 multiplicity of infection (MOI) and 2 h later transfected with 2μg of AvrII-linearized BAC transfer vector (Figure 1) using Fugene HD transfection reagent (Roche) according to the manufacturer’s instruction. MVA recombinants were isolated following six rounds of plaque purification using GFP expression of the inserted BAC vector as a marker (Figure 1). CEF cells were then infected at 5 MOI with the isolated MVA and grown in presence of 45μM Isatin-β-thiosemicarbazone (IβT) to inhibit viral hairpin resolution and to promote heat-to-tail genome concatemerization and circularization (Domi and Moss, 2002). After 5 h of incubation in presence of IβT, DNA was isolated from the infected CEF cells using the DNAeasy Blood and Tissue genome isolation Kit from Qiagen according to the manufacturer’s instructions, and purified DNA was transformed into DH10B E. coli cells (Invitrogen). Numerous BAC clones were investigated by PCR and restriction fragment length analysis. One BAC clone (#1-81) was ultimately sequenced by Illimina shotgun sequencing at the City of Hope Core Facility, and the sequence of the cloned MVA genome (excluding the large terminal repeat sequences) was found to be identical to the genome sequence of MVA strain Acambis (Accession Nr. AY603355.1).
BAC recombinants
MVA vectors expressing P2A-linked HCMV PC subunits were generated by En passant mutagenesis in GS1783 bacteria cells as described previously (Tischer et al., 2010; Wussow et al., 2013). Briefly, transfer constructs of the P2A-linked UL128/130/131A or gH/gL subunits were amplified from pGEM-T-mH5 cloning vectors with primers containing 50 bp extensions homologous to the target site, and inserted into the MVA genome via an initial Red recombination. Subsequently, the kanamycin marker within the gene sequences was seamlessly removed by a second Red recombination utilizing the engineered 50 bp gene duplications flanking the marker sequences (Tischer et al., 2006; Wussow et al., 2013). For generating MVA with P2A-linked HCMV PC subunits inserted into two separate MVA insertion sites (MVA-PC2A2, Figure 1), the UL128/130/131A and gH/gL subunit subsets were inserted by two successive En passant mutagenesis reactions into the MVA Deletion 2 site (Del2) and the intergenic region between MVA genes 69 and 70 (IGR69/70; Accession Nr. U94848), respectively. For inserting the five HCMV PC subunits all together into only one insertion site (MVA-PC2A1, Figure 1), the UL128/130/131A subunits were inserted into the IGR69/70 by a first En passant mutagenesis reaction, and the gH/gL subunits were subsequently inserted into the UL128/130/131A-containing IGR69/70 site by a second En passant recombination reaction. All inserted HCMV PC gene were confirmed by PCR, restriction fragment length analysis, and sequencing. Detailed BAC sequence maps are available upon request. Virus reconstitution from recombinant BAC DNA was performed in BHK cells as described (Cottingham et al., 2008; Domi and Moss, 2002; Wussow et al., 2014).
Immunoblot
Western blot analysis to detect HCMV PC subunits in whole cell lysates of MVA infected BHK cells was performed using standard procedures (Wussow et al., 2014). HCMV gL and UL131A were detected with peptide-specific rabbit polyclonal antisera (Wussow et al., 2014); UL128 was detected using MAb Z9G11 (Gerna et al., 2008), a kind gift from Giuseppe Gerna (Pavia University, Italy); gH was detected using MAb AP86 (Simpson et al., 1993), kindly provided by William Britt (University of Alabama at Birmingham). UL130 was detected using MAb 3C5 (Wang and Shenk, 2005), a kind gift from Thomas Shenk (Princeton University). Vaccinia virus B5R was detected using MAb 19C2 (Schmelz et al., 1994).
Flow Cytometry
Cell surface flow cytometry staining to detect HCMV PC subunits on MVC-infected cells by monoclonal NAb was performed as described previously (Chiuppesi et al., 2015). Briefly, BHK cells (70–90% confluent) were infected with the MVA vectors at MOI 5. At 4 h post infection, infected cells were collected, washed in phosphate buffered saline (PBS), and incubated for 1 h at 4°C with 10 μg/ml NAb. After washing with PBS, the cells were incubated with Alexa Fluor 647 goat anti-mouse IgG (Life Technologies) at a dilution of 1:2,000. The cells were washed again and resuspended in PBS with 0.1% bovine serum albumin (BSA). Fifteen thousand events were collected using a Gallios flow cytometer (Beckman Coulter) and analyzed with FlowJo software (Tree Star).
Mouse immunization
The Institutional Animal Care and Use Committee (IACUC) of the Beckman Research Institute of City of Hope approved protocol 98004 assigned for this study. All study procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. BALB/c mice (Jackson Laboratory) were vaccinated via intraperitoneal (i.p.) route with 5×107 PFU of MVA. Blood samples were collected by retro-orbital bleeding.
Neutralization assay
HCMV microneutralization assay was performed similar to published reports (Chiuppesi et al., 2015; Wussow et al., 2014). Heat-inactivated sera were serially two-fold diluted in 100 μl volumes using complete growth medium for ARPE-19 EC or MRC-5 FB depending on the cell type used in the assay. Dilutions ranged from 1∶25 to 1∶102400. Diluted serum was mixed with 100 μl of complete growth medium containing approximately 2400 PFU of HCMV TB40/Ewt-GFP-derived virus. After 2 h incubation, virus/sera mixtures were added in triplicate (50 μl) to ARPE-19 or MRC-5 cells seeded the day before at 1.5×104 cells/well in a clear bottom polystyrene 96-well plate (Corning) that contained 50 μl per well of complete growth medium. Cells were grown for 48 h and fixed in methanol/acetone. Infected cells were identified by immunostaining using mouse anti-HCMV IE1 Ab (p63-27 (Andreoni et al., 1989); kindly provided by William Britt (University of Alabama at Birmingham)) and the Vectastain ABC kit (VectorLabs). The substrate was 3, 3′-diaminobenzidine (DAB, VectorLabs). Plates were analyzed by an automated system using the Axio Observer Z1 inverted microscope equipped with a linear motorized stage (Carl Zeiss). IE1 positive nuclei per field of view using a 5× objective were counted using ImagePro Premier (Media Cybernetics). For each dilution the average number of positive nuclei in triplicate was calculated. The percent neutralization titer (NT) for each dilution was calculated as follows: NT = [1 − (positive nuclei number with immune sera)/(positive nuclei number with pre-immune sera)]×100. The titers that gave 50% neutralization (NT50) were calculated by determining the linear slope of the graph plotting NT versus plasma dilution by using the next higher and lower NT values that were closest to 50% neutralization.
Statistics
GraphPad Prism software version 5.0 (GraphPad) was used to compare NAb titers in the different vaccine groups by statistical analysis using Wilcoxon matched-pairs test.
Results
Construction of MVA expressing 2A-linked HCMV PC subunits
Using a novel MVA BAC, termed MVABAC-TK, with genome sequence based on the MVA isolate of the NIH (Figure 1), two different recombinant MVA vectors were generated that expressed self-processing HCMV PC subunits. Codon-optimized and 2A-linked gene sequences of the five HCMV PC subunits were inserted either as a single polycistronic expression construct into only one MVA insertion site to generate MVA-PC2A1, or as UL128/130/131A and gH/gL expression constructs into two separate MVA insertion sites to generate MVA-PC2A2 (Figure 1). All 2A peptide sequences were based on the 2A peptide of porcine teschovirus-1 (P2A), which in combination with an N-terminal GSG-linker has recently been shown to mediate highly efficient polyprotein processing in human cells and animal models (Kim et al., 2011). DNA sequences with alternate codon usage were used to encode for P2A peptides between different HCMV PC subunits to avoid instability by homologous recombination of the multiple P2A sequence signals (Figure 1). MVA-PC2A1 and MVA-PC2A2 were reconstituted and propagated in BHK cells, and purified virus stocks of these MVA recombinants were characterized in vitro and in vivo.
Expression of 2A-linked HCMV PC subunits using MVA
In order to characterize the expression of the P2A-linked HCMV PC subunits of MVA-PC2A1 and MVA-PC2A2, whole cell-lysates of MVA-infected BHK cells were investigated via Immunblot analysis following five virus passages. As control, a previously generated MVA vector, termed MVA-PC, was included, which co-expressed all five HCMV PC subunits using expression constructs inserted into five separate MVA insertion sites (Wussow et al., 2014; Wussow et al., 2013). As shown in Figure 2, both MVA-PC2A1 and MVA-PC2A2 showed comparable expression levels for the individual HCMV PC subunits during the five virus passages on BHK cells, suggesting that the PC subunits are stably maintained within these MVA vectors. In addition, the detectable protein sizes of the individual HCMV PC subunits expressed from MVA-PC2A1 and MVA-PC2A2 were consistent with anticipated molecular weight values for the HCMV PC subunits as a consequence of efficient polyprotein processing. Most of the HCMV PC subunits expressed from MVA-PC2A1 and MVA-PC2A2 showed slightly higher molecular weight values compared to their counterparts expressed from MVA-PC, which was consistent with an expected increase in protein size (~2kD) due to remaining C-terminal peptides remnants following P2A-mediated cleavage. This was in particularly evident for UL128 and UL131A of MVA-PC2A1 and UL128 of MVA-PC2A2. The two protein bands that were observed for UL128 expressed either from MVA-PC2A1 or MVA-PC2A2 appeared to be specific for these MVA vectors as they were not detected in MVA-PC-infected cells and have not been described for HCMV. The C-terminal P2A peptide remnant may have rendered the UL128 protein unstable, leading to specific UL128 degradation products associated with MVA-PC2A1 or MVA-PC2A2. Compared to MVA-PC, MVA-PC2A2 appeared to express overall slightly lower amounts of the HCMV PC subunits, and lowest expression levels of the HCMV PC subunits were observed for MVA-PC2A1. In particularly UL131A expressed from MVA-PC2A1 appeared to be expressed in only very low amounts (Figure 2). These results indicated that MVA-PC2A1 and MVA-PC2A2 stably expressed all five HCMV PC subunits that were efficiently cleaved in MVA-infected cells.
Figure 2. Expression of P2A-linked HCMV PC subunits using MVA.

MVA-PC2A1 (A) and MVA-PC2A2 (B) were passaged five times on BHK cells and whole cell lysates of infected cells of the different virus passages (1 to 5) were investigated by Immunoblot using rabbit polyclonal antisera and MAb specific for the individual HCMV PC subunits. MVA-PC, uninfected BHK cells, and BHK cells infected with MVA expressing HCMV gB were investigated for controls. Vaccinia protein BR5 was detected as a loading control. kDa = kilo Dalton. Arrows indicate expected molecular weights of the PC subunits.
Complex assembly and formation of NAb epitope by 2A-linked HCMV PC subunits
To evaluate whether the P2A-linked HCMV PC subunits expressed from MVA-PC2A1 and MVA-PC2A2 assembled into complexes that form NAb epitopes, cell surface flow cytometry staining analysis of MVA-infected BHK cells was performed using MAb targeting PC- and gH-specific neutralizing epitopes. These MAb were previously isolated from mice immunized with MVA-PC and recognized quaternary conformational neutralizing epitopes formed by the UL128/130/131A subunits, a continuous neutralizing epitope within UL128, or a neutralizing epitope within gH (Chiuppesi et al., 2017a; Chiuppesi et al., 2015). In addition, these vaccine-derived NAb afforded similar neutralizing potency as PC- and gH-specific NAb induced by HCMV during natural infection (Chiuppesi et al., 2015). MVA-PC was used as a control in the analysis. As shown in Figure 3, BHK cells infected with either MVA-PC2A1 or MVA-PC2A2, or control vector MVA-PC were stained efficiently and with similar intensity with all different NAb. Only slightly higher staining intensity was observed for MVA-PC2A2 compared to MVA-PC2A1 and control vector MVA-PC. Overall lowest staining intensity was observed for MVA-PC2A1. These results indicated that the HCMV PC subunits expressed from both MVA-PC2A1 and MVA-PC2A2 assembled efficiently and were transported to the cell surface as protein complexes that displayed NAb epitopes.
Figure 3. Cell surface staining of 2A-linked HCMV PC subunits expressed from MVA.
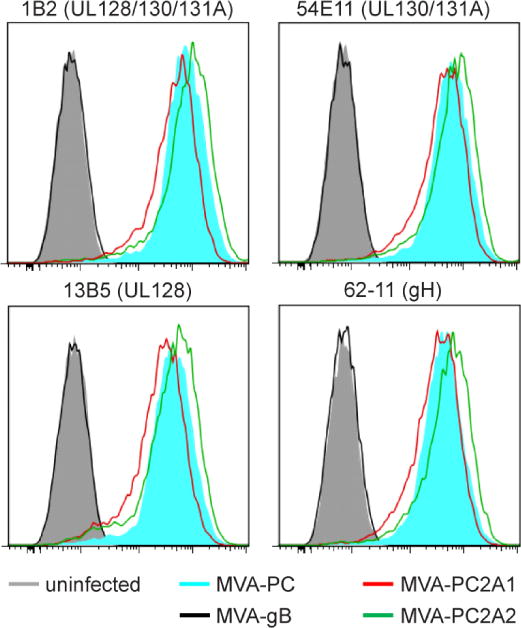
A) BHK cells infected with MVA-PC2A1 or MVA-PC2A2 (live, non-permeabilized) were investigated by cell surface flow cytometry staining using MAb specific for neutralizing epitopes of UL128/130/131A (MAb 1B2), UL130/131A (MAb 54E11), UL128 (13B5), or gH (62-11). MVA-PC, uninfected BHK cells, and BHK cells infected with MVA expressing HCMV gB were investigated for controls. Histogram axes in A and B represent fluorescence intensity (X-axis) and cell count (Y-axis).
NAb induction by 2A-linked HCMV PC subunits in mice
To determine the immunogenicity of the P2A-linked HCMV PC subunits expressed from MVA-PC2A1 and MVA-PC2A2 to stimulate HCMV NAb, HCMV NAb induction by these MVA vectors in mice using a two-dose immunization schedule was evaluated. As control MVA-PC was used, which has previously been shown to elicit high-titer and durable HCMV NAb in mice and rhesus macaques that significantly exceeded those induced by MVA expressing only PC subunits or subunit subsets or only gB (Wussow et al., 2014). In addition, MVA-PC elicited NAb that exceeded those induced by HCMV during natural infection (Chiuppesi et al., 2015). Hence, NAb induced by MVA-PC may indirectly serve as a benchmark to elicit NAb of greater potency to those induced by HCMV in infected individuals. Balb/c mice were two times vaccinated by intraperitoneal route with the MVA vectors, and HCMV NAb in mouse sera were measured against HCMV strain TB40/E on ARPE-19 EC and MRC-5 FB by microneutralization assay over a period of 6 months. Both MVA-PC2A1 and MVA-PC2A2 stimulated robust HCMV NAb responses that were similar or even slightly elevated compared to those stimulated by control vector MVA-PC (Figure 4). Potent HCMV NAb responses were induced by all MVA vectors after only one immunization, and these responses were efficiently boosted in all vaccine groups after the second immunization. In addition, NAb remained relatively stable in all vaccine groups until the end of the experiment. Consistent with previous observation for MVA-PC and other vaccine approaches employing the PC (Kabanova et al., 2014; Wen et al., 2014; Wussow et al., 2014), MVA-PC2A1 and MVA-PC2A2 elicited high-titer NAb that blocked EC infection and less potent NAb blocking FB infection. This is likely due to immunological properties of the HCMV PC to stimulate NAb targeting UL128/130/131A epitopes that potently and specifically block EC infection, and NAb of lower potency to gH epitopes that have the capacity to block both EC and FB infection (Chiuppesi et al., 2015; Kabanova et al., 2014). While EC NAb titers were generally comparable across all vaccine groups, FB NAb titers were significantly higher in MVA-PC2A2 immunized animals at week 7 and 24 and in MVA-PC2A1 immunized animals at week 7 compared to the control group (Figure 4). These results indicated that the P2A-linked HCMV PC subunits expressed from MVA-PC2A1 and MVA-PC2A2 were highly immunogenic to stimulate HCMV NAb responses in mice using only two immunizations.
Figure 4. NAb induction by MVA vectors expressing P2A-linked HCMV PC subunits.

A) NAb titers (geometric mean titer) measured over a 24 weeks period. Balb/c mice (N=4 to 6) were prime/boost vaccinated in four weeks interval (black arrows) with MVA-PC2A1, MVA-PC2A2, or control vector MVA-PC. At the indicated time points, HCMV specific NAb titer (log10 NT50) in mouse sera were measured against HCMV strain TB40/E on ARPE-19 EC (continuous lines) or on MRC-5 FB (dashed lines). The dotted line indicates the limit of NAb detection. Bars represent 95% confidence intervals B) Statistical analysis of NAb titers. Wilcoxon matched-pairs test was used to investigate differences of ARPE-19 EC and MRC-5 FB specific NAb titers at week 7 and week 24 in vaccine groups immunized with the indicated MVA vectors. P values less than 0.05 were indicated with *.
Discussion
The results of this study show in mice that the ribosomal skipping mechanism mediated by 2A peptides of picornavirus can be utilized to stimulate robust NAb responses by self-processing subunits of the HCMV PC. Using a novel MVA BAC with genome sequence identical to the MVA isolate of the NIH (excluding the terminal repeats), recombinant MVA vectors were generated that simultaneously expressed all five HCMV PC subunits either via only a single polycistonic expression construct (MVA-PC2A1) or via two separate transcripts encoding UL128/130/131A and gH/gL (MVA-PC2A2). While these MVA vectors appeared to express different amounts of the HCMV PC subunits, all five PC subunits expressed from both of these MVA vectors were efficiently cleaved and transported to the cell surface of MVA-infected cells where they formed protein complexes and displayed conformational and linear neutralizing epitopes. In addition, both of these MVA vectors were at least as potent in eliciting HCMV NAb as the previously generated MVA vector co-expressing all five PC subunits using separate insertion site, suggesting that the polycistronic MVA vectors have the potency to elicit NAb that exceed those induced by HCMV during natural infection (Wussow et al., 2014). Whether the HCMV PC subunits are stoichiometrically produced from MVA-PC2A1 and MVA-PC2A2 remains unclear. Yet, the observations that all five PC subunits were expressed in lower levels from MVA-PC2A1 compared MVA-PC2A2 may suggest that the 2A linkage tightly controls the expression levels of the individual HCMV PC subunits. While the 2A technology has been used successfully to produce soluble and highly immunogenic HCMV PC protein (Kabanova et al., 2014), this report describes the first example for the use of the 2A system to elicit robust NAb responses by self-processing subunits of a multi-subunit herpesvirus glycoprotein complex using a clinically-deployable viral vector.
Similar to the PC of HCMV, the gH/gL/gO complex of HCMV or multi-subunit envelope glycoprotein complexes of other herpesviruses such as gH/gL/gp42 of EBV play a critical role in receptor-mediated entry into host cells (Heldwein, 2016; Kabanova et al., 2016; Sathiyamoorthy et al., 2017). Considering the findings for the HCMV PC (Kabanova et al., 2014; Macagno et al., 2010; Wussow et al., 2014), it is likely that efficient vaccine-mediated induction of NAb by other multi-subunit herpesvirus glycoprotein complexes will require to express all individual complex subunits simultaneously. Because the procedure presented in this study for eliciting NAb by 2A-mediated polycistronic expression avoids the need for multiple promoter elements or insertion sites, this method could serve as a general approach for subunit vaccine development to stimulate robust NAb by multi-subunit herpesvirus glycoprotein complexes using virtually any kind of expression or delivery system. In addition, due the small size of the 2A signal sequences compared to internal ribosomal entry sites (IRES) (Kim et al., 2011; Szymczak et al., 2004), the use of 2A peptides considerably reduces the size of the element needed to combine multiple glycoprotein subunits into a single polycistronic expression construct. While the results provide proof-of-concept that the 2A cleavage system can be used to express the HCMV PC in the context of MVA, they do not a priori predict that other herpesvirus glycoprotein complexes can be expressed in a similar way using the 2A technology. The processing efficiency of other herpesviruses glycoprotein complex subunits by 2A-mediated cleavage may be significantly lower. In addition, other herpesvirus glycoprotein complex subunits may be more sensitive to negative effects of the residual C-terminal 2A peptides on subunit folding, complex assembly, stability, or translocation to the cell surface.
Because the developed procedure avoids or reduces repeated manipulation of the MVA genome to insert multiple antigens into a single MVA vector as it would be the case when using different insertion sites, this methodology may generally serve as a valuable alternative to construct multi-antigenic MVA vectors. Hence, the procedure may also be utilized for a subunit vaccine formulation to facilitate simultaneous co-expression of multiple subunits of different envelope glycoprotein complexes such as the PC and gB of HCMV to stimulate NAb that interfere with multiple steps of herpesvirus entry including virus attachment, fusion, and receptor binding. Moreover, the system could be utilized to easily combine multiple humoral and cellular immunodominant antigens into a subunit vaccine formulation to elicit potentially protective immune responses that cover both arms of adaptive immunity. Based on the large insertion capacity and numerous insertion sites of MVA and the relative small size of the 2A peptides, MVA in combination with 2A-mediated ribosomal skipping could represent an ideal system to develop multivalent subunit vaccines to prevent the infection of herpesviruses or other viruses that utilize a multi-glycoprotein complex entry machinery for the infection of host cells.
Highlights.
MVA vectors expressing 2A-linked, self-processing subunits of the HCMV pentamer
Complex assembly and neutralizing epitope formation by HCMV pentamer subunits
Induction of robust HCMV-specific neutralizing antibody responses in mice
Method could contribute to develop multi-antigenic herpesvirus subunit vaccines
Acknowledgments
We acknowledge Dr. Bernard Moss (NIAID) for providing MVA 1974/NIH clone 1, and Drs. Thomas Shenk and Eain Murphy (Stanford University) for providing HCMV TB40/Ewt-GFP BAC. We would like to thank Aline Matsuo for assisting in the submission process. This research was funded by U.S. Public Health Service grant R01 AI103960 and AI063356 to DJD and PAB. DJD was partially supported by CA077544 and CA181045. Research reported in this publication included work performed in the Light Microscopy Digital Imaging Core and the Integrative Genomics Core at City of Hope supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no competing interest exists.
References
- Andreoni M, Faircloth M, Vugler L, Britt WJ. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods. 1989;23:157–67. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. Human Herpesviruses Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge: 2007. [PubMed] [Google Scholar]
- Chandramouli S, Malito E, Nguyen T, Luisi K, Donnarumma D, Xing Y, Norais N, Yu D, Carfi A. Structural basis for potent antibody-mediated neutralization of human cytomegalovirus. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan1457. [DOI] [PubMed] [Google Scholar]
- Chiuppesi F, Kaltcheva T, Meng Z, Barry PA, Diamond DJ, Wussow F. Identification of a Continuous Neutralizing Epitope within UL128 of Human Cytomegalovirus. J Virol. 2017a;91 doi: 10.1128/JVI.01857-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuppesi F, Wussow F, Johnson E, Bian C, Zhuo M, Rajakumar A, Barry PA, Britt WJ, Chakraborty R, Diamond DJ. Vaccine-Derived Neutralizing Antibodies to the Human Cytomegalovirus gH/gL Pentamer Potently Block Primary Cytotrophoblast Infection. J Virol. 2015;89:11884–98. doi: 10.1128/JVI.01701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuppesi F, Wussow F, Scharf L, Contreras H, Gao H, Meng Z, Nguyen J, Barry PA, Bjorkman PJ, Diamond DJ. Comparison of homologous and heterologous prime-boost vaccine approaches using Modified Vaccinia Ankara and soluble protein to induce neutralizing antibodies by the human cytomegalovirus pentamer complex in mice. PLoS One. 2017b;12:e0183377. doi: 10.1371/journal.pone.0183377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C, Chandramouli S, Donnarumma D, Nikitin PA, Cianfrocco MA, Gerrein R, Feire AL, Barnett SW, Lilja AE, Rappuoli R, Norais N, Settembre EC, Carfi A. Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes. Proc Natl Acad Sci U S A. 2015a;112:1767–72. doi: 10.1073/pnas.1424818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C, Chandramouli S, Leitner A, Donnarumma D, Cianfrocco MA, Gerrein R, Friedrich K, Aggarwal Y, Palladino G, Aebersold R, Norais N, Settembre EC, Carfi A. Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies. PLoS Pathog. 2015b;11:e1005230. doi: 10.1371/journal.ppat.1005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI. Epstein-barr virus vaccines. Clin Transl Immunology. 2015;4:e32. doi: 10.1038/cti.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham MG, Andersen RF, Spencer AJ, Saurya S, Furze J, Hill AV, Gilbert SC. Recombination-mediated genetic engineering of a bacterial artificial chromosome clone of modified vaccinia virus Ankara (MVA) PLoS One. 2008;3:e1638. doi: 10.1371/journal.pone.0001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines. 2009;8:1023–35. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe P. Skipping the co-expression problem: the new 2A “CHYSEL” technology. Genet Vaccines Ther. 2004;2:13. doi: 10.1186/1479-0556-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domi A, Moss B. Cloning the vaccinia virus genome as a bacterial artificial chromosome in Escherichia coli and recovery of infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:12415–20. doi: 10.1073/pnas.192420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol. 2012;86:7444–7. doi: 10.1128/JVI.00467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, Gallina A, Baldanti F, Revello MG. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008;89:853–65. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- Gilbert SC. Clinical development of Modified Vaccinia virus Ankara vaccines. Vaccine. 2013;31:4241–6. doi: 10.1016/j.vaccine.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, Wagner M, Gallina A, Milanesi G, Koszinowski U, Baldanti F, Gerna G. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78:10023–33. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldwein EE. gH/gL supercomplexes at early stages of herpesvirus entry. Curr Opin Virol. 2016;18:1–8. doi: 10.1016/j.coviro.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, Gottlieb SL, Wald A. Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine. 2016;34:2948–52. doi: 10.1016/j.vaccine.2015.12.076. [DOI] [PubMed] [Google Scholar]
- Kabanova A, Marcandalli J, Zhou T, Bianchi S, Baxa U, Tsybovsky Y, Lilleri D, Silacci-Fregni C, Foglierini M, Fernandez-Rodriguez BM, Druz A, Zhang B, Geiger R, Pagani M, Sallusto F, Kwong PD, Corti D, Lanzavecchia A, Perez L. Platelet-derived growth factor-alpha receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat Microbiol. 2016;1:16082. doi: 10.1038/nmicrobiol.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanova A, Perez L, Lilleri D, Marcandalli J, Agatic G, Becattini S, Preite S, Fuschillo D, Percivalle E, Sallusto F, Gerna G, Corti D, Lanzavecchia A. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc Natl Acad Sci U S A. 2014;111:17965–70. doi: 10.1073/pnas.1415310111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, Kim MK, Shin BA, Choi SY. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One. 2011;6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol. 2010;84:1005–13. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr A, Malicki K. Attenuation of virulent fowl pox virus in tissue culture and characteristics of the attenuated virus. Zentralbl Veterinarmed. 1966;B 13:1–13. [PubMed] [Google Scholar]
- Nelson CS, Cruz DV, Tran D, Bialas KM, Stamper L, Wu H, Gilbert M, Blair R, Alvarez X, Itell H, Chen M, Deshpande A, Chiuppesi F, Wussow F, Diamond DJ, Vandergrift N, Walter MR, Barry PA, Cohen-Wolkowiez M, Koelle K, Kaur A, Permar SR. Preexisting antibodies can protect against congenital cytomegalovirus infection in monkeys. JCI Insight. 2017;2:pii, 94002. doi: 10.1172/jci.insight.94002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Murphy EA. A myeloid progenitor cell line capable of supporting human cytomegalovirus latency and reactivation, resulting in infectious progeny. J Virol. 2012;86:9854–65. doi: 10.1128/JVI.01278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis. 2013;56:1458–65. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
- Sathiyamoorthy K, Chen J, Longnecker R, Jardetzky TS. The COMPLEXity in herpesvirus entry. Curr Opin Virol. 2017;24:97–104. doi: 10.1016/j.coviro.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss MR. Cytomegalovirus vaccine development. Curr Top Microbiol Immunol. 2008;325:361–82. doi: 10.1007/978-3-540-77349-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss MR. Cytomegalovirus vaccines under clinical development. J Virus Erad. 2016;2:198–207. [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Sodeik B, Ericsson M, Wolffe EJ, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994;68:130–47. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA, Chow JC, Baker J, Avdalovic N, Yuan S, Au D, Co MS, Vasquez M, Britt WJ, Coelingh KL. Neutralizing monoclonal antibodies that distinguish three antigenic sites on human cytomegalovirus glycoprotein H have conformationally distinct binding sites. J Virol. 1993;67:489–96. doi: 10.1128/jvi.67.1.489-496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st Century: A Tool for Decisionmaking, Vaccines for the 21st Century: A Tool for Decisionmaking. Washington (DC): 2000. [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–94. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- Tischer BK, Smith GA, Osterrieder N. En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol. 2010;634:421–30. doi: 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]
- Tischer BK, von Einem J, Kaufer B, Osterrieder N. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques. 2006;40:191–7. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- Vanarsdall AL, Johnson DC. Human cytomegalovirus entry into cells. Curr Opin Virol. 2012;2:37–42. doi: 10.1016/j.coviro.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheust C, Goossens M, Pauwels K, Breyer D. Biosafety aspects of modified vaccinia virus Ankara (MVA)-based vectors used for gene therapy or vaccination. Vaccine. 2012;30:2623–32. doi: 10.1016/j.vaccine.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A. 2005;102:18153–8. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Monroe J, Linton C, Archer J, Beard CW, Barnett SW, Palladino G, Mason PW, Carfi A, Lilja AE. Human cytomegalovirus gH/gL/UL128/UL130/UL131A complex elicits potently neutralizing antibodies in mice. Vaccine. 2014;32:3796–804. doi: 10.1016/j.vaccine.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Wussow F, Chiuppesi F, Martinez J, Campo J, Johnson E, Flechsig C, Newell M, Tran E, Ortiz J, La Rosa C, Herrmann A, Longmate J, Chakraborty R, Barry PA, Diamond DJ. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog. 2014;10:e1004524. doi: 10.1371/journal.ppat.1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wussow F, Yue Y, Martinez J, Deere JD, Longmate J, Herrmann A, Barry PA, Diamond DJ. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J Virol. 2013;87:1322–32. doi: 10.1128/JVI.01669-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt LS, Earl PL, Xiao W, Americo JL, Cotter CA, Vogt J, Moss B. Elucidating and minimizing the loss by recombinant vaccinia virus of human immunodeficiency virus gene expression resulting from spontaneous mutations and positive selection. J Virol. 2009;83:7176–84. doi: 10.1128/JVI.00687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


