Abstract
Patients with Acute Myeloid Leukemia (AML) have compromised marrow function and chemotherapy causes further suppression. As a result complications are frequent, and patients may require admission to the intensive care unit (ICU). How codes status changes when these events occur and how those changes influence outcome are largely unknown. Outcomes for adult patients with AML, undergoing induction chemotherapy, and transferred to the ICU between January 2000 and December 2013 were analyzed. 94 patients were included. Median survival was 1.3 months. At 3 and 6 months overall survival (OS) was 27% and 18% respectively. Respiratory failure was the most common reason for transfer to ICU (88%), with 63% requiring mechanical ventilation at transfer. Other reasons included: cardiac arrest (18%), septic shock (17%), hypotension (9%), and acute renal failure (9%). The most frequent interventions were mechanical ventilation in 85%, vasopressors in 62%, and hemodialysis in 30%. Following transfer 55 patients (58%) had a change in code status. Overall, 46 patients (49%) changed from Full Code (FC) to Comfort Care (CC), 7 (7%) from FC to Do Not Resuscitate (DNR), and 2 (2%) from DNR to CC. For the entire cohort, ICU mortality (IM) was 61% and hospital mortality (HM) was 71%. For FC or DNR patients, IM was 30% and HM was 41%. For CC patients, IM was 90% and HM was 100%. Overall, 27 patients (29%) survived to discharge. Of those discharged, 22 (81%) were alive at 3 months and 17 (63%) were alive at 6 months. In conclusion, patients that required ICU admission during induction chemotherapy have a poor prognosis. Code status changed during the ICU stay for the majority of patients and always to a less aggressive status.
Keywords: acute myeloid leukemia, ICU, Code status
Introduction
Acute Myeloid Leukemia (AML), a hematologic malignancy characterized by the clonal proliferation of myeloblasts, is the most common acute leukemia in adults and is uniformly fatal without treatment [1]. The initial phase of treatment is referred to as remission induction therapy. For nearly 40 years, the use of an anthracycline with cytarabine has constituted the backbone of remission induction therapy [2]. The goal of treatment is to obtain a rapid restoration of normal hematopoiesis without morphological evidence of residual leukemia termed a complete remission (CR). However, both AML and its therapies can compromise bone marrow function resulting in prolonged periods of neutropenia and thrombocytopenia putting patients at risk for infectious complications and hemorrhages, respectively. As a result, these complications can lead to end-organ damage or multi-organ system failure requiring transfer to the intensive care unit (ICU). ICU admissions in this setting are felt to be associated with high mortality rates leading some practitioners to view these transfers as potentially inappropriate therapy. Few studies have investigated ICU intervention in AML patients specifically during induction therapy.
Sharing management decisions among oncologists and ICU physicians may be a source of conflict as the short-term prognostic impact of organ dysfunction may overshadow the overall AML prognosis[3]. Before the patient is admitted to the ICU, a decision whether or not to transfer the patient must be made considering various factors including the reversibility of the acute condition, prospect of long-term survival, facility limitations, and skill set availability. Acutely ill AML patients are a heterogeneous group and as a result vastly different outcomes have been observed in both the short-term and long-term survival between individual patients. In order to better guide treatment decisions, it would be useful to identify clinical factors that can predict favorable outcomes. Short-term mortality is usually associated with the characteristics of the acute illness and the use of vasopressors or mechanical ventilation may hold prognostic significance. Long-term prognosis appears to be associated with general AML prognostic factors including cytogenetics, age, and response to treatment [4]. Some studies have shown that patients with AML who survive the ICU have the same continuous complete remission rates and long-term survival as non-ICU patients with AML [3]. How patients and their families integrate this complex information and what decisions are made regarding the desire for continued aggressive care are largely unreported.
The purpose of this retrospective study was to describe changes in codes status, outcomes and prognostic factors for a group of patients with AML all undergoing induction chemotherapy admitted to the intensive care unit in a large academic medical center. We also describe the reasons cited for transfer, the interventions made during the stay, and disposition from the ICU.
Patients and Methods
Patients
This retrospective study was approved by the Institutional Review Board of Wake Forest University. We reviewed the medical records for all patients with the diagnosis of non-APL AML who were transferred to the ICU between January 01, 2000 and December 31, 2013 at Wake Forest University Baptist Medical Center. Data was collected by chart review. Inclusion criteria were: Age >18, not APL, and admission to the ICU during initial induction chemotherapy. The majority of cases that were excluded were admissions for acute illness, patients with APL or for re-induction/salvage chemotherapy. As the purpose of the study was to assess the change in code status of AML patients initially admitted to the leukemia service patients that were admitted to the ICU upon hospital admission and later received induction therapy were not included in this study.
Patient characteristics reviewed included age, number of comorbidities, social history, physical exam, baseline labs on admission, history of antecedent hematologic disorder, and cytogenetic risk group. Comorbidities captured included congestive heart failure, coronary artery disease, cerebrovascular disease, diabetes mellitus, chronic obstructive pulmonary disease, hypertension, renal dysfunction, previous cancer, depression, osteoporosis, rheumatologic disease (systemic lupus erythematosus, rheumatoid arthritis, scleroderma, Sjogren's disease), cognitive impairment, venous thromboembolism (deep venous thrombosis including venous catheter associated, pulmonary embolism), cardiac valve disease, and cirrhosis. Labs on the day of ICU transfer were also obtained. Review of cytogenetic status included karyotypes taken from bone marrow biopsy samples taken at initial diagnosis. Karyotypes were divided into favorable karyotypes [t(8;21), t(16;16) or i16], unfavorable karyotypes [-5, del(5q), -7, del(7q), complex karyotype (3 or more abnormalities), or 11q23 translocation], and intermediate (all other abnormalities) as described by the European LeukemiaNet (ELN) [5].
Terminologies for code status at our institution included four options. Full Code, is a term defined as the use of every effort to sustain life. Do Not Resuscitate-full scope of treatment (DNR-Full), a term meaning do not initiate cardiopulmonary resuscitation (CPR) or advanced cardiac life support (ACLS). DNR-limited scope of treatment (DNR-Limited), is a term indicating not to initiate CPR, ACLS, or intubation sequence for mechanical ventilation. Both DNR-Full and DNR-Limited allow for full resuscitative medical therapy including the use of vasopressors, antibiotics, and potentially renal replacement therapy. Comfort Care, is a term to identify the use of interventions that will sustain comfort for the patient as they transition to end of life. This terminology was not uniform throughout the timeframe of this study. Because of the change over time, our analysis was done using three terminologies as follows: Full Code, DNR (included DNR-Full and DNR-Limited where differentiated), and Comfort Care.
Objectives
The primary objective of the study was to describe overall survival and code status for the cohort. Secondary objectives included 3 month and 6 month survival, ICU survival, hospital mortality, reason for ICU transfer, duration of ICU stay, disposition after hospitalization, requirement of intubation/mechanical ventilation, requirement of vasopressors, requirement of renal replacement therapy, and requirement of surgical intervention.
Response assessment
Overall survival was defined as the time from the date of diagnosis to the date of death. If patient was alive at the date of last contact, he/she was censored at that time point. ICU survival was defined as the time from ICU admission to date of death and time from ICU discharge to date of death. The criteria used to define response following induction chemotherapy were outlined by Dohner et al. [6] including CR (neutrophils > 1000/µl, platelets>100,000/µl, and bone marrow blasts<5%) and CRi (all marrow criteria of CR but with only either complete platelet or neutrophil recovery).
Statistical analysis
Descriptive statistics were used to describe the characteristics of the sample. Median overall survival (OS) of the sample will be calculated using Kaplan-Meier estimation. A log rank test will be used to evaluate differences between predictor variable categories for OS. A Cox proportional hazards model will be used to evaluate a multivariate OS model. Chi-square tests or Fisher’s exact tests will be used to evaluate differences of predictor variables for the secondary outcomes of 30 day survival, 60 day survival, use of intubation/mechanical ventilation, new location after hospital discharge, reason for ICU transfer, requirement of vasopressors, and need of renal replacement therapy. A multivariate logistic regression model will also be used for these outcomes which are binary if multiple independent predictors show association with the outcomes.
Results
Of 246 cases reviewed, 94 met inclusion criteria. Age greater than 60 at the time of diagnosis included 69 patients (73.4%) and age 60 years or less included 25 patients (26.6%). There were 51 (54.3%) males and 43 (45.7%) females. The majority of patients were white (91.5%). 60 patients (63.8%) had de novo AML, 26 patients (27.7%) had secondary AML, and 8 patients (8.5%) had therapy related AML. 23 patients (24.5%) had zero comorbidities, 24 patients (25.5%) had one comorbidity, 23 patients (24.5%) had 2 comorbidities, and 24 patients (25.5%) had 3 or more comorbidities (Table 1).
Table 1.
Patient Characteristics
| Total N=94 |
<= 60 N=25 |
Over 60 N=69 |
|
|---|---|---|---|
| Age at Diagnosis | 65.0 (25.0–85.0) | 52.0 (25.0–60.0) | 68.0 (61.0–85.0) |
| Gender | |||
| Male | 51 (54.3%) | 16 (64.0%) | 35 (50.7%) |
| Female | 43 (45.7%) | 9 (36.0%) | 34 (49.3%) |
| Race | |||
| White | 86 (91.5%) | 22 (88.0%) | 64 (92.8%) |
| Black | 4 (4.3%) | 1 (4.0%) | 3 (4.3%) |
| Unknown | 4 (4.3%) | 2 (8.0%) | 2 (2.9%) |
| Ethnicity | |||
| Non-hispanic | 92 (97.9%) | 25 (100.0%) | 67 (97.1%) |
| Unknown | 2 (2.1%) | 0 (0.0%) | 2 (2.9%) |
| Type of AML | |||
| De novo | 60 (63.8%) | 19 (76.0%) | 41 (59.4%) |
| Secondary | 26 (27.7%) | 5 (20.0%) | 21 (30.4%) |
| Therapy related | 8 (8.5%) | 1 (4.0%) | 7 (10.1%) |
| Number of Comorbidites | |||
| 0 | 23 (24.5%) | 11 (44.0%) | 12 (17.4%) |
| 1 | 24 (25.5%) | 7 (28.0%) | 17 (24.6%) |
| 2 | 23 (24.5%) | 4 (16.0%) | 19 (27.5%) |
| 3 | 14 (14.9%) | 1 (4.0%) | 13 (18.8%) |
| 4 | 7 (7.4%) | 1 (4.0%) | 6 (8.7%) |
| 5 | 3 (3.2%) | 1 (4.0%) | 2 (2.9%) |
| Cytogenetics | |||
| Favorable | 3 (3.2%) | 1 (4.0%) | 2 (2.9%) |
| Intermediate | 33 (35.1%) | 7 (28.0%) | 26 (37.7%) |
| Unfavorable | 35 (37.2%) | 10 (40.0%) | 25 (36.2%) |
| Missing | 23 (24.5%) | 7 (28.0%) | 16 (23.2%) |
Respiratory failure was the most frequent reason for transfer to ICU and was seen in 83 patients (88%) with 59 patients (63%) requiring mechanical ventilation at the time of transfer. Other reasons for ICU transfer included: cardiac arrest in 17 patients (18.1%), septic shock in 16 patients (17%), hypotension of unclear origin in 8 patients (8.5%), acute renal failure in 8 patients (8.5%), uncontrolled bleeding in 2 patients (2.1%) and decompensated heart failure in 1 patient (1.1%). Patients may have had more than one of these indications at the time of transfer to the ICU (Table 2). Of the 17 patients listed in Table 2 as having “other” reasons for ICU transfer this was in addition to a documented reason for 14 of them. The three patients whose only reason for transfer to the ICU did not include one of the listed reasons were transferred for atrial fibrillation with rapid ventricular response, anaphylactic shock and post operatively after a small bowel resection.
Table 2.
Reasons for ICU transfer.
| Total (N = 94) |
<= 60 (N=25) |
Over 60 (N=69) |
|
|---|---|---|---|
| Cardiac Arrest | |||
| No | 76 (80.9%) | 17 (68.0%) | 59 (85.5%) |
| Yes | 17 (18.1%) | 8 (32.0%) | 9 (13.0%) |
| Missing | 1 (1.1%) | 0 (0.0%) | 1 (1.4%) |
| Respiratory failure | |||
| No | 10 (10.6%) | 0 (0.0%) | 10 (14.5%) |
| Yes | 83 (88.3%) | 25 (100%) | 58 (84.1%) |
| Intubation | 59 (62.8%) | 18 (72.0%) | 41 (59.4%) |
| No Intubation | 24 (25.5%) | 7 (28.0%) | 17 (24.6%) |
| Missing | 1 (1.1%) | 0 (0.0%) | 1 (1.4%) |
| Septic Shock | |||
| No | 77 (81.9%) | 20 (80.0%) | 57 (82.6%) |
| Yes | 16 (17.0%) | 5 (20.0%) | 11 (15.9%) |
| Missing | 1 (1.1%) | 0 (0.0%) | 1 (1.4%) |
| Hypotension of unclear origin | |||
| No | 85 (90.4%) | 23 (92.0%) | 62 (89.9%) |
| Yes | 8 (8.5%) | 2 (8.0%) | 6 (8.7%) |
| Missing | 1 (1.1%) | 0 (0.0%) | 1 (1.4%) |
| Decompensated heart failure | |||
| No | 92 (97.9%) | 24 (96.0%) | 68 (98.6%) |
| Yes | 1 (1.1%) | 1 (4.0%) | 0 (0.0%) |
| Missing | 1 (1.1%) | 0 (0.0%) | 1 (1.4%) |
| Uncontrolled bleeding | |||
| No | 91 (96.8%) | 25 (100.0%) | 66 (95.7%) |
| Yes | 2 (2.1%) | 0 (0.0%) | 2 (2.9%) |
| Missing | 1 (1.1%) | 0 (0.0%) | 1 (1.4%) |
| Acute renal failure (renal replacement) | |||
| No | 85 (90.4%) | 24 (96.0%) | 61 (88.4%) |
| Yes | 8 (8.5%) | 1 (4.0%) | 7 (10.1%) |
| Missing | 1 (1.1%) | 0 (0.0%) | 1 (1.4%) |
| Other reason | |||
| No | 77 (81.9%) | 23 (92.0%) | 54 (78.3%) |
| Yes | 17 (18.1%) | 2 (8.0%) | 15 (21.7%) |
The most frequent interventions received while in the ICU were initiation of mechanical ventilation in 80 patients (85.1%), use of vasopressor support in 58 patients (61.7%), and acute hemodialysis in 28 patients (29.8%). 13 patients (13.8%) required surgical intervention. Surgical interventions included 5 patients requiring chest tubes, 5 patients requiring tracheostomy, 1 patient required neurosurgical evacuation for subdural hematoma, 1 patient required ventriculostomy shunt and one patient received an open cholecystectomy. Median number of days on mechanical ventilation was 5 (range 1–56). Median number of days on vasopressor support was 2 (range 1–25). Median number of days in the ICU was 5 (range 1–103). Of the 94 patients, only 6 patient’s (6.4%) absolute neutrophil count recovered while in the ICU (Table 3).
Table 3.
Outcomes/Interventions received in the ICU.
| Total (N = 94) |
<= 60 (N=25) |
Over 60 N=69 |
|
|---|---|---|---|
| Died in ICU? | |||
| No | 37 (39.4%) | 11 (44.0%) | 26 (37.7%) |
| Yes | 57 (60.6%) | 14 (56.0%) | 43 (62.3%) |
| Died prior to hospital discharge? | |||
| No | 27 (28.7%) | 6 (24.0%) | 21 (30.4%) |
| Yes | 67 (71.3%) | 19 (76.0%) | 48 (69.6%) |
| Require Vasopressor support? | |||
| No | 36 (38.3%) | 7 (28.0%) | 29 (42.0%) |
| Yes | 58 (61.7%) | 18 (72.0%) | 40 (58.0%) |
| Require dialysis? | |||
| No | 66 (70.2%) | 17 (68.0%) | 49 (71.0%) |
| Yes | 28 (29.8%) | 8 (32.0%) | 20 (29.0%) |
| Require bronchoscopy? | |||
| No | 84 (89.4%) | 21 (84.0%) | 63 (91.3%) |
| Yes | 10 (10.6%) | 4 (16.0%) | 6 (8.7%) |
| Require surgical intervention | |||
| No | 81 (86.2%) | 22 (88.0%) | 59 (85.5%) |
| Yes | 13 (13.8%) | 3 (12.0%) | 10 (14.5%) |
| Did ANC recover in ICU? | |||
| No | 86 (91.5%) | 22 (88.0%) | 64 (92.8%) |
| Yes | 6 (6.4%) | 1 (4.0%) | 5 (7.2%) |
| Missing | 2 (2.1%) | 2 (8.0%) | 0 (0.0%) |
While in the ICU, 55 patients (58%) had a change in their code status. Of the 55 patients that had a change in code status, 46 patients (49%) changed from Full Code to Comfort Care, 7 patients (7%) changed from Full Code to DNR, and 2 patients (2%) changed from DNR to Comfort Care.
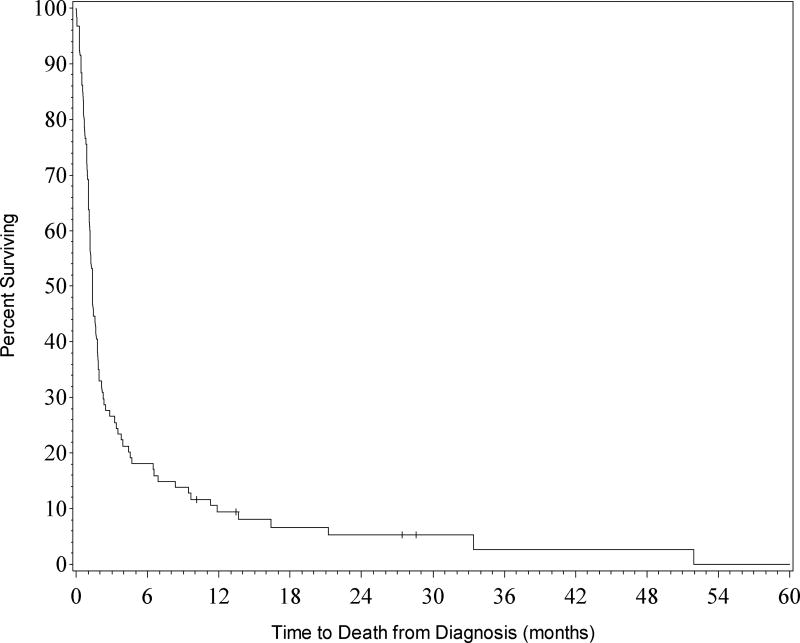
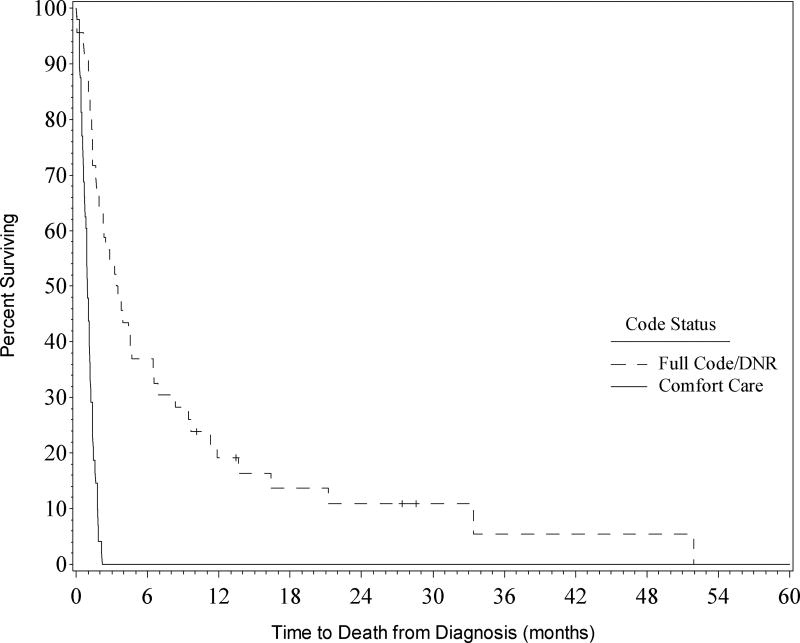
Median survival for the entire cohort was 1.3 months (Figure 1). At 3 months OS was 27% and at 6 months OS was 18%. Age greater than 60 at time of diagnosis did not significantly impact OS (p = 0.8916). For the entire cohort, ICU mortality was 61% and hospital mortality was 71%. For patients that continued treatment, defined as code status of DNR or Full Code, ICU mortality was 30% and hospital mortality was 41%. For patients that were Comfort Care, ICU mortality was 90% and hospital mortality was 100% (Figure 2).
Figure 1. Overall survival of all patients (n=94).
Survival rates for the entire cohort. Kaplan-Meier curves for all patients transferred to the ICU.
Figure 2. Overall survival by Code Status.
Survival of cohort by code status. Kaplan-Meier curves for patients are shown for patients with full code/DNR compared to those who were comfort care.
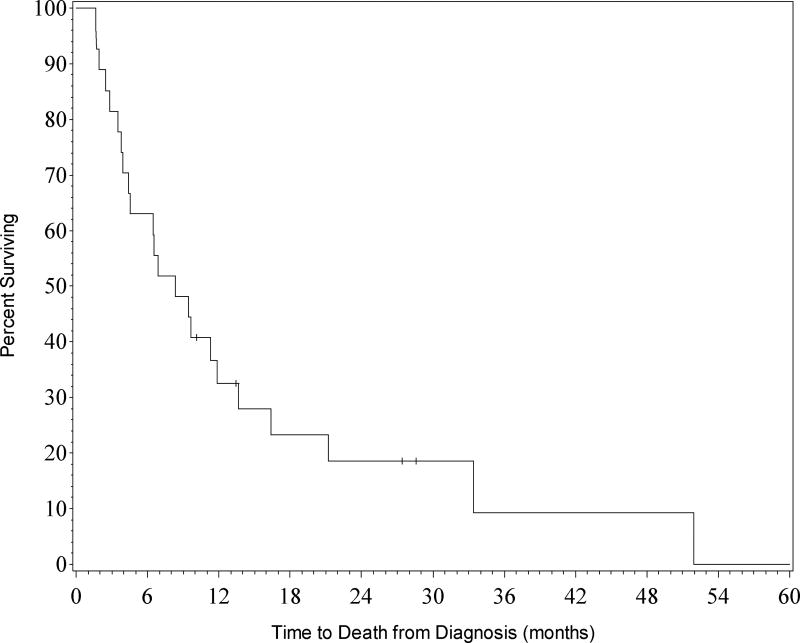
Response to treatment in the 27 patients (29%) that survived to hospital discharge was as follows: 17 patients achieved complete remission (CR), 5 patients reached complete remission with incomplete blood count recovery (CRi), and 5 patients had partial response (PR). Of those discharged, 22 patients (81%) were alive at 3 months and 17 patients (63%) were alive at 6 months (Figure 3).
Figure 3. Overall survival of patients discharged from hospital (n=27).
Survival rates for patients who were discharged from the hospital. Kaplan-Meier curves for all patients who survived to hospital discharge.
In the initial multivariate analysis of the entire cohort, the characteristics that were statistically significant between those that survived to discharge and those that died in the hospital were: type of AML [de novo, secondary, therapy related (p=0.0235)], need for mechanical ventilation (p=0.0004), need for vasopressor support (p=0.0024), and response to treatment [CR, CRi, PR (p=<0.0001)]. In a second subset multivariate analysis we included only those patients that continued care, defined as code status Full code or DNR. The characteristics that were statistically significant between those that survived to discharge and those that died in the hospital were: need for mechanical ventilation (p=0.0156), need for vasopressor support (p=0.0071), and response to treatment [CR, CRi, PR (p=0.0038) Table3].
Discussion
Patients with AML undergoing induction chemotherapy are at risk of developing life-threatening complications necessitating ICU admission. These complications arise due to disease-specific complications such as neutropenia, thrombocytopenia, as well treatment-related complications such as tumor lysis and renal dysfunction. Consequences of these include septic shock, respiratory failure, hemorrhage, and renal failure.
This retrospective study at our institution reports on 94 AML patients undergoing induction chemotherapy who were transferred to the ICU. The median OS was 1.3 months with 3 month and 6 month OS of 27% and 18% respectively. Our institution recently looked into outcomes for AML patients undergoing induction chemotherapy from 2002 to 2009 and found that patients > 60 years and < 60 years of age had a median OS of 8.7 months and 23.1 months, respectively [7]. Not surprisingly, in the current study of patients transferred to the ICU, the cohort had a far worse prognosis.
The hospital mortality for the entire cohort was 71% which is similar to previous studies for AML patients requiring ICU level care [8, 9]. If the patients who were made comfort care were excluded, hospital mortality dropped to 41%. Our multivariate analysis showed that the type of AML (de novo or therapy related), use of mechanical ventilation, use of vasopressors, and response to therapy were the only significant factors that predicted hospital mortality for the entire cohort. The use of mechanical ventilation and vasopressors were associated with poor outcomes in our study which is consistent with previous studies [9–11]. This remained true in the cohort of patients who remained Full code or DNR indicating that despite the continuation of aggressive care if these interventions are needed outcomes are poor. This may aid in counseling patients and family members that aggressive care is likely to be potentially inappropriate in these circumstances. The association of response with survival is unlikely to reflect patients in remission at time of transfer as only 4 patients in the cohort had counts compatible with remission when transferred to the ICU and 3 of the 4 died within 3 weeks of transfer. Additionally, the median ANC and platelet count at time of transfer for patients who ultimately achieved a response was 0.0/ µL and 22,000/µL respectively. Resistance to therapy is associated with poor outcomes in our study likely as a consequence of longer periods of neutropenia and thrombocytopenia as they were receiving additional lines of therapy. Of the 27 patients (29%) that survived to hospital discharge 22 of them achieved a CR or CRi, 81% were alive at 3 months, and 63% were alive at 6 months. This indicates that those patients that survived to hospital discharge were able to achieve similar outcomes compared to those patients who did not need ICU level care also consistent with prior studies [4].
Two previous studies which primarily focused on AML patients requiring mechanical ventilation reported very poor ICU survival of 3–13% [10, 11]. In a study of 83 consecutive AML patients, Rabbat et al reported ICU survival of 66% and 1-year survival of 33% [8]. Thakkar et al reported 90 patients, of which 82% had AML, admitted to the ICU had poor 6 and 12 month survival of only 18% and 16%, respectively [12]. In another study of 50 patients, 70% with AML, Park et al described 68% hospital mortality in patients admitted with septic shock to the ICU [9]. The reasons for the discrepancies among studies are unclear, but may include selection bias, heavily pretreated population, patients with other acute leukemias, inclusion of relapsed or refractory patients and facility limitations.
In addition to outcomes, we reported on the patient’s goals of care. Not surprisingly patients who opted for comfort care had 100% hospital mortality. It is unclear if the decision to pursue comfort care was based on a truly irreversible physiologic derangement, or the patient/ family/providers’ belief that an irreversible condition existed. In our study, once a patient was transferred to the ICU during induction chemotherapy, 49% of patients transitioned to comfort care and 51% remained either full code or DNR. We were not able to determine if the goals of care discussion were being led by critical care physician, the primary oncology service, or the palliative care team. Future studies are needed to clarify the optimal approach to the discussion of goals care with patients and families during this often hectic and stressful time.
Our retrospective study had limitations. Since our data was collected by chart review, we were limited by provider’s documentation of events that occurred. Medication reconciliation was available to confirm vasopressors use and duration. During the majority of the time between 2000–2013, the ICU’s daily progress notes were not in the electronic medical record (EMR) and these notes were not scanned into the EMR. This further limited our data collection as we were then only relying on dictated discharge summaries or service transfer notes summarizing their ICU stay. Also during the time period of our study, our institution had a step-down unit for our oncology patients. This unit had better nurses to patient ratio, closer respiratory care, and was able to provide interventions not available on the regular floor, most notably noninvasive positive pressure ventilation, and single vasopressor use. It was not uncommon for our ICU transfers to have come from this unit. This may have led to selection bias as a “sicker” population of patients were transferred to the ICU and may explain why the need for mechanical ventilation and cardiac arrest were high in this cohort as they failed initial treatment on this unit.
In conclusion, our study is unique in that we report on the code status changes and outcomes of AML patients undergoing induction chemotherapy who required transfer to the ICU. The choice of this cohort was deliberate and was to describe the rates of code status changes during the initial phase of illness and the associated outcomes. It is at this high acuity time when patients and families have had limited amount of time to reflect on the diagnosis, prognosis, and the morbidity associated with resuscitative care. Changes in goals of care or code status represent shared informed decision making between the patients, surrogates and provider teams. These discussions have the potential to be unduly influenced by provider’s preconceived notions of the above factors while leading these discussions. Not surprisingly, the overall survival of this cohort of patients was poor with a median OS of 1.3 months. Hospital mortality was high, as 71% of the entire cohort did not survive the hospitalization, but vastly improved to 41% in patients who did not elect comfort care. All of the patients who transitioned to comfort care died during their hospital stay. Whether all of these patients would have died with continued aggressive care or whether avoidance of life sustaining measures had previously been identified in advanced directives is unknown. However, clinical predictors in this unique cohort of patients who may, with additional time and support, recover their marrow function are lacking. Our study emphasizes the need for additional work to determine more reliable predictors of survival in this population of patients to aid providers, patients, and families in discussing goals of care.
Highlights.
AML patients transferred to the ICU have a guarded prognosis
Code status can dictate the extent of aggressive ICU care
Code status changed for 58% of patients transferred to the ICU
Full Code and DNR patients had better survival compared to comfort care
Acknowledgments
TSP is supported by NCI 1R01CA197991-01A1, SI is supported by NCI Cancer Center Support Grant (CCSG) P30CA012197, HDK is supported by K23AG038361.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 2.Yates JW, Wallace HJ, Jr, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer chemotherapy reports Part 1. 1973;57:485–8. [PubMed] [Google Scholar]
- 3.Lengline E, Raffoux E, Lemiale V, Darmon M, Canet E, Boissel N, et al. Intensive care unit management of patients with newly diagnosed acute myeloid leukemia with no organ failure. Leuk Lymphoma. 2012;53:1352–9. doi: 10.3109/10428194.2011.649752. [DOI] [PubMed] [Google Scholar]
- 4.Jackson K, Mollee P, Morris K, Butler J, Jackson D, Kruger P, et al. Outcomes and prognostic factors for patients with acute myeloid leukemia admitted to the intensive care unit. Leuk Lymphoma. 2014;55:97–104. doi: 10.3109/10428194.2013.796045. [DOI] [PubMed] [Google Scholar]
- 5.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 7.Tawfik B, Pardee TS, Isom S, Sliesoraitis S, Winter A, Lawrence J, et al. Comorbidity, age, and mortality among adults treated intensively for acute myeloid leukemia (AML) J Geriatr Oncol. 2016;7:24–31. doi: 10.1016/j.jgo.2015.10.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabbat A, Chaoui D, Montani D, Legrand O, Lefebvre A, Rio B, et al. Prognosis of patients with acute myeloid leukaemia admitted to intensive care. British journal of haematology. 2005;129:350–7. doi: 10.1111/j.1365-2141.2005.05459.x. [DOI] [PubMed] [Google Scholar]
- 9.Park HY, Suh GY, Jeon K, Koh WJ, Chung MP, Kim H, et al. Outcome and prognostic factors of patients with acute leukemia admitted to the intensive care unit for septic shock. Leuk Lymphoma. 2008;49:1929–34. doi: 10.1080/10428190802353609. [DOI] [PubMed] [Google Scholar]
- 10.Rabe C, Mey U, Paashaus M, Musch A, Tasci S, Glasmacher A, et al. Outcome of patients with acute myeloid leukemia and pulmonary infiltrates requiring invasive mechanical ventilation-a retrospective analysis. J Crit Care. 2004;19:29–35. doi: 10.1016/j.jcrc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Tremblay LN, Hyland RH, Schouten BD, Hanly PJ. Survival of acute myelogenous leukemia patients requiring intubation/ventilatory support. Clin Invest Med. 1995;18:19–24. [PubMed] [Google Scholar]
- 12.Thakkar SG, Fu AZ, Sweetenham JW, McIver ZA, Mohan SR, Ramsingh G, et al. Survival and predictors of outcome in patients with acute leukemia admitted to the intensive care unit. Cancer. 2008;112:2233–40. doi: 10.1002/cncr.23394. [DOI] [PubMed] [Google Scholar]