Abstract
Mesenchymal stem cells (MSCs) secrete endogenous factors such as vascular endothelial growth factor (VEGF) and prostaglandin E2 (PGE2) that promote angiogenesis, modulate the inflammatory microenvironment, and stimulate wound repair, and MSC spheroids secrete more trophic factors than dissociated, individual MSCs. Compared to injection of cells alone, transplantation of MSCs in a biomaterial can enhance their wound healing potential by localizing cells at the defect site and upregulating trophic factor secretion. To capitalize on the therapeutic potential of spheroids, we engineered a fibrin gel delivery vehicle to simultaneously enhance the proangiogenic and anti-inflammatory potential of entrapped human MSC spheroids. We used multifactorial statistical analysis to determine the interaction between four input variables derived from fibrin gel synthesis on four output variables (gel stiffness, degradation rate, and secretion of VEGF and PGE2). Manipulation of the four input variables tuned fibrin gel biophysical properties to promote the simultaneous secretion of VEGF and PGE2 by entrapped MSC spheroids while maintaining overall gel integrity. MSC spheroids in stiffer gels secreted the most VEGF, while PGE2 secretion was highest in more compliant gels. Simultaneous VEGF and PGE2 secretion was greatest using hydrogels with intermediate mechanical properties, as small increases in stiffness increased VEGF secretion while maintaining PGE2 secretion by entrapped spheroids. The fibrin gel formulation predicted to simultaneously increase VEGF and PGE2 secretion stimulated endothelial cell proliferation, enhanced macrophage polarization, and promoted angiogenesis when used to treat a wounded three-dimensional human skin equivalent. These data demonstrate that a statistical approach is an effective strategy to formulate fibrin gel formulations that enhance the wound healing potential of human MSCs.
Keywords: mesenchymal stem cells, spheroids, fibrin, wound healing, trophic factors
Graphical Abstract
INTRODUCTION
Wound healing is a complex process involving multiple cell types and bioactive factors combining in a well-orchestrated sequence of events to repair damaged tissue. However, this orderly progression of healing can be disrupted due to the size, location, and inflammatory milieu of the defect, as well as underlying disease [1]. Compared to bioinert wound dressings, the local delivery of recombinant growth factors such as platelet derived growth factor (PDGF), epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF) accelerates wound closure by stimulating granulation and epithelization. Accelerated repair translates to reduced scarring upon wound closure and improved collagen fiber deposition, organization, and thickness [2]. Nonetheless, the supraphysiological concentrations of these growth factors, combined with the high cost and challenges in achieving the appropriate release kinetics, diminish their long-term clinical feasibility [3].
Mesenchymal stem cells (MSCs) are under investigation for wound healing applications due to their secretion of bioactive factors that enhance granulation tissue formation, angiogenesis, and reduce inflammation [4, 5]. However, the efficacy of cell-based therapies is often impaired due to poor engraftment and high rates of cell death when transplanted into low oxygen environments. Compared to dissociated cells, MSCs exhibit increased overall function when aggregated into three-dimensional spheroids [6, 7]. Furthermore, MSC spheroids secrete more vascular endothelial growth factor (VEGF) and prostaglandin E2 (PGE2) [8–10], two key cytoactive factors in promoting angiogenesis and mediating local inflammation, respectively [11]. We recently reported a combination of microenvironmental stimuli during MSC spheroid formation that increases the production and bioactivity of VEGF and PGE2, resulting in beneficial effects within the wound environment [10].
Similar to dissociated MSCs, spheroids have been directly injected to promote vascularization in ischemic tissue [7, 12, 13]. Upon injection, cells engage the tissue extracellular matrix, migrate from the aggregate, and the therapeutic benefit of spheroid formation is quickly diminished. Alternatively, the delivery of cells within a biomaterial can localize cells at the implant site and provide continuous cues to guide cell fate in vivo. Healing is enhanced when wounds are dressed with materials that maintain a moist environment and degrade at an appropriate rate [2]. Both natural and synthetic polymer-based materials have been examined for wound healing purposes [14–16], yet there remains limited research on the appropriate material to deliver MSC spheroids. Fibrin is found naturally in the body as a scaffold for leukocytes and endothelial cells during tissue regeneration [17, 18]. Additionally, the bulk stiffness, degradability, and porosity of fibrin gels can be easily tailored to direct the lineage-specific differentiation and secretome of entrapped MSCs [19–22]. Compared to hydrogels formed from collagen that is found in mature tissues, fibrin gels direct associated cells to secrete reparative growth factors and extracellular matrix components to stimulate tissue repair [23]. Therefore, fibrin gels represent a promising biomaterial platform for cell transplantation to promote wound healing.
The overall purpose of this study was to engineer a biomaterial to deliver MSC spheroids that enhances the wound healing potential of entrapped MSCs. Wound healing potential was characterized by assessing the quantity and bioactivity of VEGF and PGE2, two key factors within the MSC secretome that exhibit potent effects on cells within the wound environment. We hypothesized that fibrin hydrogels could be formulated with appropriate biophysical properties to simultaneously promote the proangiogenic and anti-inflammatory potential of entrapped MSC spheroids. We used a Design-of-Experiments (DOE) multivariable analysis to determine the interaction between multiple input variables derived from fibrin gel synthesis to control material properties and MSC response. These data demonstrate the potential of modulating hydrogel biophysical properties to enhance the wound healing potential of MSC spheroids.
MATERIALS AND METHODS
Cell culture
Human bone marrow-derived MSCs and diabetic human microvascular cells (HMVECs) (Lonza, Walkersville, MD) were used without additional characterization. MSCs were expanded in standard culture conditions (37°C, 21% O2, 5% CO2) in α-MEM supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Flowery Branch, GA) and 1% penicillin/streptomycin (P/S, Gemini, Sacramento, CA) until use at passages 4–5. Diabetic HMVECs were expanded in standard culture conditions in EGM-2 MV media with Lonza’s SingleQuot supplements (hydrocortisone, gentamycin, VEGF, bFGF, EGF, insulin-like growth factor [IGF], and heparin) and further supplemented with 5% FBS and 1% P/S. Growth-factor deficient media (GF-Def EGM-2 MV) was prepared with serum-containing EGM-2 but lacking VEGF, FGF, and IGF [10, 22]. Raw264.7 murine macrophages (ATCC, Manassas, VA) were used without further characterization and expanded as adherent cultures in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and 1% P/S. Neonatal human epidermal keratinocytes (Lonza) were expanded in Keratinocyte Basal Medium-Gold basal medium with SingleQuot supplements (Lonza) until use at passage 8. Human umbilical cord blood endothelial colony forming cells (ECFCs) were a kind gift of Dr. Mervin Yoder and isolated using a protocol approved by the Institutional Review Board of the Indiana University School of Medicine as previously described. Adherent ECFCs were expanded in culture flasks coated with 5 μg/cm2 rat tail collagen (BD Biosciences, San Jose, CA) in EGM-2 until use [24].
Design of Experiments (DOE) model
A Box-Behnken experimental design was created with Design-Expert 8 software (Stat-Ease, Minneapolis, MN) [10, 25] to analyze the contribution of four continuous variables derived from fibrin gel synthesis (concentration of fibrinogen, sodium chloride (NaCl), calcium chloride (CaCl2), and aprotinin) on four output variables (gel stiffness, degradation rate, and secretion of VEGF and PGE2 by entrapped MSC spheroids). These four input variables were chosen due to their ability to modulate material properties such as stiffness and degradation rate. Fibrinogen and aprotinin are well-established modulators of cell response within fibrin gels [26], and we previously demonstrated that changing the ionic concentrations in the pre-gel solution alters gel stiffness, pore size, fiber diameter, and permeability [20, 21]. The four input variables were examined at low, medium, and high levels, with a centrally repeated condition used to assess system variability. The ranges for input variables were as follows: fibrinogen concentration from 5 to 20 mg/mL, total NaCl concentration from 1 to 3% (w/v), CaCl2 concentration from 10 to 40 mM, and aprotinin concentration from 0 to 500 KIU/mL. The resultant material properties for each condition were measured by quantifying the compressive modulus, gel contraction rate, and VEGF and PGE2 secretion from the same sample using cytokine-specific ELISAs (R&D Systems, Minneapolis, MN). The significance and interaction of the four input variables on the four output variables were assessed via response surface predictions generated by the Design-Expert software.
Spheroid formation
MSC spheroids were formed over 48 hrs using the hanging drop technique with 40,000 cells per 25 μL droplet, incubated in 1% oxygen, and stimulated with 1 μg/mL Pam3-Cys-Ser-Lys4 (Pam3CSK4, Invitrogen, Carlsbad, CA), a Toll-like receptor 2 (TLR2) antagonist [10]. We previously demonstrated that this combination of stimuli maximizes VEGF and PGE2 secretion by MSC spheroids. Spheroids formed under these conditions are approximately 600 μm in diameter and exhibit no discernable differences in viability compared to smaller spheroids.
Entrapment of spheroids into fibrin gels
Fibrin gels were prepared as we described by combining fibrinogen (5, 12.5, 20 mg/mL; Calbiochem, Gibbstown, NJ, USA), NaCl (1, 2, 3% (w/v); Sigma Aldrich), 2.5 U/mL thrombin (Calbiochem), CaCl2 (0, 20, 40 mM; Sigma Aldrich) and aprotinin (0, 250, 500 KIU/mL; Santa Cruz Biotechnology, Santa Cruz, CA), all in phosphate-buffered saline (PBS). Spheroids were suspended in 80 μL fibrin gels at 5 x 106 cells/mL, cast into custom polydimethylsiloxane molds, and allowed to gel for 1 hr [27, 28]. After gelation, fibrin gels were transferred into individual wells of 24-well plates, and the medium was refreshed every 3 days.
Assessment of mechanical and morphologic properties
The compressive moduli of MSC spheroid-containing fibrin gels were measured using an Instron 3345 Compressive Testing System (Norwood, MA). Gels were allowed to swell for 1 hr in PBS, blotted, and then loaded between two flat platens and compressed at 1 mm/min. Compressive moduli were calculated from the linear portions of the force–displacement graph for strain ranging from 0 to 5% [21]. The viscoelastic properties of the fibrin gels were measured using a Discovery HR2 Hybrid Rheometer (TA Instruments, New Castle, DE). An 8.0 mm diameter Peltier plate geometry was used for gels with corresponding 8.0 mm diameter. Gels were polymerized and then maintained in media under standard culture conditions for 24 hrs before measurement. An oscillatory strain sweep ranging from 0.004% to 4% strain was performed on each gel to obtain the linear viscoelastic region (LVR) before gel failure. A minimum of 10 data points were collected for the LVR and averaged to obtain gel shear storage modulus. The contraction of fibrin gels due to activity of entrapped MSCs was measured by visually following morphologic changes in gel area. MSC spheroid-containing gels were imaged over 7 days in culture using a Canon PowerShot A470 camera, and the area of each construct were measured using ImageJ [21, 22]. To visualize the gross morphology of acellular fibrin hydrogels scanning electron microscopy (Hitachi 3500-N, Hitachi Science Systems Ltd, Tokyo, Japan) was used at 3 kV.
Spheroid response to hydrogel formulations
Culture media was refreshed 24 hrs prior to collection, and the concentration of VEGF and PGE2 in the media was determined using a human VEGF or PGE2 ELISA kit according to the manufacturer’s protocol (R&D Systems). Spheroid viability was assessed by a live/dead assay (Invitrogen) and imaged after 7 days in culture using a high sensitivity confocal microscope (Leica TCS SP8 STED 3X, Leica Microsystems, Wetzlar, Germany).
Validation of DOE model
Hydrogels were formed from conditions predicted to maximize secretion of VEGF alone, PGE2 alone, or simultaneous secretion of VEGF and PGE2. We set other constraints regarding mechanical properties as follows: all hydrogels must possess a compressive modulus greater than 10 kPa and exhibit no more than 50% contraction over 7 days to ensure mechanical integrity and encourage integration of the hydrogel into the surrounding tissue. The fibrin gel formulations are described in Table 1. The resulting compressive modulus, gel contraction, VEGF, and PGE2 secretion were again measured.
Table 1.
Fibrin hydrogel formulations for optimizing cytokine secretion from MSC spheroids
| Cytokine | Fibrinogen (mg/mL) | NaCl (% w/v) | CaCl2 (mM) | Aprotinin (KIU/mL) |
|---|---|---|---|---|
| VEGF | 17.5 | 2.8 | 18.5 | 500 |
| PGE2 | 5.0 | 2.0 | 24.9 | 500 |
| VEGF & PGE2 | 16.4 | 1.9 | 26.4 | 500 |
Evaluation of cytokine bioactivity to stimulate endothelial cells and macrophages
To test the bioactivity of conditioned media (CM) from spheroids entrapped in fibrin gels, we measured the mitogenic response of diabetic HMVECs as we previously described [22]. Briefly, diabetic HMVECs were seeded at 7,500 cells/cm2 in EGM-2 MV in 12-well culture plates and allowed to attach overnight. The next day, culture media was refreshed with a 1:4 volume ratio of CM (collected at 7 days of culture in fibrin gels) to GF-Def EGM-2 MV and cultured for 72 hrs. Each well was then rinsed with PBS to remove non-adherent cells and debris. Proliferation was analyzed using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen). Cells cultured in complete EGM-2 MV served as the positive control, while cells cultured in GF-Def EGM-2 MV served as the negative control.
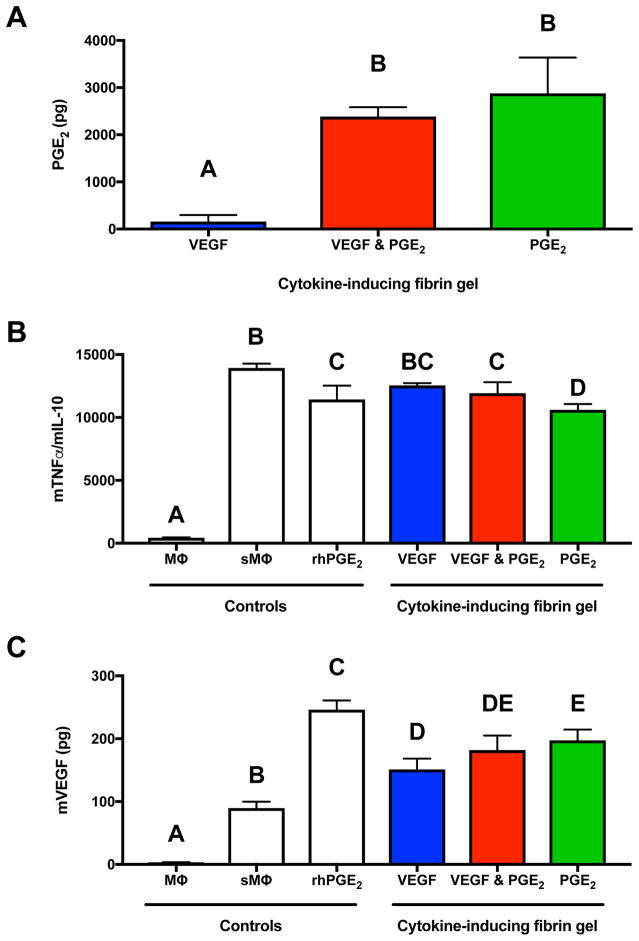
To investigate the anti-inflammatory potential of entrapped MSC spheroids, Raw264.7 mouse macrophages were suspended in DMEM and stimulated with 100 ng/mL lipopolysaccharide (LPS, Invitrogen). After 10 min, the stimulus was removed via centrifugation at 250xg for 5 min and unstimulated or stimulated macrophages were plated at 25,000 cells/cm2 in 12-well culture plates. CM from MSC-laden hydrogels was added to stimulated Raw264.7 mouse macrophages at a 1:10 dilution. After 24 hrs, the macrophage CM was collected and assessed for pro- and anti-inflammatory markers. Recombinant human PGE2 (1 ng/mL, Sigma) was used as a positive control on stimulated macrophages. Macrophage polarization was determined by measuring TNFα, IL-10, and VEGF using mouse cytokine-specific ELISA kits (R&D Systems).
Three-dimensional endothelial human skin equivalent model
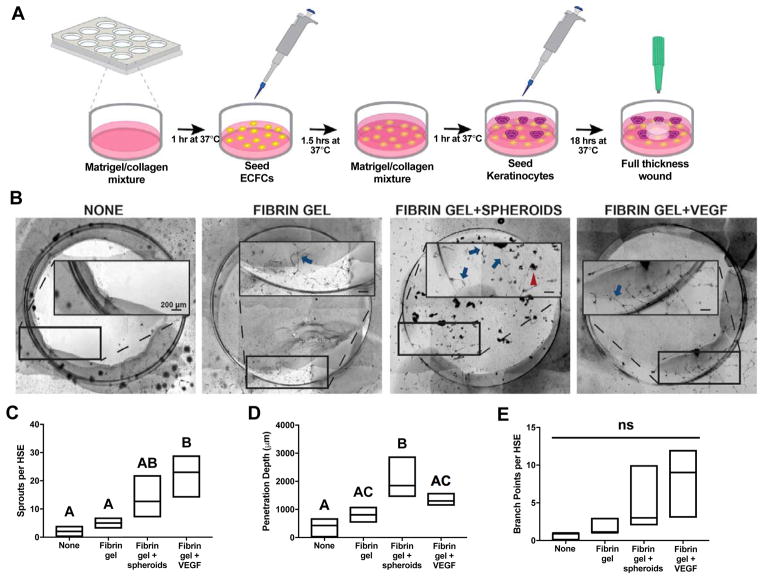
We modified a human skin equivalent (HSE) model based on a previously published protocol [29] to evaluate the capacity of entrapped MSC spheroids to promote angiogenesis in a three-dimensional skin-like environment. Briefly, 600 μL of growth factor reduced Matrigel (Corning, Corning, NY) was combined with 300 μL of collagen 1 (3 mg/mL; Advanced Biomatrix, Carlsbad, CA), added to a 12-well plate, and allowed to gel at 37°C for 1 hr. Human ECFCs were seeded on top of this layer at 200,000 cells per well in EGM-2 and allowed to attach for 1.5 hrs, after which excess media was aspirated and 100 μL EGM-2 was added to each well. 600 μL of the Matrigel/collagen mixture was added over the ECFCs and allowed to gel at 37°C for 1 hr. Keratinocytes were seeded on top of the layered construct at 250,000 cells per HSE in 75 μL of KGM-Gold media. HSE constructs were maintained in standard culture conditions (37°C, 5% CO2, 21% O2) for 18 hrs. Each HSE construct was then wounded with an 8 mm biopsy punch (Integra Miltex, Plainsboro, NJ), and defects were immediately treated with acellular fibrin gels or gels containing 10 spheroids created using the formulation predicted to synergistically increase MSC secretion of VEGF & PGE2. Fibrin gels loaded with 200 ng recombinant human VEGF (PeproTech, Rocky Hill, NJ) were included as a positive control, while untreated defects served as the negative control. Endothelial cell invasion was monitored over 15 hrs [30]. The number of endothelial cell sprouts, penetration depth of sprouts into the wound, and number of branch points per HSE were quantified using large scan brightfield microscopy at 4x magnification on a Nikon Eclipse TE2000-U microscope with an Andor Zyla Image Source camera (Belfast, Ireland, UK).
Statistical Analysis
Data are presented as mean ± standard deviation of the mean. Statistical analysis was performed using a one-way ANOVA with a Tukey correction for multiple comparisons or paired t-tests when appropriate, with p values less than 0.05 considered statistically significant. All statistical analysis was performed in Prism 7 software (GraphPad, La Jolla, CA). Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
RESULTS
DOE model reveals interplay between fibrin gel formulation conditions and MSC response
A DOE-approach was used to determine the significance and interaction of fibrinogen, NaCl, CaCl2, and aprotinin concentrations on the biophysical properties of resulting fibrin hydrogel delivery vehicles for MSC spheroids used for wound healing (Table 2). A Box-Behnken experimental design generated 25 unique fibrin gel formulations with a centrally repeated condition, and we measured the resulting compressive modulus, gel contraction, and VEGF and PGE2 secretion by entrapped MSC spheroids (Supplementary Figure 1). Each response output was influenced by one or more input variables (p ≤ 0.05), and the interactions were described as linear, quadratic, or two-factor interactions (Table 3).
Table 2.
DOE input variables and specific levels tested
| DOE Input Values | Specific Conditions Tested |
|---|---|
| A (Fibrinogen, mg/mL) | 5, 12.5, 20 |
| B (NaCl, % w/v) | 1, 2, 3 |
| C (Aprotinin, KIU/mL) | 0, 250, 500 |
| D (CaCl2, mM) | 0, 20, 40 |
Table 3.
Interplay between input variables and output response
| DOE Output Response | Significant Input Variables (p≤0.05) |
|---|---|
| Compressive modulus (kPa) | A, B, D, AB, BD, A2, B2, D2 |
| Gel Contraction (% of original area) | A, B, C, AC, A2, B2 |
| VEGF secretion | A, B, AB, A2, B2 |
| PGE2 secretion | A, B, D, AB, AD, BD, A2, B2, D2 |
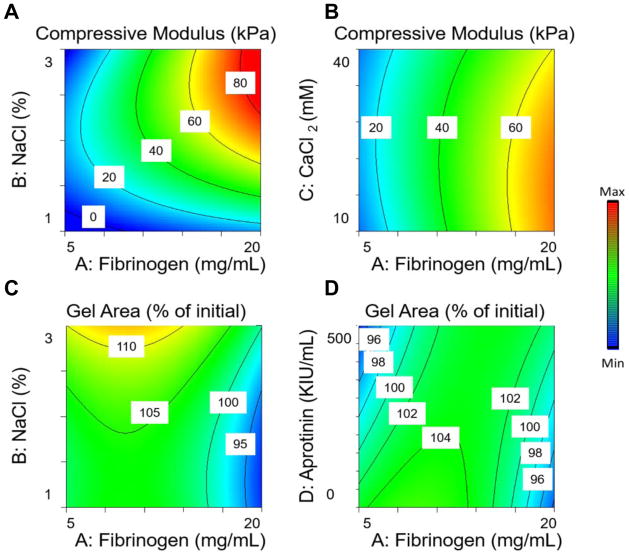
By controlling the composition of fibrin gels, we observed compressive moduli ranging from 0.2 to 81.3 kPa (over a 400-fold difference). Fibrinogen, NaCl, and CaCl2 concentrations all had a significant effect on the compressive modulus, while the aprotinin concentration had no significant effect. Fibrinogen, NaCl, and CaCl2 had strong quadratic relationships with compressive modulus, and NaCl and CaCl2 had strong interaction terms with fibrinogen and each other, resulting in low compressive moduli at high NaCl, low CaCl2, and low fibrinogen concentrations, yet high compressive moduli at high NaCl, low CaCl2, and high fibrinogen concentrations (Fig. 1A, B). The interplay of the four input variables yielded gels that shrank or swelled from 92% to 117% of their initial dimensions. Gel contraction was also a function of fibrinogen and NaCl concentrations. However, unlike compressive moduli, gel contraction did not depend on CaCl2 and varied as a function of aprotinin concentration. Gel contraction was a quadratic function of fibrinogen and NaCl, resulting in the least amount of gel contraction at the median values for those three input variables (Fig. 1C, D).
Figure 1. Response surface maps of hydrogel formulation effects on the material properties of fibrin hydrogels.
(A) Effect of NaCl and fibrinogen concentration on the compressive modulus. (B) Effect of CaCl2 and fibrinogen concentration on fibrin gel compressive modulus. (C) Effect of NaCl and fibrinogen concentration on gel contraction. (D) Effect of aprotinin and fibrinogen concentration on gel contraction.
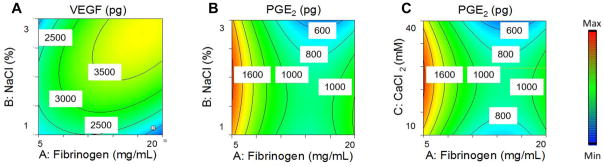
The secretory potential of the entrapped MSCs was dependent on the hydrogel formulation. Both fibrinogen and NaCl had a significant effect on VEGF secretion, while aprotinin and CaCl2 had no significant effect, resulting in a range of VEGF from 1400 to 4600 pg per gel. Both fibrinogen and NaCl concentrations had a strong quadratic relationship with VEGF secretion, as well as a significant interaction term, resulting in the maximal VEGF secretion near the median range for both input variables and the lowest VEGF secretion at low fibrinogen and NaCl (Fig. 2A). PGE2 secretion was dependent on fibrinogen, NaCl, and CaCl2, while aprotinin had no significant effect, resulting in PGE2 secretion from 312 to 1920 pg per gel. PGE2 had a strong quadratic relationship with fibrinogen, NaCl, and CaCl2. These three input variables also had strong interaction terms with each other, resulting in maximal PGE2 secretion in hydrogels containing low fibrinogen and median values of NaCl and CaCl2. Gels containing median fibrinogen and high NaCl and CaCl2 resulted in the lowest PGE2 secretion (Fig. 2B, C).
Figure 2. Response surface maps of hydrogel formulation effects on the proangiogenic and anti-inflammatory potential of entrapped MSC spheroids.
(A) Effect of NaCl and fibrinogen concentration on (A) VEGF secretion and (B) PGE2 secretion by MSC spheroids. (C) Effect of CaCl2 and fibrinogen concentration on PGE2 secretion by MSC spheroids.
Cytokine secretion by MSC spheroids is driven by hydrogel mechanical properties
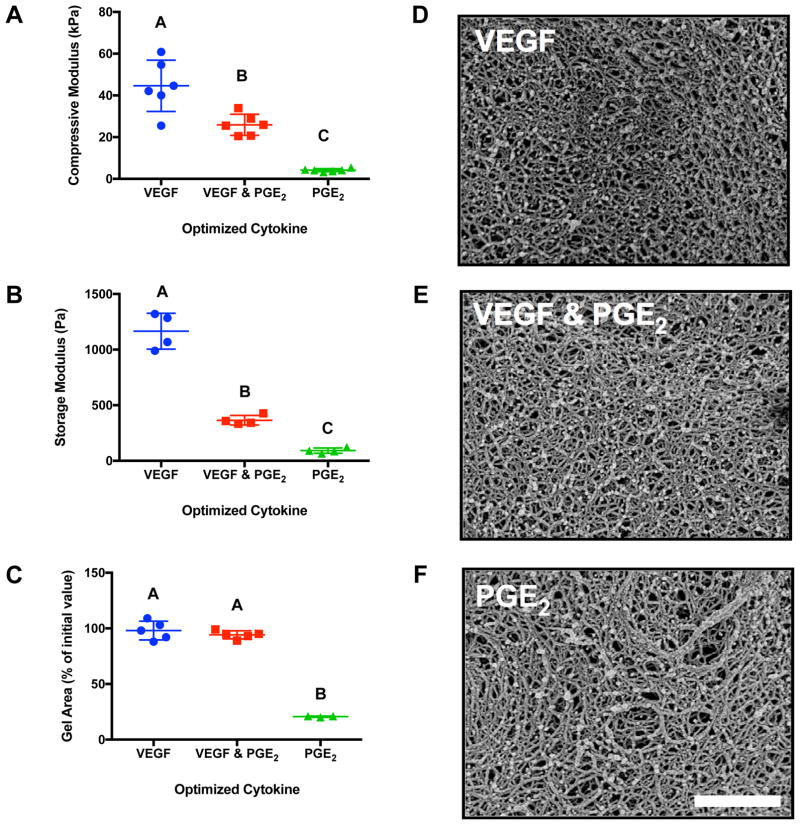
Both the compressive (Fig. 3A) and storage moduli (Fig. 3B) decreased in gels predicted to maximize PGE2 secretion compared to gels predicted to maximize VEGF secretion. The hydrogels predicted to maximize either VEGF alone or VEGF & PGE2 exhibited no significant contraction after 7 days, while the hydrogel predicted to maximize PGE2 secretion contracted to 20% of its initial area (Fig. 3C), in agreement with their weaker mechanical properties. Differences in hydrogel network morphology were apparent, as gels predicted to maximize VEGF or VEGF & PGE2 exhibited a tighter meshwork (Fig. 3D, E) than gels maximizing PGE2 (Fig. 3F).
Figure 3. Validation of the DOE model by examining material properties of the hydrogel formulations predicted to maximize VEGF, VEGF & PGE2, or PGE2 alone.
(A) Compressive moduli of acellular fibrin gels (n=6). (B) Shear storage moduli of acellular fibrin gels (n=4). (C) Gel area after 7 days of culture normalized to its Day 0 area (n=3–5). Morphology of fibrin gel optimized for (D) VEGF secretion, (E) VEGF & PGE2 secretion, (F) PGE2 secretion as observed by scanning electron microscopy. Scale bar represents 1 μm. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
MSC spheroids in fibrin gels secrete cytokines with proangiogenic and anti-inflammatory potential
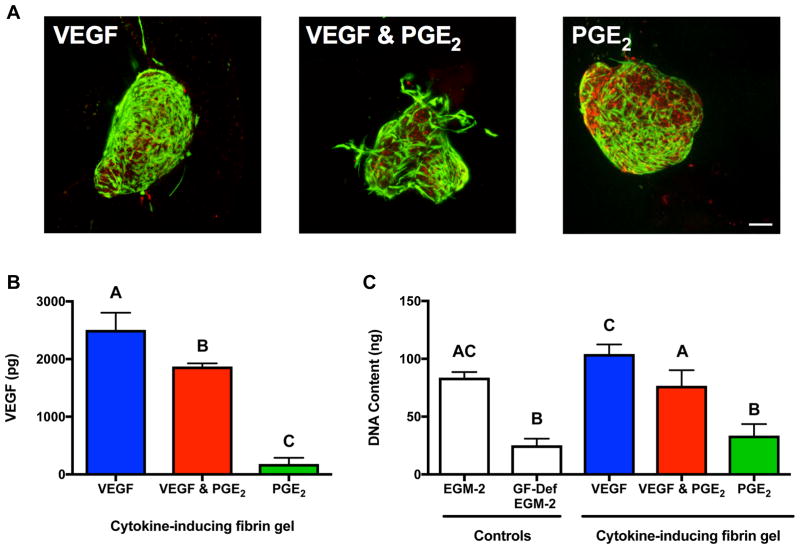
After 7 days in culture, MSC spheroids entrapped in cytokine-optimized gels exhibited differences in viability (Fig. 4A), with many spheroids changing their initial morphology as cells migrated into the fibrin gel. Spheroids entrapped in VEGF-inducing gels appeared more viable and elliptical in morphology, while spheroids in PGE2-inducing fibrin gels had lower viability by live/dead staining. After 7 days, the fibrin hydrogel predicted to maximize VEGF secretion exhibited 1.3-fold more VEGF than those predicted to maximize VEGF & PGE2 but nearly 14-fold more than those predicted to maximize PGE2 (Fig. 4B). We characterized the functional bioactivity of secreted endogenous factors by testing the ability of conditioned medium to stimulate diabetic HMVEC proliferation (Fig. 4C). Conditioned medium from hydrogels predicted to maximize VEGF or both VEGF & PGE2 supported HMVEC proliferation similar to the positive control (EGM-2 MV), and significantly better than fibrin gels predicted to maximize PGE2. There were no statistical differences between HMVECs treated with CM from hydrogels predicted to maximize PGE2 and GF-def EGM-2.
Figure 4. Validation of the DOE model examining the proangiogenic potential of MSC spheroids entrapped in hydrogels predicted to maximize VEGF, VEGF & PGE2, or PGE2 alone.
(A) Representative confocal microscopy images of live (green)/dead (red) assay revealing MSC spheroid viability when entrapped in a fibrin gel optimized for VEGF secretion, VEGF & PGE2 secretion, and PGE2 secretion after 7 days of culture. Scale bar is 100 μm; images captured using 10X objective. (B) Proangiogenic potential as measured by VEGF secretion by entrapped MSC spheroids (n=3). (C) Endothelial cell proliferation in the presence of MSC-conditioned media measured by DNA content after 3 days of culture (n=3). Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
Fibrin gels predicted to maximize PGE2 secretion exhibited 1.8-fold more PGE2 than those predicted to maximize both VEGF & PGE2 but 18.5-fold more than those predicted to maximize VEGF alone (Fig. 5A). We characterized the functional bioactivity of secreted endogenous cytokines by testing the ability of conditioned medium to polarize stimulated macrophages (Fig. 5B). Conditioned medium from hydrogels predicted to maximize PGE2 alone and VEGF & PGE2 significantly reduced the ratio of TNFα/IL-10 expression, an indication of polarizing macrophages towards the M2 phenotype. There were no statistical differences between macrophages treated with the CM from hydrogels predicted to maximize VEGF and untreated stimulated macrophages. However, conditioned medium from hydrogels predicted to maximize PGE2 alone reduced the TNFα/IL-10 ratio below that of the positive control, 1 ng/mL recombinant PGE2. To further characterize macrophage phenotype, we quantified their secretion of VEGF after CM treatment (Fig. 5C). Conditioned medium from all three hydrogel formulations elicited greater VEGF secretion compared to untreated stimulated macrophages, and CM from the hydrogel predicted to maximize PGE2 alone induced significantly more macrophage VEGF secretion compared to gels predicted to maximize VEGF alone.
Figure 5. Validation of the DOE model examining the anti-inflammatory potential of MSC spheroids entrapped in hydrogels predicted to maximize VEGF, VEGF & PGE2, or PGE2 alone.
(A) Anti-inflammatory potential as measured by PGE2 secretion by entrapped MSC spheroids (n=3). (B) Stimulated macrophage polarization in the presence of MSC-conditioned media represented by the ratio of TNFα and IL-10 secretion after 24 hours in culture (n=3). (C) VEGF secretion by stimulated macrophages in the presence of MSC-conditioned media after 24 hours in culture (n=3). MΦ denotes macrophages; sMΦ denotes stimulated macrophages. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
MSC spheroids in engineered gels enhance angiogenesis in a human skin equivalent
MSC spheroids were entrapped in fibrin gels engineered to maximize VEGF and PGE2 secretion, and this material was used to treat wounded endothelial HSE constructs (Fig. 6A). Compared to untreated gels, the use of optimized fibrin gels consistently increased endothelial cell penetration (Fig. 6B). MSC spheroid-laden hydrogels stimulated more sprouts (Fig. 6C) and greater invasion distance into the wound (Fig. 6D) compared to fibrin gels alone. On average, MSC- and VEGF-loaded hydrogels increased the number of branch points per HSE, but no statistical differences were observed among groups (Fig. 6E). Importantly, spheroid containing hydrogels performed similarly to VEGF-eluting fibrin gels and far better than acellular fibrin gels.
Figure 6. Validation of the DOE model examining the wound healing potential of MSC spheroids entrapped in hydrogels predicted to maximize VEGF & PGE2.
(A) Schematic of endothelial based human skin equivalent (HSE) model fabrication. (B) Representative images of wounded HSE models 15 hours after wounding. Insert shows ECFC sprouting into the wound. All scale bars represent 200 μm. Blue arrow indicates a sprout. Red arrowhead indicates an entrapped spheroid. (C) Number of sprouts per HSE 15 hours post-wounding (n=3). (D) Penetration depth of endothelial cells into the HSE (n=3). (E) Number of branch points per HSE (n=3). Boxes represent the 25th and 75th percentiles, with the line denoting the mean. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
DISCUSSION
By identifying a combination of variables associated with hydrogel synthesis, we formulated fibrin gels to simultaneously promote the secretion of proangiogenic and anti-inflammatory cytokines by entrapped MSC spheroids to enhance their wound healing potential. The response surface map revealed that the secretory potential of entrapped MSCs was dependent on the hydrogel formulation. Both VEGF and PGE2 secretion were dependent on fibrinogen and NaCl content, and PGE2 secretion further depended on CaCl2 content. Mechanical properties of resulting gels were significantly different due to changes in composition. The hydrogel formulation that was predicted to maximize VEGF secretion was markedly different from the formulation predicted to maximize PGE2 secretion. By modulating the constituents of fibrin gel synthesis, we engineered fibrin hydrogels to regulate the quantity of secreted angiogenic and anti-inflammatory cytokines, thereby providing a strategy to direct the contribution of cell-based therapies in tissue repair.
All four of the input variables examined (fibrinogen, NaCl, CaCl2, and aprotinin concentration) had significant effects on the hydrogel material properties. Increasing the fibrinogen concentration in fibrin gels increases fibril density, decreases pore size, and increases substrate stiffness [31, 32]. Here, we examined fibrinogen concentrations from 5 – 20 mg/mL, as we and others have shown this concentration yields hydrogels within the range of human skin compressive moduli (5–15 kPa) [33]. The supplementation of the pre-gel solution with NaCl provides a means to enhance hydrogel material properties, is rapidly eluted over 24 hours and does not alter the osmolality of the cell microenvironment, and does not impair cell viability [20]. We previously demonstrated that changing the NaCl content in the pre-gel solution alters gel stiffness, pore size, fiber diameter, and permeability [20–22]. While the mechanism of how NaCl affects material properties is not fully characterized, thrombin possesses Na+ binding sites, which may regulate activation kinetics and network organization. Similarly, the precise interplay between CaCl2 concentration and fibrin hydrogel properties is not fully characterized, although the availability of calcium ions can alter gelation kinetics by affecting the rate of fibrinopeptide release by thrombin. Aprotinin was included as an input variable due to its role as a serine protease inhibitor, thus preventing fibrinolysis and gel degradation by the entrapped cells. Wound healing occurs from the margins, and gels that significantly contract and pull away from the wound margins would be rendered obsolete. However, the host cells must be able to migrate and remodel their surroundings for the wound dressing to be effective. Thus the gel degradation rate should be kept within 40–50% over the course of 7 days [34].
A critical finding of this work is that multifactorial analysis revealed intimate interactions of these four input variables and their effect on the mechanical properties of engineered fibrin gels. While increasing fibrinogen content was expected to increase mechanical properties, we did not anticipate the strong interactions between fibrinogen, NaCl, and CaCl2 content. High fibrinogen concentration coupled with 1% (w/v) NaCl and 5 mM CaCl2 yielded mechanically weak hydrogels with a compressive stiffness of 0.2 kPa. However, maintaining constant fibrinogen concentration but increasing NaCl and CaCl2 content to 3% (w/v) and 10 mM, respectively, resulted in hydrogels with a significantly greater compressive modulus of 81.3 kPa. This resultant stiffness is over 4-fold greater than magnitudes achieved by changing one factor (NaCl content) at a time [21, 22, 27]. In agreement with our previous studies, hydrogels with lower compressive moduli were more susceptible to cellular contractile forces [21]. Overall, the effectiveness of aprotinin appeared to be enhanced by NaCl content. However, while aprotinin had a significant effect on gel contraction in our DOE model, the predicted difference in gel area was relatively small.
The manipulation of fibrin gel material properties altered the proangiogenic potential of entrapped MSC spheroids. The compressive modulus of the hydrogel predicted to maximize VEGF secretion was approximately 40 kPa and elicited a strong proangiogenic response from entrapped cells. The magnitude of this modulus differs from our previous work in which we reported that fibrin hydrogels formed with 1.2% NaCl (w/v) were more proangiogenic than hydrogels containing 2.3% or 3.9% NaCl (w/v) [21]. However, those studies analyzed the response of dissociated MSCs when we only changed one factor, NaCl content, whereas these studies investigated the interplay of multiple factors simultaneously. We and others reported that spheroids of progenitor cells secrete more VEGF and other proangiogenic cues than equal numbers of cells in monolayer culture [7, 9, 10, 28]. We recently demonstrated that spheroids and dissociated cells do not respond in the same manner to external biophysical cues [10, 28], confirming the role of the surrounding milieu on MSC response when entrapped as dissociated cells or spheroids.
While the interplay between matrix composition and the proangiogenic potential of MSCs has been reported, the effect of matrix composition on the anti-inflammatory potential of MSCs is poorly described. Substantial evidence confirms that spheroid formation upregulates the anti-inflammatory potential of MSCs through PGE2 secretion, yet these findings are the first to demonstrate the effect of a biomaterial delivery vehicle on the continued anti-inflammatory potential of MSC spheroids. MSC spheroids upregulated PGE2 secretion in softer hydrogels, and we could modulate the production of PGE2 nearly 20-fold by altering the hydrogel composition. These data provide strong evidence that the anti-inflammatory potential of MSCs is intrinsically linked to the biophysical cues communicated by their surroundings. The DOE method enables the evaluation of numerous variables and their interactions on an output measure, while the traditional One Factor at a Time (OFAT) approach does not facilitate such comparisons.
We previously demonstrated that MSC spheroids do not exhibit a hypoxic core due to a nutrient gradient, perhaps due to adaptive reductions in matrix deposition and packing density with increases in spheroid diameter [35]. However, the entrapment of MSC spheroids in hydrogels likely reduces diffusion-mediated transport from that observed with free spheroids, altering the oxygen microenvironment and potentially upregulating hypoxia-adaptive signals even further. While we did not study the oxygen gradient for entrapped MSC spheroids within these materials, others have modeled the extent of oxygen diffusion and cellular consumption in engineered hydrogels [36–38]. In fibrin hydrogels, gel thickness has the most pronounced influence on the distribution of oxygen within the system [39]. These studies employed relatively thin fibrin hydrogels, less than 2 mm, which were not particularly cell dense, thereby reducing the likelihood of large nutrient gradients that activate oxygen-mediated signaling pathways. However, the limited knowledge of oxygen gradients in hydrogels engineered to modulate MSC spheroid function is a limitation of this work. Furthermore, the underlying mechanism of action as to how MSC spheroids sense their surroundings and translate that signal to growth factor production is unknown and merits further investigation.
When used in cell-based therapies of wound healing, MSC spheroids contribute to tissue repair by secreting trophic factors that stimulate cells in the wound bed such as endothelial cells and macrophages [40]. Upon demonstrating the bioactivity of VEGF and PGE2 secreted by entrapped MSCs on diabetic HMVECs and macrophages, we investigated the potential of MSC spheroid-containing hydrogels to stimulate endothelial cell sprouting in a three-dimensional skin equivalent model. Spheroid-containing hydrogels exhibited similar capacity as VEGF-eluting fibrin gels to stimulate sprout formation and outperformed VEGF-eluting gels for sprout penetration depth into a wounded construct. MSC spheroid-containing hydrogels significantly upregulated sprouting compared to acellular optimized fibrin gels in all quantified parameters. Wounds treated with MSC-loaded gels exhibited similar number of sprouts and branch points but markedly increased depth of penetration compared to hydrogels loaded with 100x the dosage of recombinant VEGF protein. These data further emphasize the advantages of the bioactive secretome linked to cell-based approaches over local delivery of recombinant proteins. The effect of fibrin gel biophysical properties on the MSC secretome merits further investigation as a tool to further enhance wound healing. Taken together, these data confirm that we can differentially affect key cell types that may be dysregulated in chronic non-healing wounds by modulating the delivery vehicle for MSC spheroids.
CONCLUSION
These studies reveal, for the first time, the effect of hydrogel formulation to simultaneously enhance the proangiogenic and anti-inflammatory potential of entrapped MSC spheroids. Hydrogel composition dictated mechanical properties of fibrin gels, which modulated the secretion of each cytokine. MSC spheroids in stiffer gels secreted the most VEGF, while PGE2 secretion was highest in more compliant gels. Simultaneous VEGF and PGE2 secretion was greatest using hydrogels with intermediate mechanical properties, as small increases in stiffness increased VEGF secretion while maintaining PGE2 secretion by entrapped spheroids. This work is important for those attempting to develop delivery vehicles for MSC spheroid transplantation and for predicting cell behavior once entrapped. This system can be pre-cast and placed over a wound as described herein, but may also be injectable, provided the needle were larger than the spheroids to avoid undesirable shear effects on MSCs. Overall, these data demonstrate that a multifactorial statistical approach can be used to the determine fibrin gel formulations that enhance the wound healing potential of human MSC spheroids.
Supplementary Material
Predicted values versus experimentally measured values for (A) gel stiffness, (B) gel contraction, (C) VEGF secretion by entrapped MSC spheroids, and (D) PGE2 secretion by entrapped MSC spheroids.
STATEMENT OF SIGNIFICANCE.
Mesenchymal stem cells (MSCs) are under investigation for wound healing applications due to their secretion of bioactive factors that enhance granulation tissue formation, blood vessel ingrowth, and reduce inflammation. However, the effectiveness of cell-based therapies is reduced due to poor engraftment and high rates of cell death when transplanted into harsh environments characteristic of large wounds. Compared to dissociated cells, MSCs exhibit increased overall function when aggregated into three-dimensional spheroids, and transplantation of cells using biomaterials is one strategy for guiding cell function in the defect site. The present study demonstrates that the biophysical properties of fibrin hydrogels, designed for use as a cell carrier, can be engineered to dictate the secretion of bioactive factors by entrapped MSC spheroids. This strategy enables MSCs to contribute to wound healing by synergistically promoting neovascularization and modulating the inflammatory milieu.
Acknowledgments
This work was supported by the National Institutes of Health (R01 DE025475) to JKL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the decision to publish, or preparation of the manuscript. KM was supported by an American Heart Association Western States Affiliate Predoctoral Fellowship (15PRE21920010). JW was supported by a National Science Foundation Graduate Research Fellowship (1650042). SH was supported by the T32 Animal Models of Infectious Disease Training Program Kirschstein-NRSA (T32 AI060555).
Footnotes
AUTHOR DISCLOSURE STATEMENT
We have no competing interests.
Kaitlin C. Murphy: Conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing
Jacklyn Whitehead: Collection and/or assembly of data
Dejie Zhou: Collection and/or assembly of data
Steve S. Ho: Collection and/or assembly of data
J. Kent Leach: Conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee N, Robinson J, Lu H. Biomimetic strategies for engineering composite tissues. Curr Opin Biotechnol. 2016;40:64–74. doi: 10.1016/j.copbio.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Moura LI, Dias AM, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review. Acta Biomater. 2013;9(7):7093–114. doi: 10.1016/j.actbio.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–43. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balaji S, Keswani SG, Crombleholme TM. The Role of Mesenchymal Stem Cells in the Regenerative Wound Healing Phenotype. Adv Wound Care (New Rochelle) 2012;1(4):159–165. doi: 10.1089/wound.2012.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hocking AM. Mesenchymal Stem Cell Therapy for Cutaneous Wounds. Adv Wound Care (New Rochelle) 2012;1(4):166–171. doi: 10.1089/wound.2011.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhang SH, Lee S, Lee TJ, La WG, Yang HS, Cho SW, Kim BS. Three-dimensional cell grafting enhances the angiogenic efficacy of human umbilical vein endothelial cells. Tissue Eng Part A. 2012;18(3–4):310–9. doi: 10.1089/ten.tea.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhang SH, Lee S, Shin JY, Lee TJ, Kim BS. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012;18(19–20):2138–47. doi: 10.1089/ten.tea.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30(10):2283–96. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy KC, Fang SY, Leach JK. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014;357(1):91–9. doi: 10.1007/s00441-014-1830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy KC, Whitehead J, Falahee PC, Zhou D, Simon SI, Leach JK. Multifactorial experimental design to optimize the anti-inflammatory and proangiogenic potential of mesenchymal stem cell spheroids. Stem Cells. 2017;35(6):1493–1504. doi: 10.1002/stem.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusamori K, Nishikawa M, Mizuno N, Nishikawa T, Masuzawa A, Shimizu K, Konishi S, Takahashi Y, Takakura Y. Transplantation of insulin-secreting multicellular spheroids for the treatment of type 1 diabetes in mice. J Control Release. 2014;173:119–24. doi: 10.1016/j.jconrel.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Uchida S, Itaka K, Nomoto T, Endo T, Matsumoto Y, Ishii T, Kataoka K. An injectable spheroid system with genetic modification for cell transplantation therapy. Biomaterials. 2014;35(8):2499–506. doi: 10.1016/j.biomaterials.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462(7272):426–32. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vedadghavami A, Minooei F, Mohammadi MH, Khetani S, Rezaei A, Mashayekhan S, Sanati-Nezhad A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017 doi: 10.1016/j.actbio.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Ceccarelli J, Putnam AJ. Sculpting the blank slate: how fibrin’s support of vascularization can inspire biomaterial design. Acta Biomater. 2014;10(4):1515–23. doi: 10.1016/j.actbio.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barsotti MC, Magera A, Armani C, Chiellini F, Felice F, Dinucci D, Piras AM, Minnocci A, Solaro R, Soldani G, Balbarini A, Di Stefano R. Fibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cells. Cell Prolif. 2011;44(1):33–48. doi: 10.1111/j.1365-2184.2010.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Puente P, Ludena D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp Cell Res. 2014;322(1):1–11. doi: 10.1016/j.yexcr.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Jung JP, Bache-Wiig MK, Provenzano PP, Ogle BM. Heterogeneous differentiation of human mesenchymal stem cells in 3D extracellular matrix composites. Biores Open Access. 2016;5(1):37–48. doi: 10.1089/biores.2015.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis HE, Miller SL, Case EM, Leach JK. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011;7(2):691–9. doi: 10.1016/j.actbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Murphy KC, Hughbanks ML, Binder BY, Vissers CB, Leach JK. Engineered fibrin gels for parallel stimulation of mesenchymal stem cell proangiogenic and osteogenic potential. Ann Biomed Eng. 2015;43(8):2010–21. doi: 10.1007/s10439-014-1227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy KC, Stilhano RS, Mitra D, Zhou D, Batarni S, Silva EA, Leach JK. Hydrogel biophysical properties instruct coculture-mediated osteogenic potential. FASEB J. 2015;30(1):477–86. doi: 10.1096/fj.15-279984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grassl ED, Oegema TR, Tranquillo RT. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res. 2002;60(4):607–12. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 24.Decaris ML, Lee CI, Yoder MC, Tarantal AF, Leach JK. Influence of the oxygen microenvironment on the proangiogenic potential of human endothelial colony forming cells. Angiogenesis. 2009;12(4):303–11. doi: 10.1007/s10456-009-9152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decaris ML, Leach JK. Design of experiments approach to engineer cell-secreted matrices for directing osteogenic differentiation. Ann Biomed Eng. 2011;39(4):1174–85. doi: 10.1007/s10439-010-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CC, Jeon NL, Putnam AJ, George SC. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J. 2008;94(5):1930–41. doi: 10.1529/biophysj.107.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy KC, Leach JK. A reproducible, high throughput method for fabricating fibrin gels. BMC Res Notes. 2012;5:423. doi: 10.1186/1756-0500-5-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho SS, Murphy KC, Binder BY, Vissers CB, Leach JK. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 2016;5(6):773–81. doi: 10.5966/sctm.2015-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman IM, Leung A. Creation of human skin equivalents for the in vitro study of angiogenesis in wound healing. Methods Mol Biol. 2009;467:241–8. doi: 10.1007/978-1-59745-241-0_14. [DOI] [PubMed] [Google Scholar]
- 30.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5(4):628–35. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 31.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6(30):1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kniazeva E, Kachgal S, Putnam AJ. Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Eng Part A. 2011;17(7–8):905–14. doi: 10.1089/ten.tea.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pailler-Mattei C, Bec S, Zahouani H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys. 2008;30(5):599–606. doi: 10.1016/j.medengphy.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Wan J, Xia L, Liang W, Liu Y, Cai Q. Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J Diabetes Res. 2013;2013:647107. doi: 10.1155/2013/647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy KC, Hung BP, Browne-Bourne S, Zhou D, Yeung J, Genetos DC, Leach JK. Measurement of oxygen tension within mesenchymal stem cell spheroids. J R Soc Interface. 2017;14(127) doi: 10.1098/rsif.2016.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen YI, Abaci HE, Krupsi Y, Weng LC, Burdick JA, Gerecht S. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater Sci. 2014;2(5):655–665. doi: 10.1039/C3BM60274E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demol J, Lambrechts D, Geris L, Schrooten J, Van Oosterwyck H. Towards a quantitative understanding of oxygen tension and cell density evolution in fibrin hydrogels. Biomaterials. 2011;32(1):107–18. doi: 10.1016/j.biomaterials.2010.08.093. [DOI] [PubMed] [Google Scholar]
- 38.Colom A, Galgoczy R, Almendros I, Xaubet A, Farré R, Alcaraz J. Oxygen diffusion and consumption in extracellular matrix gels: Implications for designing three-dimensional cultures. J Biomed Mater Res A. 2014;102(8):2776–2784. doi: 10.1002/jbm.a.34946. [DOI] [PubMed] [Google Scholar]
- 39.Ehsan SM, George SC. Nonsteady state oxygen transport in engineered tissue: implications for design. Tissue Eng Part A. 2013;19(11–12):1433–42. doi: 10.1089/ten.tea.2012.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel S, Maheshwari A, Chandra A. Biomarkers for wound healing and their evaluation. J Wound Care. 2016;25(1):46–55. doi: 10.12968/jowc.2016.25.1.46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predicted values versus experimentally measured values for (A) gel stiffness, (B) gel contraction, (C) VEGF secretion by entrapped MSC spheroids, and (D) PGE2 secretion by entrapped MSC spheroids.