Abstract
Mixed phenotype acute leukemia (MPAL) represents a poorly characterized group of acute leukemias that lack an accepted therapeutic approach and are typically associated with poor outcomes. We present our experience of genomic profiling, pre-transplant therapy and transplant outcomes for 36 well characterized pediatric and adult patients with MPAL defined according to the 2016 WHO leukemia update. A predominance of ALL-associated mutations and cytogenetic abnormalities was noted. Remission rates after induction appeared comparable among adults (20/23) and children (11/13) and among those who received ALL (10/11) or AML-type (21/25) induction. Adults were transplanted in first remission while children were transplanted in the setting of relapse or MLL rearrangement. The median follow-up among the 25 patients who underwent transplantation was 39.6 months and median OS was not reached. Relapse after transplant was associated with MLL rearrangement (p=0.022), reduced intensity (p<0.001), and higher WBC at diagnosis (p=0.034). These data highlight differing therapeutic approaches between adult and pediatric MPAL and demonstrate favorable survival of adult MPAL patients consolidated with allogeneic hematopoietic cell transplantation.
Keywords: Mixed phenotype acute leukemia, allogeneic hematopoietic cell transplantation, somatic mutations
INTRODUCTION
Mixed phenotype acute leukemia (MPAL) is uncommon, accounting for 2–5% of all newly diagnosed acute leukemia 1. These neoplasms are thought to arise from an immature undifferentiated progenitor that expresses both myeloid and lymphoid antigens. The lack of distinguishing morphologic or genomic features means diagnosis is based solely on blast immunophenotype 2. Little is known about the biology of MPAL and as a result there is single standard induction chemotherapy approach with ALL and AML-type regimens used interchangeably.
Historically, outcomes in MPAL were thought to be inferior to AML and ALL 3, 4; however, studies drawing these conclusions may be biased by including patients with high risk myeloid malignancies which frequently have aberrant lymphoid antigen expression such as therapy-related neoplasms and AML with MDS related changes. These cases, as well as those with karyotypes that define therapy-related neoplasms, as well as cases with TP53 mutation which are typically seen in therapy-related neoplasms 5 should not be categorized as MPAL according to the WHO guidelines 6. More recent publications show favorable outcomes when patients are consolidated with allogeneic hematopoietic stem cell transplantation (HCT) 7, 8. There remains a paucity of MPAL transplant data and variables predicting transplant outcome have not been confirmed.
An improved understanding of leukemia genetics has resulted in genomic classification systems for ALL and AML 9, 10. Genomics offer an understanding of disease biology and identify therapeutic targets and biomarkers that predict relapse after conventional chemotherapy and HCT. MPAL with MLL or Philadelphia rearrangement are the only two genetically defined subgroups, but make up a minority of MPAL cases. B and T myeloid subtypes are defined on the basis of immunophenotype alone but may be better categorized into ALL or AML subgroups on the basis of expression profiling 4 or somatic molecular mutations which are characteristic of lineage-committed leukemia. Here we describe the outcomes with MPAL strictly defined according to the 2016 update to the WHO classification of myeloid neoplasms and acute leukemia 11. We observe that somatic mutations and cytogenetic abnormalities in MPAL are characterized by mutations seen frequently in ALL rather than AML. We report favorable outcomes in MPAL consolidated with HCT and identify variables associated with remission induction and relapse after HCT.
MATERIALS AND METHODS
Patients
This was a retrospective analysis of 36 MPAL patients treated at our center between 2005 and 2015. MPAL was defined according to the WHO 2016 update on myeloid neoplasms and acute leukemia 11. Pediatric patients were defined as those diagnosed below the age of 18 years.
Pathology
A single pathologist reviewed all cases. Patients with AML-defining cytogenetic abnormalities, myelodysplastic syndrome transformed to AML, AML with MDS related changes and, therapy-related neoplasms were excluded. Bone marrow cytogenetics was assessed using G-band karyotyping. Molecular sequencing (N=16) was performed using two separate next generation sequencing (NGS) assays. Bone marrow from 8 cases was sequenced using a NGS platform that sequences DNA from 405 genes and RNA from 265 genes of known oncogenic drivers in hematologic malignancies including AML and ALL, sarcomas and pediatric cancer 12. Additionally, 8 MPAL were sequenced using an institutional NGS platform, which targets 28 genes recurrently mutated in myeloid neoplasms 13. Minimal residual disease (MRD) at the time of HSCT was assessed using various methods including 10-colour flow cytometry (n=9), quantitative PCR for BCR ABL (n=5), FISH for patient specific leukemia-defining cytogenetic alteration (n=3), presence of IHG or TCR rearrangement (n=3).
Clinical parameters
Induction chemotherapy was grouped as ‘ALL-type’ if it incorporated L-asparaginase, corticosteroids or ‘AML-type’ if it included cytarabine and an anthracycline without L-asparaginase, vincristine or steroids. Transplant conditioning intensities were defined according to consensus guidelines14. Complete remission was defined as presence of less than 5% blasts on bone marrow aspirate and relapse was defined as presence of ≥ 5% blasts on bone marrow assessment 15.
Statistical analysis
Fisher’s exact test was used to examine associations between MPAL subtypes and other clinic variables, as well as between remission status after induction. Associations between these clinical variables and overall survival (OS) after transplant were examined using Kaplan-Meier method and the log rank test. Cumulative incidence of relapse (CIR) was estimated, treating death due to other causes and second transplant as competing risks. Gray’s test was used to examine associations between clinical factors and relapse. A test with p-value < 0.05 was considered statistically significant. All statistical analyses were performed in software packages SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.1 (The R Foundation for Statistical Computing).
RESULTS
Patients
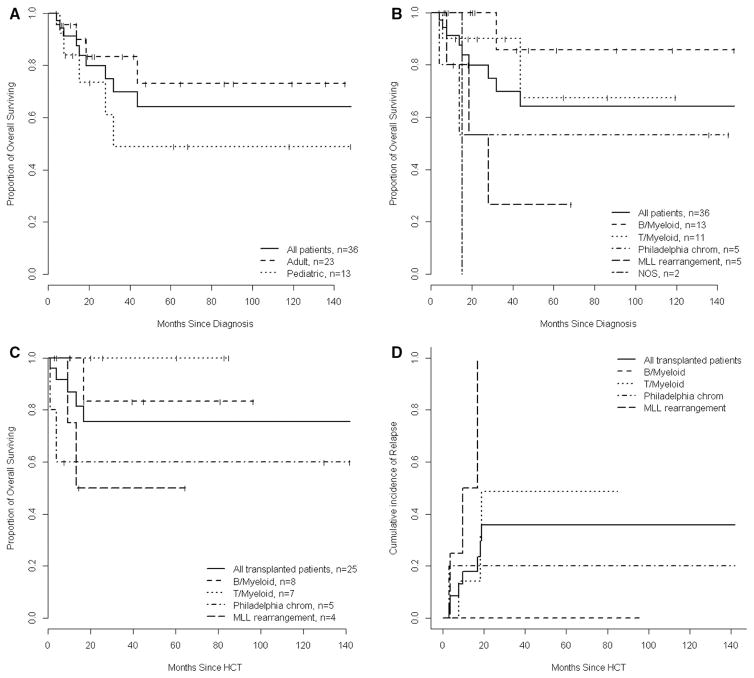
Clinical variables stratified by WHO MPAL subtype are presented in Table 1. B and T Myeloid cases made up 67% of all patients. The median age was 27 years (range: 1–69) and 13 (36%) were children. Patients with Ph+ MPAL were older while those with MLL MPAL were younger. Five patients had histologically confirmed extramedullary disease at diagnosis (gingival, lymph node, breast, muscle and pericardium) and seven had CNS disease. The median follow-up of all survivors (n=27, 75%) was 42.1 months (95% CI: 19.4–68.4) and median OS after diagnosis was not reached (95% CI: 32 months-NR). OS was not different between adult and pediatric patients (p=0.195) or between MPAL subtypes (p=0.080) (Figure 1A and 1B).
Table 1.
Clinical characteristic of MPAL at diagnosis
| Total | B/Myeloid | T/Myeloid | Ph+MPAL | MLL MPAL | MPAL NOS | |
|---|---|---|---|---|---|---|
| N | 36 | 13 | 11 | 5 | 5 | 2 |
| Men | 21 (58%) | 7 (54%) | 6 (55%) | 4 (80%) | 2 (40%) | 2 (100%) |
| Median age, years (range) | 27 (1–69) | 17 (1–65) | 37 (14–69) | 45 (18–60) | 15 (2–29) | 15 (3–27) |
| Age <18 | 13 (36%) | 7 (54%) | 2 (18%) | 0 (0%) | 3 (60%) | 1 (50%) |
| Clinical features | ||||||
| Splenomegaly | 9 (25%) | 4 (33%) | 1 (9%) | 2 (50%) | 1 (20%) | 1 (50%) |
| Adenopathy | 11 (32%) | 2 (17%) | 6 (55%) | 1 (25%) | 0 (0%) | 2 (100%) |
| Sarcoma | 5 (15%) | 0 (0%) | 3 (27%) | 0 (0%) | 2 (40%) | 0 (0%) |
| CNS involvement | 7 (19%) | 4 (31%0 | 1 (9%) | 0 (0%0 | 1 (20%) | 1 (50%) |
| CBC, median (range) | ||||||
| WBC (x10^9/L) | 13.5 (0.5–200) | 9.9 (0.5–71) | 37.2 (8.1–143) | 60.2 (34.7–130.5) | 169.3 (1.9–200) | 13.5 |
| Hb (g/dL) | 10 (5.5–14.1) | 9.4 (5.5–13.1) | 10.7 (6.6–13.3) | 10.8 (10–14.1) | 7 (6.5–7.3) | 12.7 (12.7–12.7) |
| Plt (x10^9/L) | 114 (9–341) | 109.5 (28–341) | 104.5 (14–333) | 139 (123–327) | 11 (9–26) | 235 (235–235) |
| Cytogenetics | ||||||
| Normal | 4 (11%) | 2 (15%) | 2 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Complex | 14 (40%) | 3 (23%) | 4 (36%) | 4 (80%) | 2 (40%) | 1 (50%) |
| -7/7q | 5 (14%) | 1 (8%) | 2 (18%) | 0 (0%) | 1 (20%) | 0 (0%) |
| t 14(q31-32) | 4 (11%) | 0 (0%) | 4 (36%) | 0 (0%) | 0 (0%) | 0 (0%) |
| +21 | 6 (17%) | 5 (38%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) |
| Molecular | ||||||
| Rearranged TCR | 10/15 (67%) | 3 (75%) | 4 (67%) | 1 (33%) | 1 (100%) | 1 (100%) |
| Rearranged BCR | 3/8 (38%) | 1 (50%) | 0/2 | 2 (100%) | 0/1 | 0/0 |
| Induction type | ||||||
| AML-type# | 25 (69%) | 9 | 7 | 4 | 3 | 1 |
| ALL-type^ | 11 (31%) | 4 | 4 | 1 | 2 | 1 |
Figure 1.
(A) OS for all adult and pediatric MPAL from time of disease diagnosis. (B) OS for all patients by MPAL subtypes from time of disease diagnosis. (C) OS by MPAL who underwent HCT by MPAL subtype from time of transplant. (D) Estimated cumulative incidence of relapse after HCT by MPAL subtype. In figure C and D a separate curve for the single MPAL NOS patient who underwent HCT is not presented; however, this patient is included in the curve showing HCT outcome of all MPAL.
Cytogenetics and molecular typing
Diagnostic cytogenetics was available for 35 patients. Translocations were the most common structural abnormality seen in 20 (56%) patients. Four patients had a normal karyotype, 14 (40%) had a complex karyotype defined as 3 or more structural abnormalities, 6 (17%) had monosomies and ten (29%) had polysomies of which +21 was seen in 7 (20%) and 6 of these had B/myeloid antigen expression. There were no AML, MDS or therapy-related myeloid neoplasm defining cytogenetic abnormalities 11. Chromosomal changes seen in ALL were common and included high-hyperdiploidy (>50 chromosomes) (n=2) with polysomy of chromosomes typically duplicated in hyperdiploid ALL (chromosomes 4, 7, 11 and 21) and hypodiploidy (<44 chromosomes) in one case. The ALL-associated translocation t(12;21)(p13;q22) was identified in 2 cases. Aside from MLL and BCR-ABL rearrangement, 4/36 patients (11%) had translocations involving 14(q31-q32) and in 3 of these patients this was the only abnormality. All 4 cases with t(v;14)(v;q31-32) had T/myeloid antigen expression. The region on 14(q31-32) encodes several genes associated with B cell Non Hodgkin Lymphoma (IGH, CL11B) and T cell leukemia (TCL1A, TCL6) and is very frequently rearranged in ALL 16, 17 but not in AML. Rearrangements of 14(q31-32) have been reported in MPAL 18. One patient had t(10;11)(p15;q21) (PICALM-MLL10) rearrangement which has been described in MPAL 18, 19. MLL, located on 11(q23) was always rearranged with 4(q21). Rearrangement of MLL is seen in both ALL and AML; however, t(4;11)(q21;q23) is rarely seen in AML 20 but is the most common MLL rearrangement in ALL and the second most common translocation overall in ALL 21. Ph+ MPAL was seen only in adults and in 4/5 it was associated with a complex karyotype.
Genes recurrently mutated in AML were infrequently altered relative to mutations seen frequently in ALL. There were no mutations in NPM1, IDH2, TP53 and no biallelic CEBPA mutations, which define non-overlapping genomic AML subtypes 9. Alterations associated with myeloproliferative disorders: JAK2 and MPL mutations were not seen in any patient. There was an overwhelming predominance of ALL-associated mutations among the 8 MPAL who had sequencing using the AML and ALL specific NGS panel (Table 2). Alterations occurred in specific functional pathways including transcription factors critical for lymphoid maturation (IKZF1 n=1/8, NOTCH n=4/8, ETV6 n=1/16); kinases involved in JAK-STAT signaling (FLT3-ITD n=3/16, IL7R n=1/8, JAK1 n=1/16, JAK3 n=2/16), RAS-pathway mutations (PTPN11 n=2/8, NF1 n=1/8, RAS n= 1/16), tumor suppressor genes (CDKN2A n=2/8, PHF6 n=3/16) and epigenetic regulators (MLL2 mutation n=3/8, TET2 n=4/16, RUNX1 n=3/16, DNMT3A n=2/16, and IDH1 n=1/16). There were no TP53 mutations (0/16) which are strongly associated with therapy-related myeloid neoplasms and no CRLF2 rearrangements (n=0/8) or alterations in PAX5 (n=0/8), which appear to be ALL-specific mutations.
Table 2.
Genomic variants in 8 patients who had extended DNA and RNA sequencing for ALL and AML type somatic mutations.
| Age | MPAL | Cytogenetics | Molecular mutations |
|---|---|---|---|
| 1 | B/myeloid | 46,XY,?inv(11)(q22q23),der(16)t(1;16)(q21;q12) | PASK R451* |
| 3 | MPAL, NOS rare type | 46,XY,t(1;5;9)(p32;q33;p22) |
IL7R L243_T244insGES GPCL, NOTCH1 P2415fs*5, IKZF1 K91fs*3, PHF6 Y105fs*38 |
| 4 | B/myeloid | 46,XY,del(6)(q?23q?25),add(12)(p13)[12] 46,idem,add(4)(p?14)[4]/46,XY[4] FISH positive for t(12;21)(p13;q22) |
ETV6-RUNX1 fusion |
| 15 | MLL/MPAL | 53,X,+X,-Y,+1,der(1)inv(1)p12q12)del(1)(p12); t(4;11)(q21;q23),+der(1)t(4;11),+8,+10,+13,+21, +22 | PTPN11 p.E76K, CDKN2A p.16INK4a CDKN2A p.H63Y, CDKN2A p.14ARF CDKN2A p.A97V, MLL-AFF1 fusion, CD36 N53fs*24, ETV6 p.E392* |
| 25 | MLL/MPAL | 46,XX,t(4;11)(q21;q23) | MLL-AFF1 fusion |
| 27 | MPAL, NOS rare type | 47,XY,+8,del(12)(p12)[8]47,idem,del(9)(q?34)[4] 46,XY[8] |
RUNX1 R320*, JAK3 p.A573V, JAK3 p.M511I, JAK3 p.V674A, NOTCH1 truncation intron 2, NUP214 SET-NUP214 fusion, PHF6 R128*, PTPN11 p.G503V, SF3B1 p.E862K, SUZ 12 S53fs*32, TYK2 p.V15A |
| 29 | B/myeloid | 45,XX,der(13;14)(q10;q10)[8]51,idem,+10,+11,+ 17,+18,+21x2 | FLT3 p.Y589S, CDKN2A p16INK4a loss, CDKN2A p14ARF loss exon 2–3, EP300 C14orf119 - EP300 fusion, MLL2 R1702* |
| 37 | T/myeloid | 45,XY,add(1)(p?22),der(3)t(1;3)(p22;q21),-9, add(10)(p11.2),del(11)(q23),del(12)(p11.2), add(14)(q32),i(17)(q10),der(18)t(9;18)(q13;q23) | NF1 deletion exon 31–35, MLL PICALM-MLL T10 fusion^, NOTCH2 p.I1549M, MLL3 p.M711T, FANCE p.R141* # |
In bold are alteration recurrently identified in ALL.
MLL PICALM-MLL T10 fusions are described to occur in acute leukemia with mixed phenotype and typically in patients with T/myeloid phenotype as in this case 51.
Mutations in FANCE are associated with Fanconi anemia 52 but to the best of our knowledge they have never been reported in MPAL.
Induction and consolidation chemotherapy
ALL-type induction regimens were used in 11 patients (31%) and AML-type in 25 patients (69%) (Table 1). There were no deaths during induction and 31/36 (86%) attained complete remission (CR). There was no association between MPAL subtype or antigen expression (MPO, CD3, CD19) and use of ALL or AML-type induction regimens. All Ph+ MPAL patients received multi-agent induction chemotherapy in combination with a tyrosine kinase inhibitor (imatinib n=2, dasatinib n=3). AML-type regimens were used more frequently in all MPAL subtypes and among adult (16/23, 70%) and pediatric (9/13, 70%) cases. The most frequently used induction regimen in adults was cytarabine plus high dose mitoxantrone 22 (CR in 10/10) and DCTER 23 in children (CR in 7/8). CR rate was significantly lower in patients with T/Myeloid MPAL (7/11, 64%) than B/Myeloid (13/13, 100%), Ph+ MPAL (5/5, 100% and MLL MPAL (5/5, 100%) (p=0.022) (Supplementary table). CR rate after first induction was similar after ALL-type (10/11, 91%) and AML-type regimens (21/25, 84%) (p=0.999) and among adults (20/23, 87%) and children (11/13, 85%) (p=0.999). CR rates were not different following induction with regimens containing a steroid (18/20), L-asparaginase (9/9), anthracycline (30/35), anti-metabolite (20/25), vinca-alkaloid (11/11), alkylating agent (2/2) or topoisomerase inhibitor (7/10).
The majority of adults (n=14/23) and children (n=9/13) received consolidation therapy. Adults were typically treated according to the administered induction regimen, while children were switched from AML-type induction to ALL-type consolidation. Eight children were consolidated with an ALL-type regimen (NY-2)24 despite 6/8 achieving remission with AML-type induction (DCTER). Seven of 8 adults who achieved remission with an AML-type induction were consolidated with an AML-type consolidation (high dose cytarabine) and 5/7 were consolidated using an ALL-type regimen after initially achieving remission following ALL-type induction. In adults, only 1/14 who received consolidation did not proceed to HCT due to death from relapsed disease. Twelve patients required salvage therapy for relapse. AML-type was used in 9: HIDAC (n=2), ALL-2 (n=5), 7+3 idarubicin (n=1) and 5+2 idarubicin (n=1). ALL-type reinduction was used once (COG AALL-1131) and two children received TVTC (n=2) 25. CR2 was achieved in 7/9 following AML-type salvage, 1/2 following TVTC and in 1/1 after COG AALL-1131.
Transplantation
Twenty-five patients underwent HCT of which 21 (84%) were adults (Table 3). The median follow-up of transplant survivors (n=20) was 39.6 months (95%CI: 14.7–81.1) after transplant and median OS was not reached (Figure 1C). Twenty-one of 23 (91%) adults underwent HCT of which 19 proceeded directly to transplant in first remission. Two adults (both with T/Myeloid MPAL) did not undergo transplant, the first due to comorbidity and the second due to death from refractory disease. The median time to transplant among adult MPAL was 2 months (2–34 months). Only 4/13 (31%) pediatric patients underwent transplant and only 2 proceeded to HCT directly following remission induction (MPAL with MLL and B/Myeloid MPAL), and the other 2 (MPAL with MLL and B/Myeloid MPAL) underwent HCT in second remission. Two of three pediatric patients with MLL rearrangement underwent HCT and the third died due to disease progression before transplant. Six (5 with B/Myeloid and 1 with T/Myeloid) of 9 pediatric patients who did not undergo transplant were alive and in remission at a median of 16 months (7–118) follow-up, while 3 (MLL MPAL, MPAL NOS and T/myeloid) died due to refractory disease.
Table 3.
Variables associated with transplant outcome
| N | Death N |
2-year OS (95% CI) | Relapse N |
2-year CIR (95% CI) | |
|---|---|---|---|---|---|
| Remission status at BMT1 | |||||
| Non-CR1 | 4 | 3 | 25% (1–67%) | 2 | 50% (0–100%) |
| CR1 | 21 | 2 | 88% (60–97%) | 5 | 34% (8–60%) |
| Conditioning intensity1,2 | |||||
| Ablative | 22 | 3 | 83% (56–94%) | 4 | 26 (3–49%) |
| Reduced | 3 | 2 | NA (NA) | 3 | NA (NA) |
| TBI containing conditioning1 | |||||
| No | 10 | 4 | 53% (17–79%) | 5 | 56% (19–93%) |
| Yes | 15 | 1 | 92% (54–99%) | 2 | 19% (0–45%) |
| Induction type2 | |||||
| ALL-type | 8 | 3 | 43% (6–78%) | 5 | NA (NA) |
| AML-type | 17 | 2 | 87% (58–97%) | 2 | 15% (0–36%) |
| MPAL Subtype2 * | |||||
| B/Myeloid | 8 | 1 | 83% (27–98%) | 0 | 0% (0–0%) |
| T/Myeloid | 7 | 0 | 100% (100–100%) | 3 | 49% (3–94%) |
| Ph+ MPAL | 5 | 2 | 60% (13–88%) | 1 | 20% (0–60%) |
| MLL MPAL | 4 | 2 | 50% (6–85%) | 3 | NA (NA) |
| Age (years) | |||||
| ≥18 | 21 | 3 | 83% (55–94%) | 5 | 32% (7–58%) |
| <18 | 4 | 2 | 50% (6–85%) | 2 | 50% (0–100%) |
| Diagnostic WBC2 | |||||
| ≤13.5x10^9/L | 10 | 0 | 100% (100–100%) | 0 | 0% (0–0%) |
| >13.5x10^9/L | 11 | 5 | 80% (39–95%) | 4 | 49% (10–89%) |
| Cytogenetics | |||||
| No complex karyotype | 16 | 3 | 77% (44–92%) | 4 | 29% (3–55%) |
| Complex karyotype | 9 | 2 | 76% (33–94%) | 3 | 51% (3–99%) |
| No monosomy | 20 | 4 | 74% (44–90%) | 4 | 26% (2–49%) |
| Monosomy | 5 | 1 | 80% (20–97%) | 3 | 60% (6–100%) |
| GVHD prophylaxis | |||||
| Non-TCD | 13 | 2 | 82% (44–95%) | 5 | 48% (13–83%) |
| TCD | 12 | 3 | 69% (30–89%) | 2 | 22% (0–51%) |
| Donor | |||||
| HLA Mismatched | 10 | 2 | 73% (28–93%) | 1 | 11% (0–33%) |
| HLA Matched | 15 | 3 | 77% (44–92%) | 6 | 50% (19–80%) |
| Cell source | |||||
| Peripheral blood or bone marrow | 19 | 5 | 69% (40–86%) | 7 | 44% (18–69%) |
| Cord blood | 6 | 0 | 100% (100–100%) | 0 | 0% (0–0%) |
| MRD at time of transplant | |||||
| No | 13 | 4 | 65% (31–86%) | 3 | 26% (0–54%) |
| Yes | 9 | 0 | 100% (100–100%) | 1 | 25% (0–74%) |
A significant variable in OS;
A significant variable in relapse.
Ablative conditioning was busulfan based (n=4); clofarabine, melphalan and thiotepa (n=2); TBI based (n=9); or other.
One patient with NOS MPAL was not included in the survival analysis.
CIR: Cumulative incidence rate; NA: not available; MRD: minimal residual disease, TBI: total body irradiation.
Overall 7 patients relapsed after transplant, 5 died including two from treatment-related mortality and 3 from disease relapse. Variables associated with transplant outcome are described in Table 3. Factors associated with shorter OS included reduced intensity conditioning (p=0.010), non TBI containing conditioning (p=0.048), HCT not in CR1 (p=0.006) while relapse was associated with MLL rearrangement (p=0.022) (Figure 1D), reduced intensity conditioning (p<0.001), receiving ALL type induction (p=0.001) and higher diagnostic WBC (above the median 13.5x10^9/L) (p=0.034). Expression of MPO, CD19 and CD3 did not affect transplant outcomes, nor did pre transplant MRD status.
DISCUSSION
AML and ALL have distinct and non-overlapping profiles of molecular and cytogenetic alterations. Our data indicated a strong bias in favor of ALL-associated abnormalities in MPAL. Yan et al 19 reported on somatic mutations in 31 MPAL patients identifying ALL-type mutations including: IKZF1 (4/31), NOTCH (1/31), CDKN2A (4/12), EZH2 (3/31), ASLX1 (3/31) while no patient had mutations in common AML associated genes: NPM1, FLT3, DNMT3A, IDH1 or IDH2. Eckstein et al 26 identified mutations in 21/23 MPAL cases using whole exome sequencing and found that DNMT3A was the most frequently mutated gene (6/23). Investigators grouped MPAL alleles into three functional groups including cell signaling pathways (RAS, NF1, JAK); tumor suppressors (TP53, WT1) and transcription factor (NOTCH1, RUNX1 and GATA2). Notably, mutations in NPM1 and PAX5, which are the most common mutations in AML 27 and ALL 28, respectively, were not seen in any patient in either of the described cohorts or in the cases presented in this report suggesting that these mutations may confer lineage specificity, an observation made by other recent investigators 29. MPAL with TP53 mutations may represent inappropriate classification of therapy-related myeloid neoplasms as MPAL given that mutations in this gene are strongly associated with prior cytotoxic exposure and rarely seen in de novo leukemia 5, 30. Mutations in genes coding for epigenetic regulators (IDH1 and 2, DNMT3A, TET2, MLL and ASXL1) that are common in clonal hematopoiesis states, and do not appear to be associated with lineage specificity given they are described in AML, ALL and MPAL29. Here, mutation in DNMT3A and TET2 were only identified in adult patients. In our cohort we noted a strong predominance of ALL-associated alterations characteristic of genomic ALL subtypes: ‘Philadelphia-like ALL’ (IKZF1, RAS and components of the JAK-STAT pathway 31), hyperdiploid ALL (n=2); ALL with ETV6-RUNX1 fusion (n=2), MLL rearranged ALL, of which t(4;11) translocations are most frequent (n=5), ALL with t(v;14q) rearrangement (n=4), PHF-6 (n=3) and NOTCH (n=3) mutations which are typical of T-ALL32. Other ALL associated mutations identified in this report included: IL7R, MLL2, CDKN2A, NF1 and PTPN11. Gene expression profiling of MPAL was able to categorize most MPAL into either AML or ALL based on gene expression patterns seen in lineage committed leukemia 4. As we move to a genomic characterization of AML 9 and ALL 33, it is likely that many MPAL cases may be better classified by identification of AML, ALL and therapy-related neoplasm defining mutations and cytogenetic changes rather than by immunophenotype alone.
We identified a high CR rate following ALL (91%) and AML-type (84%) induction regimens with no benefit for regimens containing a particular chemotherapeutic agent. This CR rate was higher than previously published estimates that vary between 22–70% 34, 35. The difference may be due to strict exclusion of therapy-related neoplasms and secondary AML which often have aberrant lymphoid antigen expression, poor prognosis and can be misclassified as MPAL 36. Patients who underwent transplantation after receiving ALL-type induction had a higher incidence of post-transplant relapse (5/8 versus 2/17 with AML-type); however, the number of patients in this analysis is small. The difference may reflect a selection bias for higher risk patients undergoing treatment with ALL-type regimens. The optimal induction therapy for MPAL is unknown with both ALL 37, 38 and AML-type regimens reported 4. It is unclear from retrospective studies on what basis investigators selected between AML and ALL-type induction and this was also the case in this report. We noted high CR rate with cytarabine and high dose mitoxantrone (n=10/10) administered according to the induction phase of the ALL-2 protocol 39, which is the preferred induction approach for adult MPAL at our center. Ph+/MPAL are historically associated with poor outcomes likely due to omission of tyrosine kinase inhibitors (TKI) in published cohorts 38. Here all Ph+/MPAL achieved CR and proceeded to transplant. None received post-transplant TKI maintenance with 1 relapse and 1 treatment-related mortality noted. Patients with MLL rearrangement had equally high CR rates with relapse being the major barrier to long-term survival (Figure 1). Investigation into inhibiting the aberrantly recruited DOT1L methyltransferase in MLL leukemia is being pursued as a maintenance therapy and results are awaited 40. MPAL who underwent HCT had favorable OS (Figure 1), supporting recent publications 1, 3, 37. Adults were referred for allograft in CR1 while pediatric patients were referred in the event of MLL rearrangement or relapse. A recent CIBMTR analysis 3 of 95 carefully defined MPAL showed no difference in survival after transplant with B/Myeloid or T/Myeloid MPAL and similar OS to matched ALL and AML controls, suggesting that MPAL itself may not confer a high transplant specific disease risk as previously thought 41. The CIBMTR investigators did not find a worse survival for MPAL with MLL or Philadelphia chromosome rearrangement. In the present cohort, MLL rearrangement was associated with a poor prognosis, with only 1/5 alive and disease free at last follow-up (Figure 1). Variables associated with favorable survival after HCT included ablative and TBI-containing conditioning and transplant in CR1. Conditioning intensity for MPAL was previously associated with favorable transplant outcome 41. MRD status was not associated with transplant outcome. Although MRD was assessed using different methods, with variable sensitivities, we found no relapses among patients who were transplanted with detected MRD, contrary to recent findings in MPAL 42. Six patients received cord blood allografts with no mortality or relapse identified highlighting the potent anti-leukemia affect of cord blood in acute leukemia 43.
It is important to note that these results are derived from a univariate analysis of a small sample size derived from a single center. The number of patients in this analysis precluded a multivariate model to account for potential confounding patient or treatment characteristics. For these reasons, the results presented here should be interpreted with caution and require validation in larger series of patients that would allow for multivariate modeling.
As we move to a genomic classification of acute leukemia where treatment can be personalized based on the spectrum of somatic alterations rather than immunophenotype we are likely to find that some mixed-phenotype leukemias may be more strictly classified into ALL or AML categories by identification of lineage specific somatic mutations and gene expression. Survival analysis after careful classification of adult MPAL and exclusion of high-risk AML subtypes that may have aberrant lymphoid antigen expression suggests that MPAL cases have favorable outcome with either ALL or AML-type induction followed by ablative HCT. HCT remains the standard of care for Ph+ MPAL in the absence of data with a non-transplant approach. It is unclear that HCT benefits MLL rearranged leukemia typically seen in younger MPAL patients. Children with MPAL can be managed expectantly with chemotherapy unless high-risk cytogenetics such as MLL rearrangement or Ph-like genomic alterations are identified 44.
Supplementary Material
HIGHLIGHTS.
MPAL had a predominance of ALL-type mutations and cytogenetic abnormalities.
Remission induction rates were high with both ALL and AML-type induction regimens
Survival was high after allogeneic transplant with ablative conditioning
MPAL with MLL rearrangement has an unfavorable prognosis
Acknowledgments
This research was supported in part by National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: Authors have no conflict of interest to declare.
FINANCIAL DISCLOSURE STATEMENT
The authors declare no financial interests relevant to the work presented in this manuscript.
BMG, MR, BS collected and analyzed data and wrote manuscript. JZ analyzed data and wrote manuscript. MT, JHP, EMS, RL, EBP, NK, PS, RJO, MAP, SG wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yan L, Ping N, Zhu M, et al. Clinical, immunophenotypic, cytogenetic, and molecular genetic features in 117 adult patients with mixed-phenotype acute leukemia defined by WHO-2008 classification. Haematologica. 2012;97:1708–1712. doi: 10.3324/haematol.2012.064485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porwit A, Bene MC. Acute leukemias of ambiguous origin. American journal of clinical pathology. 2015;144:361–376. doi: 10.1309/AJCPSTU55DRQEGTE. [DOI] [PubMed] [Google Scholar]
- 3.Munker R, Brazauskas R, Wang HL, et al. Allogeneic Hematopoietic Cell Transplantation for Patients with Mixed Phenotype Acute Leukemia. Biol Blood Marrow Transplant. 2016;22:1024–1029. doi: 10.1016/j.bbmt.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113:5083–5089. doi: 10.1182/blood-2008-10-187351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen-Bjergaard J, Christiansen DH, Desta F, Andersen MK. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 6.Ossenkoppele GJ, van de Loosdrecht AA, Schuurhuis GJ. Review of the relevance of aberrant antigen expression by flow cytometry in myeloid neoplasms. Br J Haematol. 2011;153:421–436. doi: 10.1111/j.1365-2141.2011.08595.x. [DOI] [PubMed] [Google Scholar]
- 7.Tian H, Xu Y, Liu L, et al. Comparison of outcomes in mixed phenotype acute leukemia patients treated with chemotherapy and stem cell transplantation versus chemotherapy alone. Leuk Res. 2016;45:40–46. doi: 10.1016/j.leukres.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu H, Saitoh T, Machida S, et al. Allogeneic hematopoietic stem cell transplantation for adult patients with mixed phenotype acute leukemia: results of a matched-pair analysis. European journal of haematology. 2015;95:455–460. doi: 10.1111/ejh.12516. [DOI] [PubMed] [Google Scholar]
- 9.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. New England Journal of Medicine. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts KG, Mullighan CG. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nat Rev Clin Oncol. 2015;12:344–357. doi: 10.1038/nrclinonc.2015.38. [DOI] [PubMed] [Google Scholar]
- 11.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia. Blood. 2016 [Google Scholar]
- 12.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature biotechnology. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng DT, Cheng J, Mitchell TN, et al. Detection of mutations in myeloid malignancies through paired-sample analysis of microdroplet-PCR deep sequencing data. The Journal of molecular diagnostics : JMD. 2014;16:504–518. doi: 10.1016/j.jmoldx.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giralt S, Ballen K, Rizzo D, et al. Reduced-Intensity Conditioning Regimen Workshop: Defining the Dose Spectrum. Report of a Workshop Convened by the Center for International Blood and Marrow Transplant Research. Biology of Blood and Marrow Transplantation. 15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Chapiro E, Russell L, Radford-Weiss I, et al. Overexpression of CEBPA resulting from the translocation t(14;19)(q32;q13) of human precursor B acute lymphoblastic leukemia. Blood. 2006;108:3560–3563. doi: 10.1182/blood-2006-03-010835. [DOI] [PubMed] [Google Scholar]
- 17.Dyer MJS, Akasaka T, Capasso M, et al. Immunoglobulin heavy chain locus chromosomal translocations in B-cell precursor acute lymphoblastic leukemia: rare clinical curios or potent genetic drivers? Blood. 2010;115:1490–1499. doi: 10.1182/blood-2009-09-235986. [DOI] [PubMed] [Google Scholar]
- 18.Manola KN. Cytogenetic abnormalities in acute leukaemia of ambiguous lineage: an overview. British journal of haematology. 2013;163:24–39. doi: 10.1111/bjh.12484. [DOI] [PubMed] [Google Scholar]
- 19.Yan L, Ping N, Zhu M, et al. Clinical, immunophenotypic, cytogenetic, and molecular genetic features in 117 adult patients with mixed-phenotype acute leukemia defined by WHO-2008 classification. Haematologica. 2012;97:1708–1712. doi: 10.3324/haematol.2012.064485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mrozek K, Heinonen K, Lawrence D, et al. Adult patients with de novo acute myeloid leukemia and t(9; 11)(p22; q23) have a superior outcome to patients with other translocations involving band 11q23: a cancer and leukemia group B study. Blood. 1997;90:4532–4538. [PubMed] [Google Scholar]
- 21.Marks DI, Moorman AV, Chilton L, et al. The clinical characteristics, therapy and outcome of 85 adults with acute lymphoblastic leukemia and t(4;11)(q21;q23)/MLL-AFF1 prospectively treated in the UKALLXII/ECOG2993 trial. Haematologica. 2013;98:945–952. doi: 10.3324/haematol.2012.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberg OK, Seetharam M, Ren L, Alizadeh A, Arber DA. Mixed phenotype acute leukemia: A study of 61 cases using World Health Organization and European Group for the Immunological Classification of Leukaemias criteria. Am J Clin Pathol. 2014;142:803–808. doi: 10.1309/AJCPPVUPOTUVOIB5. [DOI] [PubMed] [Google Scholar]
- 23.Woods WG, Kobrinsky N, Buckley J, et al. Intensively timed induction therapy followed by autologous or allogeneic bone marrow transplantation for children with acute myeloid leukemia or myelodysplastic syndrome: a Childrens Cancer Group pilot study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11:1448–1457. doi: 10.1200/JCO.1993.11.8.1448. [DOI] [PubMed] [Google Scholar]
- 24.Steinherz PG, Redner A, Steinherz L, Meyers P, Tan C, Heller G. Development of a new intensive therapy for acute lymphoblastic leukemia in children at increased risk of early relapse. The Memorial Sloan-Kettering-New York-II protocol. Cancer. 1993;72:3120–3130. doi: 10.1002/1097-0142(19931115)72:10<3120::aid-cncr2820721038>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Steinherz PG, Shukla N, Kobos R, Steinherz L. Remission re-induction chemotherapy with clofarabine, topotecan, thiotepa, and vinorelbine for patients with relapsed or refractory leukemia. Pediatric blood & cancer. 2010;54:687–693. doi: 10.1002/pbc.22321. [DOI] [PubMed] [Google Scholar]
- 26.Eckstein OS, Wang L, Punia JN, et al. Mixed-phenotype acute leukemia (MPAL) exhibits frequent mutations in DNMT3A and activated signaling genes. Experimental hematology. 2016;44:740–744. doi: 10.1016/j.exphem.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. The New England journal of medicine. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 29.Eckstein OS, Wang L, Punia JN, et al. Mixed-phenotype acute leukemia (MPAL) exhibits frequent mutations in DNMT3A and activated signaling genes. Experimental hematology. 2016;44:740–744. doi: 10.1016/j.exphem.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ok CY, Patel KP, Garcia-Manero G, et al. TP53 mutation characteristics in therapy-related myelodysplastic syndromes and acute myeloid leukemia is similar to de novo diseases. Journal of hematology & oncology. 2015;8:45. doi: 10.1186/s13045-015-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. The New England journal of medicine. 2014;371:1005–1015. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Qiu H, Jiang H, et al. Mutations of PHF6 are associated with mutations of NOTCH1, JAK1 and rearrangement of SET-NUP214 in T-cell acute lymphoblastic leukemia. Haematologica. 2011;96:1808–1814. doi: 10.3324/haematol.2011.043083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts KG, Mullighan CG. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nat Rev Clin Oncol. 2015;12:344–357. doi: 10.1038/nrclinonc.2015.38. [DOI] [PubMed] [Google Scholar]
- 34.Killick S, Matutes E, Powles RL, et al. Outcome of biphenotypic acute leukemia. Haematologica. 1999;84:699–706. [PubMed] [Google Scholar]
- 35.Matutes E, Pickl WF, Van’t Veer M, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood. 2011;117:3163–3171. doi: 10.1182/blood-2010-10-314682. [DOI] [PubMed] [Google Scholar]
- 36.Venditti A, Del Poeta G, Buccisano F, et al. Prognostic relevance of the expression of Tdt and CD7 in 335 cases of acute myeloid leukemia. Leukemia. 1998;12:1056–1063. doi: 10.1038/sj.leu.2401067. [DOI] [PubMed] [Google Scholar]
- 37.Heesch S, Neumann M, Schwartz S, et al. Acute leukemias of ambiguous lineage in adults: molecular and clinical characterization. Annals of Hematology. 2013;92:747–758. doi: 10.1007/s00277-013-1694-4. [DOI] [PubMed] [Google Scholar]
- 38.Matutes E, Pickl WF, van’t Veer M, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood. 2011;117:3163–3171. doi: 10.1182/blood-2010-10-314682. [DOI] [PubMed] [Google Scholar]
- 39.Weiss M, Maslak P, Feldman E, et al. Cytarabine with high-dose mitoxantrone induces rapid complete remissions in adult acute lymphoblastic leukemia without the use of vincristine or prednisone. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14:2480–2485. doi: 10.1200/JCO.1996.14.9.2480. [DOI] [PubMed] [Google Scholar]
- 40.Stein EM, Tallman MS. Mixed lineage rearranged leukaemia: pathogenesis and targeting DOT1L. Current opinion in hematology. 2015;22:92–96. doi: 10.1097/MOH.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 41.Liu QF, Fan ZP, Wu MQ, et al. Allo-HSCT for acute leukemia of ambiguous lineage in adults: the comparison between standard conditioning and intensified conditioning regimens. Ann Hematol. 2013;92:679–687. doi: 10.1007/s00277-012-1662-4. [DOI] [PubMed] [Google Scholar]
- 42.Etan Orgel MO, Malvar Jemily, O’Gorman Maurice. ASH. San Diego, CA: 2016. Predictive Value of Minimal Residual Disease in WHO2016-Defined Mixed Phenotype Acute Leukemia (MPAL) [Google Scholar]
- 43.Milano F, Gooley T, Wood B, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. New England Journal of Medicine. 2016;375:944–953. doi: 10.1056/NEJMoa1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JA, Ghim TT, Bae K, et al. Stem cell transplant in the treatment of childhood biphenotypic acute leukemia. Pediatric blood & cancer. 2009;53:444–452. doi: 10.1002/pbc.22105. [DOI] [PubMed] [Google Scholar]
- 45.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stock W, Yu D, Sanford B, et al. Incorporation of Alemtuzumab into Front-Line Therapy of Adult Acute Lymphoblastic Leukemia (ALL) Is Feasible: A Phase I/II Study from the Cancer and Leukemia Group B (CALGB 10102) Blood. 2005;106:145–145. [Google Scholar]
- 47.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating Dose Intravenous Methotrexate without Leucovorin Rescue during Interim Maintenance Is Superior to Oral Methotrexate for Children with Standard Risk Acute Lymphoblastic Leukemia (SR-ALL): Children’s Oncology Group Study 1991. Blood. 2008;112:9–9. [Google Scholar]
- 48.Winter SS, Dunsmore KP, Devidas M, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0434. Pediatric blood & cancer. 2015;62:1176–1183. doi: 10.1002/pbc.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 50.Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children’s Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:2222–2230. doi: 10.1200/JCO.1997.15.6.2222. [DOI] [PubMed] [Google Scholar]
- 51.Savage NM, Kota V, Manaloor EJ, et al. Acute leukemia with PICALM-MLLT10 fusion gene: diagnostic and treatment struggle. Cancer genetics and cytogenetics. 2010;202:129–132. doi: 10.1016/j.cancergencyto.2010.07.126. [DOI] [PubMed] [Google Scholar]
- 52.Taniguchi T, D’Andrea AD. The Fanconi anemia protein, FANCE, promotes the nuclear accumulation of FANCC. Blood. 2002;100:2457–2462. doi: 10.1182/blood-2002-03-0860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.