Abstract
Environmental temperatures influence cardiovascular physiology. However, the majority of time is spent indoors, making outdoor-ambient temperatures inaccurate estimates of true exposures encountered by most individuals. We evaluated in 50 healthy adults the associations between previous 7-day outdoor-ambient (4 occasions) and prior 24-hour personal-level (2 occasions) environmental temperature exposures with blood pressure, heart rate variability, sleep parameters, and endothelial-dependent vasodilatation (brachial flow-mediated dilatation [FMD]) using generalized estimating equations. Participants (34 females; age, 32.1±9.6 years) had normal blood pressures (107.8±13.3/70.2 ± 9.4 mm Hg), FMD (7.4±2.8%), as well as sleep and HRV parameters. Mean 7-day outdoor-ambient (4.6±9.7 °C) differed from personal-level temperature exposures (22.0±3.0 °C). Colder outdoo r-ambient temperatures (per -10°C) over the previous 1-6 days (rolling averages) were associated with decreases in FMD: -0.57% (95% confidence interval [CI] -1.14% to 0.01%, p=0.055) to -0.62% (95%CI -1.07% to -0.18%, p=0.006). However, a 10°C decrease in personal-leve l temperature during the prior 24-hours was associated with a greater decrement in FMD: -2.44% (95%CI -4.74% to -0.13%, p=0.038). Both were also linearly related to FMD during all seasons and without a threshold temperature. Other endpoints were not significantly related to either temperature level in this study. Short-term exposures to colder environmental temperatures reduced endothelial-dependent vasodilatation, supporting the epidemiological associations with heightened cardiovascular risk. We show here for the first time that temperature exposures characterized at the personal-level may be more robust predictors of endothelial function than outdoor-ambient levels.
Keywords: Environment, Hypertension, Endothelial Function
Numerous studies conducted worldwide have demonstrated that environmental temperatures during the prior few days are associated with all-cause as well as cardiovascular morbidity and mortality1-4. While extreme heat is linked to adverse health effects, a large body of evidence demonstrates that colder temperatures are strongly predictive of increased cardiovascular events2,4. This issue is likely to become an even greater public health problem due to global climate change which not only heightens summer heat waves but can promote larger overall variations in temperature and more pronounced winter cold spells across temperate zones5.6.
Several biological mechanisms may underlie the linkage between temperature and cardiovascular risk1. Cold exposure causes adrenal and sympathetic nervous system (SNS) activation as well as thermoregulatory vasoconstriction which may explain the well-established association with elevations in arterial blood pressure (BP)1,7,8. In addition, a few studies have shown that temperature and season are related to changes in brachial flow-mediated dilation (FMD), an established measure of endothelial-dependent vasodilatation9-11. FMD is an independent predictor of cardiovascular events and as such temperature-induced endothelial dysfunction could be a key pathway explaining the epidemiological associations12.
It is important to note that the relationship with FMD in the published literature has thus far relied on ambient (i.e., prevailing regional outdoor) temperature values. However, ambient levels are known to be inaccurate metrics of “true” temperature exposures given that most individuals spend the majority (∼90%) of their time indoors in regulated environments where the temperature is held relatively constant, particularly in colder regions/seasons and urban locations13. We and others have reported greater predictive value of arterial BP by using personal-level environmental temperature (PET) exposures compared to ambient-outdoor levels8,14-16. On the other hand, only one study has yet evaluated the differing associations with FMD14. Give that endothelial dysfunction plays a fundamental role in cardiovascular diseases12, we aimed to explore the impact of recent temperature exposures measured by PET versus outdoor-ambient levels on several physiological parameters with a specific focus on FMD.
Methods
This study is a post hoc exploratory analysis of results obtained from the human project component of the Great Lakes Air Center for Integrated Environmental Research (GLACIER) at the University of Michigan. The protocol was designed and powered to evaluate the effect of personal-level PM2.5 exposures on primary cardio-metabolic health endpoints. The study was approved by the Institutional Review Board of the University of Michigan and all participants signed a written informed consent document during a screening visit. Participant inclusion criteria were healthy nonsmoking adults living in nonsmoking households aged 18-50 years without a history of cardiovascular disease or risk factors (screening visit BP < 140/90 mm Hg and fasting glucose < 126 mg/dL). Body mass index (BMI) was calculated from height and weight measured at a screening visit. Subjects were also excluded if they were taking any medication or over-the-counter pill (e.g., cholesterol or BP-lowering medication, fish oil, anti-oxidant) on a routine basis that might alter study outcomes.
Qualifying participants (n=50) were enrolled into a repeated measures panel study, each fully-completing the protocol within a 2 to 3 week-long period (Online supplement, Figure S1). The study was conducted among individuals living in southeast Michigan within a 2-year period during the months of August through October 2014; January through May 5th 2015; November through December 2015; and January through February 2016. There were 2 study blocks, each consisting of 2 visit days in a row when participants came fasting ≥8 hours at 8 am to a temperature-controlled (21-22 °C) outpatient Clinic al Translational Science Award (CTSA) research facility of the University of Michigan (Domino's Farms, Ann Arbor, MI). On visit day 1, cardiovascular outcomes were measured in the order provided in Figure S1 and afterwards participants were discharged with instructions to proceed with usual daily activities (excluding exercise and travel) while wearing a personal environmental monitor. They were also provided with a portable home sleep monitor and instructions on its usage during the upcoming night. The following morning (visit day 2), participants returned to the CTSA. Cardiovascular outcomes were repeated in the same sequence as day 1. Participants were then discharged home and underwent a 6-day (minimum) to 3-week (maximum) washout period. Thereafter, each participant repeated the same process during study block 2.
Cardiovascular Outcomes
Study outcome methods were performed as detailed in our prior experiments and are only be briefly outlined here14,17. Seated right upper arm blood pressure (BP) using an appropriate sized cuff was measured by an automated device (BPM-100; http://www.bptru.com/) after participants rested unattended in the exam room for 5 minutes with their arm supported at mid-sternal level. The average of 5 BP readings (2nd to 6th levels using 1 minute intervals) was defined as the BP outcome. Participants next rested supine on a patient exam bed and continuous electrocardiogram monitoring was performed for 6 minutes using a Spacelabs evo Holter system. Time domain (standard deviation of normal-to-normal intervals; SDNN) and frequency domain (high (HF); low (LF) frequency) heart rate variability (HRV) metrics were analyzed using the Spacelabs Pathfinder system (http://www.spacelabshealthcare.com/). Thereafter, resting basal longitudinal brachial artery diameter (BAD) images were measured at a standardized site on the right upper arm using a portable Terason ultrasound system and a 10 mHz linear array transducer (http://www.terason.com/). All images were captured by an electrocardiogram triggered on the R-wave. Digital images were analyzed using a software package employing an edge-detection system (Brachial Analyzer, Medical Imaging Applications; http://www.mia-llc.com/). Conduit artery endothelial-dependent vasodilation was then measured using the standardized flow-mediated dilatation (FMD) method employing a 5-minute long upper arm cuff occlusion technique. Brachial artery images were captured continuously for 2 minutes during reactive hyperemia. FMD was calculated as the percent increase in BAD from baseline at the largest percent change (FMD-peak). Finally, sleep quality parameters were measured by the portable finger arterial tonometry technique following the manufacturer's directions (http://www.itamarmedical.com/watchpat-main/) starting at bedtime during the night between visit day 1 and 2 on both study blocks. The outcomes analyzed included the apnea-hypopnea index (AHI) and respiratory disturbance index (RDI).
Environmental Exposure Outcomes
The Michigan Department of Environmental Quality operates tapered element oscillating microbalance samplers (Rupprecht & Patashnick TEOM 1400a) to monitor continuous PM2.5 levels across Michigan. Outdoor ambient temperature and PM2.5 data for this study were averaged over the proceeding 24-hour period from the monitoring site in Ypsilanti, Michigan (ID: 261610008; 42.2406 -83.59972 http://www.michigan.gov/deq/). For assessing outdoor-ambient exposure, each patient had their own unique multiple 24-hour long ambient exposure epochs calculated starting retrospectively from the time in the morning at the start of each of the 4 study visit days. Seven individual 24-hour lag periods were calculated for each patient from each study visit day. In addition, the rolling average of exposures from 1 to 7 days in duration was calculated.
Personal-level particulate exposures and PET were recorded using a battery-powered active personal monitor (Thermo Scientific pDR-1500). Averages were recorded for the 24-hour period prior to the start of visit day 2 in each study block. Average personal exposure to PM2.5 mass was calculated from gravimetric determinations using a microbalance (MT-5, Mettler Toledo, Columbus OH) in a temperature/humidity-controlled environment. Sample handling, processing, and analysis took place in a Class 100 ultraclean room at the University of Michigan Air Quality Laboratory.
Statistical Methods
Summary statistics were computed for continuous measures as mean ± standard deviation (SD), as well as median (interquartile range, IQR), and for categorical variables as frequency and proportion (%). All outcomes were evaluated for normality of distribution using the Shapiro-Wilk Normality Test. Correlations between environmental exposures were calculated by Pearson's correlation analysis. Tests for differences between mean values were performed by independent samples t-tests. We evaluated the associations of longitudinal health measurements repetitively obtained with 24-hour average outdoor ambient (4 associations per patient for each lag day and rolling average lag periods) and personal-level temperature exposures (2 associations per patient) using generalized estimating equations (GEEs), where we assumed a very general unstructured correlation structure to account for within subject correlations, and GEEs use data to estimate the within subject correlation matrix. The outcomes of this model are the longitudinal health parameters (e.g., FMD, or BAD). Models were fit with a pool of potential predictors and confounders available in our data collection (such as patient age, sex, BMI, study block, PM2.5) and then we perform a backward model selection procedure with a cut-off p-value as 0.1 to select the final model, which is relatively parsimonious and more stable given the sample size of this study. Our final models, as selected through the backward selection procedure, were adjusted for patient age and prior 24-hour ambient PM2.5. Potential non-linear relationships between temperature exposures and FMD-peak were also explored using local polynomial regression (LOESS) analysis, and our results show that assuming this relationship as linear is reasonable in our study. All analyses were performed using the statistical software package R (version 3.3.3).
Results
Study participants were healthy without cardiovascular disease or risk factors. All physiological endpoints including BP, basal FMD, HRV, and sleep parameters were within normal ranges (Table 1). Personal-level versus outdoor-ambient exposures differed for both temperatures and PM2.5 concentrations (Table 2). PET was on-average warmer and less variable. The correlations between exposure variables on lag day 1 are provided in Table 3.
Table 1. Descriptive Statistics on Study Participant Parameters.
| Variable | Obs | Mean ± SD | Min | 25th percentile | Median | 75th percentile | Max |
|---|---|---|---|---|---|---|---|
| Age (years) | 50 | 32.1 ± 9.6 | 19.0 | 24.0 | 28.5 | 41.0 | 50.0 |
| BMI (kg/m2) | 50 | 26.1 ± 5.7 | 19.0 | 21.7 | 24.9 | 29.1 | 43.5 |
| SBP (mm Hg) | 200 | 107.8 ± 13.3 | 86.0 | 99.0 | 105.0 | 112.0 | 161.0 |
| DBP (mm Hg) | 200 | 70.2 ± 9.4 | 50.0 | 65.0 | 69.0 | 75.0 | 107.0 |
| HR (beats/min) | 200 | 70.6 ± 11.3 | 44.0 | 63.0 | 71.0 | 78.0 | 111.0 |
| SDNN | 194 | 79.0 ± 37.1 | 19.1 | 53.9 | 70.1 | 92.0 | 225.8 |
| LF | 189 | 1829 ± 1900 | 66 | 597 | 1174 | 2325 | 14329 |
| HF | 189 | 2224 ± 4364 | 23 | 403 | 949 | 1758 | 38256 |
| LF/HF | 189 | 2.1 ± 2.7 | 0.1 | 0.7 | 1.3 | 2.6 | 28.2 |
| FMD 50-90 (%) | 185 | 4.1 ± 2.4 | 0.0 | 2.3 | 3.9 | 5.8 | 10.7 |
| FMD peak (%) | 185 | 7.3 ± 2.8 | 1.5 | 4.9 | 7.2 | 9.6 | 14.8 |
| BAD (cm) | 185 | 3.7 ± 0.8 | 2.3 | 3.1 | 3.6 | 4.4 | 5.2 |
| AHI | 93 | 3.4 ± 4.5 | 0.0 | 0.6 | 1.6 | 5.0 | 23.1 |
| RDI | 93 | 11.6 ± 5.6 | 2.7 | 7.8 | 10.5 | 14.4 | 32.8 |
Obs, number of observations; min, minimal value; max, maximal value; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SDNN, standard deviation of normal-to-normal intervals; LF, low-frequency power variability; HF, high frequency power; FMD, flow-mediated dilatation; BAD, brachial artery diameter; AHI, apnea-hypopnea index; RDI, respiratory disturbance index
Table 2. Descriptive Statistics on Environmental Exposures.
| Variable | Obs | Mean ± SD | Min | 25th percentile | Median | 75th percentile | Max | |
|---|---|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | Personal | 95 | 12.2 ± 17.0 | 0.2 | 4.0 | 6.9 | 13.0 | 94.0 |
| Outdoor 7-day average | 100 | 9.2 ± 1.9 | 5.5 | 7.7 | 9.1 | 10.7 | 14.2 | |
| Temp (°C) | Personal | 98 | 22.0 ± 3.0 | 13.8 | 20.4 | 22.2 | 24.2 | 28.7 |
| Outdoor 7-day average | 100 | 4.6 ± 9.7 | -13.4 | -3.8 | 3.1 | 12.8 | 23.2 |
Personal PM2.5 and temperature levels were averaged from all available 24-hour long period measurements performed on lag day 1 prior to visit 2 during both study blocks. Outdoor PM2.5 and temperature levels were averaged over 7-day long lag periods prior to all study visits.
Table 3. Correlations among Environmental Exposures on Lag Day 1.
| Outdoor ambient PM2.5 | Personal-level PM2.5 | Outdoor ambient temperature | Personal-level temperature | |
|---|---|---|---|---|
| Outdoor ambient PM2.5 | 0.23 (p=0.001) | 0.02 (p=0.77) | 0.13 (p=0.07) | |
| Personal-level PM2.5 | 0.23 (p=0.001) | -0.06 (p=0.38) | -0.16 (p= 0.03) | |
| Outdoor ambient temperature | 0.02 (p=0.77) | -0.06 (p=0.38) | 0.59 (p<.0001) | |
| Personal temperature | 0.13 (p=0.07) | -0.16 (p= 0.03) | 0.59 (p<.0001) |
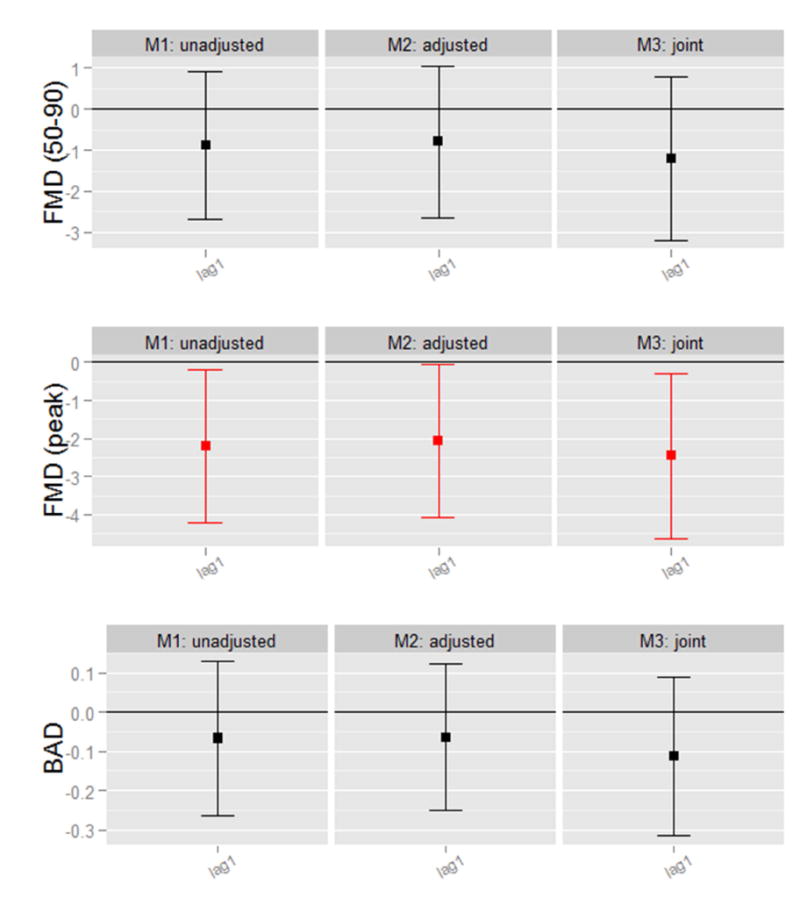
Exposures to colder outdoor-ambient temperatures (per -10°C) over the previous week, measured on individual lag days and over rolling averages, were associated with reductions in FMD (Figure 1). Reductions in FMD ranged from -0.57% (95% confidence interval [CI] -1.14% to 0.01%, p=0.055) to -0.62% (95%CI -1.07% to -0.18%, p=0.006) for 1-6 days rolling average temperature changes. However, the same 10°C decreas e in PET exposure during the prior 24-hours (individual lag day 1) was associated with a substantially larger reduction in FMD -2.44% (95%CI -4.74% to -0.13%, p=0.038) (Table 4). Given that PET varied considerably less than outdoor-ambient temperatures, PET is a more precise measurement and more stable predictor of FMD changes. We also compared lag day 1 associations per interquartile range (IQR) change in both exposures. We found similar effects per IQRs, which are representative exposure variations that are likely to occur in the “real world setting”. FMD decreased by -1.01% (95%CI -1.74% to -0.29%, per -16.3 °C) and by -0.93 % (95%CI -1.80% to -0.05%, per -3.8 °C) for each IQR reduction in ambient-outdoor and PET temperatures, respectively. The moderately high correlation (r=0.59) between temperature levels together with our limited sample size make identifying the variable more strongly associated with FMD statistically difficult. Nonetheless, these results taken altogether support a strong predictive ability of PET decreases in relation to FMD reductions and the superiority of PET with regard to measurement precision. Thus, more robust associations with FMD changes can be observed using PET rather than outdoor-ambient temperatures for any given absolute degree change in temperature exposure.
Figure 1. Associations of Colder Ambient-Outdoor Temperatures with Endothelial-Dependent Vasodilatation.
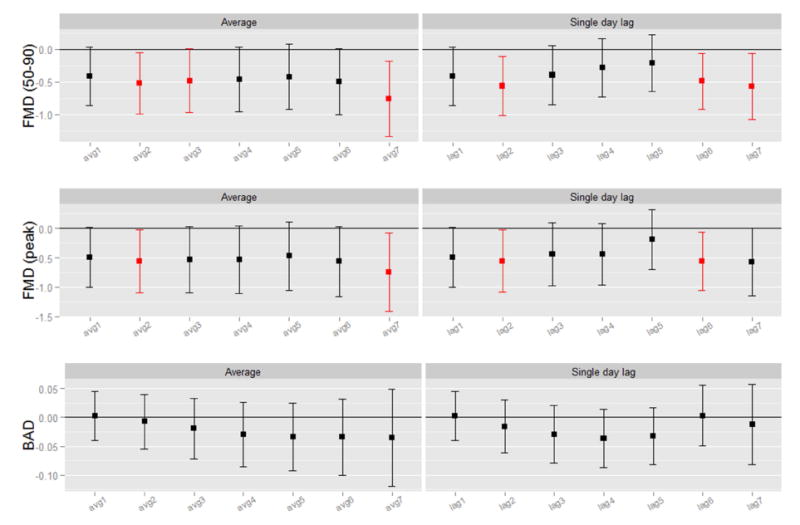
Results provided per (-10°C). Average, rolling mean temperature averaged over all exposure days up through lag day(s) 1-6; Single day lag, individual lag day temperature, for lag day 1-6; All models adjusted for age and ambient PM2.5 (particulate matter level) during the past 24 hours. FMD (peak), peak flow-mediated dilatation during 120 seconds of reactive hyperemia; BAD, brachial artery diameter at a basal resting state.
Table 4. Associations of Colder Prior 24-hour Personal-Level Temperature Exposures with Endothelial-Dependent Vasodilatation, adjusted for age and prior 24-hour ambient PM2.5 exposures.
| Outcome Variable | Covariate in the Model | Coefficient Estimate | Lower CI | Upper CI | P-Value |
|---|---|---|---|---|---|
| FDM (peak) | |||||
| Age | -0.091 | -0.152 | -0.029 | 0.004 | |
| Prior 24-hour Ambient PM2.5 | -0.119 | -0.255 | 0.017 | 0.086 | |
| Prior 24-hour Personal Temperature | -2.438 | -4.743 | -0.133 | 0.038 | |
|
| |||||
| BAD | |||||
| Age | 0.028 | 0.008 | 0.047 | 0.005 | |
| Prior 24-hour Ambient PM2.5 | 0.004 | -0.004 | 0.011 | 0.331 | |
| Prior 24-hour Personal Temperature | -0.010 | -0.158 | 0.139 | 0.899 | |
Results provided per (-10°C). Model is adjusted for age and ambient PM2.5 (particulate matter level) during the past 24 hours. FMD (peak), peak flow-mediated dilatation during 120 seconds of reactive hyperemia; BAD, brachial artery diameter at a basal resting state.
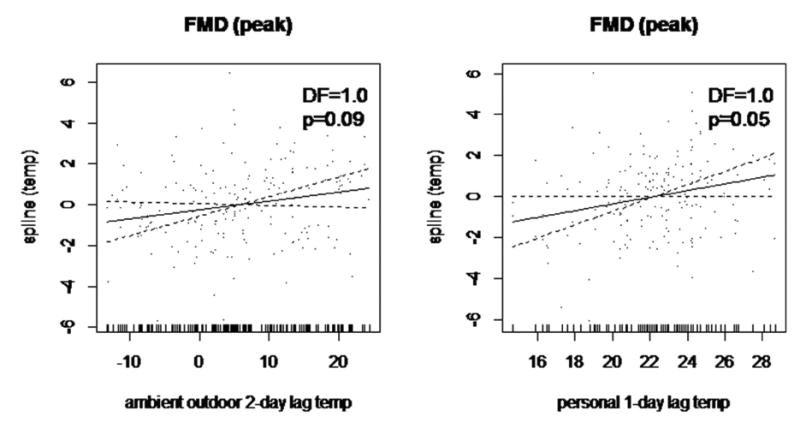
We also explored the potential nonlinear relationship between temperature exposures and FMD through LOESS nonparametric regression method. The graphical associations between changes in both temperature exposures with FMD are presented in Figure 3. The LOESS curves illustrate near-linear associations across the entire exposure ranges (throughout both seasons as well), which confirmed that our model assumptions in GEEs estimations above are reasonable.
A total of 18 participants visited the site during April to October, while 32 participants visited the site during November to March. No participant had visit records across different seasons. During cold seasons (November through March), FMD was significantly lower among participants (6.7 ± 2.7%) compared to other the season (8.4 ± 2.6%) (p<0.001). Lag day 1 outdoor ambient temperature (-1.1 ± 7.6 versus 14.8 ± 4.8 °C) and PET (21.0 ± 2.6 versus 24.0 ± 2.6 °C) were also both significantly (p<0.001) lo wer during the cold season. There was also no significant effect modification (i.e. non-significant interaction terms in the models) by “cold season” on the PET or outdoor-ambient temperature associations with FMD. These results support that variations in real-world temperatures encountered in both cold-to-temperate (0-23.2°C) and freezing (-13.4 to 0°C) periods remain an influence on FMD. This is despite the fact that PET varied less overall and individuals likely spent even more time indoors during colder seasons. This is supported by the weaker correlation between PET and outdoor-ambient temperature on lag day 1 during cold (r=0.30, p=0.016) versus the other season (r=0.71, p<0.001). There was also no significant effect modification by “warm-season”. Finally, we found no significant associations between changes in either temperature levels and other health outcomes studied including BP levels, HRV metrics, and sleep parameters (data not shown).
Discussion
Short-term exposures to colder environmental temperatures were associated with reductions in endothelial-dependent vasodilatation during the ensuing few days. Our results corroborate prior findings10,11 and provide further mechanistic support for the epidemiological linkages between colder temperatures and increased cardiovascular risk1-4. Here, we show for the first time that exposures characterized at the personal-level are likely superior predictors of endothelial function compared with outdoor-ambient levels. This is likely explained by the fact that individuals spend most of their time indoors, particularly in colder environments13. PET levels are therefore not only less variable but also represent more reliable estimates of “true” exposures encountered throughout any given day. Nevertheless, there were moderately positive associations between PET and outdoor-ambient temperatures throughout the year. This likely explains why relatively “inaccurate” exposure estimates captured by outdoor temperatures can still significantly predict FMD across all seasons in this as well as prior studies9-11.
A few studies have reported a relationship between ambient temperature and endothelial function9-11. Narwot et al. demonstrated that warmer temperatures were related to impaired FMD. However, in at least 2 other studies, including the Framingham Offspring Cohort10, FMD was significantly lower in winter after adjusting for clinical covariates, baseline BAD and arterial flow. In the only prior study to evaluate the effect of PET, we did not find an association of same-day levels with FMD14. This is likely due to differences in the previous study design, as FMD was performed non-fasting in the late afternoon.
The relationship between environmental temperature exposure and endothelial function is not fully-understood. Possible mechanistic linkages are that colder temperatures activate the sympathetic nervous system and hypothalamic-pituitary-adrenal axis and can also directly trigger thermogenic vasoconstriction. Alone or together these responses could impair endothelium-dependent vasodilatation1,10. While we showed trends to arterial constriction (reduced BAD) in this study, we did not observe cold-induced changes in HRV or BP to support these pathways. This is most likely because that this was a post hoc exploratory analysis of a study not specifically designed to assess the impact of temperature exposures on these outcomes. Future trials will be required to better elucidate the responsible mechanisms.
Cardiovascular mortality is higher during cold and winter months2-4. Understanding the underlying reasons is of great clinical and public health importance to help develop preventive strategies given the millions of at-risk individuals worldwide. Our findings support that cold-induced endothelial dysfunction may be at least partially responsible as FMD is a known independent predictor of cardiovascular events12. Our observations also accord with a prior meta-analysis showing that indoor is a stronger predictor of BP than outdoor temperature level8. This suggests that if personal-level rather than outdoor temperatures were evaluated in epidemiological studies, the associations with cardiovascular events may be even more robust. Our results further suggest that reducing personal-level exposures to cold temperature, regardless of the outdoor-ambient temperatures or season, might be an effective approach to help combat cardiovascular disease. We posit that improved indoor heating and insulation to maintain a stable indoor living temperature, along with avoiding outdoor cold exposures as much as possible in high-risk individuals, may help mitigate excess cardiovascular events.
We recognize a limitation of this study is that it is a post hoc observational analysis and the results should be considered hypothesis-generating. We also examined several outcomes, raising the possibility for type 1 errors. Only FMD was significantly related to temperature levels and the expected association with BP was not observed likely due to the relatively small size of the cohort. Nevertheless, the statistically robust, reproducible, expected, and near linear associations of both temperature values with FMD supports the veracity of our findings. Future studies need to better define the underlying biological mechanisms involved and to test whether interventions to reduce personal-level exposures to colder temperatures can mitigate against endothelial dysfunction. Additional research is also required to determine if the observed temperature-induced changes in FMD are due to alterations in microvascular responses to hyperemia which thereby change the stimuli (shear-stress) induced post cuff release, or due to changes in intrinsic brachial reactivity, or both. Whether these findings extend to high-risk patients with cardiovascular disease or risk factors also remains to be clarified. Finally, in addition to cold temperatures, heat waves are also known to increase cardiovascular morbidity and mortality. We had few days with extreme elevations in temperature in our study (maximum PET: 28.7 degrees Celsius). Whether very hot days (e.g., >35-40 degrees Celsius) trigger adverse events via impaired endothelial function, or by other mechanisms, remains to be determined.
Colder environmental temperatures are associated with reduced endothelial function. Our results show for the first time that exposures characterized at the personal-level are superior predictors of endothelial-dependent vasodilatation than outdoor-ambient temperatures.
Supplementary Material
Figure S1. Study Protocol Flow Chart
Figure 2. Graphical Associations of Temperature Exposures and Endothelial-Dependent Vasodilatation Using Nonparametric Modeling.
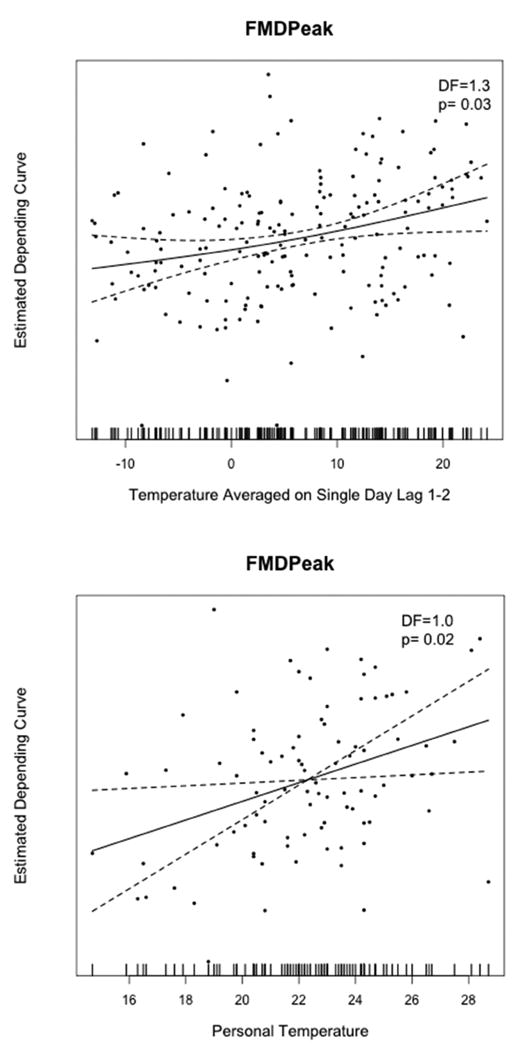
Local polynomial regression (LOESS) curve and 95% confidence intervals (hashed lines) for (a) how peak FMD depends on the change of ambient-outdoor temperature averaged over lag day 1 and lag day 2; and (b) how peak FMD depends on the change of personal-level temperature measured during the prior 24 hours.
Acknowledgments
This study was funded by grants from the NIH (UL1RR024986), EPA (RD83479701 and R833740), and University of Michigan-Peking University Joint Institute for Translational and Clinical Research.
The authors thank the Michigan Department of Environmental Quality for their air monitoring data.
Footnotes
Competing Financial Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brook RD, Weder AB, Rajagopalan S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich) 2011;13:836–42. doi: 10.1111/j.1751-7176.2011.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient temperature and morbidity: a review of epidemiological evidence. Environ Health Perspect. 2012;120:19–28. doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Gasparrini A, Armstrong BG, Tawatsupa B, Tobias A, Lavigne E, Coelho MS, Pan X, Kim H, Hashizume M, Honda Y, Guo YL, Wu CF, Zanobetti A, Schwartz JD, Bell ML, Overcenco A, Punnasiri K, Li S, Tian L, Saldiva P, Williams G, Tong S. Temperature Variability and Mortality: A Multi-Country Study. Environ Health Perspect. 2016;124:1554–1559. doi: 10.1289/EHP149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryti NR, Guo Y, Jaakkola JJ. Global Association of Cold Spells and Adverse Health Effects: A Systematic Review and Meta-Analysis. Environ Health Perspect. 2016;124:12–22. doi: 10.1289/ehp.1408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown A. North American cold spells. Nature Climate Change. 2015;5:515. [Google Scholar]
- 6.Cohen JL, Furtado JC, Barlow MA, Alexeev VA, Cherry JE. Article warming, increasing snow cover and widespread boreal winter cooling. Environ Res Lett. 2012;7:014007. [Google Scholar]
- 7.Giorgini P, Rubenfire M, Das R, Gracik T, Wang L, Morishita M, Bard RL, Jackson EA, Fitzner CA, Ferri C, Brook RD. Particulate matter air pollution and ambient temperature: Opposing effects on blood pressure in high risk cardiac patients. J Hypertens. 2015;33:2032–8. doi: 10.1097/HJH.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Li C, Guo Y, Barnett AG, Tong S, Phung D, Chu C, Dear K, Wang X, Huang C. Environmental ambient temperature and blood pressure in adults: A systematic review and meta-analysis. Sci Total Environ. 2017;575:276–286. doi: 10.1016/j.scitotenv.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Nawrot TS, Staessen JA, Fagard RH, Van Bortel LM, Struijker-Boudier HA. Endothelial function and outdoor temperature. Eur J Epidemiol. 2005;20:407–10. doi: 10.1007/s10654-005-1068-x. [DOI] [PubMed] [Google Scholar]
- 10.Widlansky ME, Vita JA, Keyes MJ, Larson MG, Hamburg NM, Levy D, Mitchell GF, Osypiuk EW, Vasan RS, Benjamin EJ. Relation of season and temperature to endothelium-dependent flow-mediated vasodilation in subjects without clinical evidence of cardiovascular disease (from the Framingham Heart Study) Am J Cardiol. 2007;100:518–23. doi: 10.1016/j.amjcard.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata M, Miyashita Y, Kumagai H. Seasonal variation of endothelium-dependent flow-mediated vasodilation measured in the same subjects. Am J Cardiovasc Dis. 2012;2:111–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2015;4:e002270. doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–52. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 14.Brook RD, Shin HH, Bard RL, Burnett RT, Vette A, Croghan C, Williams R. Can Personal Exposures to Higher Nighttime and Early Morning Temperatures Increase Blood Pressure? J Clin Hypertens. 2011;13:881–888. doi: 10.1111/j.1751-7176.2011.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modesti PA, Morabito M, Massetti L, Rapi S, Orlandini S, Mancia G, Gensini GF, Parati G. Seasonal blood pressure changes: an independent relationship with temperature and daylight hours. Hypertension. 2013;61:908–14. doi: 10.1161/HYPERTENSIONAHA.111.00315. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Nicolas A, Meyer M, Hunkler S, Madrid JA, Rol MA, Meyer AH, Schötzau A, Orgül S, Kräuchi K. Daytime variation in ambient temperature affects skin temperatures and blood pressure: Ambulatory winter/summer comparison in healthy young women. Physiol Behav. 2015;149:203–11. doi: 10.1016/j.physbeh.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Brook RD, Bard RL, Burnett RT, Shin HH, Vette A, Croghan C, Phillips M, Rodes C, Thornburg J, Williams R. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus the community level. Occup Environ Med. 2011;68:224–30. doi: 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study Protocol Flow Chart


