Abstract
The environmental factors driving the increase in food allergies are unclear and possibly involve dual exposure to allergens, microbiome-driven effects or other mechanisms. Until they can be better understood, early intervention aiming at establishing oral tolerance provides an effective way to decrease the window-of-risk when children may develop allergic sensitisation to foods due to the absence of a protective immune response. Thus, the recent LEAP (Learning Early About Peanut allergy) and LEAP-On studies achieved a high level of peanut allergy prevention by early introduction of peanuts in the infants diet and conveyed more information regarding the evolution of IgE and IgG4 antibody responses to food antigens over time.
Introduction
Food allergies are increasing in developed countries [1], reaching in young children a prevalence around 4.2% (Germany), 6.8% (Norway), and 8% in the UK [2●] and US [3]. Notably, recent studies in China, where food allergy was infrequent in the past, found a prevalence of allergy to at least one food between 3.2–7.7% [1].
Given that genes do not change over short periods of time, it must be one or several environmental factors which drive this allergy epidemic. Several non-mutually exclusive hypotheses regarding the mechanisms underpinning this allergy epidemic have been formulated. Apart from the vitamin D hypothesis, which is comprehensively discussed in a recent review [4], other key hypotheses are the dual allergen exposure hypothesis and the hygiene hypothesis (including the potential role of microbiota diversity for establishing oral tolerance to foods).
Immune mechanisms of allergy and early prevention
Until the precise environmental drivers can be disentangled, primary prevention strategies have to rely upon early ‘natural’ tolerance induction, which then counters allergic sensitisation. Food allergy is induced when gut (or eventually skin) antigen presenting cells drive T helper cell differentiation into Th2 cells that consequently induce B cells to switch and mature into predominantly IgE-producing cells [5]. Conversely, food tolerance results when antigen presentation in the Gut-Associated Lymphoid Tissue (GALT) leads to the development of regulatory T cells that drive B cells to produce predominantly IgG antibodies to foods, as well as potentially regulatory B cells that secrete IL10 and drive IgG4 production. [5] (Table 1)
Table 1.
Regulatory immune effectors involved in food allergy pathogenesis.
| Site/Effector | Expected association with allergy | Mechanism and potential role for allergy prevention |
|---|---|---|
| Regulatory T cells | ||
| Th1 cells | Th1 responses suppress Th2 | Use of Th1-inducing adjuvants for immunotherapy [43] |
| Induced Tregs | Suppress Th2 | Multiple subsets have been described. More recently, Duhen et al [44] described Tregs subsets ‘mirroring’ effector subsets ie Th1reg, Th2reg, Th17reg etc. Tregs may inhibit allergy at multiple levels, acting upon the antigen presentation process and directly on T effectors. [5] |
| LAP+FoxP3- Treg | Gastrointestinal-homing subset | TGF beta-dependent suppression of allergic reactions [5] |
| Regulatory B cells | ||
| B cells producing ‘blocking’ IgG/IgG4 | Suppress allergy | IgG/IgG4 cross-link the inhibitory Fc gamma type 2 receptor on antigen presenting cells [40] |
| CD5+ Bregs | Suppress allergy | Suppress Th2 responses by secreting IL10 [45] |
It is still unclear how dendritic cells (DC) could select between the tolerance and allergy responses. It has been known that gut CD103+ DC, that migrate to the mesenteric lymph nodes of mice and humans, drive tolerance because they induce gut-homing Foxp3+ Treg cells due to their production of retinoic acid [6]. More recently, additional DC subsets that drive pro-inflammatory responses rather than tolerance have been identified; one of those subsets, the type 2 DC (DCTh2) has a distinct transcription signature expressing IRF4 and STAT5 [7]. Since STAT5 is a downstream target of TSLP, this supports a Th2 skewed response. Other immune effectors driving allergic responses are the tissue-resident group 2 innate lymphoid cells (ILC2) that secrete locally high amounts of cytokines, especially IL5 and IL13 [8]. Therefore one possible mechanism leading to food allergy increase is an altered balance between pro-tolerogenic and pro-allergenic DC.
The interplay between antagonistic T cell subsets, especially the effector T cells and the regulatory T cells (Treg) is crucial for determining the allergic/tolerant phenotype in animal models of food allergy. Thus, allergy to ovalbumin can be transferred by injecting Th2 effector cells from an allergic mouse into a naive mouse [9]. On the other hand, mice rendered tolerant to ovabumin by oral administration were protected against subsequent allergic sensitization, suggesting that, once established, oral tolerance is difficult to breach [10]. The antagonism between the Tregs underpinnig oral tolerance and food allergy effectors is demonstrated by the finding that transferring CD25+ mesenteric lymph node Tregs from a mouse orally tolerized to ovalbumin suppressed allergy symptoms [11]. In humans the role of Tregs is mainly known by studying the IPEX syndrome (Immune dysregulation, polyendocrinopathy, enteropathy, X linked), resulting from mutations of the FOXP3 gene that lead to a loss of Treg-mediated tolerance [12]. Nevertheless, peanut allergy could be transferred from an allergic donor after liver and kidney transplantation. Subsequent investigation demonstrated the persistence microchimerism from the donor in the skin but not in the blood of the recipient, presumably due to post-transplantation immunosuppression [13].
Therefore it appears that the balance between food tolerance and allergy could be influenced at multiple levels during the development of an immune response. Recently three cytokines, TSLP, IL25 and IL33, were each found to be necessary for the induction of food allergy in a mouse egg white allergy model [14]. Thus the injection, at the time of sensitisation, of a blocking antibody to any of these three cytokines was sufficient to block the development of allergy. Conversely, once egg white allergy was established, only a blocking cocktail against all three (anti-TSLP+anti-IL33R+anti-IL25 antibodies) but not single antibodies or combinations of two of those were able to suppress allergic responses [14].
Conversely, mesenteric lymph node ablation or deficiency of the chemokine receptor CCR7 that is involved in DC and T cell subset migration prevents oral tolerance induction [15]. Other immune effectors, such as secreted IgA antibodies, do not seem to play a role since sIgA-deficient piglets are able to develop oral tolerance and transfer it with their memory T cells [16].
Once allergy or tolerance is established, this state is relatively difficult to breach since these strongly antagonize each other [10, 11]. The absence of a clinical reaction in the presence of allergen-specific IgE (i.e. sensitisation) represents a set of distinct phenotypes of tolerance in atopic individuals. Five types of sensitisation can be identified: antenatal sensitisation, stable sensitisation, pre-allergic sensitisation, post-allergic sensitisation and desensitisation [17–19]. Understanding the mechanisms of tolerance despite IgE sensitisation may shed light into ways to modify and prevent the food allergic response. Different types of sensitisation may be underpinned by distinct immune mechanisms. The absence of clinical reactivity could be due to passive processes (e.g. elaboration of low-affinity IgE, low epitope spreading or IgE that only binds to clinically irrelevant epitopes) or to active inhibitory and/or regulatory processes, such as the production of IgG4 antibodies with the same specificity as IgE and the induction of Treg cells [20]. The strategy of early oral tolerance induction prevents this antagonism between the mechanisms of food allergy and oral tolerance as it decreases the temporal window of risk when children can be sensitised to allergens due to the absence of a protective immune response. This antagonism underpins the prevention strategy of early oral tolerance induction that decreases the temporal window of risk when children can be sensitised to allergens due to the absence of a protective immune response.
Dual Allergen Exposure Hypothesis
Routes of exposure to food antigens appear to be crucial in determining whether food allergy or tolerance develops. There is a clear association between eczema and the development of food allergy, with up to 50% of children with early-onset, severe eczema developing challenge proven allergy to peanut, egg or sesame seed allergy [21]. In children with moderate to severe eczema recruited to the LEAP study (Learning Early About Peanut study at http://www.leapstudy.co.uk/) even by 4 months of age there was demonstrable specific IgE to common food allergens such as egg (50%), milk (28%) and peanut (21%). [22] Animal and human observational and in-vitro studies support the hypothesis of transcutaneous sensitization to food allergens through inflamed eczematous skin [23, 24]. Epicutaneous exposure to 100 μg of peanut protein or ovalbumin (OVA) in mice after tape stripping the skin induces a potent allergic Th2-type response, high levels of peanut or OVA specific IgE respectively and anaphylaxis on subsequent oral exposure [24]. More recently, the TSLP-basophil axis was shown in a mouse model to be responsible for the promotion of food allergy resulting from. exposure to food allergens through inflamed skin [25].
In humans, observational studies have found that topical application of Arachis (peanut) oil onto eczematous skin during infancy is significantly associated with the development of peanut allergy; among peanut allergic children with eczema in the ALSPAC study, 90% had been topically exposed to creams containing Arachis (peanut) oil in the first 6 months of life [17]. In human studies, environmental exposure to peanut during infancy (assessed by household peanut consumption) increased the risk of peanut allergy; however, if the infant had consumed peanut in the first year of life then they were protected against developing peanut allergy [26]. More recent studies found that eczema severity amplifies the risk of peanut sensitization and likely allergy resulting from exposure to peanut antigen in household dust [27]. A similar increase of peanut sensitisation and allergy risk was seen in children with filaggrin loss-of-function mutations exposed to high levels of peanut allergens in the household dust [28]. This provides a good example of gene-environment interactions leading to the development of peanut allergy in young infants.
Thus, the dual allergen exposure hypothesis suggests that through this balance of exposures during the first year of life (depending on whether the initial exposure to peanut is through the skin or gut), the immune system is then primed to develop allergy versus tolerance respectively. There is therefore a narrow window of opportunity, during the child’s first year of life, where this balance could be influenced towards a tolerogenic response.
The hygiene hypothesis and microbiome role in food allergy and its prevention
During early life, environmental exposures are associated with protection from food allergy. For instance, analysis of the Finnish national database of state-supported hypoallergenic cow’s milk formula prescription demonstrated that having already had more than four deliveries and multiple pregnancy were associated with protection from cow’s milk allergy [29]. Hygiene factors in the Melbourne based Healthnuts cohort have own that egg allergy is less likely amongst children with older siblings and those with a pet dog at home [30].
Germ-free mice have a strong disposition towards developing food allergy. They harbour a profoundly under-developed immune system, with less local and lymphoid IgA, fewer plasma cells in their gastro-intestinal mucosa and a dramatic reduction in their secondary mesenteric lymphoid tissue [31]. Mice with normal gut microbiota (commensal communities) are protected from allergic sensitisation by regular oral feeding with ovalbumin. By contrast, sensitised germ-free mice are unable to produce the Interferon-γ and IgG2a associated with tolerance induction, and instead produce increased IgE and Interleukin-4 (IL-4) on ovalbumin challenge [32]. The reconstitution of germ-free mice with Bifidobacterium infantis before sensitisation can ameliorate this disposition towards allergy, showing that the gut microbiota can facilitate and promote tolerance.
This model has been developed to more closely reflect findings amongst humans. For instance, increasing the diversity and richness of gut microbiota donated to germ-free models allows greater capacity for the induction of oral tolerance [33]. Similarly in infants, lower gut microbiota richness and or diversity is associated with greater sensitisation when age matched comparisons are undertaken between those with food sensitisation and controls [34]. The introduction of commensal gut microbiota strains to germ-free mouse strains induces de novo generation of mucosal CD4+CD25+ Foxp3+ cells, allowing the local production IL-10 [35]. By removing these T-regulatory cells from a model of oral tolerance, we see a relapse into allergy. Furthermore, specific pathogen-free mice receiving PC61 anti-CD25 monoclonal antibody are no longer able to support tolerance after oral β-lactoglobulin gavage, and instead demonstrate raised β-lactoglobulin specific IgE and reduced ability to suppress IL-5 and IL-13 production from splenic preparations [36]. Oral tolerance and Treg cells are likely promoted by short-chain fatty acid metabolites such as butyrate, released by commensal Clostridia constituents derived taxa locally at the mucosal surface. Greater short chain fatty acid production has also been noted amongst probiotic formula supplementation used to treat milk allergy [37]. Whilst environmental exposures may promote Treg activity, biomarkers that are correlated with the establishment of tolerance to foods are difficult to measure in infants. However, longitudinal assessment of peanut specific IgE, IgG, IgG4 and potentially other immunological biomarkers allow some insight into the continued balance of sensitisation and oral tolerance amongst infants undertaking early introduction of peanut in their diets.
Immune mechanistic insights resulting from peanut allergy prevention by early introduction of peanut in the diet
The LEAP [38] and LEAP-On [39] peanut allergy prevention studies have increased our insight into the changes that occur with IgE, IgG and IgE:IgG4 ratios over time, when developing allergy or tolerance to peanuts (Figure 1). In the LEAP study, 640 high-risk children were randomized into two groups – a peanut consumption group who ate peanut products at least 3 times a week (average of 6 grams of peanut protein a week) and the peanut avoidance group who avoided any peanut products until 60months of age. Peanut allergy was determined by oral food challenges. Subsquently, in the LEAP-On study, all participants stopped eating for one year and were then reassessed, in order to determine whether the protective effect of early peanut consumption persisted in the long term. In the LEAP study we saw an early increase in IgG4 production in the consumption group (already evident after a few months of consumption). Surprisingly, there were no differences between the consumption and avoidance groups regarding their mean levels of IgE to peanut throughout the LEAP study, all the way to 5 years of age. However, when children were followed after one year of peanut avoidance to 6 years of age (LEAP-On) a statistically significant decline in peanut-specific IgE was observed in the initially peanut-consuming population, especially in IgE to Ara h 2, the major peanut component. This reflects two important points relevant for our understanding of the immunology of food allergy prevention.
Figure 1.
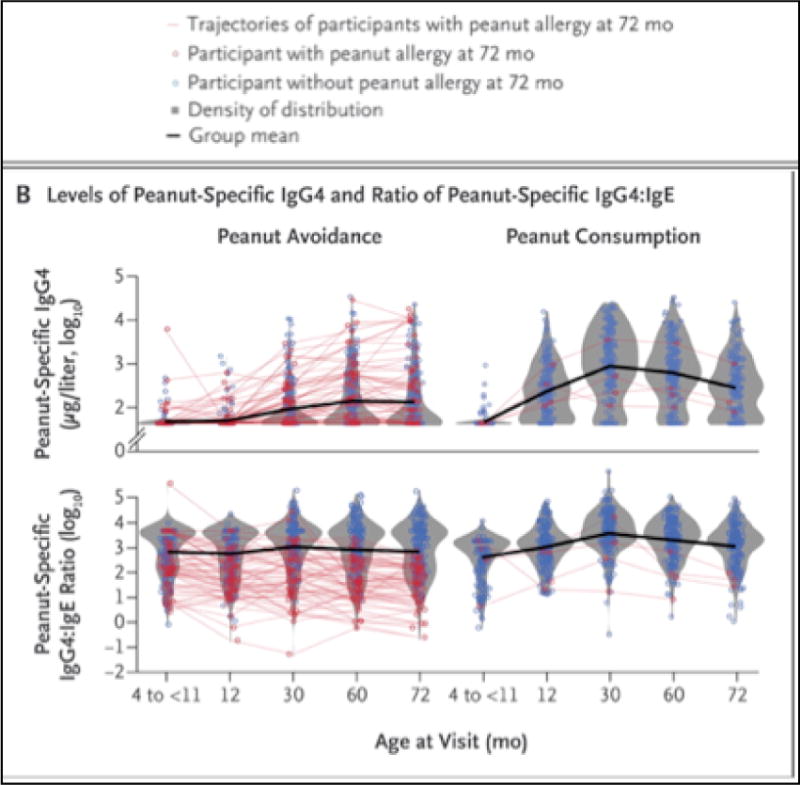
Changes that occur with IgE, IgG and IgE:IgG4 ratios over time in children who consumed or avoided peanuts in the frame of the LEAP and LEAP-On studies [39]. The red lines outline the evolution of the levels of peanut-specific IgG4 and respectively the evolution of the ratio of peanut-specific IgG/IgE in children who were subsequently found to be peanut allergic at 72 months of age.
The first point is that the elaboration of IgE antibodies to foods occurs early in infancy and may take a very long time to switch off, likely due to the presence of long-lived memory B and plasma cells committed to IgE production. Hence it is extremely important to follow children in such studies for a long time in order to gain an insight into the evolution of immune responses to food allergens over time.
Children who were allergic to peanut at 60months of age already had higher peanut-specific IgE at 12months, 30months and 60months. Peanut-specific IgG and IgG4 levels also increased over time in both the peanut consumption and avoidance groups, however the peanut consumption group, who were subsequently protected against peanut allergy, had a significantly greater and earlier increase (i.e. by 12 months).
In sensitised patients, the interplay between IgE and other allergen-specific antibodies of different isotypes (such as IgG4) may determine whether exposure to the allergen causes the activation of mast cells and basophils with degranulation and release of vasoactive and pro-inflammatory mediators that lead to the allergic symptoms. This could explain the absence of clinical responses seen in the five types of sensitisation described above. In the LEAP study, the overall balance between peanut-specific IgG4 and peanut-specific IgE reflected the participants’ allergic status to peanut. In another peanut study, peanut sensitised tolerant patients had higher levels of specific IgE compared to IgG4 to peanut and the three peanut major allergens as assessed by IgG4/IgE ratios [40]. The plasma samples of peanut sensitised but tolerant children were able to inhibit IgE-mediated peanut-induced basophil and mast cell activation to a similar extent as plasma from peanut allergic patients that had been submitted to OIT. Depletion of this antibody isotype led to an increase in peanut-induced mast cell activation, indicating that this effect was partially mediated by IgG4 (Figure 2).
Figure 2.
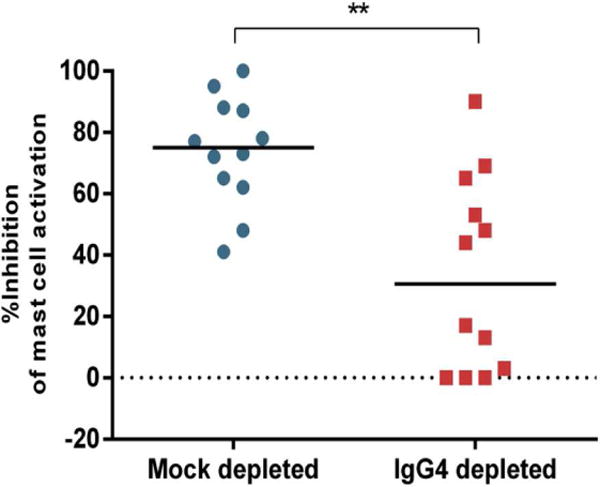
Depletion of IgG4 decreased the ability of plasma from peanut-sensitized but clinically tolerant patients to inhibit peanut-induced activation of mast cells. **p<0.01 [17] In these inhibition experiments, LAD2 mast cells were sensitized with plasma from a patient with peanut allergy, then were activated with peanut allergens in the presence of either IgG4-depleted or mock-depleted plasma from 12 peanut-sensitized but clinically tolerant individuals. The vertical axis shows the percentage of inhibition of mast cell activation achieved by adding the respective plasma samples.
In children with peanut allergy in the avoidance group of the LEAP study, almost all of their IgG4/IgE ratios fell below the mean ratio for the group and it was found that unless IgE levels were very high, elevated IgG4 levels were associated with the absence of an allergic reaction to peanuts. In the LEAP-On study, after a yearlong period of peanut avoidance in previous peanut consumers, peanut-specific IgG4 levels (p<0.001) and peanut-specific IgG4/IgE ratios continued to be higher in the peanut consumption group than in the peanut avoidance group. However, IgG4 levels started to slowly drift down after 30 months, even in the peanut consumption group. In the participants whose became allergic in the LEAP-On study (1.1% of the peanut consumption group and 1.1% of the peanut avoidance group), the ratio of peanut-specific IgG4/IgE declined between 60 and 72 months. The children from the peanut consumption group who were able to tolerate peanut continued to have low levels of peanut-specific IgE and high ratios of IgG4/IgE at 60 months in the primary trial but this was maintained at 72 months even after a 12-month period of not consuming peanut.
The second point relevant for the immunology of food allergy prevention is related to the dilution effect seen in prevention studies. Although there was no difference in mean peanut-specific IgE during the LEAP Study, extreme value analysis revealed that high level IgE production to peanut was increasingly observed over time in the group that went on to become peanut allergic. Nevertheless, since these would represent a minority of study participants, their data would be ‘diluted’ by the majority of study participants, who remain tolerant. This sampling effect has the potential to influence the way in which we interpret results on an immunological level and must be considered in future prevention study design. As discussed by Bahnson et al [41], most children start as ‘normal’ and without the disease of interest (i.e. peanut allergy) so that if a study has a high proportion of healthy participants, statistical power decreases causing a dilution effect. Thus, in LEAP, 17% of the peanut avoidance group and 3% of the peanut consumption group developed peanut allergy, respectively. If peanut consumption were responsible for altering IgE production, it would have only been the cause for 14% of the consumption group who would have developed peanut allergy. If this was true and immunological differences existed in IgE between children consuming peanut and those avoiding peanut, the differences could be “diluted” by the 86% children who were likely to be non-allergic to peanut.
Conclusions
The establishment of oral tolerance to foods is a complex process, resulting from the balance between immune effectors that drive the development of ‘healthy’ Treg and IgG/IgG4 responses and antagonistic immune effectors that promote Th2 dominant responses leading to IgE production. This balance may be altered due to environmental factors at the point of allergen presentation in the gut or at subsequent levels of the acquired immune response, possibly involving Treg cells. Promising areas of intervention have been defined through the dual allergen exposure hypothesis and the investigation of the role of microbiota. Nevertheless, until the environmental factors that drive the current allergy epidemic are identified, early food allergy prevention through the induction of oral tolerance remains the most promising approach at a population level. This has been demonstrated by the LEAP and LEAP-On studies for peanut allergy and there is further evidence that this could apply for egg allergy [42]. More recently, the outcome of the LEAP and LEAP-On studies further increased our knowledge of the longitudinal evolution of the immune responses to foods that were introduced early in the infant diet.
Highlights.
-
-
Skin exposure to foods may promote allergy whereas tolerance develops in the gut
-
-
Local microbiota may influence the balance of tolerance/allergy gut immune responses
-
-
Early intervention reduces the window-of-risk when children are not protected by tolerance
-
-
Prevention studies require long-term immunological and clinical follow-up
-
-
The dilution effect needs to be considered when assessing prevention biomarkers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1●.Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JKh, Fiocchi A, Ebisawa M, Sampson HA, Beyer K, Lee BW. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013 Dec 4;6(1):21. doi: 10.1186/1939-4551-6-21. This paper provides a comprehensive overview of food allergy epidemiology in the world. It outlines the methodology used and the recent evolutions of food allergy prevalence in different countries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, Brough H, Marrs T, Radulovic S, Craven J, Flohr C, Lack G, EAT Study Team Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. New Engl J Med. 2016 May 5;374(18):1733–43. doi: 10.1056/NEJMoa1514210. [DOI] [PubMed] [Google Scholar]
- 3.Gupta RS, Lau CH, Sita EE, Smith B, Greenhawt MJ. Factors associated with reported food allergy tolerance among US children. Ann Allergy Asthma Immunol. 2013 Sep;111(3):194–198. doi: 10.1016/j.anai.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 4●●.Peters RL, Neeland MR, Allen KJ. Primary Prevention of Food Allergy. Curr Allergy Asthma Rep. 2017 Aug;17(8):52. doi: 10.1007/s11882-017-0718-x. This paper outlines very clearly the main hypotheses regarding the triggers of the current increase of food allergies. It covers the most recent findings related to the dual allergen exposure, the vitamin D and the hygiene hypotheses. [DOI] [PubMed] [Google Scholar]
- 5.Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol. 2013 May 6;23(9):R389–400. doi: 10.1016/j.cub.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011 Jul 22;35(1):13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tjota MY, Sperling AI. Distinct dendritic cell subsets actively induce Th2 polarization. Curr Opin Immunol. 2014 Dec;31:44–50. doi: 10.1016/j.coi.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8●.Bernink JH, Germar K, Spits H. The role of ILC2 in pathology of type 2 inflammatory diseases. Curr Opin Immunol. 2014 Dec;31:115–20. doi: 10.1016/j.coi.2014.10.007. This paper describes the role of ILC2 as a key component of innate immunity that may play an essential role in inducing food allergy. ILC2 may act directly upon T cells or indirectly by modulating DC presentation of allergens. [DOI] [PubMed] [Google Scholar]
- 9.Knight AK, Blázquez AB, Zhang S, Mayer L, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1234–G1243. doi: 10.1152/ajpgi.00323.2007. [DOI] [PubMed] [Google Scholar]
- 10.Perrier C, Thierry AC, Mercenier A, Corthésy B. Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice. Clin Exp Allergy. 2010 Jan;40(1):153–62. doi: 10.1111/j.1365-2222.2009.03329.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita H, Takahashi K, Tanaka H, Nagai H, Inagaki N. Overcoming food allergy through acquired tolerance conferred by transfer of Tregs in a murine model. Allergy. 2012 Feb;67(2):201–9. doi: 10.1111/j.1398-9995.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 12.d’Hennezel E, Bin Dhuban K, Torgerson T, Piccirillo CA. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2012 May;49(5):291–302. doi: 10.1136/jmedgenet-2012-100759. [DOI] [PubMed] [Google Scholar]
- 13.Legendre C, Caillat-Zucman S, Samuel D, Morelon S, Bismuth H, Bach JF, Kreis H. Transfer of symptomatic peanut allergy to the recipient of a combined liver-and-kidney transplant. N Engl J Med. 1997 Sep 18;337(12):822–4. doi: 10.1056/NEJM199709183371204. [DOI] [PubMed] [Google Scholar]
- 14●●.Khodoun MV, Tomar S, Tocker JE, Wang YH, Finkelman FD. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J Allergy Clin Immunol. 2017 May 26; doi: 10.1016/j.jaci.2017.02.046. pii: S0091-6749(17)30842-4. This paper outlines the necessary versus the sufficient cytokine factors involved in the development and persistence of food allergy in mice. It shows that suppression of all three cytokines is necessary for the blocking established food allergy. [DOI] [PubMed] [Google Scholar]
- 15.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Förster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006 Mar 20;203(3):519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson MR, Johansen FE, Kahu H, Macpherson A, Brandtzaeg P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy. 2010 May;65(5):561–70. doi: 10.1111/j.1398-9995.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 17.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–985. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 18.Schnabel E, Sausenthaler S, Schaaf B, Schafer T, Lehmann I, Behrendt H, Herbarth O, Borte M, Kramer U, von Berg A, et al. Prospective association between food sensitization and food allergy: results of the LISA birth cohort study. Clin Exp Allergy. 2010;40:450–457. doi: 10.1111/j.1365-2222.2009.03400.x. [DOI] [PubMed] [Google Scholar]
- 19.Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, Dawson P, Mayer L, Burks AW, Grishin A, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133:492–499. doi: 10.1016/j.jaci.2013.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savilahti EM, Rantanen V, Lin JS, Karinen S, Saarinen KM, Goldis M, Makela MJ, Hautaniemi S, Savilahti E, Sampson HA. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J Allergy Clin Immunol. 2010;125:1315–1321 e1319. doi: 10.1016/j.jaci.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. 2014;69:17–27. doi: 10.1111/all.12268. [DOI] [PubMed] [Google Scholar]
- 22.Du Toit G, Roberts G, Sayre P, Plaut M. Identifying infants at high risk of peanut allergy - The LEAP Screening Study. J Allergy Clin Immunol. 2013;131:135–43. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 23.van Reijsen FC, Felius A, Wauters EA, Bruijnzeel-Koomen CA, Koppelman SJ. T-cell reactivity for a peanut-derived epitope in the skin of a young infant with atopic dermatitis. J Allergy Clin Immunol. 1998;101:1–9. doi: 10.1016/s0091-6749(98)70410-5. [DOI] [PubMed] [Google Scholar]
- 24.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol. 2004;34:2100–9. doi: 10.1002/eji.200425196. [DOI] [PubMed] [Google Scholar]
- 25●●.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, Sattentau QA, Comeau MR, Spergel JM, Artis D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 2014 May;133(5):1390–9. doi: 10.1016/j.jaci.2014.01.021. This paper clarifies a key effector mechanism that underpins the induction of food allergy due to skin exposure to food antigens. It shows that epicutaneous sensitisation on a disrupted skin barrier is associated with the accumulation of TSLP-elicited basophils which are necessary and sufficient to promote food allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox AT, Sasieni P, Du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–23. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 27●.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, Stephens AC, Irwin McLean WH, Turcanu V, Wood RA, Jones SM, Burks W, Dawson P, Stablein D, Sampson H, Lack G. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015 Jan;135(1):164–70. doi: 10.1016/j.jaci.2014.10.007. This showed that peanut household consumption, which correlates strongly with peanut protein levels in household dust, is correlated with a higher risk of developing peanut allergy. It also shows that this risk is further increased for children with a history of atopic dermatitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, Belgrave DC, Penagos M, Stephens AC, McLean WH, Turcanu V, Nicolaou N, Custovic A, Lack G. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014 Oct;134(4):867–875. doi: 10.1016/j.jaci.2014.08.011. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metsälä J, Lundqvist A, Kaila M, Gissler M, Klaukka T, Virtanen SM. Maternal and perinatal characteristics and the risk of cow’s milk allergy in infants up to 2 years of age: a case-control study nested in the Finnish population. Am J Epidemiol. 2010 Jun 15;171(12):1310–6. doi: 10.1093/aje/kwq074. [DOI] [PubMed] [Google Scholar]
- 30.Koplin JJ, Dharmage SC, Ponsonby AL, Tang ML, Lowe AJ, Gurrin LC, Osborne NJ, Martin PE, Robinson MN, Wake M, Hill DJ, Allen KJ, HealthNuts Investigators Environmental and demographic risk factors for egg allergy in a population-based study of infants. Allergy. 2012 Nov;67(11):1415–22. doi: 10.1111/all.12015.. [DOI] [PubMed] [Google Scholar]
- 31.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19(2):59. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159(4):1739–45. [PubMed] [Google Scholar]
- 33.Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe. 2013;14(5):559–70. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CC, Chen KJ, Kong MS, Chang HJ, Huang JL. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr Allergy Immunol. 2016 May;27(3):254–62. doi: 10.1111/pai.12522. [DOI] [PubMed] [Google Scholar]
- 35.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34(5):794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Adel-Patient K, Wavrin S, Bernard H, Meziti N, Ah-Leung S, Wal JM. Oral tolerance and Treg cells are induced in BALB/c mice after gavage with bovine betalactoglobulin. Allergy. 2011;66(10):1312–21. doi: 10.1111/j.1398-9995.2011.02653.x. [DOI] [PubMed] [Google Scholar]
- 37.Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016;10(3):742–50. doi: 10.1038/ismej.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38●●.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G, LEAP Study Team Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. This paper shows that early peanut introduction in the infant diet leads to significant levels of peanut allergy prevention, when compared with peanut avoidance as an alternative strategy. It also demonstrates that primary prevention is moiré effective than secondary prevention (where participants were already sensitised but not allergic to peanuts when they entered the trial). In terms of immunological biomarkers of food allergy, it shows that a lower ratio of peanut specific IgG4/peanut-specific IgE is correlated with peanut allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39●●.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, Brough HA, Santos AF, Harris KM, Radulovic S, Basting M, Turcanu V, Plaut M, Lack G, LEAP-On Study Team Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med. 2016;374:1733–43. doi: 10.1056/NEJMoa1514209. This paper shows that peanut tolerance induced by early peanut introduction in the infant diet was not significantly affected after one year of stopping peanut consumption. In terms of immunological biomarkers of tolerance to food, it shows that children who had eaten peanuts early in life continued to have a higher level of peanut-specific IgG4 and a higher ratio of peanut specific IgG4/peanut-specific IgE than children who avoided peanut consumption early in life. [DOI] [PubMed] [Google Scholar]
- 40.Santos AF, James LK, Bahnson HT, Shamji MH, Couto-Francisco NC, Islam S, Houghton S, Clark AT, Stephens A, Turcanu V, Durham SR, Gould HJ, Lack G. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249–56. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41●●.Bahnson HT, du Toit G, Lack G. Statistical Considerations of Food Allergy Prevention Studies. J Allergy Clin Immunol Pract. 2017 Mar-Apr;5(2):274–282. doi: 10.1016/j.jaip.2016.12.007. This paper shows that in population-wide interventional allergy prevention studies measured outcomes may be ‘diluted’ because most of the participants remain tolerant. The authors propose ‘extreme value’ statistical analysis as a potential solution for identifying biomarkers for food allergy prevention studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42●.Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, Robinson Z, Geoghegan N, Jarrold K, Reeves T, Tagiyeva-Milne N, Nurmatov U, Trivella M, Leonardi-Bee J, Boyle RJ. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis. JAMA. 2016 Sep 20;316(11):1181–1192. doi: 10.1001/jama.2016.12623. This paper is a systematic meta-analysis of studies on the effect of early egg or peanut introduction to the diet of infants. It revealed that this approach was associated with a lower risk of developing egg or peanut allergy. [DOI] [PubMed] [Google Scholar]
- 43.Fonseca DE, Kline JN. Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv Drug Deliv Rev. 2009 Mar 28;61(3):256–62. doi: 10.1016/j.addr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 44●.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012 May 10;119(19):4430–40. doi: 10.1182/blood-2011-11-392324. This paper identified distinct populations of Tregs in human blood on the basis of their chemokine expression. Each population was suppressive but they displayed unique patterns of cytokines and responses differently to antigens associated with Th1 and Th17 responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Veen W, Stanic B, Wirz OF, Jansen K, Globinska A, Akdis M. Role of regulatory B cells in immune tolerance to allergens and beyond. J Allergy Clin Immunol. 2016 Sep;138(3):654–65. doi: 10.1016/j.jaci.2016.07.006. [DOI] [PubMed] [Google Scholar]


