Abstract
The U.S. National Pain Strategy calls for increased population research on “high impact chronic pain,” i.e., longstanding pain that substantially limits participation in daily activities. Using data from the nationally-representative Health and Retirement Study (HRS), we investigated the prevalence of high-impact chronic pain in U.S. adults over age 50 overall and within population subgroups. We also explored sociodemographic variation in pain-related disability within specific activity domains. Data are from a subsample of HRS respondents (n=1,925) who were randomly selected for a supplementary pain module in 2010. Our outcome was operationalized as pain duration of ≥7 months and a disability rating of ≥7 (0 to 10 scale) in at least one domain: family/home, leisure, social activities, work, or basic activities. Overall, 8.2% (95% C.I. = 6.7 to 10.1%) of adults over age 50 met criteria for high-impact chronic pain. This proportion rose to 17.1% (95% C.I. = 12.3 to 23.4%) among individuals in the lowest wealth quartile. Prevalence differences by education, race/ethnicity and age were not significant. Arthritis and depression were significantly associated with high-impact pain in multivariable analysis. Among adults with any chronic pain, African Americans and individuals in the lowest wealth quartile reported more pain-related disability across activity domains.
INTRODUCTION
Approximately one-third of Americans experience chronic pain, a condition that costs society over $500 billion each year.18 A seminal 2011 report from the Institute of Medicine (IOM) presented chronic pain as a pressing public health issue and offered recommendations to improve its prevention and treatment. These recommendations formed the basis for a comprehensive National Pain Strategy (NPS), developed with input from experts in pain management, research, insurance, and policy.29 One NPS objective is to improve decision-making via the collection and analysis of epidemiologic data on “high-impact chronic pain,” i.e., pain experienced for over six months that causes substantial limitations in work, social, and/or self-care activities. This definition identifies those unable to maintain normal activities due to chronic pain, and who have experienced pain longer than the three-month threshold typically used to indicate the transition from acute to chronic pain.18
Many conditions that may cause high-impact chronic pain are age-related, including osteoarthritis, diabetic neuropathy, and post-stroke pain.12 Studies conducted among individuals over age 50 demonstrate a strong link between chronic pain and decrements in physical, psychological, cognitive and social functioning.7,9,37 Chronic pain also increases the likelihood of falls.42 Pain may be more closely linked to disability in older adults than in their younger counterparts,10 and chronic pain can impede the ability of older adults to remain independent.31 Prior prevalence estimates of chronic pain in U.S. older adults range from 16% to 31%.19,37,38 However, these estimates are based on definitions that did not consider pain’s duration and/or impact on the activity domains specified in the NPS definition of high-impact chronic pain.
While high-impact chronic pain is found throughout the midlife and older population, it may disproportionately affect African American adults and those of low socioeconomic status (SES). Disparities across a wide range of health-related outcomes disadvantaging these subgroups of Americans have been observed over decades of research.1 Health inequalities may be most pronounced in midlife and early old age,1 owing to an accelerated aging process thought to result from social disadvantage and chronic stress.13,20 Both socioeconomic and racial/ethnic minority status are linked to the chronic pain experience and its treatment, for reasons that include greater vulnerability to chronic conditions, exposure to occupational hazards, and reduced access to care.43, 14, 16, 26 Although non-Hispanic whites typically report a similar or higher overall prevalence of chronic pain compared to other groups,19,35,38 African Americans and Hispanics tend to report greater pain severity15,16 and African Americans report more pain-related disability.14,35,37 Lower educational attainment and fewer economic resources are also associated with chronic pain and pain severity;15,35,37,38 however, socioeconomic patterns are not consistent across studies.19
The goal of the present study is to conduct secondary data analysis to estimate the population prevalence of high-impact chronic pain, as recommended in the 2016 National Pain Strategy. We assess whether the prevalence of high-impact pain in community-dwelling Americans over age 50 varies across groups defined by race/ethnicity and SES (indicated by education and household wealth), as well as gender and age. We also examine potential health correlates of high-impact chronic pain. Finally, we assess variation in pain intensity and impact by race/ethnicity and SES among those who report any chronic pain, to explore differences in the pain experience across the entire range of pain impact.
METHODS
Study population
Since 1992, the Health and Retirement Study (HRS) has conducted biannual telephone and face-to-face surveys of a nationally-representative sample of community-dwelling Americans over age 50.40 HRS was approved by the University of Michigan Institutional Review Board and informed consent was obtained from each respondent. HRS uses a multi-stage area probability sample design, involving stratification, clustering, and oversampling of African American and Hispanic adults. From over 20,000 HRS respondents surveyed in 2010, HRS investigators selected a random subsample of 1,925 self-respondents who were subsequently screened for the presence of pain using an item asking whether during the past year they had experienced pain that lasted one week or longer. Respondents answering yes to this question (N=778) were then given the following instructions: “If you have had more than one week-long or longer episode of pain in the past year, please think about the one that was most severe. The remaining questions will be about that episode of pain.” They were next asked a series of questions about pain duration, intensity and impact. Because of the random selection process, Pain Module respondents are representative of the larger HRS sample and—with the use of HRS-provided sampling weights—of the U.S. population of midlife and older adults.
Pain-related outcomes
Domain-specific pain impact and “high impact chronic pain”
Using items from the Pain Disability Index,44 respondents rated the impact of pain on a 0–10 scale (0=no disability and 10=total disability) in the following seven areas: family and home responsibilities; recreation/leisure activities; social activities with friends; paid and non-paid work activities; frequency and quality of sex life; “doing basic things for yourself” (e.g., showering, fixing meals, getting dressed, driving); and “essential activities” (eating, breathing, sleeping). In addition, one yes/no question was asked about the financial impact of pain: “Due to your pain did you have financial difficulty such that it interfered with your ability to pay for things you need?”
Respondents were categorized as having high-impact chronic pain based on the NPS definition29 if they reported a pain episode of ≥ 7 months duration (response categories did not permit identification of those with pain for 6 months), and if they had a mean pain impact score of ≥ 7 in one or more of the following domains: family and home, recreation/leisure, social activities, paid and non-paid work, and basic daily activities. Although the HRS pain measures differ from those included in the NPS (e.g., NPS-suggested questions assess duration of participation restrictions with ordinal response categories ranging from never to always), the operational definition used in the present study is consistent with the NPS in that it captures adults with long-lasting pain that substantially interferes with daily life in at least one major life domain. Our cutoff score of 7 for pain impact was chosen based on similar thresholds used elsewhere to operationalize severe pain intensity or impact.19,29,35
Pain intensity
Respondents were asked, in reference to their most severe pain episode in the last year: “On a 0–10 scale, how would you rate your pain on average?”
Demographic correlates
Age, sex, race (non-Hispanic black, non-Hispanic white, Hispanic, other), education (less than high school, high school diploma or equivalent, more than high school) and quartiles of total household wealth (1st: < $16,000; 2nd: < $131,000; 3rd: < $410,700, 4th:≥$410,700), based on a variable in the data set that represents the sum of household assets and liabilities, incorporating spouse data.8
Health correlates
Several health factors associated with chronic pain in prior studies were examined: Self-reported doctor-diagnosis of the following chronic conditions (yes/no): arthritis, cancer, diabetes, heart disease, high blood pressure, and lung disease. Smoking status: current vs. former/never smoker. Obesity: Body Mass Index ≥ 30 (obese)36 vs. <30, based on self-reported weight and height. Depression (yes/no): A score ≥ 4 on the 8-item Center for Epidemiologic Studies Depression Scale (CES-D), indicating a clinically significant level of depressive symptoms.41
Statistical analysis
Analyses were conducted using Stata/SE 14.2. Data were weighted to reflect the U.S. population of community-dwelling adults 51 years and older using respondent-level weights provided by HRS. Standard errors were adjusted for the clustering and stratification inherent to the HRS sampling design. Health and demographic variables had <1% missing data with the exception of obesity (2% missing). Pain impact variables were missing less than 2.5% of cases with the exception of impact on sex life (8% missing). These were not imputed.
We first estimated the prevalence of high-impact chronic pain in the population, both overall and within subgroups defined by race/ethnicity, education, household wealth, age, and gender. Next, we used bivariate logistic regression models to examine the association between each demographic and health variable and the presence of high-impact chronic pain. We then entered the main predictors of interest – race/ethnicity, education, and wealth – into an initial multiple logistic regression model (Model 1), with high-impact chronic pain (yes versus no) as the outcome and age and gender as covariates. In a subsequent model (Model 2), health indicators were added.
To assess whether health variables, as a group, explained some of the association between sociodemographic factors and high-impact chronic pain, we compared the design-adjusted Wald tests for sociodemographic factors (race/ethnicity, education, and wealth) between Models 1 and 2. A change from statistical significance to non-significance would suggest that health variables helped to explain this relationship. Goodness of fit for both models was tested using Archer and Lemeshow’s design-adjusted test; a non-significant F statistic suggests an adequate fit.2
To examine group differences (defined by education, wealth, and race/ethnicity) in mean pain intensity, we selected all respondents who reported a pain episode lasting “2 to 3 months” or longer. Within this subsample, we also assessed the mean impact of pain on seven activity domains and on the ability to pay for needs. These analyses were not limited to the subgroup of individuals with “high impact chronic pain”, as everyone in that subgroup, by definition, already had at least one domain highly impacted (≥7 on a 0–10 scale) by pain. Instead, for this analysis, we specifically chose all people who were likely to have chronic pain and then assessed differences using the entire potential range of impact (0 to 10). Pairwise comparisons were made among groups with an adjusted alpha (.01) to account for multiple comparisons. Analyses incorporated survey weights and the complex sample design.
RESULTS
In the U.S. population over 50 years of age, 8.2% (95% C.I. = 6.7 to 10.1%) met criteria for high-impact chronic pain in 2010 (Table 1). Differences in prevalence among White (8.0%), Black (9.2%), and Hispanic adults (9.1%) were non-significant (p=0.92). A trend toward declining prevalence with increasing education was observed (p=0.06); from 11.2% among adults without a high school diploma to 6.3% in adults with education beyond high school. Prevalence decreased significantly (p <.001) with increasing wealth, from 17.1% in the bottom wealth quartile to 5.6% in the highest. Prevalence was higher among those 65 and older (8.9%) compared to those under 65 (7.7%), but this difference was not significant (p=.54).
Table 1.
Prevalence of high-impact chronic pain in adults age 51+ in the United States, 2010 (unweighted n=1796)
| Population prevalence (weighted percentage) 95% Confidence Interval |
p-value for between-group differencesa |
N (unweighted) |
|
|---|---|---|---|
| Overall | 8.2 (6.7, 10.1) | – | 176 |
| Gender | .16 | ||
| Female | 9.2 (7.4, 11.2) | – | 112 |
| Male | 7.0 (4.9, 10.0) | – | 64 |
| Race/ethnicity | .92 | ||
| Non-Hispanic White | 8.0 (6.5, 10.0) | – | 114 |
| Non-Hispanic Black | 9.2 (6.1, 13.6) | – | 36 |
| Hispanic | 9.1 (4.3, 18.4) | – | 22 |
| Other | 8.1 (2.5, 23.0) | – | 4 |
| Education | .06 | ||
| Less than high school | 11.2 (7.6, 16.2) | – | 48 |
| High school/equivalent | 10.0 (6.8, 14.4) | – | 64 |
| More than high school | 6.3 (4.6, 8.7) | – | 64 |
| Total household wealth quartile | .00 | ||
| Quartile 1 (lowest) | 17.1 (12.3, 23.4) | – | 73 |
| Quartile 2 | 7.7 (4.9, 12.9) | – | 38 |
| Quartile 3 | 5.8 (4.0, 8.3) | – | 36 |
| Quartile 4 (highest) | 5.6 (4.2, 7.4) | – | 29 |
| Age group | .54 | ||
| 51–64 years | 7.7 (5.5, 10.7) | – | 81 |
| 65+ years | 8.9 (6.9, 11.3) | – | 87 |
Rao-Scott Design-based F
A number of health conditions had a significant (p<.05) bivariate relationship with high-impact chronic pain (Table 2): arthritis (OR=7.67), cancer (OR=1.94), depression (OR=3.30), diabetes (OR=1.61), heart disease (OR=1.94), high blood pressure (OR=1.70), lung disease (OR=2.72), and smoking (OR=2.01).
Table 2.
Predictors of high-impact chronic pain among U.S. adults age 51+, unadjusted logistic regression models (unweighted n=1796)
| Odds Ratio | (95% Confidence Interval) | P value | |
|---|---|---|---|
| Age 65+ | 1.15 | (0.72, 1.83) | .546 |
| Female | 1.34 | (0.89, 2.00) | .157 |
| Race/ethnicity | |||
| Non-Hispanic White | reference | reference | |
| Non-Hispanic Black | 1.16 | (0.77, 1.75) | .472 |
| Hispanic | 1.15 | (0.51, 2.61) | .735 |
| Other | 1.01 | (0.29, 3.47) | .987 |
| Education | |||
| High school | reference | reference | |
| Less than high school | 1.13 | (0.65, 1.98) | .662 |
| More than high school | 0.61 | (0.34, 1.07) | .088 |
| Household wealth | |||
| Quartile 1 (lowest) | reference | reference | |
| Quartile 2 | 0.40 | (0.22, 0.73) | .004 |
| Quartile 3 | 0.30 | (0.18, 0.50) | .000 |
| Quartile 4 (highest) | 0.29 | (0.18, 0.46) | .000 |
| Health Correlatesa | |||
| Arthritis | 7.67 | (4.27, 13.75) | .000 |
| Cancer | 1.94 | (1.15, 3.26) | .014 |
| Depression | 3.30 | (2.23, 4.87) | .000 |
| Diabetes | 1.61 | (1.02,2.56) | .041 |
| Heart Disease | 1.94 | (1.26, 2.99) | .003 |
| High Blood Pressure | 1.70 | (1.12, 2.59) | .013 |
| Lung Disease | 2.72 | (1.49, 4.96) | .001 |
| Obese | 1.27 | (0.82, 1.98) | .283 |
| Current smoker | 2.01 | (1.10, 3.67) | .024 |
All health correlates coded as dichotomous (yes vs. no; “no” is referent group).
Results from the initial multiple logistic regression model are displayed in Table 3. Compared to U.S. adults in the lowest wealth quartile, those in the highest three quartiles had significantly lower odds of having high-impact chronic pain, controlling for race/ethnicity, education, age, and gender. The Wald F test for wealth was significant (F(3,54)=9.06, p=.000), indicating a significant overall association of this variable with pain. No significant association was evident for race/ethnicity, education, age or gender.
Table 3.
Predictors of high-impact chronic pain among U.S. adults age 51+, multiple logistic regression models
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age 65+ | 1.24 (0.76, 2.03) | .384 | 0.78 (0.45, 1.36) | .380 |
| Female | 1.34 (0.89, 2.01) | .163 | 1.08 (0.69, 1.69) | .738 |
| Race/ethnicityc,d,e | ||||
| Non-Hispanic White | reference | – | reference | |
| Non-Hispanic Black | 0.73 (0.44, 1.23) | .236 | 0.66 (0.38, 1.16) | .146 |
| Hispanic | 0.77 (0.32, 1.82) | .541 | 1.28 (0.60, 2.73) | .508 |
| Educationd,e | ||||
| High school | reference | – | reference | |
| Less than high school | 0.98 (0.51, 1.87) | .944 | 1.06 (0.56, 1.98) | .861 |
| More than high school | 0.73 (0.41, 1.31) | .284 | 0.88 (0.48, 1.58) | .657 |
| Household wealthd,e | ||||
| Quartile 1 (lowest) | reference | reference | ||
| Quartile 2 | 0.39 (0.21, 0.72) | .003 | 0.53 (0.28, 1.00) | .050 |
| Quartile 3 | 0.28 (0.16, 0.49) | .000 | 0.42 (0.24, 0.73) | .003 |
| Quartile 4 (highest) | 0.27 (0.16, 0.46) | .000 | 0.48 (0.29, 0.79) | .004 |
| Comorbidities | – | – | ||
| Arthritis | – | – | 6.65 (3.54, 12.49) | .000 |
| Cancer | – | – | 1.61 (0.94, 2.78) | .081 |
| Depression | – | – | 1.92 (1.17, 3.17) | .011 |
| Diabetes | – | – | 1.23 (0.75, 2.04) | .396 |
| Heart Disease | – | – | 1.42 (0.95, 2.14) | .086 |
| High Blood Pressure | – | – | 1.07 (0.68, 1.69) | .771 |
| Lung Disease | – | – | 1.50 (0.79, 2.86) | .208 |
| Obesity | 1.15 (0.72, 1.83) | .541 | ||
| Current smoker | 1.54 (0.84, 2.81) | .157 |
Unweighted n= 1788
Unweighted n=1736
Non-Hispanic White respondents were combined with those of “Other” race/ethnicity, due to the nearly identical prevalence of high-impact chronic pain in these two groups
Model 1 Wald tests for overall significance of categorical variables with ≥2 levels: Race/ethnicity: F(2,55)=0.85, p=.432 Educational attainment: F(2,55)=0.69, p=.506 Household wealth: F(3,54)=9.06, p=.000.
Model 2 Wald tests: Race/ethnicity: F(2,55)=1.28, p=.286 Educational attainment: F(2,55)=0.23, p=.799 Household wealth: F(3,54)=3.68, p=.017.
When health predictors were added (Model 2), the Wald F statistic for wealth was reduced (F(3,54)=3.68, p=.017), suggesting that health factors partially explain the association between wealth and pain. Adjusting for sociodemographic and other health variables, the only health conditions retaining statistical significance were arthritis (OR=6.65; p <.001) and depression (OR=1.92; p =.011). The non-significant F statistics for Archer and Lemeshow’s design-adjusted test indicate acceptable fit for both models.
Figures 1 and 2 show differences by race/ethnicity and wealth in mean ratings of average pain intensity and impact on specific domains, among individuals experiencing pain for at least 2–3 months. Pain intensity and impact across all domains decreased monotonically with increasing wealth, with statistically significant pairwise contrasts found between the highest quartile and the two lowest quartiles. White adults reported significantly less average pain than Black or Hispanic adults. Across activity domains, Black adults reported the highest pain impact, and Hispanic adults second-highest, though contrasts did not always reach significance. The impact of chronic pain tended to decrease with increasing education (Supplementary Figure 1), with some significant pairwise comparisons between respondents who completed “more than high school” and other groups. Only 1% of individuals in the highest wealth quartile and 12% of White adults with chronic pain reported that pain affects their ability to “pay for needs.” In contrast, nearly half (45%) of adults in the lowest wealth quartile and more than one-third of Black (35%) and Hispanic (38%) adults reported that it does so (Supplementary Table 1).
Figure 1.
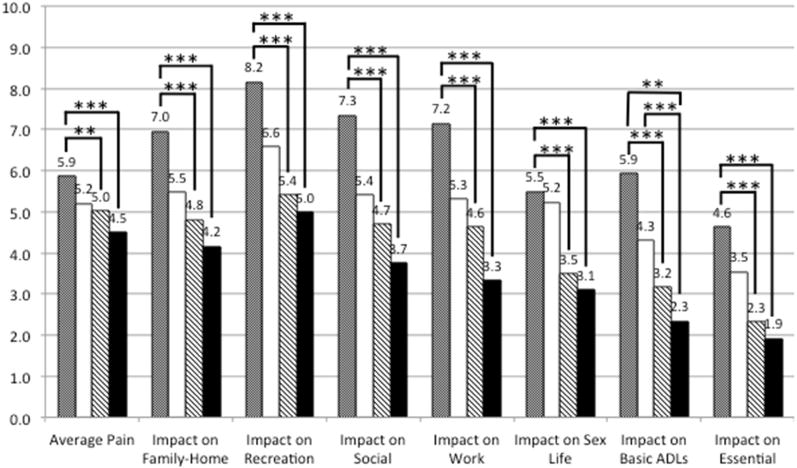
Average pain intensity and domain-specific impact by race/ethnicity
Race/ethnicity (White non-Hispanic, Black non-Hispanic, Hispanic)

Brackets indicate statistically significant pairwise comparison, * p < .05, ** p < 0.01, *** p < 0.001
Figure 2.
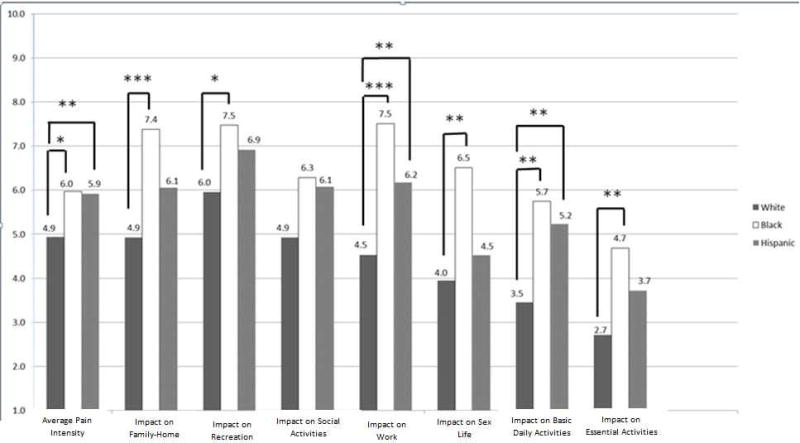
Average pain intensity and domain-specific impact by household wealth quartile
Household wealth quartile

Brackets indicate statistically significant pairwise comparison, ** p < 0.01, *** p < 0.001
DISCUSSION
Chronic pain is typically reported to affect about one-third of the U.S. population. We found that high-impact chronic pain – defined not by pain intensity but by its protracted duration and substantial negative impact on daily life - affects 8% of the U.S. population over age 50. This estimate falls within the 6% to 14% range for severe, disabling pain that has been reported in previous population studies.27, 45 Present findings help identify priority groups for efforts to alleviate the burden of chronic pain in the middle-aged and older population.
Our analysis revealed striking wealth disparities in high-impact chronic pain, with the percentage of adults living with the condition greatest among those with the least wealth. Although high impact pain was more common among women, Black adults, individuals with a high school education or less, and adults over age 65 than their respective counterparts, differences were nonsignificant. Among people with any chronic pain (i.e., 2 to 3+ months’ duration), average pain intensity and pain-related disability were greater among Black than White respondents, and decreased as wealth increased.
Wealth differences
To our knowledge, this is only the second U.S. population-based study to link household wealth, an indicator of a person’s financial resources amassed over a lifetime,33 with disparities in chronic pain. Compared to income, wealth may more accurately reflect the economic resources of older people, who are less likely to be in the paid labor force,33 and is less subject to gender, race and cohort effects than education.3 Using 1998–2010 HRS data, Grol-Prokopczyk15 also reported wealth-based disparities in pain prevalence and pain-related disability. While highly informative, these findings are based on three general pain items (e.g., “Are you often troubled with pain?”) that capture neither pain’s duration nor its impact on specific life domains.
Our work extends Grol-Prokopczyk’s by revealing that midlife and older adults with more wealth are less likely to experience high-impact chronic pain using a definition informed by the NPS. Moreover, among people with any chronic pain, we found that pain interference across multiple life domains (family/home, recreation, social activities, paid and unpaid work, sexual behavior, and basic and essential daily activities) decreases monotonically with increasing wealth. We propose that the observed wealth differences likely stem from the web of factors that have been posited to explain the relationship between SES and pain.5
Biological factors include an increasing likelihood of poor health as wealth decreases.34 We found that the relationship between wealth and pain was attenuated after accounting for chronic diseases and risk factors for these conditions (smoking and obesity). Psychological risk factors for pain, such as mood disorders, stress and a history of trauma, are also more prevalent among people with fewer economic resources.32, 33, 23 In our study, depression was strongly associated with the high-impact pain, though the association was weaker when wealth was in the model, indicating overlap of the two conditions.
Certain environmental factors linked to wealth can influence the development of chronic pain and its impact. These include past or current occupational hazards, such as physically taxing jobs offering low autonomy.32 Pain over the life course may also inhibit wealth accumulation, as pain is associated with work-related disability.21 Wealth enables modification of one’s living environment to facilitate functioning (e.g., replacing steps with a ramp, moving to a more accessible home, hiring assistance). It allows individuals with chronic pain to pay for conveniences that enable leisure activities like travel.
Wealthier older adults benefit from past and current access to higher-quality health care, which may prevent the onset of chronic pain or curtail its downstream consequences on daily functioning.26 Also, non-pharmacological treatments that are potentially efficacious for reducing pain’s impact (e.g., mindfulness training)6 may not be covered by insurance, placing such therapies out of reach for many older Americans.28 Among older adults with any chronic pain, almost half of individuals in the lowest wealth quartile, and 35% of African Americans, reported that pain impacted their ability to “pay for needs”–suggesting that even in a population largely insured through Medicare, chronic pain presents a financial hardship.
Racial differences
In both unadjusted and adjusted analysis, we found no significant difference in the odds of experiencing high-impact chronic pain for Black or Hispanic adults compared to Whites. After controlling for socioeconomic variables in her aforementioned analysis of HRS data, Grol-Prokopczyk15 found no Hispanic-White difference in being “often troubled with pain”–a more general indicator of chronic pain than used in the present study–and a Black advantage in this regard. Among individuals with any chronic pain in our study, White adults reported lower average pain intensity than other groups, which is congruent with Grol-Prokopczyk’s finding that White adults were least likely to report severe pain. We also found that White adults experienced less pain-related disability in most activity domains compared to Black adults, with fewer White-Hispanic differences.
Overall, our study adds to evidence15,35,37 suggesting that socioeconomic disadvantage has a stronger negative impact on the prevalence of chronic pain than race/ethnicity per se. However, Meghani & Chittami25 rightly caution against the conclusion that “race does not matter but wealth does,” given that race is a key determinant of SES. White Americans as a group have vastly more accumulated wealth than Black Americans,3 due in large part to institutional discrimination and segregation in housing.46 In the present study, African American and Hispanic respondents together made up only 3% of the top wealth quartile compared with 36% of the lowest (see Supplementary Table 2). Given the strong link between wealth and pain-related disability observed in the present study, our findings suggest that racial inequalities in wealth— along with other established factors such as inequitable pain care14 – may be a major contributor to the pain burden among older African Americans.
Age differences
As in prior studies,27,37 we found that the prevalence of high-impact chronic pain does not rise sharply from middle to older adulthood. Although many painful conditions are more prevalent in older adulthood, others (e.g., fibromyalgia, migraine) are more common in middle age,24,39 and the onset of certain age-associated conditions like arthritis often occurs in midlife. Also, work and family demands tend to peak during midlife, potentially increasing the impact of pain on these aspects of daily life. In light of recent evidence presented by Grol-Prokopczyk, however, it should be acknowledged that a lack of age differences in cross-sectional studies can be misleading, as mortality selection may mask age-related increases in chronic pain prevalence.15
Association with health variables
After adjusting for covariates, people with arthritis had nearly seven times the odds of experiencing high-impact chronic pain than those without arthritis. Our findings therefore support expanding efforts to prevent and manage this debilitating illness, including dissemination of evidence-based physical activity and other arthritis self-management interventions.17
Limitations
As noted, the survey items used to identify people with high-impact chronic pain did not align perfectly with the NPS definition29 (e.g., pain of ≥ 7 months duration, instead of 6, was examined). Also, when answering items, respondents were instructed to consider their most severe episode of pain lasting one week or longer. Because of this wording, our findings may overestimate the usual impact of chronic pain in the lives of Americans over age 50. The subgroup with any chronic pain, in which we assessed differences in domain-specific interference, included those with pain of “2 to 3 months” duration. Because chronic pain is typically defined as pain lasting ≥ 3 months, we may have included some respondents in this analysis whose pain was not chronic per the usual definition.
Other limitations include the fact that the Hispanic subgroup was small, resulting in large standard errors. Moreover, Hispanic Americans are a heterogeneous group and acculturation and country of origin could affect the pain experience.16 We did not examine interactive effects of race, SES, and gender, and age on pain outcomes, though there is evidence that complex patterns are present in the links between these factors and health outcomes.4 We encourage exploration of these intersections in future investigations. Last, we are unable to estimate pain prevalence among Asian Americans, a diverse and growing group of older adults. Limitations notwithstanding, the publicly-available Health and Retirement Study dataset has notable strengths including a sampling design that permits extrapolation to the U.S. population of the same age group and high-quality economic data.
Implications
Reducing the prevalence of high-impact chronic pain in the United States is a Healthy People 2020 goal.45 This type of pain results when individuals are unable to sufficiently manage their pain and/or environment. Fortunately, there is a solid evidence base of interventions that can reduce pain’s impact on daily functioning, independent of addressing its underlying cause. For example, cognitive behavioral therapy for pain improves function and reduces distress by teaching self-management skills.11 Although few older adults with chronic pain currently receive such non-pharmacologic treatments,11,22 access may be expanded via new delivery modes (e.g., internet) and greater reimbursement by insurers. Ultimately, such changes may help curb widespread opioid misuse and overuse.30
Conclusion
We found that 8.2% of Americans over age 50 experience high-impact chronic pain, which has marked adverse effects on functioning and quality of life. Among older adults in the bottom wealth quartile, this proportion more than doubles. Efforts to reduce high-impact chronic pain should be directed toward socioeconomically vulnerable groups to minimize disability and suffering.
Supplementary Material
Supplementary Figure 1: Average pain intensity and domain-specific impact by educational attainment (less than high school, high school diploma or equivalent, more than high school)

Brackets indicate statistically significant pairwise comparison, ** p < 0.01, *** p < 0.001
Perspective.
High-impact chronic pain is unequally distributed among midlife and older U.S. adults. Efforts to reduce the burden of disabling chronic pain should prioritize socioeconomically vulnerable groups, who may have the least access to multi-modal pain treatment to improve function.
Highlights.
The National Pain Strategy calls for population research on high-impact chronic pain.
We assessed the prevalence of such pain in the U.S., overall and by race and SES.
About 8% of U.S. adults over age 50 have high-impact chronic pain.
Wealth was more strongly associated with high-impact pain than race/ethnicity.
In adults with any chronic pain, Black adults had most disability across domains.
Acknowledgments
This work was supported in part by the National Institute on Aging (K01 AG050706 to MRJ). The Health and Retirement Study is sponsored by the National Institute on Aging (U01AG009740) and is conducted by the University of Michigan. Support was also provided by the National Institutes of Health (P30DK092926). JDP is a VA Research Career Scientist.
We thank Brett Slajus for help with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no personal or financial conflicts.
Author Contributions: Janevic: study conception and design, drafting the manuscript, statistical analysis, interpretation of data. McLaughlin, Heapy, Thacker, Piette: study conception and design, interpretation of data, revising manuscript.
Contributor Information
Mary R. Janevic, Center for Managing Chronic Disease and Department of Health Behavior and Health Education, University of Michigan School of Public Health.
Sara J. McLaughlin, Department of Sociology and Gerontology and Scripps Gerontology Center, Miami University.
Alicia A. Heapy, VA Connecticut Healthcare System Pain Research, Informatics, Multimorbidities, and Education (PRIME) Health Services Research and Development Center of Innovation, Yale University School of Medicine.
Casey Thacker, Department of Health Behavior and Health Education, University of Michigan School of Public Health.
John D. Piette, Center for Managing Chronic Disease and Department of Health Behavior and Health Education, University of Michigan School of Public Health.
References
- 1.Adler NE, Stewart J. Health disparities across the lifespan: Meaning, methods, and mechanisms. Annals of the New York Academy of Sciences. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- 2.Archer KJ, Lemeshow S, Hosmer DW. Goodness-of-fit tests for logistic regression models when data are collected using a complex sampling design. Computational Statistics & Data Analysis. 2007;51:4450–4464. [Google Scholar]
- 3.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Meltzer M, Posner S. Socioeconomic status in health research: One size does not fit all. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 4.Brown TH, Richardson LJ, Hargrove TW, Thomas CS. Using multiple-hierarchy stratification and life course approaches to understand health inequalities: The intersecting consequences of race, gender, SES, and age. J Health Soc Behav. 2016;57:200–222. doi: 10.1177/0022146516645165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell LC, Robinson K, Meghani SH, Vallerand A, Schatman M, Sonty N. Challenges and opportunities in pain management disparities research: Implications for clinical practice, advocacy, and policy. The Journal of Pain. 2012;13:611–619. doi: 10.1016/j.jpain.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherkin DC, Sherman KJ, Balderson BH, Cook AJ, Anderson ML, Hawkes R, Hansen K, Turner J. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: A randomized clinical trial. JAMA. 2016;315:1240–1249. doi: 10.1001/jama.2016.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherry BJ, Zettel-Watson L, Shimizu R, Roberson I, Rutledge DN, Jones CJ. Cognitive performance in women aged 50 years and older with and without fibromyalgia. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2014;69:199–208. doi: 10.1093/geronb/gbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien S, Campbell N, Chan C, Hayden O, Hurd M, Bugliari D, Main R, Mallett J, McCullough C, Meijer E, Moldoff M, Pantoja P, Rohwedder S, Calir P. RAND Health and Retirement Study (HRS) Data Documentation. RAND Center for the Study of Aging; 2016. [Google Scholar]
- 9.Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009;57:1556–1561. doi: 10.1111/j.1532-5415.2009.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards RR. Age differences in the correlates of physical functioning in patients with chronic pain. J Aging Health. 2006;18:56–69. doi: 10.1177/0898264305280976. [DOI] [PubMed] [Google Scholar]
- 11.Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am Psychol. 2014;69:153–166. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- 12.Ferrell BR, Ferrell BA. Pain in the Elderly. IASP Press; 1996. Overview of aging and pain; pp. 1–10. [Google Scholar]
- 13.Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US Black women experience stress-related accelerated biological aging?: A novel theory and first population-based test of Black-White differences in telomere length. Human Nature (Hawthorne, NY) 2010;21:19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Medicine. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 15.Grol-Prokopczyk H. Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain. 2017;158:313–322. doi: 10.1097/j.pain.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingshead NA, Ashburn-Nardo L, Stewart JC, Hirsh AT. The Pain Experience of Hispanic Americans: A critical literature review and conceptual model. The Journal of Pain. 2016;17:513–528. doi: 10.1016/j.jpain.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hootman JM, Helmick CG, Brady TJ. A public health approach to addressing arthritis in older adults: The most common cause of disability. Am J Public Health. 2012;102:426–433. doi: 10.2105/AJPH.2011.300423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, D.C: National Academies Press; 2011. p. xvii.p. 364. [PubMed] [Google Scholar]
- 19.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. The Journal of Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Kapteyn A, Smith JP, van Soest A. Dynamics of work disability and pain. Journal of Health Economics. 2008;27:496–509. doi: 10.1016/j.jhealeco.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keefe FJ. Translational behavioral pain management: New directions and new opportunities. Translational Behavioral Medicine. 2012;2:19–21. doi: 10.1007/s13142-012-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lantz PM, House JS, Mero RP, Williams DR. Stress, life events, and socioeconomic disparities in health: Results from the Americans’ Changing Lives Study. J Health Soc Behav. 2005;46:274–288. doi: 10.1177/002214650504600305. [DOI] [PubMed] [Google Scholar]
- 24.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 25.Meghani SH, Chittams J. Controlling for socioeconomic status in pain disparities research: All-else-equal analysis when “all else” is not equal. Pain Medicine. 2015;16:2222–2225. doi: 10.1111/pme.12829. [DOI] [PubMed] [Google Scholar]
- 26.Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: Directions for health policy, education, practice, and research. Pain Medicine. 2012;13:5–28. doi: 10.1111/j.1526-4637.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 27.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. The Journal of Pain. 2015;16:769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahin RL, Stussman BJ, Herman PM. Out-of-pocket expenditures on complementary health approaches associated with painful health conditions in a nationally representative adult sample. The Journal of Pain. 2015;16:1147–1162. doi: 10.1016/j.jpain.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Pain Strategy. US Department of Health and Human Services. 2016 [Google Scholar]
- 30.Olsen Y. The CDC Guideline on Opioid Prescribing: Rising to the Challenge. JAMA. 2016;315:1577–1579. doi: 10.1001/jama.2016.1910. [DOI] [PubMed] [Google Scholar]
- 31.Podlasek S. Pain management to promote independence in older adults. J Am Geriatr Soc. 2010;58:1195–1196. doi: 10.1111/j.1532-5415.2010.02878.x. [DOI] [PubMed] [Google Scholar]
- 32.Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. 2008;136:235. doi: 10.1016/j.pain.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollack CE, Chideya S, Cubbin C, Williams B, Dekker M, Braveman P. Should health studies measure wealth? A systematic review. Am J Prev Med. 2007;33:250–264. doi: 10.1016/j.amepre.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Pollack CE, Cubbin C, Sania A, Hayward M, Vallone D, Flaherty B, Braveman PA. Do wealth disparities contribute to health disparities within racial/ethnic groups? J Epidemiol Community Health. 2013;67:439–445. doi: 10.1136/jech-2012-200999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: Differences among white, African American, and Hispanic subjects. The Journal of Pain. 2004;5:317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Practical Guide to Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health; Oct, 2000. [Google Scholar]
- 37.Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: Non-Hispanic whites, non-Hispanic blacks, and Hispanics. The Journal of Pain. 2007;8:75–84. doi: 10.1016/j.jpain.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riskowski JL. Associations of socioeconomic position and pain prevalence in the United States: Findings from the National Health and Nutrition Examination Survey. Pain Medicine. 2014;15:1508–1521. doi: 10.1111/pme.12528. [DOI] [PubMed] [Google Scholar]
- 39.Rustoen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: Differences in health and quality of life among younger, middle-aged, and older adults. Clin J Pain. 2005;21:513–523. doi: 10.1097/01.ajp.0000146217.31780.ef. [DOI] [PubMed] [Google Scholar]
- 40.Sonnega A, Weir D. The Health and Retirement Study: A Public Data Resource for Research on Aging. Open Health Data. 2014 [Google Scholar]
- 41.Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study. Survey Research Center, University of Michigan; Ann Arbor, Michigan: p. 2000. [Google Scholar]
- 42.Stubbs B, Schofield P, Binnekade T, Patchay S, Sepehry A, Eggermont L. Pain Medicine. Vol. 15. Maldenm MA: 2014. Pain is associated with recurrent falls in community-dwelling older adults: Evidence from a systematic review and meta-analysis; pp. 1115–1128. [DOI] [PubMed] [Google Scholar]
- 43.Tait RC, Chibnall JT. Racial/ethnic disparities in the assessment and treatment of pain: Psychosocial perspectives. American Psychologist. 2014;69:131. doi: 10.1037/a0035204. [DOI] [PubMed] [Google Scholar]
- 44.Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Archives of Physical Medicine and Rehabilitation. 1987;68(7):438–1. [PubMed] [Google Scholar]
- 45.Von Korff M, Scher AI, Helmick C, Carter-Pokras O, Dodick DW, Goulet J, Hamill-Ruth R, LeResche L, Porter L, Tait R, Terman G, Veasley C, Mackey S. United States National Pain Strategy for population research: Concepts, definitions, and pilot data. The Journal of Pain. 2016 doi: 10.1016/j.jpain.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Williams DR, Collins C. Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Reports. 2001;116:404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Average pain intensity and domain-specific impact by educational attainment (less than high school, high school diploma or equivalent, more than high school)

Brackets indicate statistically significant pairwise comparison, ** p < 0.01, *** p < 0.001


