Abstract
Following infection with almost any given microorganism other than an emerging pathogen, only a minority of individuals develop life-threatening clinical disease, implying that these individuals have some form of immunodeficiency. A growing number of inherited and acquired immunodeficiencies have been deciphered over the last 50 years. HIV infection is probably the best-known acquired immunodeficiency. It emerged about 40 years ago and precipitates various severe infections, the occurrence of which is associated with a fall in circulating CD4+ T cells after HIV infection, in the presence or absence of antiretroviral treatment (ART). However, despite the strength of this correlation, infection rates differ between patients with similar levels and durations of CD4+ T lymphopenia. Moreover, a few infections seem to be less dependent on total CD4+ T-cell levels. The fine detail of the mechanisms underlying these infections is unknown. We discuss here how studies of the human genetics and immunology of some of these infections in patients with primary immunodeficiencies (PIDs) have provided unique insights into their molecular and cellular basis. Defects of specific CD4+ Th-cell subsets independently account for some of these infections, as best exemplified by Th1* for mycobacteriosis and Th17 for candidiasis. PIDs are individually rare, but collectively much more common than initially thought, with new disorders being discovered at an ever-increasing pace and a global prevalence worldwide approaching that of HIV infection. Studies of known and new PIDs should make it possible to dissect the pathogenesis of most human infections at an unprecedented level of molecular and cellular precision. The predictive, preventive, and therapeutic implications of studies of immunity to infection in PIDs may extend to HIV-infected patients and patients with infectious diseases in other settings.
Introduction
Since its discovery in 1984, HIV has claimed more than 35 million lives. At the end of 2015, there were 36.7 million individuals living with HIV, including 2.1 million newly infected in 2015 (WHO, http://www.who.int/mediacentre/factsheets/fs360/en/). This corresponds to about 0.5% of the human population, with proportions ranging from less than 1/1,000,000 in some countries to 50% in others. HIV-associated immunodeficiency is probably the single most common known human immunodeficiency, manifesting in the most severe cases as acquired immunodeficiency syndrome (AIDS) [1, 2]. Unlike HIV infection, primary immunodeficiencies (PIDs), defined as monogenic inborn errors of immunity, are individually rare. However, they have proved to be collectively much more common than initially thought [3-8], as both new phenotypes and new genotypes are being discovered at an ever-increasing pace [6, 9]. Totally 330 gene defects have already been shown to cause PIDs, some of which have been reported in fewer than 10 patients, with some described in only one patient [10, 11], whereas others have been identified in thousands of patients [4]. The prevalence of individual PIDs in human populations varies between ethnic groups, from less than 1/1,000,000 to over 1/10,000. Based on the current definition of PIDs and the conditions known, their collective prevalence can be conservatively estimated to at least 1/1,000, a figure not far from the global prevalence of HIV immunodeficiency. Their actual prevalence is probably even higher [12-14]. Interestingly, the outcome of HIV infection itself is strongly influenced by human genetic makeup: homozygosity for the CCR5 deletion mutation CCR5Δ32 protects against HIV infection, and several variants within or near the genes encoding class I HLA molecules or, to a lesser extent, the CCR5 locus, influence clinical outcome after HIV-1 infection [15, 16]. HIV immunodeficiency and PIDs appear to be very different etiologically and demographically, but they have one key clinical hallmark in common: abnormally high susceptibility to various severe infections.
HIV-infected patients suffer from both common infections (CIs) and opportunistic infections (Ols), this second group of infections being caused by pathogens that strike preferentially by taking advantage of a detectable immunological deficit, an opportunity not normally available in other hosts. Most of the CIs and Ols observed in HIV patients are closely associated with decreases in the patient's CD4+ T-cell count [17]. However, the decrease in CD4+ T-cell count would probably underlie infections caused by a number of different mechanisms, rather than susceptibility to diseases caused by a single mechanism. This would explain why HIV-infected individuals with the same global CD4+ T-cell counts, sometimes even with the same duration of CD4+ T lymphopenia, can suffer from different infections. [18]. The disruption of specific CD4+ T-cell functions is probably more critical for the development of specific CIs and Ols than the fall in total CD4+ T-cell count per se. Moreover, there are at least six known subsets of CD4+ T cells, including Tregs and five Th subsets, Th1, Th1*, Th2, Th17, TFH, each of which has been predicted or shown to fight specific infections [19, 20]. For example, the newly described Th1* cells share chemokine receptors and transcriptional factors with both classical Th1 cells and Th17 cells, yet they are the preferential producers of IFN-γ in response to mycobacterial infections, whereas the classical Th1 cells produce IFN-γ in response to viral infections [20]. HIV targets these Th CD4+ subsets differently, resulting in the loss of certain subsets and the expansion of others [18]. In this context, studies of PIDs involving defects of the development or function of specific Th CD4+ subsets can be used to analyze the pathogenesis of specific infections also seen in HIV patients [13, 14, 21]. The decline of specific Th subsets in HIV patients may be a better indicator of infection risk than total CD4+ T-cell counts. This approach may also shed light on the reasons for which a few infections are not well correlated with total CD4+ T-cell count in HIV-infected patients. We will consider here a few infections seen in HIV-infected children and adolescents, the molecular and cellular pathogenesis of which has been clarified by the study of PIDs, which also typically strike young patients.
Infections and immunological abnormalities in HIV-infected patients
HIV-infected patients display various CIs and Ols, including viral, bacterial, fungal, and parasitic infections. The most frequent infections in children and adolescents are listed in Table 1. The decrease in CD4+ T-cell count is the main immunological abnormality observed, and most infections in these patients (disseminated Mycobacterium avium complex, cytomegalovirus retinitis, histoplasmosis, and cerebral toxoplasmosis) are closely associated with low CD4+ T-cell counts [17]. During the acute phase of infection, HIV specifically targets CCR5+ CD4+ TEM, triggering the activation and proliferation of TCM to replace the lost TEM. At later stages, progressive HIV infection slowly depletes the TCM pool, resulting in a failure to maintain sufficient numbers of TEM, which precipitates immunodeficiency and leads to infections [18]. interestingly, HIV does not seem to infect the different subsets of CD4+ T cells equally. The Th17 and TFH subsets seem to be more permissive to HIV infection than other CD4+ Th cells [22-27]. During the acute phase, HIV preferentially targets Th17 and TFH cells, leading to a considerable decrease in the number of Th17 cells. By contrast, TFH cell numbers increase, and the proportion of TFH cells increases with follicular hyperplasia [18]. Other immunological abnormalities observed in HIV patients include the hyperactivation of CD8+ T cells, expansion of the CD4+ Treg cell population, the hyperactivation of B cells, impaired memory B-cell function, a crippled antibody responses to vaccines, decreases in the numbers of myeloid and plasmacytoid dendritic cells, NK cells, and T-cell receptor-invariant CDld restricted T cells [28-31]. Highly active ART (HAART) in children generally rescues most of these abnormalities [30, 31]. A few infections, such as pulmonary tuberculosis and pneumococcal pneumonia [17], do not seem to be strongly correlated with very low CD4+ T-cell counts and may, therefore, be favored by some of these other immunological abnormalities. These infections are often observed in untreated HIV patients with CD4+ T-cell counts exceeding 200/μl [17]. They also remain common in HIV patients receiving HAART [32-35], consistent with a role for immunological mechanisms other than the decrease in total CD4+ T-cell count. Finally, even with HAART, some patients do not achieve a full reconstitution of their immune system, particularly in cases of persistent HIV viremia, early HIV transmission, and late initiation of treatment [30, 36]. These patients with suboptimal immune reconstitution often suffer from residual infections, which pose new challenges in the post-HAART era. It would therefore be useful to clarify the fine molecular, cellular, and immunological pathogenesis of infections in HIV patients in both untreated patients and patients on HAART.
Table 1.
Ols and Sis in HIV-infected children, listed in order of estimated incident risk [31].
| IR of ART-naïve children | Occurs with high CD4* | Major relevant PIDs | |
| Bacterial pneumonia | 25.01 | + (pneumococcol pneumonia) | Complement deficiencies, XLA, CD40/CD40L, ICOS deficiency, NEMO and NFKBIA mutations, IRAK-4 and MYD88 deficiencies |
| Mycobacterium tuberculosis (unspecified) | 12.36 | ||
| Pulmonary tuberculosis | 9.78 | + | Defects of IFN-γ immunity: ISG15, IL12B, IL12RB1, TYK2, IFNGR1, IFNGR2, JAK1, STAT1 |
| Oral and esophageal candidiasis | 8.29 | ||
| Extrapulmonary tuberculosis | 7.26 | ||
| Varicella zoster virus | 4.69 | ||
| Bacterial sepsis | 3.95 | ||
| Pneumocystis pneumonia | 3.48 | ||
| Cerebral toxoplasmosis | 3.06 | ||
| Cryptosporidium diarrhea | 2.92 | ||
| Herpes simplex | 1.59 | ||
| Bacterial meningitis | 0.95 | ||
| CMV retinitis | 0.82 | ||
| Cryptococcus neoformans meningitis | 0.55 |
IR: incident risk;
over 25% patients with these infections had a CD4 T-cell count >200/μ [15].
Infections and immunological abnormalities in patients with PIDs
The current international classification of PIDs is based on the principal detectable immunological phenotype [4]. A large number of PIDs affect CD4+ T cells, and this diverse group of diseases can be classified into at least three groups: severe combined immunodeficiency (SCID), in which T cells are completely absent, combined immunodeficiencies (CID), which are defined by defective T-cell responses to antigens in vitro with at least some T cells present in the blood, and subtler T-cell defects limited to disruption of the development or function of specific Th subsets [37]. Autosomal recessive RAG1 and RAG2 deficiencies, which also prevent the development of B cells [9], are SCIDs. HLA-II deficiency, which prevents the development of HLA-ll-restricted antigen-specific CD4+ T cells [38], is a CID. RLTPR deficiency, which crippled the development of Th1, Th17, TFH, and Treg [39], also present as CID. An example of the impaired development of a single Th subset is provided by the absence of Th17 cells in patients with various inborn errors, as detailed below [40]. There are currently 16 known genetic etiologies of SCID, 79 known etiologies of CID, and 13 known etiologies of selective disruption of the development or function of a specific Th subset [4]. As an aside, various PIDs are known to disturb the development or function of leukocyte subsets other than CD4+ T cells, which may also be affected by HIV infection in other patients. At any rate, the range of infections seen in patients with PIDs affecting CD4+ T cells can be very broad in cases in which the patient has no T cells, more limited when only CD4+ Th cells are lacking, or narrow when the development or function of a specific Th subset is deficient. In all cases, the occurrence of a given infection in patients with a specific PID indicates that the product of the gene that has been mutated is essential for protective immunity against this pathogen, in at least some individuals. The more frequent the infection among cases with mutations of the gene concerned from different genetic backgrounds, the more robust the inference of causality.
Mucocutaneous candidiasis in patients with PIDs
Children with a lack of functional CD4+ T cells have long been known to display chronic mucocutaneous candidiasis (CMC) [40]. The key role of Th17 cells in host defense against Candida was established by the discovery of inborn errors of IL-17 immunity. Pathogens recognized by phagocytes and epithelial cells at mucosal and cutaneous barriers trigger the secretion of IL-1, IL-6, IL-21, IL-23, and TGFβ. These cytokines induce the differentiation of CD4+ T cells into IL-17A- and IL-17F-producing cells through the action of at least two transcription factors, STATS and RORI t. IL-17 cytokines control fungi by activating endothelial cells and attracting neutrophils to infection sites (Figure 1). Mutations of 10 genes have recently been shown to cause CMC due to the disruption of Th17 development or function [41-45]. Mutations of various genes, including AIRE, STAT3 (loss-of function mutations), STAT1 (gain-of-function mutations), and RORC, underlying “syndromic CMC”, with a broad spectrum of phenotypes, infectious and otherwise, are listed in Table 2. By contrast, patients with IL17F or IL17RC mutations have isolated CMC, with no other clinical manifestations. The immunity mediated by IL-17A and IL-17F dimers (homodimers or heterodimers) is selectively impaired in patients with monoallelic mutations of IL17F [46, 47] and biallelic mutations of IL17RC [44]. Two thirds of patients with biallelic mutations of IL17RA develop S. aureus infections in addition to CMC [48]. The IL17RA mutations also block IL-17E/IL-25 dimer signaling, potentially accounting for the slightly broader range of infections associated with these mutations [48]. A combination of Candida and S. aureus infections was also found in two ACTl-deficient patients [42]. These two patients carry a homozygous loss-of-function mutation affecting the FESIR domain of ACT1 and disrupting the interaction of ACT1 with IL-17 receptors, thus impairing cellular responses to IL-17A and IL-17F. Mutations of these four genes cause “isolated” CMC. The contribution of Th17 cells has not been formally demonstrated in patients with genetically determined CMC, because IL-17A and IL-17F are also produced by other cell types, including CD8+ T cells, γ/δ T cells, NKT cells, and ILC3 [49]. However, the occurrence of CMC in patients with HLA-II deficiency strongly suggests that Th17 cells are key players in this process. These experiments of Nature indicate that Th17 cells are essential for mucocutaneous immunity against Candida albicans, but the narrow range of infections associated with this disruption of an entire arm of Th immunity implies that Th17 cells are otherwise redundant in host defense.
Figure 1.
PID gene defects in the Th17 pathway. Fungal infections recognized by phagocytes and epithelial cells trigger the secretion of cytokines, which induce Th17 cells and Th17 production. Genetic defects of this pathway have been identified for IL12B, IL12RB1, STAT3, STAT1 (GOF), RORC, IL17F, IL17RA, IL17RC, ACT1. Autoantibodies made in APECED target IL-17A, IL-17F and IL-21, thereby blocking the pathway.
Table 2.
Overlapping Ols in AIDS and PIDs, by pathogen. Classic SCID and MHC deficiencies are excluded here due to the lack of specificity of the disease mechanism.
| Pathogen | PID | Accompanying infections | T-cell subset decreased | |
|---|---|---|---|---|
| Candida | Isolated CMC | IL17F | ||
| IL17RC | ||||
| IL17RA | S. aureus | |||
| ACT1 | S. aureus | |||
| Syndromic CMC | STATS (AD) | S. aureus, aspergillosis | Th17 cell | |
| RORC | Mycobacteria | Th17 cell | ||
| STAT1 (GOF) | Broad-spectrum infections | Th17 cell | ||
| IL12B | Mycobacteria, other fungi | |||
| IL12RB1 | Mycobacteria, other fungi | |||
| AIRE (APECED) | ||||
| Mycobacteria | IRF8 | Candidiasis | ||
| GATA2 | Viruses, other fungi | |||
| ISG15 | ||||
| IL12B | Fungi | |||
| IL12RB1 | Fungi | |||
| TYK2 | Fungi, viruses | |||
| IFN-γR1 | Fungi, viruses | |||
| IFN-γR2 | Viruses | |||
| JAK1 | ||||
| STAT1 (AD, LOF) | Viruses | |||
| CGD | Fungi | |||
| CD40L | Broad-spectrum infections | |||
| NEMO | Broad-spectrum infections | |||
| NFKBIA | Broad-spectrum infections | |||
| IKK2 | Broad-spectrum infections | Pan-T cell | ||
| Pneumococcus | NEMO | Broad-spectrum infections | ||
| NFKBIA | Broad-spectrum infections | |||
| IRAK-4 | Staphylococcus | |||
| MYD88 | Staphylococcus | |||
| CD40L | Broad-spectrum infections | |||
| BTK | Other bacterial infections | |||
| ICOS | Other bacterial infections | |||
| Complement | Pyogenic infections | |||
| HHV8 | Isolated KS | OX40 | CD4+ TEM | |
| Others | WAS | Other viruses | Pan-T cell | |
| IFN-γR1 | Fungi, viruses, mycobacteria | |||
| STIM1 | Broad-spectrum infections | |||
| MAGT1 | EBV | CD4 T cell | ||
| HSV | HSE | TLR3 | ||
| UNC93B | ||||
| TRIF | ||||
| TRAF3 | ||||
| TBK1 | ||||
| IRF3 | ||||
| Cutaneous herpes | DOCKS | Other viruses | TRM | |
| Cryptosporidium | CD40L | Broad-spectrum infections | ||
| CD40 | Broad-spectrum infections | |||
| NIK | Broad-spectrum infections | TFH | ||
| IL21R | Broad-spectrum infections | |||
Mucocutaneous candidiasis in patients infected with HIV
The most common fungal infection in HIV-infected children is caused by Candida spp. Studies of inborn errors disrupting IL-17 immunity have shown that CD4+ Th17 cells are essential for the control of Candida at the mucosal and skin barriers. Th17 cells are of particular importance in HIV infection, as HIV-infected individuals display a preferential loss of these cells [22-26]. Studies in transgenic mouse models have also shown that Th17 cells are intrinsically highly permissive to HIV [50, 51]. Several plausible mechanisms for this preferential permissiveness have been put forward, including high levels of expression of CCR5 and CCR6 on the cell surface and a lack of HIV-inhibitory RNases [23, 52]. The early massive loss of Th17 cells during acute HIV infection may account for the high prevalence of CMC in patients in the early stages of HIV disease. Moreover, the high permissiveness of Th17 cells may favor the persistence of the virus after HAART. A subset of Th17 cells has been shown to carry replication-competent integrated virus in ART-treated patients [53]. Overall, Th17 cell counts might be a better indicator of the risk of CMC than total CD4+ T-cell counts in HIV-infected patients, either before or after HAART. This hypothesis is testable. However, preventive approaches based on increasing the number of Th17 cells in HIV-infected individuals are not particularly attractive, as they might entail a risk of increasing viral persistence due to the permissiveness of Th17 cells for the virus. It might be safer to test the possible use of IL-17A or IL-17F cytokines or their downstream effectors in patients with CMC despite HAART, in combination with existing antifungal drugs, which are safe and potent but may lead to the development of fungal resistance if used in the long term.
Mycobacterial infections in patients with PIDs
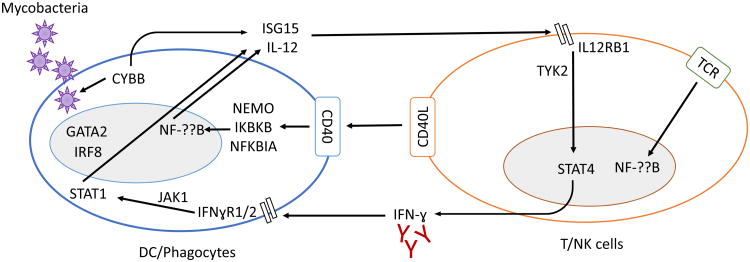
Patients with a lack of T cells (SCID) have long been known to be prone to severe disease caused by weakly virulent BCG vaccines [54]. Intriguingly, patients with HLA-II deficiency are prone to CMC but not to infections caused by BCG or environmental mycobacteria (EM) [55]. A small number of global defects of CD4+ T cells underlie severe disease caused by weakly virulent mycobacteria or by Mycobacterium tuberculosis, and they invariably also affect other types of T cells [56]. Engulfed pathogens trigger the secretion of IL-12 and IL-23 (which have the IL-12p40 subunit in common) by mononuclear phagocytes. These cytokines bind to the IL-12 and IL-23 receptors (which have the IL-12Rβl subunit in common) on NK and T cells. Signaling through TYK2 induces the secretion of IFN-γ, which activates phagocytes [57]. Pathogens also trigger the secretion of ISG15 by phagocytes, which induces IFN-γ production by lymphocytes through as yet undetermined mechanisms [58]. The signals mediated by IFN-γR further activate JAK1 and STAT1 and reinforce the IFN-γ responses (Figure 2). Phagocytes and T cells are connected by a loop involving IFN-γ and IL-12/IL-23. Recent studies have demonstrated the crucial role of IFN-γ immunity for the control of mycobacterial infections. Eight gene defects in the IFN-γ circuit underlie Mendelian susceptibility to mycobacterial disease (MSMD) (a selective vulnerability to BCG and EM) or a somewhat broader range of infections [59]. Biallelic mutations of ISG15, IL12B (IL-12p40), IL12RB1, TYK2, IFNGR1, IFNGR2, or JAK1, and monoallelic mutations of STAT1 confer susceptibility to mycobacterial infections [56, 59]. Moreover, anti-IFN-γ antibodies in other patients have been shown to underlie similar susceptibilities to mycobacterial infections [56, 59-61]. Finally, biallelic mutations of RORC underlie not only CMC, but also mycobacterial disease, as they disrupt the production of IFN-γ by γ/δ T cells and Th1* in response to mycobacteria [45]. Thus, human IFN-γ is undoubtedly essential for host defense against mycobacterial infections, but this cytokine is surprisingly largely redundant otherwise. However, by contrast to CMC, the specific contribution of CD4+ T cells, and mycobacterium-specific Th1* cells in particular, relative to the other cells capable of producing IFN-γ, such as Th1 cells, CD8+ T cells, γ/δ T cells, and NK cells, is unclear. Finally, it is relevant to HIV that patients with inborn errors of IFN-γ immunity are also vulnerable to tuberculosis (TB). Indeed, inborn errors of IFN-γ immunity have been shown to underlie critical illness not only patients with BCG/EM disease, but also in patients with TB [56, 59].
Figure 2.
PID gene defects in the IFN-γ pathway. Intracellular mycobacterial infection activates the loop of the IFN-γ pathway connecting phagocytes and T cells. In addition to its crucial functions in TCR signaling, the NF-??B pathway also enhances IFN-γ responses by inducing the secretion of IL-12. Genetic defects of this pathway have been identified for ISG15, IL12B, IL12RB1, TYK2, IFNGR1, IFNGR2, JAK1, STAT1, IRF8, GATA2, CD40L, NEMO, IKBKB, NFKBIA, and CYBB.
Mycobacterial infections in patients with HIV
HIV and TB are among the leading causes of death from infectious disease worldwide [1], partly because HIV is a major risk factor for TB and has made a major contribution to the re-emergence of this disease in some parts of the world, including in individuals not themselves infected with HIV [62]. The regional prevalence of TB has a major impact on the incidence of co-infection. In countries with a low prevalence of HIV infection, such as the the USA and UK, only 3-6% of HIV-infected individuals also have clinically diagnosed TB. By contrast, in countries with a high prevalence of HIV infection, such as South Africa, an estimated 23% of HIV-positive children also have TB [62]. However, the decline of CD4+ T-cell counts does not seem to be the only cause of this susceptibility to TB. HIV-positive individuals with CD4+ T-cell counts >200/μl are susceptible to pulmonary TB [17, 35], suggesting that subtler mechanisms are at work in the pathogenesis of TB in HIV patients. HIV-infected individuals are also susceptible to less virulent mycobacteria, including BCG and EM in particular, when their CD4+ T-cell counts fall below 100/μl [17]. Recent studies of PIDs have shown that susceptibility to BCG and EM results from an impairment of IFN-γ immunity, which can also underlie TB. This impairment of IFN-γ immunity may be due to defects of myeloid or lymphoid cells, disrupting their development or function (Figure 2). The PIDs underlying severe mycobacterial infection display a high degree of genetic and allelic heterogeneity, but are highly homogeneous physiologically, as they all involve an impairment of IFN-γ immunity. Moreover, IFN-γ immunity seems to be a quantitative trait, with more common and severe mycobacterial infections observed in patients with the most severe defects. The parallel with HIV is striking, with BCG and EM disease almost invariably striking patients with CD4+ T-cell counts below 100/μl [17], whereas TB can affect patients with higher CD4+ T-cell counts. It is possible that a subtle impairment of IFN-γ immunity, perhaps a decline of Th1* cells [20], favors pulmonary TB during the course of HIV infection, whereas a more profound defect of this subset underlies BCG or EM disease. If this were indeed the case, then studies of HIV could provide insight into the cellular basis of mycobacterial disease in patients with inborn errors of immunity.
Pneumococcal disease in patients with PIDs
Pneumococcus (Streptococcus pneumoniae) is an encapsulated pyogenic bacterium that often causes benign infections in children. Recent studies of PIDs have revealed that defects disrupting the destruction of opsonized bacteria by splenic macrophages, including congenital asplenia, defects of complement or antibody responses, and defects of the NF-κB or IRAK-4/MYD88 pathway, underlie invasive pneumococcal disease [63]. Disorders affecting antibody responses also underlie pneumococcal infections of the upper and lower respiratory tract, and affected patients frequently experience such infections earlier and more frequently than invasive infections [63]. Patients with many of the known phagocyte defects, such as chronic granulomatous disease, congenital neutropenia, or leukocyte adhesion deficiency, are not particularly prone to pneumococcal disease, despite their susceptibility to other pyogenic bacteria. Classic antibody deficiencies, such as X-linked agammaglobulinemia (XLA), CD40, CD40L, and ICOS deficiency, can lead to respiratory infections. Patients with hypomorphic NEMO and hypermorphic NFKBIA mutations are also susceptible to pneumococcal respiratory infections. They lack anti-polysaccharide antibodies, even after challenge with polyvalent pneumococcal polysaccharide vaccine [63]. By contrast, patients with complement deficiencies are more susceptible to invasive pneumococcal disease [14]. Likewise, patients with IRAK-4 and MYD88 deficiencies are susceptible to invasive pneumococcal infections, and to infections with other pyogenic bacteria, including Staphylococcus, but apparently not to respiratory infections. IRAK-4 and MYD88 conduct signals from IL-IRs and TLRs to downstream pathways, including the NF-κB pathway [64]. It is unclear why patients with IRAK-4 and MYD88 deficiencies are selectively susceptible to so few bacteria despite the recognition of many different pathogens by the upstream receptors. The TIRAP- and MyD88-dependent pathway downstream from TLR2 was recently shown to be essential for systemic immunity to Staphylococcus, but otherwise redundant [65]. No such specific pathway has yet been identified for pulmonary or systemic immunity to pneumococcus. Overall, studies of PIDs have shown that antibody defects underlie susceptibility to pneumococcal pneumonia, whereas more profound disorders of the splenic phagocytosis of opsonized pneumococci underlie invasive disease.
Pneumococcal disease in patients with HIV
Pneumococcal pneumonia is often seen in HIV-infected patients with CD4+ T-cell counts exceeding 200/μl, whereas invasive pneumococcal disease only occurs in patients with full-blown AIDS (CD4+ T-cell counts <200/μl) [17, 66]. Nevertheless, bacterial pneumonia remains the most common infection in HIV-infected children with or without HAART (Table 1) [35]. Pneumonia can be caused by many different bacteria, but we focus here on the pneumococcal disease. HIV-infected patients have small numbers of memory B cells and defective antibody responses, but may nevertheless display hyper-gammaglobinemia and the expansion of some subsets of B cells. Antibody responses to pneumococcal vaccine, specifically the IgG response to pneumococcal polysaccharides, are weak in HIV-infected patients, with or without HAART [67]. This defective specific antibody response is associated with impaired ICOS+ TFH cell functions [67]. TFH is the key cell type inducing B-cell differentiation and class-switch in germinal centers through the production of IL-21, IL-4, CD40L, and ICOS [27]. These cells are highly permissive to HIV infection and serve as the major site of viral replication. Indeed, CD4+ TFH cells are 40 times more likely to be infected by HIV than peripheral CD4+ T cells in general. Despite their high permissiveness to HIV, the percentage of TFH cells often increases early in HIV infection, together with follicular hyperplasia in the lymph nodes [27]. Regardless of their expansion, their function is impaired, as shown by their low levels of IL-21 production, and of CD40L and ICOS expression [27]. As inferred from inborn errors affecting antibody (Ab) responses, such as IL-21, IL-21R, CD40, CD40L, and ICOS deficiencies in particular, which disrupt specific communication between CD4+ TFH and B cells, the decrease in the number of functional TFH cells in HIV patients may account for their poor Ab responses to vaccines, and their susceptibility to recurrent pneumococcal respiratory diseases. Further decreases in TFH cell counts may lead to a more profound defect of Ab responses, contributing to subsequent invasive pneumococcal disease.
Peripheral and invasive HSV infections in patients with PIDs or HIV
HIV patients with CD4+ T-cell counts <200/μl often develop cutaneous lesions caused by HSV1, disseminated infections involving multiple organs, and, more rarely, herpes simplex encephalitis (HSE), at least in adults, as HSE is no more common among children with HIV than in those of the general population [17, 68]. PIDs may confer predisposition to two forms of HSV infection: recurrent mucocutaneous lesions and isolated HSE. These two distinctive presentations suggest that there are different mechanisms of host defense against mucocutaneous and cerebral HSV infections. HSE occurs in some patients with specific defects of the TLR3-IFN-α/β pathway, including mutations of TLR3, UNC93B1, TRIF, TRAF3, TBK1, 1RF3, NEMO, and STAT1 (Table 2) [69]. The last two of these genetic defects underlie HSE and mycobacterial disease, whereas the others underlie isolated HSE. By contrast, recurrent mucocutaneous HSV lesions are seen in patients with various inborn errors of T cells [4]. Recent studies have shown that both CD4+ and CD8+ tissue-resident memory T cells (TRM) are critical for HSV control in situ [70-72]. TRM is a subset of T cells that migrate to tissues, where they can remain for years. They function as first-line responders when infection re-emerges, through cytokine production or direct cytotoxic effects on infected cells [70-73]. TRM display a certain degree of plasticity and, depending on the first pathogen they encounter, they may differentiate into IFN-γ- or IL-17-producing cells [73], enabling them to combat different types of infections. Defects in the migration or survival of TRM can lead to susceptibility to cutaneous HSV infections. This mechanism has been demonstrated in DOCKS-deficient patients and in the corresponding mouse models, in which TRM levels decreased rapidly in HSV-infected skin due to cytothripsis and defective migration [74, 75]. The recurrent cutaneous HSV infections seen in patients with DOCKS deficiency are similar to those seen in HIV-infected patients, who are also susceptible to cutaneous infections caused by various viruses, such as HSV-1, HSV-2, VZV, and HPV. HIV infection may decrease the number of TRM not only by reducing total CD4+ T-cell counts, but also by targeting local TRM in the mucosa and its draining lymph nodes, because HSV and HIV share the same transmission routes in mucosal tissues. The predominant depletion of CD4+ T cells in the gastrointestinal tract, another tissue with high density of CCR5+ TRM, suggests that tissue-specific depletion of TRM occurs in the course of HIV infection [76]. It would be interesting to further determine whether HIV-infected patients with cutaneous viral infections, such as HSV, VZV, and HPV infections, suffer from a major loss of TRM in the mucosa and skin.
Cryptosporidiosis in patients with PIDs or HIV
Liver and intestinal cryptosporidiosis is another condition listed among the defining infections for AIDS in the 1980s. It is seen in HIV patients with CD4+ T-cell counts below 100/μl and improves significantly with HAART (Table 1) [17, 35, 77]. It is also common in some, but not all PID patients with CD4+ T cells defects. Less is known about the pathogenesis of cryptosporidiosis than about the other infections reviewed here, particularly in terms of its cellular basis. Well-known cases of cryptosporidiosis in CD40- and CD40L-deficient patients, and more recent findings in IL-21, IL-21R-, and NIK-deficient patients have implicated specific T-cell pathways in host defense against Cryptosporidium. Patients with CD40, CD40L, IL-21, IL-21R, or NIK deficiency are susceptible to cryptosporidiosis despite having normal peripheral T-cell counts [78, 79]. Both CD40 and IL-21R mediate signals through NIK and the non-canonical NF-κB pathway [78], suggesting the likely role for this pathway in the pathogenesis of cryptosporidiosis. Moreover, CD40L and IL-21 are highly expressed in TFH cells [20], However, the cellular mechanism by which this Th subset controls Cryptosporidium is unclear. Patients with severe antibody deficiencies rarely develop cryptosporidiosis, so it is unlikely that the infections in these patients are caused by disruption of the interaction between their TFH and B cells, which accounts for their lack of Ig class-switched memory B cells. It seems more likely that CD40L- and IL-21-expressing TFH cells interact with dendritic cells (DCs) or macrophages expressing both CD40 and the IL-21R, especially in the gut-associated lymphoid tissue (GALT) close to the site of Cryptosporidium infection [78]. IL-21R is also expressed in non-hematopoietic cells, such as epithelial cells, fibroblasts, and keratinocytes. A loss of IL-21 signaling could, therefore, also affect the functions of the gut mucosal barrier directly [78]. HIV-infected patients have been reported to have low IL-21 levels and dysfunctional IL-21-producing TFH cells, together with a drastic loss of CD4+ T cells in GALT. Moreover, TFH cells are highly permissive to HIV and are the main virus-producing cells in chronic HIV infections. Considering the evidence from both PIDs and AIDS, it appears likely that TFH cells control Cryptosporidium in a manner independent of their helper role in B-cell responses. Instead, it seems likely that the interaction between TFH and myeloid or non-hematopoietic cells mediated by CD40/CD40L and IL-21/IL21R signals plays a key role in cryptosporidiosis.
Pneumocystis pneumonitis in patients with PIDs or HIV
Pneumocystis pneumonitis is another defining infection of AIDS, mostly reported in patients with CD4+ T-cell counts below 100/μl [17]. It is also seen in PID patients with SCID, HLA-II deficiency, or with CD40, CD40L, IL-21, IL-21R, NEMO, DOCKS, or WASP loss-of-function mutations, or with NFKBIA gain-of-function mutations [43]. Pneumocystis pneumonitis is not common in other PIDs with T-cell defects. Almost half the known CD40- or CD40L-deficient patients developed Pneumocystis pneumonitis, indicating that the interaction between CD40 and CD40L is essential to host control of the infection. Patients with classic humoral defects, such as XLA, do not develop Pneumocystis. It therefore seems likely that the CD40-CD40L signaling between T and B cells is dispensable for the control of Pneumocystis in pulmonary alveoli. Instead, the signaling between T cells and CD40+ pulmonary DCs or macrophages, and other CD40+ non-hematopoietic cells, such as epithelial cells and endothelial cells, probably plays an important role in the control of Pneumocystis [80, 81]. Moreover, the CD40L expressed on T cells can activate both the canonical and non-canonical NF-κB pathways in CD40+ cells, potentially accounting for cases of Pneumocystis in NEMO and NFKBIA patients, who have defective NF-κB signaling in a broad spectrum of cells. We can speculate that CD40-CD40L-NEMO-NFKBIA signaling between T and myeloid cells or non-hematopoietic cells is the key to Pneumocystis pathogenesis. Intriguingly, two of the defining infections for AIDS, cryptosporidiosis and Pneumocystis, are both seen in CD40-, CD40L-, and IL-21, IL-21R-deficient patients. Thus, both CD40-CD40L and IL-21-IL-21R interactions seem to play a key role in the pathogenesis of an intestinal parasitic disease (caused by Cryptosporidium) and a fungal pulmonary disease (caused by Pneumocystis). It would be interesting to determine whether infections with these two almost ubiquitous pathogens are closely correlated in HIV patients, and whether they are associated with impaired development of TFH cells. Both molecular bridges seem to operate against these two pathogens not by connecting TFH cells with B cells, promoting class-switch and antibody responses, but by connecting TFH cells with other myeloid or non-hematopoietic cells.
HHV8 infection and Kaposi sarcoma (KS) in patients with PIDs or HIV
HHV8-driven KS in HIV-infected individuals, also known as epidemic KS, is one of the most characteristic illnesses observed during the course of HIV infection. HIV infection has an enormous impact on both susceptibility to HHV8 infection and progression to KS [82-84]. HHV8 infection and KS develop in patients with CD4+ T-cell counts <100/μl and their incidence decreased significantly after the introduction of HAART [85, 86]. However, in countries with long-standing access to HAART, approximately one third of HIV-infected individuals presenting with KS are already on HAART and have fully suppressed HIV replication and CD4+ T-cell counts >200/μl [87]. These findings suggest that our understanding of the disease mechanism is incomplete. KS is rare, other than in HIV-infected patients (iatrogenic KS) and transplant recipients (iatrogenic KS), although it is also observed in sub-Saharan Africans (endemic KS) and in the Mediterranean Basin (classic KS), where HHV8 infection is particularly common. KS is very rare in children, and this is particularly true for classic KS, less than 50 cases of which have been reported in children [88]. Six different genetic etiologies of KS have been reported, all in children with classic KS [88]: IFNγRl deficiency [89], Wiskott-Aldrich syndrome (WAS) [90], STIM1 deficiency [91], OX40 deficiency [92], MAGT1 deficiency [93], and a large deletion on chromosome 7 [94], each in a single patient. It is difficult to draw any firm conclusions for such a small sample of patients displaying such a high degree of genetic heterogeneity. However, four of the five patients with a monogenic disorder had a T-cell dysfunction. The patient with OX40 deficiency was the only one of these cases to present isolated KS without other unusually severe infections, highlighting the essential role of OX40-OX40L signaling in host defense against HHV8. This patient also suffered from a common form of leishmaniasis, which may or may not be related to the OX40 deficiency. OX40 is expressed by activated T cells and is important for the generation of memory CD4+ Th cells [95]. The OX40-deficient patient had a small number of TEM cells, the main target of HIV during early stages of infection [92]. Moreover, KS biopsies from both AIDS patients and the OX40-deficient patient revealed abundant OX40L expression on primary lymphatic and blood endothelial cells [92]. A decrease in or loss of OX40 expression on TEM cells may be the common mechanism behind KS in patients with OX40 deficiency and patients with HIV infection. OX40 is also expressed on CD8+ T cells, which are involved in cytotoxicity directed against HHV8-infected tumor cells. Moreover, KS has not been reported in patients with inherited HLA-I or HLA-II deficiency. The possible direct role of CD4+OX40+ TEM cells in controlling HHV8 and KS in HIV-infected patients thus requires further investigation.
Concluding remarks
The comparison of infectious diseases and immunological deficits in patients with PIDs and HIV suggests that the same immunological mechanisms are causal in PID-afflicted and HIV-infected individuals struck by the same infection, although this hypothesis remains to be confirmed. Different mechanisms may account for a specific infection in patients with different PIDs, and the pathogenesis of a given infection in HIV patients may be different before and after HAART. Moreover, it can be difficult to compare the immunological abnormalities in HIV and PID patients rigorously. However, the parallels between PIDs and HIV strongly suggest that similar mechanisms may be at work, particularly for infections with a higher frequency or severity in individuals with CD4+ T-cell deficits. Studies of infections in patients with PIDs for whom there is a clear causal relationship between the immune genotype and infectious phenotypes, can provide plausible hypotheses concerning the underlying mechanisms that can be tested in HIV-infected individuals, in whom it is more difficult to disentangle causes and consequences, particularly in the absence of hypotheses based on the observation of PIDs [13, 14]. If validated, the identification of the mechanisms involved would make it possible to predict the risk of occurrence of a given infection, thereby paving the way for personalized approaches to the prevention or treatment of these infections in HIV-infected individuals at high risk. Last, but not least, the interindividual variability of infections in HIV-infected patients may reflect human genetic variability. Resistance and susceptibility alleles, including some encoding proteins involved in pathways identified as affected in PID patients, may contribute to the different infectious patterns of HIV patients with the similar levels of CD4+ T cells and even of specific Th subsets [15, 16].
Beyond this comparison of PIDs and HIV, the study of PIDs is of more general value for improving our understanding of infections in settings other than HIV. This review does not deal with anti-HIV immunity itself and its control by human genetics. Instead, it focuses on other infections that can be precipitated by HIV or inborn immunodeficiency. Infections can strike patients with other virus-induced immunodeficiencies, patients with drug-induced immunosuppression, or even elderly patients with age-related immunodeficiency. Moreover, severe infections may strike otherwise healthy patients with no known immunodeficiency. “Idiopathic” infections with pathogens classified as opportunistic on the basis of their frequent occurrence in other patients with overt immunodeficiency may also occur. The affected patients probably have a covert immunodeficiency that has yet to be discovered; these patients may carry mutations in genes related to known PID genes, affecting related immunological pathways, or mutations affecting other genes and pathways [13, 14]. In all settings, improvements to understanding of the pathogenesis of human infections through studies of their human genetic basis can be both insightful and useful [96]. Indeed, some infections cannot be prevented by vaccines (due to a lack of vaccine or a lack of response to it), others cannot be treated with anti-infection drugs, and others will inevitably become resistant to current anti-infection agents, will display the gradual selection of variants not covered by vaccines, or both. It is thus essential to understand the pathogenesis of infectious diseases before they become uncontrollable. Infectious diseases pose a considerable medical threat to mankind, as illustrated by the HIV pandemic and the range of infections accompanying it [21]. Molecular and cellular analyses of the pathogenesis of severe infections through the genetic and immunological study of PIDs have considerable implications in the fields of PIDs and HIV, and beyond, for the field of infectious diseases as a whole.
Highlights.
In the post-ART era, HIV infections still leads to a variety of infections.
Some PIDs share the same pathogens and mechanisms with HIV-infected individuals.
Specific pathways and T cell subsets are responsible for these infections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References with annotation
- 1.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430(6996):242–9. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339(1):33–9. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 3.Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317(5838):617–9. doi: 10.1126/science.1142963. [DOI] [PubMed] [Google Scholar]
- 4*.Picard C, et al. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35(8):696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Bousfiha A, et al. The 2015 IUIS Phenotypic Classification for Primary Immunodeficiencies. J Clin Immunol. 2015;35(8):727–38. doi: 10.1007/s10875-015-0198-5. Two useful reviews for scientists and clinicians to look up the latest classification of primary immunodeficiencies. The phenotypic classification gives the readers a broad view of the infections associated with primary immunodeficiencies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Zhang Y, Su HC, Lenardo MJ. Genomics is rapidly advancing precision medicine for immunological disorders. Nat Immunol. 2015;16(10):1001–4. doi: 10.1038/ni.3275. An excellent review that illustrates both the genetic and immunological principles that govern the study of primary immunodeficiency diseases, and the medical and biological impacts of these studies. [DOI] [PubMed] [Google Scholar]
- 7.Notarangelo LD, Fleisher TA. Targeted strategies directed at the molecular defect: Toward precision medicine for select primary immunodeficiency disorders. J Allergy Clin Immunol. 2017;139(3):715–723. doi: 10.1016/j.jaci.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousfiha AA, et al. Primary immunodeficiency diseases worldwide: more common than generally thought. J Clin Immunol. 2013;33(1):1–7. doi: 10.1007/s10875-012-9751-7. [DOI] [PubMed] [Google Scholar]
- 9.Notarangelo LD, et al. Human RAG mutations: biochemistry and clinical implications. Nat Rev Immunol. 2016;16(4):234–46. doi: 10.1038/nri.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casanova JL, et al. Guidelines for genetic studies in single patients: lessons from primary immunodeficiencies. J Exp Med. 2014;211(11):2137–49. doi: 10.1084/jem.20140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picard C, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee report on Inborn Errors of Immunity. J Clin Immunol. doi: 10.1007/s10875-017-0464-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcais A, et al. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann N Y Acad Sci. 2010;1214:18–33. doi: 10.1111/j.1749-6632.2010.05834.x. [DOI] [PubMed] [Google Scholar]
- 13*.Casanova JL. Human genetic basis of interindividual variability in the course of infection. Proc Natl Acad Sci U S A. 2015;112(51):E7118–27. doi: 10.1073/pnas.1521644112. This review provides the minimal historical background that is necessary to appreciate the notion that there is a human genetic basis for interindividual variability in the course of infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Casanova JL. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc Natl Acad Sci U S A. 2015;112(51):E7128–37. doi: 10.1073/pnas.1521651112. This review discusses the emerging hypothesis that severe infectious diseases striking otherwise healthy individuals, in the course of primary infection, can be monogenic, although rarely fully penetrant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaren PJ, Carrington M. The impact of host genetic variation on infection with HIV-1. Nat Immunol. 2015;16(6):577–83. doi: 10.1038/ni.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulder PJ, Walker BD. HIV and HLA class I: an evolving relationship. Immunity. 2012;37(3):426–40. doi: 10.1016/j.immuni.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan J, et al. USPHS/IDSA Guidelines for the Prevention of Opportunistic Infections in Persons Infected with Human Immunodeficiency Virus: Introduction. Clinical Infectious Diseases. 1995;21:Sl–11. [PubMed] [Google Scholar]
- 18.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254(1):54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Ma CS, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136(4):993–1006 el. doi: 10.1016/j.jaci.2015.05.036. An elegant and comprehensive study of TFH cells in patients with a variety of PIDs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Sallusto F. Heterogeneity of Human CD4(+) T Cells Against Microbes. Annu Rev Immunol. 2016;34:317–34. doi: 10.1146/annurev-immunol-032414-112056. An excellent review on the heterogeneity of human CD4+ T cells, their diversity and roles in host defense against different pathogens. [DOI] [PubMed] [Google Scholar]
- 21.Casadevall A. Crisis in Infectious Diseases: 2 Decades Later. Clin Infect Dis. 2017;64(7):823–828. doi: 10.1093/cid/cix067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Hed A, et al. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J Infect Dis. 2010;201(6):843–54. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosselin A, et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol. 2010;184(3):1604–16. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez Y, et al. Preferential HIV infection of CCR6+ Th17 cells is associated with higher levels of virus receptor expression and lack of CCR5 ligands. J Virol. 2013;87(19):10843–54. doi: 10.1128/JVI.01838-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bixler SL, Mattapallil JJ. Loss and dysregulation of Th17 cells during HIV infection. Clin Dev Immunol. 2013;2013:852418. doi: 10.1155/2013/852418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, et al. Th1/17 Polarization of CD4 T Cells Supports HIV-1 Persistence during Antiretroviral Therapy. J Virol. 2015;89(22):11284–93. doi: 10.1128/JVI.01595-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miles B, Miller SM, Connick E. CD4 T Follicular Helper and Regulatory Cell Dynamics and Function in HIV Infection. Front Immunol. 2016;7:659. doi: 10.3389/fimmu.2016.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9(4):235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis. 2016;214(2):S44–50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cotugno N, et al. Suboptimal immune reconstitution in vertically HIV infected children: a view on how HIV replication and timing of HAART initiation can impact on T and B-cell compartment. Clin Dev Immunol. 2012;2012:805151. doi: 10.1155/2012/805151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Montagnani C, et al. Long-term effect of highly active antiretroviral therapy on immunologic features in children. Pediatr Infect Dis J. 2015;34(5 Suppl 1):S3–6. doi: 10.1097/INF.0000000000000659. A brief and informative study of the long-term effect of HAART on the immunological functions of HIV-infected children. [DOI] [PubMed] [Google Scholar]
- 32.Gona P, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296(3):292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 33.Cohen C, et al. Influenza-related mortality among adults aged 25-54 years with AIDS in South Africa and the United States of America. Clin Infect Dis. 2012;55(7):996–1003. doi: 10.1093/cid/cis549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harboe ZB, et al. Incidence and risk factors for invasive pneumococcal disease in HIV-infected and non-HIV-infected individuals before and after the introduction of combination antiretroviral therapy: persistent high risk among HIV-infected injecting drug users. Clin Infect Dis. 2014;59(8):1168–76. doi: 10.1093/cid/ciu558. [DOI] [PubMed] [Google Scholar]
- 35*.B-Lajoie MR, et al. Incidence and Prevalence of Opportunistic and Other Infections and the Impact of Antiretroviral Therapy Among HIV-infected Children in Low- and Middle-income Countries: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62(12):1586–94. doi: 10.1093/cid/ciw139. This study reports the incidence and prevalence of infections in HIV-infected children and the impact of ART. It is useful for scientists and clinicians interested in this important topic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pursuing Later Treatment Options, l.l.p.t.f.t.C.o.O.H.I.V.E.R.E. et al. Risk of triple-class virological failure in children with HIV: a retrospective cohort study. Lancet. 2011;377(9777):1580–7. doi: 10.1016/S0140-6736(11)60208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer A. Recent advances in understanding the pathophysiology of primary T cell immunodeficiencies. Trends Mol Med. 2015;21(7):408–16. doi: 10.1016/j.molmed.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Picard C, Fischer A. Hematopoietic stem cell transplantation and other management strategies for MHC class II deficiency. Immunol Allergy Clin North Am. 2010;30(2):173–8. doi: 10.1016/j.iac.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, et al. Dual T cell- and B cell-intrinsic deficiency in humans with biallelic RLTPR mutations. J Exp Med. 2016;213(11):2413–2435. doi: 10.1084/jem.20160576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puel A, et al. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22(4):467–74. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puel A, et al. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616–22. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boisson B, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39(4):676–86. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanternier F, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25(6):736–47. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling Y, et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212(5):619–31. doi: 10.1084/jem.20141065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada S, et al. IMMUNODEFICIENCIES Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349(6248):606–13. doi: 10.1126/science.aaa4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marodi L, et al. Molecular mechanisms of mucocutaneous immunity against Candida and Staphylococcus species. J Allergy Clin Immunol. 2012;130(5):1019–27. doi: 10.1016/j.jaci.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy R, et al. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci U S A. 2016;113(51):E8277–E8285. doi: 10.1073/pnas.1618300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gladiator A, LeibundGut-Landmann S. Innate lymphoid cells: new players in IL-17-mediated antifungal immunity. PLoS Pathog. 2013;9(12):el003763. doi: 10.1371/journal.ppat.1003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goupil M, et al. Defective IL-17- and IL-22-dependent mucosal host response to Candida albicans determines susceptibility to oral candidiasis in mice expressing the HIV-1 transgene. BMC Immunol. 2014;15:49. doi: 10.1186/s12865-014-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Repentigny L, Goupil M, Jolicoeur P. Oropharyngeal Candidiasis in HIV Infection: Analysis of Impaired Mucosal Immune Response to Candida albicans in Mice Expressing the HIV-1 Transgene. Pathogens. 2015;4(2):406–21. doi: 10.3390/pathogens4020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen-Quick A, et al. Human Th17 Cells Lack HIV-lnhibitory RNases and Are Highly Permissive to Productive HIV Infection. J Virol. 2016;90(17):7833–47. doi: 10.1128/JVI.02869-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wacleche VS, et al. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology. 2016;13(1):59. doi: 10.1186/s12977-016-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casanova JL, et al. Immunological conditions of children with BCG disseminated infection. Lancet. 1995;346(8974):581. doi: 10.1016/s0140-6736(95)91421-8. [DOI] [PubMed] [Google Scholar]
- 55.Hanna S, Etzioni A. MHC class I and II deficiencies. J Allergy Clin Immunol. 2014;134(2):269–75. doi: 10.1016/j.jaci.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 56*.Boisson-Dupuis S, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev. 2015;264(1):103–20. doi: 10.1111/imr.12272. A review of the predisposing factors underlying tuberculosis in children, including HIV infection and primary immunodeficiencies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nathan CF, et al. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158(3):670–89. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bogunovic D, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337(6102):1684–8. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bustamante J, et al. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol. 2014;26(6):454–70. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boisson B, Quartier P, Casanova JL. Immunological loss-of-function due to genetic gain-of-function in humans: autosomal dominance of the third kind. Curr Opin Immunol. 2015;32:90–105. doi: 10.1016/j.coi.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eletto D, et al. Biallelic JAK1 mutations in immunodeficient patient with mycobacterial infection. Nat Commun. 2016;7:13992. doi: 10.1038/ncomms13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venturini E, et al. Tuberculosis and HIV co-infection in children. BMC Infect Dis. 2014;14(1):S5. doi: 10.1186/1471-2334-14-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Picard C, et al. Primary immunodeficiencies associated with pneumococcal disease. Curr Opin Allergy Clin Immunol. 2003;3(6):451–9. doi: 10.1097/00130832-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Picard C, et al. Inherited human IRAK-4 deficiency: an update. Immunol Res. 2007;38(1-3):347–52. doi: 10.1007/s12026-007-0006-2. [DOI] [PubMed] [Google Scholar]
- 65*.Israel L, et al. Human Adaptive Immunity Rescues an Inborn Error of Innate Immunity. Cell. 2017;168(5):789–800 el0. doi: 10.1016/j.cell.2017.01.039. An elegant study, showing how adaptive immunity can rescue an inborn error of innate immunity against staphylococcus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merchan EC, et al. Septic arthritis in HIV positive haemophiliacs Four cases and a literature review. Int Orthop. 1992;16(3):302–6. doi: 10.1007/BF00182717. [DOI] [PubMed] [Google Scholar]
- 67.Abudulai LN, et al. Production of IgG antibodies to pneumococcal polysaccharides is associated with expansion of ICOS+ circulating memory T follicular-helper cells which is impaired by HIV infection. PLoS One. 2017;12(5):e0176641. doi: 10.1371/journal.pone.0176641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mofenson LM, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58(RR-11):1–166. [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang SY, Abel L, Casanova JL. Mendelian predisposition to herpes simplex encephalitis. Handb Clin Neurol. 2013;112:1091–7. doi: 10.1016/B978-0-444-52910-7.00027-1. [DOI] [PubMed] [Google Scholar]
- 70.Ariotti S, et al. T cell memory Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346(6205):101–5. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 71.lijima N, Iwasaki A. T cell memory A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346(6205):93–8. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schenkel JM, et al. T cell memory Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21(7):688–97. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Q, et al. DOCKS regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211(13):2549–66. doi: 10.1084/jem.20141307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flesch IE, et al. Delayed control of herpes simplex virus infection and impaired CD4(+) T-cell migration to the skin in mouse models of DOCK8 deficiency. Immunol Cell Biol. 2015;93(6):517–21. doi: 10.1038/icb.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Connor RM, et al. Cryptosporidiosis in patients with HIV/AIDS. AIDS. 2011;25(5):549–60. doi: 10.1097/QAD.0b013e3283437e88. [DOI] [PubMed] [Google Scholar]
- 78.Kotlarz D, et al. Human IL-21 and IL-21R deficiencies: two novel entities of primary immunodeficiency. Curr Opin Pediatr. 2014;26(6):704–12. doi: 10.1097/MOP.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 79.Willmann KL, et al. Biallelic loss-of-function mutation in NIK causes a primary immunodeficiency with multifaceted aberrant lymphoid immunity. Nat Commun. 2014;5:5360. doi: 10.1038/ncomms6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bustamante J, et al. Genetic lessons learned from X-linked Mendelian susceptibility to mycobacterial diseases. Ann N Y Acad Sci. 2011;1246:92–101. doi: 10.1111/j.1749-6632.2011.06273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chatzigeorgiou A, et al. CD40/CD40L signaling and its implication in health and disease. Biofactors. 2009;35(6):474–83. doi: 10.1002/biof.62. [DOI] [PubMed] [Google Scholar]
- 82.Butler LM, et al. Human herpesvirus 8 infection in children and adults in a population-based study in rural Uganda. J Infect Dis. 2011;203(5):625–34. doi: 10.1093/infdis/jiq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olsen SJ, et al. Increasing Kaposi's sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi's sarcoma endemic region, Zambia in 1985. AIDS. 1998;12(14):1921–5. doi: 10.1097/00002030-199814000-00024. [DOI] [PubMed] [Google Scholar]
- 84.Dedicoat M, Newton R. Review of the distribution of Kaposi's sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi's sarcoma. Br J Cancer. 2003;88(1):1–3. doi: 10.1038/sj.bjc.6600745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rezza G, Padian N. AIDS 2000 Epidemiology: overview. AIDS. 2000;14(3):S45–6. [PubMed] [Google Scholar]
- 86.Bohlius J A.I.-d.C.P.W.G.f. leDEA, and C.i. EuroCoord. Comparison of Kaposi sarcoma risk in HIV-positive adults across five continents: a multiregional multicohort study. Clin Infect Dis. 2017 doi: 10.1093/cid/cix480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dittmer DP. Restricted Kaposi's sarcoma (KS) herpesvirus transcription in KS lesions from patients on successful antiretroviral therapy. MBio. 2011;2(6):e00138–11. doi: 10.1128/mBio.00138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jackson CC, et al. Kaposi Sarcoma of Childhood: Inborn or Acquired Immunodeficiency to Oncogenic HHV-8. Pediatr Blood Cancer. 2016;63(3):392–7. doi: 10.1002/pbc.25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Camcioglu Y, et al. HHV-8-associated Kaposi sarcoma in a child with IFNgammaRl deficiency. J Pediatr. 2004;144(4):519–23. doi: 10.1016/j.jpeds.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 90.Picard C, et al. Kaposi's sarcoma in a child with Wiskott-Aldrich syndrome. Eur J Pediatr. 2006;165(7):453–7. doi: 10.1007/s00431-006-0107-2. [DOI] [PubMed] [Google Scholar]
- 91.Byun M, et al. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med. 2010;207(11):2307–12. doi: 10.1084/jem.20101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Byun M, et al. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med. 2013;210(9):1743–59. doi: 10.1084/jem.20130592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brigida I, et al. Large Deletion of MAGT1 Gene in a Patient with Classic Kaposi Sarcoma, CD4 Lymphopenia, and EBV Infection. J Clin Immunol. 2017;37(1):32–35. doi: 10.1007/s10875-016-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jackson CC, et al. Kaposi sarcoma, oral malformations, mitral dysplasia, and scoliosis associated with 7q34-q36.3 heterozygous terminal deletion. Am J Med Genet A. 2017 doi: 10.1002/ajmg.a.38275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishii N, et al. OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv Immunol. 2010;105:63–98. doi: 10.1016/S0065-2776(10)05003-0. [DOI] [PubMed] [Google Scholar]
- 96.Casanova JL, Abel L, Quintana-Murci L. Immunology taught by human genetics. Cold Spring Harb Symp Quant Biol. 2013;78:157–72. doi: 10.1101/sqb.2013.78.019968. [DOI] [PubMed] [Google Scholar]