Abstract
Schizophrenia is a severe mental illness that causes major functional impairment. Current pharmacologic treatments are inadequate, particularly for addressing negative and cognitive symptoms of the disorder. Oxytocin, a neuropeptide known to moderate social behaviors, has been investigated as a potential therapeutic for schizophrenia in recent years. Results have been decidedly mixed, leading to controversy regarding oxytocin's utility. In this review, we outline several considerations for interpreting the extant literature and propose a focused agenda for future work that builds on the most compelling findings regarding oxytocin effects in schizophrenia to date. Specifically, we examine underlying causes of heterogeneity in randomized clinical trials (RCTs) conducted thus far and highlight the complexity of the human oxytocin system. We then review evidence of oxytocin's effects on specific deficits in schizophrenia, arguing for further study using objective, precise outcome measures in order to determine whether oxytocin has the potential to improve functional impairment in schizophrenia.
Keywords: Oxytocin, schizophrenia, negative symptoms, social cognition
1. Introduction
Schizophrenia is a severe neurodevelopmental disorder that affects nearly 1% of the population worldwide (McGrath et al., 2008) and results in marked functional impairment. In recent years, the neuropeptide oxytocin, known to play a key role in bonding and social behavior, has been heralded as an important player in the etiology, symptom severity, and possible treatment of schizophrenia. Interest in this area of research stems from numerous studies suggesting pro-social effects of intranasal oxytocin in both non-clinical and clinical human populations. However, initial enthusiasm about oxytocin has now given way to doubt and controversy. Mounting evidence suggests that the oxytocin system is highly complex and has multifaceted influences on behavior. The human oxytocin literature is limited by small sample sizes, failures to replicate (Nave et al., 2015), disputed statistical approaches (Conlisk, 2011; Walum et al., 2015), incomplete understanding of pharmacodynamics (Leng and Ludwig, 2015), possible publication bias (Lane et al., 2016), and variable study design. The subset of this literature focused on schizophrenia is limited by the same challenges (Oya et al., 2015). As a result, understanding of oxytocin's role in schizophrenia remains insufficient and we continue to lack consensus regarding its place, if any, in future treatment protocols.
Others have already published thorough and excellent reviews regarding oxytocin and schizophrenia (Bartholomeusz et al., 2015; Feifel et al., 2015; K. Macdonald and Feifel, 2012; Meyer-Lindenberg et al., 2011). In this review, we aim to contribute to the existing body of work in a few specific ways. First, we summarize the literature on oxytocin effects on positive and negative symptoms of schizophrenia and explore potential causes of heterogeneity in these studies, including study design factors as well as individual-level factors such as antipsychotic dosage. Second, we review evidence of oxytocin effects on social cognition and other deficits in schizophrenia, arguing that these areas warrant further study. Specifically, we highlight promising early findings regarding oxytocin's effects on mentalizing, as well as on non-social cognition, facial expressivity, and olfaction. Finally, we propose an agenda for future research, emphasizing the importance of more objective, precise outcome measures in order to rigorously characterize oxytocin's role in schizophrenia and explore its utility as a treatment.
2. Functional impairment in schizophrenia
Schizophrenia is characterized by three symptom domains: positive, negative, and cognitive (American Psychiatric Association, 2013). Positive symptoms include disorganized behavior, delusions, and perceptual aberrations such as auditory hallucinations. Negative symptoms are a multi-dimensional construct, referring to a cluster of deficits that affect motivation (asociality, avolition, anhedonia) and emotional expressivity (alogia, blunted affect) (Blanchard and Cohen, 2006). Cognitive deficits span multiple areas, affecting both social (Green et al., 2015) and non-social (Green and Harvey, 2014) domains. Together, these symptoms make schizophrenia a particularly devastating illness that ranks among the top 25 leading causes of disability worldwide (Chong et al., 2016) and costs over $60 billion annually in the U.S. alone (Marcus and Olfson, 2008). Affected individuals suffer from high levels of unemployment, limited ability to function independently, and social isolation (World Health Organization, 2008). These impairments lead to productivity losses that are the greatest contributor to the overall societal cost of schizophrenia (Wu et al., 2005).
Unfortunately, the functional impairment associated with schizophrenia has changed little over the past several decades (Hegarty et al., 1994; Jääskeläinen et al., 2013). Although there is widespread use of antipsychotic medications to treat the illness, these medications typically only ameliorate positive symptoms and fail to improve negative or cognitive symptoms (Carpenter and Koenig, 2008; Fusar-Poli et al., 2015; Kirkpatrick, 2000). Developing treatments to improve negative and cognitive symptoms in schizophrenia is essential, as severity of impairment in these domains is consistently associated with quality of life and functional outcomes (Mancuso et al., 2011; McGlashan and Fenton, 1992; Rabinowitz et al., 2012). Given that the costs associated with psychiatric illness continue to escalate (Bloom et al., 2012) while development of new therapeutics in the field has slowed (Hyman, 2012; Miller, 2010), investigating novel potentially effective treatments is a critical task to reduce morbidity.
3. The promise of oxytocin and current challenges
Oxytocin, a highly conserved neuropeptide produced in the hypothalamus, is widely recognized as a moderator of affiliation, stress, memory, and learning in animals and humans (Caldwell, 2012; Churchland and Winkielman, 2012; Sarnyai and Kovács, 2014). Research involving administration of oxytocin has increased dramatically over the last decade, and studies in non-human primates demonstrating that intranasal oxytocin can elevate oxytocin concentrations in the cerebrospinal fluid (CSF) (Chang et al., 2012; Dal Monte et al., 2014; Modi et al., 2014a) have supported the widespread adoption of intranasal administration in human populations. Administration of a single dose of oxytocin to healthy individuals has been shown to improve retention of social information (Guastella et al., 2012), reduce anxiety associated with social threat (Meyer-Lindenberg et al., 2011), facilitate interpretation of faces expressing complex mental states and social emotions (Domes et al., 2007; Leknes et al., 2013), and promote trust during interpersonal economic transactions with human (versus computer) partners (Kosfeld et al., 2005). Neuroimaging studies have implicated oxytocin in a variety of social brain processes and shown that the amygdala, medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), insula, and temporal regions are modulated by exogenous oxytocin (Adolphs, 2009; Bethlehem et al., 2013; Wigton et al., 2015a). Oxytocin has also been linked with non-social cognitive processes, such as spatial and episodic memory and cognitive flexibility (Chini et al., 2014). These promising findings have generated enthusiasm for oxytocin's potential as a therapeutic in multiple psychiatric disorders.
A dramatic rise in the number of oxytocin studies in clinical populations over the past decade reflects this enthusiasm (Quintana et al., 2015b). In addition to targeting deficits in schizophrenia, oxytocin has been investigated as a treatment for deficits in autism (Alvares et al., 2016; Ooi et al., 2016; Guastella and Hickie, 2016), alexithymia (Luminet et al., 2011), Prader-Willi Syndrome (Einfeld et al., 2014; Tauber et al., 2011) and social anxiety (Guastella et al., 2009; Labuschagne et al., 2010; Tabak et al., 2016). Results of these clinical studies have been notably inconsistent, however. A growing body of evidence now suggests that oxytocin's effects are more complex than previously thought: rather than being simply “pro-social,” it appears to modulate social interaction in a context-specific manner (Bartz et al., 2011) that is impacted by individual differences (K. Macdonald and Feifel, 2012; Tabak, 2013). In addition, there is debate about intranasal oxytocin's ability to consistently reach neural targets, lack of clarity about its pharmacodynamics (Bakermans-Kranenburg and van I Jzendoorn, 2013; Leng and Ludwig, 2015; Quintana and Woolley, 2015), and incomplete understanding of oxytocin receptor distribution in the human brain (Freeman and L. J. Young, 2016). Perhaps not surprisingly, there have been recent failures to replicate some of the early oxytocin findings in healthy humans (Nave et al., 2015). These issues present major challenges to investigating oxytocin's effects in schizophrenia, and have tempered the excitement that followed early work. Still, given oxytocin's critical role in socialization and the marked impairment that results from schizophrenia-associated deficits, tackling such challenges may prove to be a worthwhile effort.
4. Oxytocin and the pathophysiology of schizophrenia
Evidence from animal and human models suggests that oxytocin system dysregulation may play a role in the pathophysiology of schizophrenia. Results from rodent studies suggest that oxytocin could influence both positive (Caldwell et al., 2009; Feifel and Reza, 1999) and negative symptomatology (Meziane et al., 2015; Peñagarikano et al., 2015) (for a review see Feifel at al. (2015)). One neurofunctional model posits that abnormal oxytocinergic and dopaminergic signaling in the amygdala influences emotional salience processing, potentially leading to some of the social cognitive deficits observed in schizophrenia (Rosenfeld et al., 2010). Neuroimaging studies have shown that the amygdala and other social brain regions such as the PFC as well as the temporal gyri and sulci are modulated by exogenous oxytocin in healthy individuals (for a review see Bartholomeusz et al. (2015)). Taken together, these findings suggest a relationship between the oxytocin system and social brain function that may have important implications for treatment of schizophrenia.
Understanding of the link between impairments in schizophrenia and endogenous oxytocin system functioning, however, remains limited. Multiple studies have examined central and peripheral oxytocin levels in individuals with schizophrenia, with mixed results. CSF oxytocin levels correlated with negative symptoms in one study (Sasayama et al., 2012), but another found no difference in levels between individuals with and without schizophrenia (Glovinsky et al., 1994). Others have observed correlations between plasma oxytocin levels and facial affect identification (Goldman et al., 2008; Rubin et al., 2011), perception of emotion in dynamic body expressions (Strauss et al., 2015b), and less severe negative symptoms (Kéri et al., 2009) in schizophrenia. However, elevated plasma oxytocin levels in individuals with schizophrenia have also been associated with more severe positive symptoms (Rubin et al., 2014; Walss-Bass et al., 2013) and social cognitive impairment (Walss-Bass et al., 2013). Moreover, the utility of peripheral oxytocin measurement has been called into question: it is unclear whether plasma levels consistently reflect levels in the brain (Carson et al., 2014; Kagerbauer et al., 2013; Takagi et al., 1985). Thus, though the oxytocin system may play an important role in terms of etiology and symptom severity, its relationship with the deficits associated with schizophrenia is far from clear.
5. Oxytocin effects on positive and negative symptoms of schizophrenia
Despite this lack of clarity, a number of studies have investigated oxytocin's ability to treat symptoms of schizophrenia (see Table 1). The majority of RCTs conducted thus far have assessed the effects of intranasal oxytocin on positive and negative symptoms, with mixed results. In a within-subject cross-over study of 15 outpatients, Feifel et al. (2010) found improvement in both positive and negative symptoms after three weeks of twice daily 40 IU doses of oxytocin. Pedersen et al. (2011) found significant within-subject improvement in positive symptoms, paranoia, and general psychopathology in the oxytocin group after two weeks of 24 IU twice daily in a between-subject study of a mixed sample of 20 inpatients and outpatients. Similarly, Lee et al. (2013) administered 20 IU twice daily to a sample of 28 inpatients and outpatients in a between-subject study. They found that oxytocin improved negative symptoms only in the subset of inpatient participants. Modabbernia et al. (2013) administered 40 IU twice a day in a between-subject study to a sample of 40 inpatients for eight weeks, and found that oxytocin improved both positive and negative symptoms. Gibson et al. (2014) evaluated the effects of six weeks of twice daily 24 IU of oxytocin in fourteen outpatients, finding a significant reduction in negative symptoms in the oxytocin group relative to placebo. Davis et al. (2014) and Cacciotti-Saija et al. (2015) both investigated the impact of oxytocin combined with social cognition training. Davis et al. (2014) administered 40 IU of oxytocin twice per week and conducted social cognitive training sessions in 27 individuals with schizophrenia in a between-subject study. The authors found no significant oxytocin effects on symptoms post-treatment or at one month follow-up, though all testing occurred off of oxytocin. Cacciotti-Saija et al. (2015) investigated the effect of twice-daily administration of 24 IU in a cohort of 52 patients diagnosed with an early psychotic illness in a between-subject study, and saw no oxytocin-induced improvements in symptoms after six weeks of treatment.
Table 1.
Studies evaluating oxytocin effects on symptom rating scales.
| Authors | N | Gender | Mean age (SD) | Duration | Design | Setting | Dosing | Administration | Symptom measures | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Cacciotti-Sajia et al., 2015 | 52 SZ OT=27, PL=26 | M+F | OT: 21.5 (4.2) PL: 22.3 (4.4) | 6 weeks | Between | Outpt | 24 IU BID | Self | SAPS SANS | OT did not improve positive symptoms Increased use of OT correlated with less severe negative symptoms |
| Dagani et al., 2016 | 32 SZ | 26 M 6F | 30.4 (6.7) | 16 weeks | Within | Outpt | 40 IU daily | Self | PANSS | OT did not improve positive, negative, or general symptoms |
| Davis et al., 2014 | 27 SZ OT=13, PL=14 | M | OT: 37 (10.8) PL: 37 (10.8) | 6 weeks | Between | Outpt | 40IU 2× per week | Supervised | BPRS CAINS | OT did not improve positive or negative symptoms |
| Feifel et al., 2010 | 15 SZ | M+F | 48 (8.9) | 3 weeks | Within | Outpt | 40IU BID | Self | PANSS | OT improved positive and negative symptoms |
| Gibson et al., 2014 | 14 SZ OT=8, PL=6 | M+F | OT: 38.9 (7.22) PL: 35.6 (9) | 6 weeks | Between | Outpt | 24IU BID | Self | PANSS | OT improved negative symptoms |
| Lee et al., 2013 | 28 SZ OT=13, PL=15 | M+F | OT: 44.7 (11.7) PL: 35.1 (8.2) | 3 weeks | Between | Inpt/Outpt | 20IU BID | Self | BPRS SANS | OT improved negative symptoms in inpatients only |
| Modabbernia et al., 2013 | 40 SZ OT=20, PL=20 | M+F | OT: 32.3 (7.4) PL: 33.2 (6.9) | 8 weeks | Between | Inpt | 40IU BID | Supervised | PANSS | OT improved positive and negative symptoms |
| Pedersen et al., 2011 | 20 SZ OT=11, PL=9 | M+F | OT: 39.0 (11.2) PL: 35.8 (9.5) | 2 weeks | Between | Inpt/Outpt | 24IU BID | Self | PANSS | OT improved positive symptoms, general psychopathology, & paranoia |
A meta-analysis of these RCTs (Oya et al., 2015) with a combined sample size of N=206 found that oxytocin was superior to placebo only on a subset of general symptom measures, and not on positive or negative symptoms specifically. Significant heterogeneity among studies was noted, however, and the authors emphasized the need for further work. Dagani et al. (2016) subsequently conducted the longest trial to date, an 8-month within-subject study in 32 outpatients with a relatively short duration of illness (<11 years). They found no treatment effects on negative or positive symptoms. Recently, a second meta-analysis (Williams and Burkner, 2016) used a Bayesian approach to analyze all eight longitudinal RCTs conducted thus far. With a combined sample size of N=238, the authors found no effects on either negative or positive symptoms (again noting significant heterogeneity). Finally, in addition to these longitudinal RCTs, one single-dose, between-subject study has evaluated clinical symptoms: Davis et al. (2013) found no effect of 40 IU of oxytocin on symptom severity in a sample of 23 outpatients. In summary, while early work was promising, recent findings regarding oxytocin's effects on clinical symptoms of schizophrenia have generally been discouraging with multiple studies failing to find meaningful impact.
6. Potential sources of heterogeneity in extant studies
The mixed results described above have made it challenging to draw conclusions about oxytocin's potential to improve positive and negative symptoms of schizophrenia. Multiple authors have made the important observation that small, underpowered studies may make it difficult to detect any effects of oxytocin (Oya et al., 2015; Shilling and Feifel, 2016; Williams and Burkner, 2016). In addition to these limitations, several moderating factors arising from study design and from differences at the individual level appear to modulate oxytocin's influence. These factors are critical to consider as potential sources of the heterogeneity between and within extant studies. Below, we summarize study and individual-difference factors that may significantly influence results.
6.1 Study factors
Use of symptom rating scales
An identified challenge in the meta-analyses described above (Oya et al., 2015; Williams and Burkner, 2016) is synthesizing outcomes generated by the variety of metrics used across studies. The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) is the most established scale to track changes in symptom severity schizophrenia (Obermeier et al., 2010) and has been used most frequently in the literature. The PANSS is conducted by a clinician using a semi-structured interview and consists of a total of 30 items divided into negative, positive, and general psychopathology subscales (the general psychopathology subscale is a collection of non-specific symptoms distinct from the positive and negative categories) (Kay et al., 1987). To date, four oxytocin studies have assessed schizophrenia symptoms using the PANSS (Feifel et al., 2010; Gibson et al., 2014; Modabbernia et al., 2013b; Pedersen et al., 2011a). Two others (M. C. Davis et al., 2014a; Lee et al., 2013) have used the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962), an 18-item instrument used to assess general psychopathology. The Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1984) and the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984), 25-item tools that evaluate symptom factors using a Likert scale, have also been used (Lee et al., 2013) (Cacciotti-Saija et al., 2015). Finally, one study (M. C. Davis et al., 2014a) used the Clinical Assessment Interview for Negative Symptoms (CAINS), a 13-item, interview-based tool (Kring et al., 2013) to assess symptoms. Despite significant overlap in terms of content, these instruments are distinct—there are no agreed-upon methods to compare or convert scores between any of them. Even the PANSS and BPRS, which contain several identically-named items, are not interchangeable (BELL et al., 1992). Use of these different instruments therefore presents a challenge to effectively comparing outcomes across studies.
Furthermore, clinical assessment tools themselves may limit our ability to adequately capture the effects of oxytocin in schizophrenia. The widely-used PANSS, for instance, has several drawbacks including a 7-point rating scale that may not reflect clinically meaningful change (e.g., examiners may not actually be able to distinguish between auditory hallucinations that are a “4 - moderate” versus a “5 – moderate severe” in severity) (Levine et al., 2011), item redundancy (Lehoux et al., 2009), incomplete measurement of positive symptoms (Aboraya and Nasrallah, 2016), and reliance on a structured clinical interview (a requirement that many studies do not meet) (Nicotra et al., 2015). In addition, the PANSS may not capture the most salient elements of psychotic illness. For instance, core symptoms of schizophrenia per the DSM-V are represented by only eight of the thirty total PANSS items (P1-delusions, P2-conceptual disorganization, P3-hallucinatory behavior, P5-grandiose delusions, P6-persecutory delusions, G1-somatic delusions, P6-persecutory delusions, G1-somatic delusions, G3-delusions of guilt, and G9-unusual thought content). A lower PANSS score, then, does not necessarily reflect reduced severity of psychosis (Aboraya and Nasrallah, 2016).
The PANSS and other symptom rating scales may be particularly problematic when it comes to assessing negative symptoms. As discussed above, negative symptoms are multidimensional and challenging to evaluate. Existing rating scales can be divided into older, first-generation and newer, second-generation tools (Marder and Kirkpatrick, 2014). First-generation tools, such as the PANSS negative subscale, BPRS, and SANS are limited in that they do not capture all negative symptoms (such as asociality, avolition, and anhedonia), and rely more heavily on behavior for assessment rather than internal experiences (Paz Garcia-Portilla et al., 2015). The PANSS, a comprehensive scale to asses psychopathology (Kay et al., 1987), was not designed to measure negative symptoms independently (Marder and Kirkpatrick, 2014). Furthermore, the PANSS and the SANS include constructs outside of the five negative symptom domains (blunted affect, alogia, asociality, anhedonia and avolition) recognized by the National Institute of Mental Health (NIMH) consensus statement (Kirkpatrick et al., 2006). Though frequently used, these first-generation tools reflect an incomplete and outdated approach to negative symptom assessment.
Second-generation assessment scales, such as the Brief Negative Symptom Scale (BNSS) (Kirkpatrick et al., 2011) and the CAINS, were developed to address limitations of earlier tools. They are organized to reflect an updated understanding of negative symptoms, including the five negative symptom domains and distinguishing between internal experience versus expressive behavior (Kane, 2013; Marder and Kirkpatrick, 2014). The BNSS and CAINS have consistently shown a similar factor structure (Marder and Kirkpatrick, 2014), and evidence supports their inter-rater reliability, test-retest reliability, and external validity (Kring et al., 2013). Comparison of the BNSS with the SANS and BPRS (Strauss and J. M. Gold, 2016; Strauss et al., 2012) indicates that although BNSS total scores correlate with the scores from both of these first-generation tools, the BNSS is distinct and not redundant with either. In particular, items relating to motivation and pleasure demonstrate lower levels of convergence between the BNSS and the BPRS (Strauss and J. M. Gold, 2016). A comparison of the CAINS with the BPRS showed that the CAINS Expression subscale does correlate strongly with BPRS negative symptom ratings; however, the Experience subscale correlates to a much lesser degree (Horan et al., 2011). Although the BNSS and CAINS share a similar framework and overlap significantly, they are still not considered equivalent (Marder and Kirkpatrick, 2014) and there is no conversion between them. As both are in relatively early stages of development, they will also likely be adapted in the future. Thus far, the CAINS has only been used in one trial of oxytocin in schizophrenia (M. C. Davis et al., 2014a) and the BNSS has not yet been used in any such studies. Importantly, even implementation of these newer, improved tools may not mean better capture of oxytocin effects. All of the symptom-rating scales discussed here rely on a clinical interview, which requires subjective judgment of symptom severity rather than objective measurement. This approach may introduce significant variability even within each assessment tool, and limits our ability to rigorously examine oxytocin's effects on specific symptoms in schizophrenia.
Dosing and duration of treatment
Studies in both animals and humans have shown divergent responses to oxytocin at different doses. In rodents, low doses of exogenous oxytocin have pro-social effects but high doses can be socially impairing (Benelli et al., 1995; Popik et al., 1992). The vast majority of oxytocin studies in humans have administered 20 to 40 IU intranasally (K. Macdonald and Feifel, 2012), following precedent rather than empirical evidence (Quintana et al., 2015b)—no study has systematically evaluated dose-response and clearance of oxytocin from the CSF and blood (Striepens et al., 2011). A handful of small studies, however, have directly compared multiple dosages of oxytocin. Administration of a single dose of 24 IU to 17 healthy men attenuated cortisol levels relative to placebo, while 48 IU did not (Cardoso et al., 2013). In contrast, administration of a single dose of oxytocin in a sample of 46 healthy women resulted in significantly elevated salivary oxytocin levels, but the dose received (16 IU versus 24 IU) did not appear to matter (van IJzendoorn et al., 2012). Hall et al. (2012) administered a single dose of oxytocin to a sample of eight men with Fragile X syndrome, and found that eye gaze frequency improved significantly in response to 24 IU, but not 48 IU. Using four treatment conditions (two different single doses of intranasal oxytocin, intravenous oxytocin, and placebo), Quintana et al. (2016) compared amygdala response to emotional faces by fMRI in a within-subject study of 16 healthy men. The lower dosage (8 IU) of intranasal oxytocin, but not the higher dosage (24 IU), dampened amygdala activation. The cause of these divergent responses is unclear, though the authors highlight cross-reactivity between oxytocin and arginine vasopressin (AVP) receptors at higher doses as a potential explanation (Quintana et al., 2015b).
Oxytocin acts primarily through the oxytocin receptor (OXTR), but also has affinity for arginine vasopressin (AVP) receptors (Manning et al., 2012). The balance between oxytocin and AVP, which tends to enhance anxiogenic and depressive effects in the brain, is thought to be key for the regulation of social behavior and psychopathology (Neumann and Landgraf, 2012). Different doses of exogenous oxytocin may exert different effects on the equilibrium between AVP and oxytocin, which could lead to divergent behavioral outcomes. In the only intranasal oxytocin study to compare multiple dosages in individuals with schizophrenia, divergent responses were observed, but in the opposite direction seen in Quintana et al. (2015b). Goldman et al. (2011) administered 10 IU, 20 IU, and placebo in a between-subject study in 13 individuals with schizophrenia and 11 healthy controls. Emotion recognition worsened in individuals with schizophrenia following the 10 IU dose, and only improved in a subgroup—those with polydipsic schizophrenia—following the higher 20 IU dose. Dysregulation of the antidiuretic hormone AVP in polydipsic schizophrenia has been linked to deficits in CNS oxytocin activity (Goldman, 2009), highlighting the possibility that activity of the endogenous oxytocin and AVP systems may have implications for exogenous oxytocin dosage in schizophrenia. Additional work is needed to clarify neuroendocrine dynamics in schizophrenia in order to implement evidence-based, optimal dosing of oxytocin in clinical trials.
Furthermore, preclinical studies in healthy animals suggest that oxytocin has markedly different effects when administered acutely versus chronically. In prairie voles, a single dose of oxytocin enhances social behavior, whereas long-term treatment with oxytocin during adolescence causes a deficit in partner preference behavior in adulthood (Bales et al., 2013). A single dose of oxytocin in rats significantly increased pre-pulse inhibition (PPI) (Feifel et al., 2012b), a measure of sensorimotor gating deficient in individuals with schizophrenia that may underlie positive symptoms (Greenwood et al., 2013). Chronic dosing in mice, however, had no effect on PPI and even reduced OXTR throughout the brain (Huang et al., 2014). In another mouse study, administration of chronic oxytocin at high doses induced an anxiogenic phenotype that correlated with reduced OXTR binding. Administering low doses chronically, however, was protective against the effects of stress (Peters et al., 2014). Finally, a study in infant macaques showed that chronic administration of intranasal oxytocin resulted in decreased attention to the eye region of faces (Parr et al., 2016). Taken together, these studies suggest that prolonged oxytocin administration may disrupt social behavior in healthy animals, possibly via down-regulation of OXTR expression in social brain regions.
In the setting of neuropsychiatric illness, chronic oxytocin treatment may produce notably different effects. In a rat model of post-traumatic stress disorder (PTSD), for example, chronic low doses of oxytocin were shown to have cumulative and persistent anxiolytic effects (Janezic et al., 2016). The implications of such animal studies for humans are unclear, as oxytocin's role varies significantly between species (Insel, 2016), but they suggest the need to thoroughly investigating chronic oxytocin treatment at clinically effective doses in individuals with schizophrenia. Almost all pharmacologic treatments for neuropsychiatric disorders involve chronic daily administration, and many require weeks of use before significant changes are observed (K. Macdonald and Feifel, 2013). Some agents, such as selective serotonin reuptake inhibitors (SSRIs) can have notably different acute versus chronic effects (Burghardt and Bauer, 2013). Studies of oxytocin have shown positive effects following treatment of a variety of regimens ranging from a single dose to several weeks, but it is a challenge to interpret these findings as long as optimal administration protocols and clinically effective dosages remain unknown. Developing clear dose-response curves and conducting longitudinal studies in larger samples are essential steps in ultimately understanding oxytocin's effects in schizophrenia.
Administration protocols
Poor bioavailability by oral administration and rapid metabolism in peripheral circulation present challenges for using neuropeptides as therapeutics (Insel, 2016). Almost all human studies have therefore relied on intranasal administration using a nasal spray to deliver oxytocin in order to maximize delivery to the CNS. In non-human primates, intranasal oxytocin has been shown to elevate CSF oxytocin levels (Chang et al., 2012; Dal Monte et al., 2014; Freeman et al., 2016; Modi et al., 2014b), offering promising evidence that oxytocin is able to reach the CNS via the intranasal route. However, the consistency of this method has not been fully examined (Quintana et al., 2015a) and may represent another source of heterogeneity both between and within studies. First, the pharmacokinetics of intranasally administered oxytocin are not yet well understood. In non-human primate studies, elevated CSF oxytocin levels have been observed at different time points following intranasal administration, ranging from 15 (Freeman et al., 2016) to 120 minutes (Modi et al., 2014b). In the only study in humans examining CSF oxytocin levels following intranasal administration (Striepens et al., 2013), a dose of 24 IU of oxytocin administered to eleven individuals led to elevation of CSF levels after 75 minutes. Protocols in human oxytocin studies have generally included a 30-minute delay after administration of oxytocin before beginning assessments. This convention arose from studies showing robust oxytocin-induced behavioral and physiological responses using this delay in healthy individuals (K. Macdonald and T. M. Macdonald, 2010). The peak and duration of oxytocin's CNS effects, however, are unknown. Studies examining oxytocin in schizophrenia have used a variety of assessment protocols with a range of lengths. Differences in the timing of oxytocin administration relative to assessments may impact whether oxytocin effects are captured, or not, in a given study.
Second, intranasal administration poses several challenges. Guastella et al. (2013) and Quintana et al. (2015) have published excellent reviews detailing many of the considerations regarding intranasal oxytocin administration. Briefly, although multiple pathways from the nose to the central nervous system (CNS) have been described, the nasal cavity has complex anatomy that makes drug administration by this route less than ideal (Guastella et al., 2013). Specifically, dimensions of the nasal valve vary over time and across individuals, and additional variability in terms of drug delivery can be introduced by body position, breathing patterns during delivery, and operator coordination (Djupesland, 2013). Furthermore, standard nasal spray devices generally deposit drug anterior to the nasal valve, though oxytocin must access the upper portion of the cavity beyond the valve to ultimately reach the brain (Djupesland, 2013). A study in macaques found different effects of oxytocin on CSF levels when the drug was delivered via intranasal spray versus nebulizer (Modi et al., 2014b), and Quintana et al. (2015) demonstrated in humans that nasal valve dimensions were associated with oxytocin treatment response on a measure of social cognition. These findings suggest that nasal cavity anatomy and delivery method may significantly influence the effects of oxytocin, and that improving intranasal delivery of oxytocin to target sites in the nasal cavity could lead to better and more reliable CNS penetration. Recently, there have been promising developments in intranasal delivery device technology (e.g. OptiNose (Djupesland, 2013) and Impel (Lochhead and Thorne, 2012)), offering potential alternatives to the traditional nasal spray used in studies thus far and an opportunity to improve standardization of oxytocin administration.
A related issue is the variation in intranasal administration protocols between, and even within, studies that may result in significant differences in oxytocin bioavailability. In several studies, a trained technician administered oxytocin in the laboratory or inpatient setting (Feifel et al., 2010; Fischer-Shofty et al., 2013b; Guastella et al., 2015; Lee et al., 2013; Modabbernia et al., 2013b). In other studies, participants self-administered the drug, with ((Averbeck et al., 2012; Pedersen et al., 2011b; Woolley et al., 2014); inpatient subgroup in Pedersen et al. (2011)) or without (Cacciotti-Saija et al., 2015; Lee et al., 2013; Pedersen et al., 2011a) supervision. These different protocols may have led to variability in adherence that, in turn, impacted oxytocin effects. For instance, in the study conducted by Lee et al. (2013), only the inpatient subgroup, which received drug administered by a technician, showed significant improvement in negative symptoms with oxytocin. There was no improvement in the outpatient subgroup, which self-administered the drug without supervision. In addition, Cacciotti-Saija et al. (2015) found that the amount of nasal spray used by participants significantly correlated with a reduction in negative symptoms. The range of administration techniques used in oxytocin trials thus far, incomplete understanding of the pharmacokinetics of intranasal oxytocin, and the variability of nasal drug delivery are likely important to consider when interpreting study results. All represent potential sources of heterogeneity that cloud our understanding of oxytocin effects in schizophrenia.
Context
Oxytocin acts as a modulator of complex social interactions and may promote pro-social, group-differentiating, or even aggressive behaviors depending on the situation (Bakermans-Kranenburg and van IJzendoorn, 2014; Shamay-Tsoory and Abu-Akel, 2016). Thus, the context in which oxytocin is administered may have an important influence on study results (for a review see Bartz et al., (2011)). In healthy individuals, intranasal administration has been shown to increase feelings of envy and schadenfreude when other players won more money (Shamay-Tsoory et al., 2009). De Dreu et al. conducted a series of studies in which oxytocin increased in-group, but not out-group, trust during various games (De Dreu and Kret, 2016). Similarly, during a prisoner's dilemma task, effects of oxytocin depended on whether or not participants interacted with one another prior to the experiment (Declerck et al., 2014). A critical implication of these findings is the fact that oxytocin's pro-social effects within groups may ultimately contribute to intergroup tension and conflict (De Dreu and Kret, 2016). Oxytocin has even been shown to increase inclinations towards intimate partner violence in individuals with high trait aggression (DeWall et al., 2014). Reconciling these findings with oxytocin's pro-social effects, one model proposes that oxytocin impacts the salience of various social stimuli, regardless of valence (Shamay-Tsoory and Abu-Akel, 2016). Oxytocin's role as a modulator of social salience may be important to consider when designing clinical studies and interpreting results that are likely to be influenced by context. Furthermore, there may be particularly complex implications for schizophrenia research, as individuals with schizophrenia tend to demonstrate impaired salience processing (Hahn et al., 2010; Holt et al., 2006b). Improved understanding of the ways in which context impacts oxytocin's effects is needed in order to thoroughly test its ability to improve deficits in schizophrenia. In the extant literature, influence of different contexts between and within studies may be another significant contributor to heterogeneous findings.
6.2 Individual factors
Gene-environment interactions and early life experiences
Differences in genetic expression and early life events may account for some of the observed variability in individuals' responses to oxytocin. In healthy individuals, certain OXTR variants have been associated with social phenotypes such as empathy, prosocial behavior, stress reactivity, and mentalizing (see Meyer-Lindenberg et al., (2011) for a review). Lower DNA methylation of the structural gene for oxytocin (OXT) has been associated with more secure attachment style, improved ability to detect facial expressions, and greater superior temporal sulcus activity during social tasks (Haas et al., 2016). Early life events may influence the oxytocin system through such epigenetic mechanisms: abuse or neglect in childhood, for example, has been associated with lower central oxytocin levels in rhesus monkeys (Winslow et al., 2003) and in women (Heim et al., 2009). In individuals with schizophrenia, there is early evidence to suggest that certain OXTR and OXT variants may correlate with symptom severity (Montag et al., 2013; Souza et al., 2010; Watanabe et al., 2012) as well as social cognitive impairments (M. C. Davis et al., 2014b; for a review see Bartholomeusz et al., (2015)). Furthermore, OXTR methylation has been associated with cognitive performance in individuals with psychosis (Grove et al., 2016). Taken together, these findings suggest that genotype, early life experiences, and epigenetic signatures likely impact development of the human oxytocin system.
Endogenous oxytocin system functioning may, in turn, have important implications for responsiveness to oxytocin administration. For example, intranasal oxytocin administration has been shown to attenuate stress responses in individuals with poor emotion regulation abilities, but not in those with normal emotion regulation (Cardoso et al., 2012; Quirin et al., 2011). However, there is also evidence to suggest that, in individuals who faced childhood adversity, oxytocin administration fails to produce pro-social effects (Bakermans-Kranenburg et al., 2012; van IJzendoorn et al., 2011; for a review see Bakermans-Kranenburg and van IJzendoorn, (2013)). Clearly, further work is needed to characterize the complex interactions between genetic variation in oxytocin pathway genes, environmental conditions during development, and oxytocin system function. Improved understanding of the ways that genotype, epigenetic signatures, and developmental events ultimately affect sensitivity to oxytocin in schizophrenia is critical to the interpretation of clinical trial results.
Sex
Sex differences in oxytocin modulatory effects, which have been observed in both animal and human studies (Dumais and Veenema, 2016), represent another source of heterogeneity in the extant literature. Neuroimaging studies have demonstrated divergent activation in the temporal lobes of men and women in response to oxytocin administration (for a review see Wigton et al., (2015)), sex hormones influence expression of OXTR in the brain as well as central oxytocin release (Gabor et al., 2012), and intranasal oxytocin administration may have differing effects on amygdala reactivity (Domes et al., 2010; Lischke et al., 2012) and salience of social cues (Gao et al., 2016) in men and women. Women's higher circulating levels of oxytocin and the hormonal shifts involved in the menstrual cycle may complicate their responsiveness to exogenous oxytocin (Bakermans-Kranenburg and van I Jzendoorn, 2013). Unfortunately, the impact of sex differences on oxytocin responsiveness is poorly understood, as the vast majority of clinical studies have enrolled only male participants.
The sex differences seen in schizophrenia—men are more likely to have the illness (Hafner, 2003), develop symptoms at an earlier age (Aleman et al., 2003), and tend to have a more severe course (Desai et al., 2013; Halbreich and Kahn, 2003)—complicate the picture further. It is unclear how sex differences in schizophrenia and in oxytocin responsiveness relate to one another, but there is early evidence to suggest that sex may be an important modulating factor. For example, greater OXTR methylation has been associated with social cognitive impairment in women with schizophrenia, but not in men (Rubin et al., 2016). Furthermore, OXTR methylation and peripheral levels of oxytocin were positively correlated in women, but negatively correlated in men. Thus far, several studies of oxytocin in schizophrenia have used samples with varying percentages of both men and women (Feifel et al., 2010; Fischer-Shofty et al., 2013b; Gibson et al., 2014; Goldman et al., 2011; Lee et al., 2013; Modabbernia et al., 2013b; Pedersen et al., 2011b; Woolley et al., 2015), while others have included only male participants (Averbeck et al., 2012; M. C. Davis et al., 2014a; 2013; Horta de Macedo et al., 2014; Michalopoulou et al., 2015; Woolley et al., 2014). Differences between these study samples, and between individual men and women in terms of their responsiveness to oxytocin, add another challenge to interpreting findings. Better characterization and accounting for sex differences may be a critical step in predicting individuals' responsiveness to oxytocin and ultimately reducing heterogeneity in the literature.
Age
Age may also be an important moderator of oxytocin effects that has the potential to introduce heterogeneity. Oxytocin's role in social cognition and behavior may be regulated by age-related factors such as shifts in gonadal hormone levels and neuroendocrine function (Bos et al., 2012). In healthy humans, complex age-related changes in social abilities are observed: some abilities, such as emotion regulation, tend to improve with age, while others, such as emotion recognition, decline (see Isaacowitz et al., (2007) and Ruffman et al., (2008) for reviews). These changes are not fully explained by the changes in visual processing or neurocognition that occur with aging (Samanez-Larkin and Carstensen, 2011). Whether alterations in the oxytocin system could underlie changes in social abilities is unclear, as research on oxytocin and aging is extremely limited (Ebner et al., 2013). The few studies involving exogenous oxytocin administration in humans, however, suggest that age may be an important moderator to consider: in healthy individuals, intranasal oxytocin has been shown to improve emotion recognition in older men, but not in older women or young adults (Campbell et al., 2014), and to increase attention to one's own feelings only in older men and young women (Ebner et al., 2015a). Furthermore, intranasal oxytocin's ability to modulate resting-state connectivity between the amygdala and mPFC, regions involved in social cognitive processing, is impacted by both age and sex in healthy individuals (Ebner et al., 2016). In line with these intriguing findings, there has been growning interest in examining the impact of aging on oxytocin's role in social behavior (Ebner et al., 2013; 2015b; Huffmeijer et al., 2013). In fact, Ebner et al. (2013) articulated an Age-Related Genetic, Neurobiological, Sociobehavioral Model of Oxytocin (AGeNeS-OT), proposing that oxytocin research be considered from a developmental perspective and thoroughly explore age-related variation.
In the extant literature on oxytocin in schizophrenia, no studies have examined age-related changes in the endogenous oxytocin system or compared oxytocin effects in younger versus older individuals. Furthermore, no studies have taken into account the age of symptom onset in individuals with schizophrenia. Schizophrenia is not a monolithic disorder, and there is some evidence that early age of onset (during adolescence) is associated with a more severe form of the illness (Hollis, 2000). A recent meta-analysis of the longitudinal trials evaluating oxytocin effects on clinical symptoms (Williams and Burkner, 2016) found that variability in participant age did not explain a significant amount of heterogeneity between studies; however, it is unclear whether age contributes to heterogeneity within studies in the extant literature. Given the relevance of age to symptom development and severity in schizophrenia, it may be particularly important to determine whether age is a moderator of oxytocin effects at the level of the individual.
Antipsychotic medication
Anti-dopaminergic agents present another challenge to understanding the role of oxytocin in schizophrenia, as their interaction with the oxytocin system is not yet well understood (Liu and Wang, 2003). There is evidence to suggest that dopamine may play a role in social behavior known to be modulated by oxytocin, such as mother-infant bonding. For example, Atzil et al. (2017) used a combined fMRI-PET scanner to explore mothers' dopamine reponses to their infants as well as connectivity between the nucleus accumbens (NAcc), the amygdala, and the medial prefrontal cortex (mPFC), an intrinsic network that supports social functioning. The authors found that synchronous behavior between mothers and infants as well as greater network connectivity were associated with increased dopamine reponses. There is also evidence that oxytocin may impact reward-related dopaminergic activity (Mickey et al., 2016), potentially influencing social salience and valence assignment as well as inhibiting defensive behaviors (Skuse and Gallagher, 2011; 2009). Studies showing oxytocin effects on attraction to faces (Theodoridou et al., 2009) and enhanced activation of the striatum with increasing facial attractiveness (Aharon et al., 2001; Cloutier et al., 2008; Liang et al., 2010; Winston et al., 2007), for instance, suggest that oxytocin may modulate striatal dopaminergic reward mechanisms. However, the relationship between the oxytocin and dopamine systems is far from clear. In a study measuring dopamine release by positron emission tomography (PET), Striepens et al. (2014) found no evidence that oxytocin-induced increased attractiveness ratings for faces were associated with increased dopaminergic activity in reward-related brain areas. Further work in this area is essential to understand the interface between oxytocin and dopaminergic circuits.
Interactions between oxytocin and dopamine are are also unclear in the setting of schizophrenia. In animal models of psychosis, oxytocin administration has been shown to reduce dopaminergic hyperactivity in the nucleus accumbens and striatum (Qi et al., 2008), and animal work has also suggested that atypical antipsychotic agents such as clozapine and amperozide, but not the typical agent haloperidol (Uvnäs-Moberg et al., 1992), are associated with an increase in plasma oxytocin concentration. In humans, there is evidence that higher doses of antipsychotic medication are associated with lower levels of plasma (Goldman et al., 2008) and CSF oxytocin levels (Sasayama et al., 2012) in individuals with schizophrenia. It is unclear whether antipsychotic medication leads to lower endogenous oxytocin levels, possibly via antipsychotic-induced disinhibition of prolactin secretion leading to suppressed oxytocin secretion (Sirzen-Zelenskaya et al., 2011), or whether patients with lower oxytocin levels respond less well to antipsychotics and therefore tend to take higher doses of medication (Sasayama et al., 2012). Furthermore, little is understood about how endogenous oxytocin may moderate responses to exogenous oxytocin administration.
To explore the relationship between antipsychotic dosages and oxytocin sensitivity, Woolley et al. examined whether antipsychotic dosage moderated oxytocin effects in three recent studies. Chlorpromazine (CPZ) equivalents were calculated for patients using a standardized conversion table (Andreasen et al., 2010), and oxytocin-induced changes were calculated by subtracting the placebo day performance from the oxytocin day performance for each study task. Using nonparametric rank correlations, the authors found that lower antipsychotic dosage was associated with greater oxytocin-induced improvements in mentalizing (Figure 1A; CPZ equivalents ρ = -0.34; p=0.042; (Woolley et al., 2014) see supplemental materials), and facial expressivity (Figure 1B; ρ = -0.48; p=0.004; (Woolley et al., 2017) see supplemental materials). The authors did not find a significant association between antipsychotic dosage and olfactory detection thresholds (Figure 1C; ρ = -0.118, p=0.535; (Woolley et al., 2015) see supplemental materials). These findings offer further support for the idea that antipsychotic medications may interact with oxytocin effects, and highlight the need for additional study to clarify predictors of oxytocin sensitivity in schizophrenia.
Fig. 1. Relationships between oxytocin effects and antipsychotic dosage.
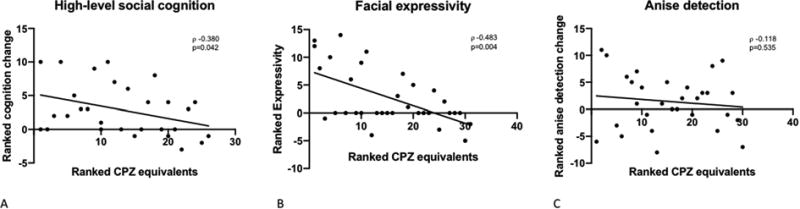
The magnitude of oxytocin-induced changes in high-level social cognition (A), and facial expressivity (B), are significantly associated with lower antipsychotic dosage. Anise odor detection (C) is not significantly associated with lower antipsychotic dosage, although the relationship is in the right direction.
Differences in antipsychotic medication dosages and the other individual factors described in this section likely combine with study factors to explain a significant amount of the observed heterogeneity in the literature on oxytocin in schizophrenia (see Figure 2). Without improved understanding of how such factors impact clinical oxytocin studies, our ability to draw conclusions from their results remains limited.
Fig. 2. Sources of heterogeneity in studies of oxytocin in schizophrenia.
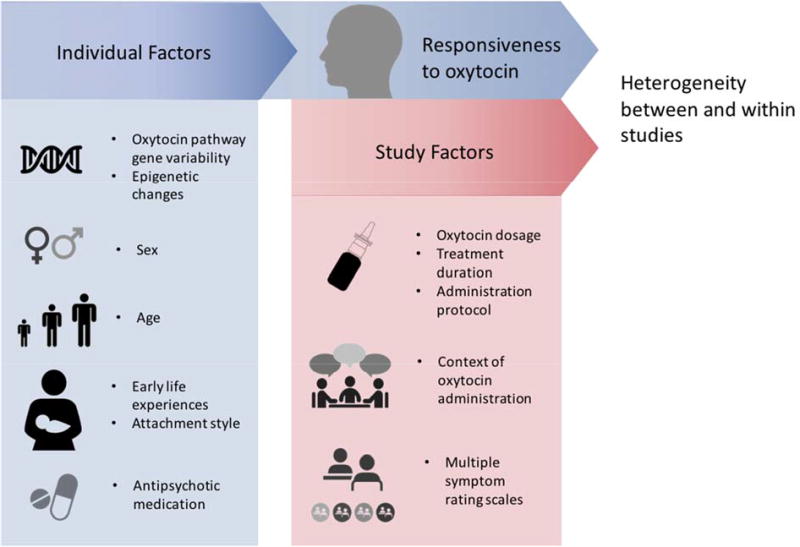
Individual factors affect responsiveness to exogenous oxytocin, which, combined with study factors, lead to heterogeneous results.
7. Oxytocin effects on social cognitive deficits in schizophrenia
In addition to the RCTs that have primarily assessed oxytocin's effects on symptom ratings in schizophrenia, several studies have examined its effects on social cognition. In this section, we summarize a model of social cognition in schizophrenia, and discuss study findings that point to a potential role for oxytocin in ameliorating deficits in a specific social cognitive domain.
7.1 Social cognition in schizophrenia
Increasingly, social cognitive deficits are recognized as a core feature of schizophrenia. Individuals with schizophrenia commonly demonstrate impairments in the ability to interpret the intentions and behavior of others (Pinkham et al., 2003), and social cognitive impairment has a greater impact on an individual's level of functioning than non-social cognitive impairment (Fett et al., 2011). Neuroimaging studies have found that individuals with first-episode psychosis and schizophrenia tend to have structural abnormalities in social brain regions (Jung et al., 2010; Mechelli et al., 2011; Pantelis et al., 2003; 2009; Takahashi et al., 2009) and display hypoactivations in these regions during social cognitive tasks (Das et al., 2007; Reske et al., 2009; Sugranyes et al., 2011). Specific social cognitive deficits tend to develop prior to the onset of positive symptoms, remain stable across the course of illness (Fett et al., 2011; Green et al., 2015), and are seen in individuals at ultra-high risk for developing psychosis as well as unaffected family members (Bora and Pantelis, 2013). Taken together, these findings suggest that social cognitive deficits may be an endophenotype for schizophrenia. Furthermore, individuals' ability to cope with the threatening experiences that arise from positive symptoms (such as paranoia and hallucinations) may be limited by poor social cognitive skills (Gumley et al., 2014). Despite their clinical importance, there are no currently available pharmacological treatments for social cognitive deficits.
There is growing consensus that social cognition is made up of distinct domains, and that some but not all social processes are impaired in schizophrenia. The model articulated by Green et al. (2015) (see Figure 3) defined four main subcomponents of social cognition: 1) social cue perception, 2) mentalizing, 3) experience and regulation of emotion, and 4) experience sharing. 1) Social cue perception involves the ability to recognize information embedded in others' faces, voices, and body movements, and is known to be impaired in schizophrenia. Deficits in the perception of facial expressions, for example, are correlated with aberrant neural activity (Delvecchio et al., 2013; Li et al., 2010; Taylor et al., 2012), and neuroimaging studies have suggested that individuals with schizophrenia tend to perceive neutral faces as threatening (Holt et al., 2006a; Morris et al., 2009). Studies using behavioral paradigms (R. Gold et al., 2012; Leitman et al., 2005) have also found deficits in the perception of voices, particularly pitch and rhythm. 2) Mentalizing (also known as theory of mind, or mental state attribution) is the ability to infer the mental states of others (Baron-Cohen et al., 2001) and is also known to be impaired in schizophrenia (Brüne et al., 2009; Harrington et al., 2005; Savla et al., 2013). Mentalizing deficits result in misinterpretation of hints, intentions, deception, metaphor, and irony (Penn et al., 2008). Impairments may also lead to the over-attribution of intention, or “hypermentalizing,” which has been linked to paranoid ideation in schizophrenia (Ciaramidaro et al., 2015; Frith, 2004). Neuroimaging studies have implicated a dispersed network of brain regions that play a role in mentalizing. In schizophrenia, dysfunction appears to be related to hypoactivation in the medial prefrontal cortex (mPFC), thalamus, middle and superior temporal regions, temporoparietal junction (TPJ), as well as hyperactivation in the posterior cingulate cortex (PCC), precuneus, and somatosensory cortices (Sugranyes et al., 2011; Walter et al., 2009). 3) Experience and regulation of emotion refers to individuals' adaptive responses to social complexities, and 4) experience sharing to the vicarious neural activation triggered when an individual observes someone else's actions (Iacoboni, 2009). As these have been well-reviewed (Green et al. 2015) and are less relevant to this discussion, they will not be detailed here. The four subcomponents articulated in this model reflect important neural and functional separations within the broad area of social cognition, providing a framework for more precise examination of oxytocin effects.
Fig. 3. Social cognitive processes in schizophrenia (adapted from Green et al. 2015) and potential oxytocin effects.
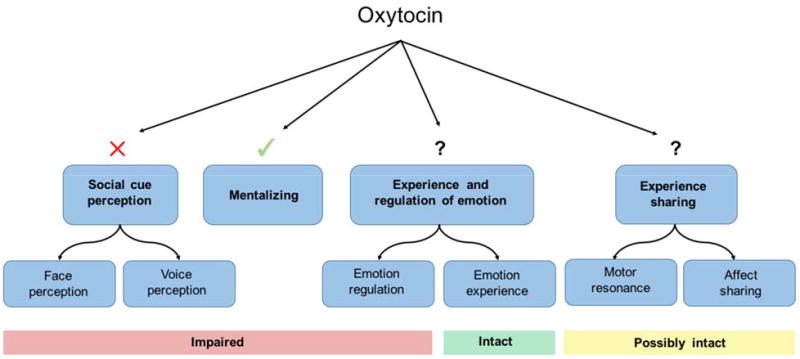
In studies of oxytocin effects on social cognition in schizophrenia, oxytocin does not consistently influence social cue perception deficits. However, more promising effects are seen on mentalizing deficits. Oxytocin effects on the other domains of social cognition, experience and regulation of emotion and experience sharing, have not yet been investigated.
7.2 Oxytocin effects on social cue perception
Studies that have investigated the effects of oxytocin on social cue perception in schizophrenia have generally found no effects or the effects have failed to replicate (see Table 2). Two within-subject studies using single doses of 20 IU (Goldman et al., 2011) and 24 IU (Averbeck et al., 2012) found some small improvements in facial affect identification, but the effect seen by Goldman et al. was only in a small subgroup of patients with polydipsia, and lower doses (10 IU) actually worsened social cue perception. Another within-subject study administered a single dose of 48 IU of oxytocin and found no improvement on facial emotion matching (Horta de Macedo et al., 2014).
Table 2.
Studies evaluating oxytocin effects on social cognition.
| Authors | N | Gender | Duration | Design | Setting | Dosing | Administration | Social cognition measures | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Averbeck et al., 2012 | Exp 1: 30 SZ, 29 HC Exp 2: 21 SZ | Exp 1: M+F Exp 2: M | Single dose | Exp 1: SZ vs HCs Exp 2: crossover | Outpt | 24IU | Supervised | Hexagon emotion discrimination test | OT improved emotion recognition in SZ |
| Davis et al., 2013 | 23 SZ OT=11, PL=12 | M | Single dose | Between | Outpt | 40IU | Self | TASIT EPTT Half-PONS Ekman stimuli | OT improved high level social cognition in SZ No improvement in low level or overall social cognition |
| Fischer-Shofty et al., 2013 | 35 SZ 48 HC | M+F | Single dose | Within | Outpt | 24IU | Supervised | IPT | OT improved kinship recognition in SZ |
| Goldman et al., 2011 | 13 SZ 11 HC | M+F | Single dose | Within | Outpt | 10 IU 20IU | Supervised | Ekman stimuli (perceived intensity) Benton Facial Discrimination Test | 10 IU decreased emotion recognition 20 IU improved emotion recognition in polydipsic SZ |
| Guastella et al., 2015 | 21 SZ | M | Single dose | Within | Outpt | 24 IU | Supervised | DANVA FEEST RMET FBPSTL Hinting task Faux Pas Recognition | OT improved performance on higher order social cognition Improved paralinguistic subscomponent of DANVA |
| Horta de Macedo et al., 2014 | 20 SZ 20 HC | M | Single dose | Within | Outpt | 48 IU | Supervised | Facial Emotion Matching | OT did not improve emotion matching in SZ or HCs |
| Shin et al., 2015 | 16 SZ 15 HC | M | Single dose | Within | Outpt | 40IU | Supervised | Emotion Recognition Test (C-KFEE stimuli) | OT attenuated amygdala activity for emotional faces in SZ OT increased amygdala activity in HCs |
| Woolley et al., 2014 | 29 SZ 31 HC | M | Single dose | Within | Outpt | 40 IU | Supervised | RMET TASIT | OT improved controlled social cognition in SZ |
Brambilla et al. (2016), notably, did observe decreased reaction times for facial affect recognition using an Emotional Priming Paradigm (EPP) in their longitudinal study (see Dagani et al., (2016)), though there were no changes in implicit emotional priming effects or for the differences between emotional categories. Gibson et al. (2014) included additional social cue perception tasks in their study: the Emotion Recognition 40 (ER-40) (Kohler et al., 2004), and a trustworthiness task (Adolphs et al., 1998). They found a significant oxytocin effect only in fear recognition within the ER-40 (there was no improvement in recognition of anger, sadness, happiness, or neutrality) in the oxytocin group. There was no oxytocin effect on the trustworthiness task. Woolley et al. (2017) also failed to find an effect of oxytocin on the same trustworthiness task in a recent study of 33 participants with schizophrenia spectrum disorders and 35 age-matched healthy controls.
Multiple studies (Cacciotti-Saija et al., 2015; Gibson et al., 2014; Brambilla et al., 2016) have used the Reading the Mind in the Eyes Test (RMET) to assess social cognition (see Table 3). In RMET, participants identify mental states depicted in photographs of the eye region of faces (Baron-Cohen et al., 2001). Though the mental states are semantically complex (e.g., “jealous”), the task does not require the participant to integrate a state with other contextual information or social cues (e.g., who is the target of jealousy and why) and thus is best categorized as a measure of social cue perception. None of these studies found an effect of oxytocin on RMET performance. Taken together, these results suggest that oxytocin may not have a meaningful impact on social cue perception in schizophrenia.
Table 3.
Social cognition tasks used in studies of oxytocin in schizophrenia.
| Task | Description | Domain assessed | Citation | Used by |
|---|---|---|---|---|
| Facial Affect Recognition Task | Still photographs of faces depicting basic emotions; participants select corresponding emotions from a list. | Social cue perception | Ekman and Friesen, 1976 | Goldman et al., 2011 |
| Emotion Matching Task | Still photographs of faces depicting basic emotions; participants select corresponding emotions from a list. | Social cue perception | Ekman and Friesen, 1978 | Horta de Macedo et al., 2014 |
| Facial Expression of Emotions Task (FEEST) | Still photographs of faces depicting basic emotions; participants select corresponding emotions from a list. | Social cue perception | Young et al., 2002 | Guastella et al., 2015 Cacciotti-Sajia et al., 2014 |
| Emotion Recognition (ER-40) | Still photographs of faces depicting basic emotions; participants select corresponding emotions from a list. | Social cue perception | Kohler et al., 2004 | Gibson et al., 2014 |
| Emotion Recognition Test | Still photographs of faces depicting basic emotions; participants select corresponding emotions from a list. | Social cue perception | Shin et al., 2015 | Shin et al., 2015 |
| Movie Stills Task | Stills from scenes depicting emotional interactions shown first with all facial expressions erased, second with facial expressions; participants select characters' emotions from a list. | Social cue perception | Adolphs et al., 2003 | Cacciotti-Saija et al., 2014 |
| Hexagon Emotion Discrimination Task | Still photographs of faces depicting basic emotions, including morphs between pairs of emotions; particpants select corresponding emotions from a list. | Social cue perception | Calder et al., 1996 | Averbeck et al., 2011 |
| Profile of Nonverbal Sensitivity (PONS) | 2-second videos containing facial expressions, voice intonations, & bodily gestures of a female; participants select which of two labels best describes a situation that would generate the social cues. | Social cue perception | Rosenthal et al, 1979 | Davis et al., 2014 |
| Half-Profile of Nonverbal Sensitivity (Half-PONS) | Abbreviated version of PONS | Social cue perception | Rosenthal et al., 1979; Ambady et al., 1995 | Davis et al., 2013 |
| Reading the Mind in the Eyes Task (RMET) | Still photographs of eye region of faces; participants select corresponding emotion from a list. | Social cue perception | Baron-Cohen, 2001 | Cacciotti-Saija et al., 2014 Woolley et al., 2014 Guastella et al., 2015 Gibson et al., 2014 |
| Trustworthiness Task | Still photographs of faces; participants rate faces on approachability or trustworthiness. | Social cue perception | Adolphs et al., 1998 | Pedersen et al., 2011 Gibson et al., 2014 |
| Diagnostic Analysis of Non-Verbal Accuracy (DANVA) | Still photographs of faces & audio clips; participants use facial expressions, postures, gestures, & paralanguage to infer corresponding emotion from a list. | Social cue perception | Nowicki and Duke, 1994 | Guastella et al., 2015 |
| The Awareness of Social Inference Task (TASIT) | Brief audiovisual scenes depicting social interactions between 2-3 characters; participants answer yes/no questions regarding emotions, beliefs, & intentions following each scene. | Part I (EET): Social cue perception Part 2 (SI-M): Social cue perception Part 3 (SI-E): Mentalizing | McDonald et al., 2006 | Davis et al., 2013 Davis et al., 2014 Woolley et al., 2014 |
| Brune Theory of Mind Picture Stories Task | Cartoon picture story depicting an interaction between two characters; participants answer questions about characters' beliefs and intentions. | Mentalizing | Brune 2003 | Pedersen et al., 2011 Gibson et al., 2014 |
| False Belief Picture Sequencing Task (FBPST) | Cartoon picture story depicting an interaction between two characters; participants infer characters' beliefs in order to correctly sequence panels. | Mentalizing | Langdon et al., 1997; Langdon and Coltheart, 1999 | Cacciotti-Saija et al., 2014 Guastella et al., 2015 |
| FBPST: The Faux Pas Recognition Task | Brief written stories; participants evaluate whether a character said something they shouldn't have and, if so, why. | Mentalizing | Baron-Cohen et al., 1999 | Cacciotti-Saija et al., 2014 Guastella et al., 2015 |
| FBPST: Hinting Task | Brief written passages presenting a verbal exchange between characters involving hinting; participants evaluate intentions of the characters. | Mentalizing | Corcoran et al., 1995; Marjoram et al., 2005 | Guastella et al., 2015 |
| Emotional Perpective Taking Task (EPTT) | Still photographs depicting two characters in a social interaction with one character's face masked; participants infer emotional expression of masked face from two choices. | Mentalizing | Derntl et al., 2009 | Davis et al., 2013 |
| Interpersonal Perception Task | Brief audiovisual scenes depicting realistic social behavior; participants infer belief & intentions of characters to answer questions about their relationships following each scene. | Mentalizing | Costanzo and Archer, 1989 | Fischer-Shofty et al., 2013 |
| Empathic Accuracy Task | Brief audiovisual clips of characters discussing positive or negative autobiographical events; participants rate how positive/negative the character is feeling & how positive/negative they themselves are feeling. | Empathy/Mentalizing | Lee et al., 2011 | Davis et al., 2014 |
| Empathy Quotient | Self-report questionnaire; participants rate their level of empathy across 40 items. | Empathy/Mentalizing | Baron-Cohen et al., 2004 | Cacciotti-Saija et al., 2014 |
| Interpersonal Reactivity Index (IRI) | Self-report measure consisting of four subscales, each addresses an aspect of empathy: Perspective Taking, Fantasy, Empathic Concern, Personal Distress. | Empathy/Mentalizing (Perspective Taking subscale) | Davis et al., 1983 | Gibson et al., 2014 |
| Ambiguous Intentions Hostility Questionnaire (AIHQ) | Brief written vignettes describing social situations; participants answer questions about intentions of the characters & how participants themselves would respond to the situation. | Attributional style/Mentalizing | Combs et al., 2007 | Cacciotti-Saija et al., 2014 Gibson et al., 2014 |
| Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT). | Assesses four components of emotional processing: Perceiving Emotions, Using Emotions to Facilitate Thinking, Understanding Emotions, and Managing Emotions. | Global emotional Intelligence/Empathy/Social cue perception/Mentalizing | Mayer et al, 2003 | Davis et al., 2014 Brambilla et al., 2016 |
7.3 Oxytocin effects on mentalizing
In contrast, studies measuring oxytocin effects on mentalizing in schizophrenia have been more consistently promising. Several have used The Awareness of Social Inference Test (TASIT), an audiovisual tool that allows for assessment of multiple domains of social cognitive processing (McDonald et al., 2003). In TASIT, participants make social inferences after viewing video clips of actors engaging in various scenarios. Divided into three parts, the task tests progressively more complex aspects of social cognition. Part I, the Emotion Evaluation Test (EET), and part II, Social Inference-Minimal (SI-M), require basic emotion recognition and immediate social cue detection. Part III, Social Inference-Enriched (SI-E), involves additional contextual information to help the viewer comprehend the speakers' intentions, perspective, and emotional state. SI-E is further divided into two categories: White Lies and Complex Sarcasm, allowing for more specific evaluation of participants' comprehension. TASIT has strong test-retest reliability and discriminant validity in patients with neuropsychiatric illness (McDonald et al., 2006), making it a strong candidate for use in social cognition studies, and its design allows for objective examination of oxytocin's effects on different levels of social cognition.
In their study, (M. C. Davis et al., 2013) derived two social cognition scores, one measuring “lower-level” social cognition (facial affect perception, social perception, detection of lies) and one measuring “higher-level” social cognition (detection of sarcasm and deception, empathy). The lower-level score was calculated from performance on multiple social cue perception tasks (the Half-Profile of Non-Verbal Sensitivity (Half-PONS) (Rosenthal et al., 1979), the Ekman facial affect recognition task (Ekman, 2007; Ekman and Friesen, 1976), and the Lie detection subsection of TASIT III). The higher-level social cognition score was calculated from tasks requiring mentalizing ability: the Emotional Perspective Taking Task (EPTT) (Derntl et al., 2009) and the Complex Sarcasm detection subsection of TASIT III. The authors found that a single dose of 40 IU of oxytocin selectively improved higher-level but not lower-level social cognition scores in a sample of 23 male outpatients. In another study administering 40 IU of oxytocin in a sample of 29 male participants with schizophrenia and 31 age-matched, healthy controls, Woolley et al. (2014) saw similar complexity in terms of oxytocin effects: while oxytocin significantly improved performance on mentalizing ability as assessed by TASIT III, it had no effect on tasks measuring social cue detection (RMET, TASIT I and TASIT II performance).
In addition to TASIT, several other tools have been used to examine oxytocin's effect on multiple levels of social cognition. The study by Pedersen et al. (2011) found significant improvement on the Brune second order false belief test (Brüne, 2003), a measure of mentalizing ability, but no effect on a facial trustworthiness task. Guastella et al. (2015) administered a single dose of oxytocin in a within-subject study of 21 men with schizophrenia and assessed multiple domains of social cognition as well. They tested emotion recognition using the Diagnostic Analysis of Non-Verbal Accuracy (DANVA) (Nowicki and Duke, 1994) as well as the Facial Expressions of Emotions Task (FEEST) (A. W. Young et al., 2002), another emotion recognition measure using still photographs, and the RMET. Oxytocin improved accuracy for detecting vocal intonations of affect, a subcomponent of the DANVA, but otherwise had no effect on these measures of social cue perception. Three additional tasks were used to assess mentalizing: the False Belief Picture Sequencing Task (FBPST) (Langdon et al., 1997), a verbal hinting task (Corcoran et al., 1995; Marjoram et al., 2005), and the Faux Pas Recognition Task (Baron-Cohen et al., 1999). Oxytocin improved performance on both the hinting task and the non-faux condition of the Faux Pas Recognition task, again suggesting that oxytocin may selectively improve complex social processing that involves integration of social nuances.
Gibson et al. (2014) included multiple tasks related to mentalizing in their study, described above. One of these is performance-based task, the Brune False Belief Test, and two others are self-report tools: the Ambiguous Intentions Hostility Questionnaire (AIHQ) (Combs et al., 2007), and the Interpersonal Reactivity Index (IRI) (M. H. Davis, 1980). The authors saw no drug effect on mentalizing as assessed by the Brune task. They also failed to find a drug effect on the AIHQ, which quantifies hostile social cognitive biases. The oxytocin group did, however, show a significant increase in perspective taking, one of the four subscales of the IRI and the one that relates most closely to mentalizing ability. Fischer-Schofty et al. (2013) administered a single dose of 24 IU of oxytocin to 35 patients with schizophrenia and 46 healthy controls in a within-subject study that evaluated complex social judgments as assessed by the Interpersonal Perception Task (IPT) (Costanzo and Archer, 1989). The authors found that oxytocin significantly improved accuracy on measures of complex perception that involve processing of subtle, ambiguous social interactions. Brambilla et al. (2016) reported that four months of oxytocin administration improved performance on the Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT), which requires participants to solve problems related to emotion (Mayer et al., 2003). Specifically, they observed improvement in patients' scores on the Understanding Emotion component of the MSCEIT, which assesses ability to understand the causes of emotions and how they may change and develop depending on context.
Not all studies have found a positive effect of oxytocin on the mentalizing domain. For example, Cacciotti-Saija et al. (2015) saw no improvement on the FBPST or the Faux Pas Task. In this study, however, both oxytocin and placebo groups received social cognition training, and significant improvements were seen in both groups over the study period. It is possible, then, that the effects of social cognitive training masked an oxytocin effect. Taken together, the findings summarized here suggest that oxytocin may have important effects specifically on mentalizing. Given the associations between mentalizing and functional outcomes in schizophrenia (Sergi et al., 2007), this is a particularly compelling area for future research. Further study with larger samples is needed to better characterize oxytocin effects on mentalizing deficits in schizophrenia.
8. Oxytocin effects on other deficits in schizophrenia
In addition to playing a role in social cognition, oxytocin may have important effects on other clinically important, but less often studied, deficits in schizophrenia. Here, we highlight findings in three of these areas: non-social cognition, facial expressivity, and olfaction.
8.1 Facial expressivity
Of the trials assessing oxytocin effects on symptoms, some reported significant oxytocin-induced improvements on negative symptom scales (Feifel et al., 2010; Gibson et al., 2014; Modabbernia et al., 2013b) while others found an ambiguous effect or none at all (Cacciotti-Saija et al., 2015; Dagani et al., 2016; M. C. Davis et al., 2014a; Lee et al., 2013; Pedersen et al., 2011b). These conflicting results are not surprising given that negative symptoms represent a heterogeneous group of deficits affecting a variety of functions: emotion, social ability, goal-directed behavior, and communication. Oxytocin may impact some of these deficits but not others, in which case investigating specific symptoms could prove useful. This is consistent with growing support in recent years for dividing negative symptoms into two distinct elements: 1) an experiential dimension comprised of avolition, anhedonia, and asociality; and, 2) an expressivity dimension comprised of restricted affect and poverty of speech (Reddy et al., 2015).
Decreased or blunted facial expressivity is a core component of the latter element (Blanchard and Cohen, 2006) that disrupts the ability to communicate emotions. It is commonly present many years before the development of frank psychotic symptoms, and typically continues into the chronic phase of the illness after treatment with antipsychotic medications (Gur et al., 2006). It is associated with difficulties navigating social situations, more severe anxiety, depression, and functional outcomes, and is predictive of poor prognosis (Kring and Moran, 2008). Blunted affect is included as an item on the symptom rating scales commonly used in studies of oxytocin in schizophrenia: the PANSS negative subscale, the BPRS, the SANS, and the CAINS (note that we contacted authors of the studies using each of these scales, hoping to analyze oxytocin effects on blunted affect specifically; unfortunately none were able to provide that data). As is the case for other negative symptoms, currently available pharmacological agents are ineffective at remediating impaired facial expressivity (Gur et al., 2006). Evidence that oxytocin may specifically normalize the neural circuitry dysfunction that is believed to underlie blunted facial affect (Kirsch et al., 2005; Shin et al., 2015; Sripada et al., 2013) has raised the question of whether oxytocin could improve this deficit in schizophrenia. In a within-subject study described above, (Woolley et al., 2017), individuals with schizophrenia and healthy controls were video recorded while they viewed emotionally evocative photos. Using an objective coding system (Kring and Sloan, 2007), the authors found that a single intranasal dose of oxytocin increased facial expressivity in both groups. Further work investigating facial expressivity specifically is needed to clarify the effects of oxytocin on this deficit in schizophrenia.
8.2 Non-social cognition
The association between cognition and functional outcomes in schizophrenia is well-documented (Green et al., 2000), but current treatments do not successfully address these deficits (Feifel et al., 2012a). Non-social cognitive deficits are widespread in individuals with schizophrenia, affecting visual and verbal learning and memory, attention/vigilance, working memory, reasoning and problem solving, and information processing speed (J. W. Young and Geyer, 2015). Given that social and non-social cognition interact and influence one another (Pessoa, 2008), it is not surprising that oxytocin has been linked to multiple aspects of non-social cognition in animals, including cognitive flexibility and spatial and episodic memory (Chini et al., 2014). Animal work suggests that low dosages of exogenous oxytocin improve memory when administered specifically during consolidation (see Chini et al., (2014) for a review). In the few studies conducted in healthy humans, however, oxytocin administration has generally resulted in no effect, or even an impairing effect, on various non-social memory tests (Bruins et al., 1992; Heinrichs et al., 2004; Herzmann et al., 2012) (notably, oxytocin has not been administered during the memory consolidation period, nor have a range of dosages been explored in the human literature). The relationship between oxytocin and cognition in healthy humans remains poorly understood, but no apparent benefit of oxytocin administration has been observed thus far.
However, there is some evidence of positive effects of oxytocin on aspects of memory in schizophrenia. Feifel et al. (2012) found that chronic oxytocin treatment improved short-term verbal memory but not long-term verbal memory or working memory as assessed by the California Verbal Learning Test (Delis, 2000) and the Letter Number Sequence task (Wechsler, 1997). In a within-subject study of 21 patients with schizophrenia, Michalopoulou et al. (2015) examined the effect of a single dose of 24 IU of oxytocin versus placebo on working memory and processing speed using the Digit Span and Digit Symbol Coding (DSC) tasks, respectively. The Digit Span task, which tests the “executive component” of working memory that involves maintenance and manipulation of information, is known to be more sensitive to deficits seen in schizophrenia (Kim, 2004). While oxytocin had no effect on processing speed, it did significantly improve the executive component of working memory. Cacciotti-Saija et al. (2015) and Guastella et al. (2015), however, used The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., 1998) to test other aspects of non-social cognition (immediate memory, language, visuospatial/constructional, attention, delayed memory); neither study found an effect of oxytocin. Taken together, these findings suggest that oxytocin's effects on non-social cognition may be highly selective and beneficial only in the setting of specific underlying cognitive deficits. Whether oxytocin has pro-cognitive effects or observed changes in performance are secondary to reduction in other symptoms of schizophrenia remains to be determined; studies conducted thus far have small sample sizes and used only a limited cognitive battery. It is essential that future work investigate whether oxytocin has effects on specific domains of non-social cognition known to be impaired in schizophrenia.
8.3 Olfaction
Olfactory cues play a role in a variety of mammalian social processes, such as emotion contagion, bonding, and mate selection (Stevenson, 2010). Individuals with schizophrenia have been found to have widespread olfactory deficits including impairments in odor identification, sensitivity, and discrimination that tend to worsen over the course of the illness and are present in unaffected family members (Moberg et al., 2014). Furthermore, deficits in odor identification have been shown to correlate with negative symptoms (Malaspina and Coleman, 2003; Moberg et al., 2006). These findings have led to interest in olfactory deficits as an endophenotype of schizophrenia. However, little is understood about the underlying neurobiology of olfactory impairment and there are currently no treatments to address these deficits.
Studies in animals and humans suggest that oxytocin plays a role in olfactory-based social processing. Animal studies have linked oxytocin signaling with olfactory and social functions (Larrazolo-Lopez et al., 2008; Wacker and Ludwig, 2012). Oxytocin receptors are found in the olfactory bulbs of rodents and sheep, and oxytocin is necessary for retaining olfactory social memories (Wacker and Ludwig, 2012). Furthermore, oxytocin receptors have been found in high concentrations on human olfactory neurons (Loup et al., 1991), and oxytocin has been implicated in modulating olfactory memory in the limbic system of rodents (L. J. Young and Wang, 2004). A study by Strauss et al. (2015) directly investigated the association between plasma oxytocin, social functioning, and olfaction in a sample of 39 individuals with schizophrenia and 21 healthy controls. Lower endogenous oxytocin levels predicted poorer olfactory identification as assessed by the University of Pennsylvania Smell Identification test (UPSIT) (Doty et al., 1984). The authors also observed an association between lower oxytocin levels and greater severity of negative symptoms. These findings suggest a possible relationship between oxytocin and olfactory deficits in schizophrenia.
Two studies have investigated the effects of intranasal oxytocin administration on olfactory deficits in schizophrenia. Lee et al. (2013) found that chronic oxytocin administration improved odor identification of pleasant, but not unpleasant, odors. Woolley et al. (2015) administered a single dose of 40 IU of oxytocin or placebo in a within-subject study in 31 individuals with schizophrenia and 34 age-matched healthy controls. The authors selected an olfactory detection threshold test to minimize confounding effects of higher order cognitive processes (detection thresholds do not require subjects to engage in memory retrieval or verbal labeling), and the synthetic fragrance compound lyral, as patients with schizophrenia have shown specific deficits in lyral detection (Turetsky et al., 2009). Oxytocin significantly and selectively improved detection of lyral in individuals with schizophrenia, but not in healthy participants. These preliminary findings suggest that the oxytocin system may play a role in the olfactory deficits in schizophrenia, but further work is needed to determine whether exogenous oxytocin could offer therapeutic benefit for such impairment.
9. Conclusions and agenda for future research
Schizophrenia represents a complex collection of neuropsychiatric disorders, and multiple neurotransmitter and neuropeptide systems have been implicated in its pathophysiology and symptomology. Recent investigations highlighting oxytocin's role in the deficits associated with schizophrenia, along with its high level of tolerability when administered intranasally, have led to excitement about its potential as a therapeutic. Thus far, however, clinical studies have yielded inconsistent findings that limit our ability to draw clear conclusions about oxytocin's utility. The heterogeneity of results seen in the literature is not surprising given that the neurobiological underpinnings of both schizophrenia and the human oxytocin system are inadequately characterized at this point. Furthermore, issues such as the use of various clinical assessment tools, differences in dosing and administration protocols, and failure to account for individual factors affecting oxytocin sensitivity have likely contributed to heterogeneity both between and within studies. In particular, antipsychotic dosage may be an important predictor of response to oxytocin in schizophrenia that has seldom been included in published reports.
Improved understanding of social cognition as a multidimensional construct has facilitated a closer examination of oxytocin's effects, and suggests that it may impact one specific domain, mentalizing. There are other intriguing findings regarding oxytocin's effects non-social cognition, olfaction, and facial expressivity that also warrant further exploration. Alvares et al. (2016) suggest that the current phase of oxytocin research be considered an “enlightenment” following the initial burst of interest and, subsequently, a period of disillusionment. This enlightenment should ideally be characterized by improvements in the consistency and rigor of research.
To meet our goal of elucidating oxytocin's role in schizophrenia and determining whether it has a place among therapeutics, future work must address limitations in the literature described in this review. First, assessment tools comprised of objective, specific symptom measures should be employed to quantify outcomes. As oxytocin's potential to improve negative symptoms is a particular area of interest, we recommend that future studies use the CAINS or BNSS, rating scales that reflect our current understanding of this domain. However, given that exclusive use of symptom rating scales to capture oxytocin effects has clear drawbacks, additional assessment tools should be incorporated into clinical trials. Measures that focus on signs linked to the neurobiological underpinnings of specific deficits, rather than subjective experiences, may allow for more thorough and precise evaluation of oxytocin's effects. For example, TASIT, which uses immersion in real-world social situations to assess deficits in specific domains of social cognition, may capture important changes that symptom rating scales do not. Objective facial expressivity coding, such as the system developed by Kring and Sloan (Kring and Sloan, 2007), is another example of a tool that may prove more useful in capturing clinically relevant oxytocin effects (e.g., as used in (Woolley et al., 2017)). Second, improved intranasal drug delivery systems, standardized administration protocols, and dose-response curves would likely increase consistency in oxytocin system engagement and allow for appropriate comparisons between studies. Specifically, replacing standard nasal spray bottles with newer devices that offer more consistent drug delivery (e.g. OptiNose (Djupesland, 2013), Impel (Lochhead and Thorne, 2012)) and using standardized instructions for administration (as outlined by Guastella et al. (2013) may significantly decrease heterogeneity introduced by administration protocols in oxytocin studies. In addition, the optimal dosages and treatment duration for intranasal oxytocin must be established. Trials that directly compare multiple dosages as well as acute versus chronic effects are essential to rigorously evaluating oxytocin as a potential therapeutic for schizophrenia.
Finally, perhaps the greatest challenge arises from the complexity of the oxytocin system itself, and our limited understanding of the mechanisms by which it modulates neural circuits at the individual level. Continued efforts to map the expression of OXTR receptors in the human brain (Freeman et al., 2017), as well as clarifying the effects of OXTR genotype and epigenetic signatures, are critical to identifying factors that predict responsiveness to oxytocin at the individual level. Furthermore, key potential moderators, such as age, sex, context of administration, and use of antipsychotic medications, must be considered in the design and data analysis phases of future trials. Elucidating the mechanisms underlying oxytocin's effects and understanding why individuals may be more or less responsive to exogenous oxytocin requires that we move beyond conducting efficacy trials. An approach that focuses instead on target identification and engagement will likely yield more informative findings that ultimately clarify oxytocin's role in schizophrenia treatment. Through this approach, oxytocin-induced changes in behavior can be linked to functional impact on neural systems (e.g., as in Aoki et al. (2014)). Studies designed to test specific targets of oxytocin treatment will provide essential insights regarding oxytocin's mechanisms of action.
To determine whether oxytocin has a role in the treatment of schizophrenia, future investigations must tackle multiple challenges. In addition to the issues discussed in this review, it is critical to highlight the fact that oxytocin has been tested as an add-on to stable treatment regimens in all clinical schizophrenia studies conducted thus far. The multiple limitations of addon designs and particular challenges of examining oxytocin effects in schizophrenia using this approach have been well-articulated in the review by Shilling and Feifel (2016). As the authors discuss, only monotherapy RCTs can test the hypothesis that oxytocin has inherent therapeutic properties in schizophrenia. Without trials that directly test the efficacy of oxytocin in the absence of antipsychotics, we are at risk of failing to capture oxytocin effects and dismissing a much-needed potential treatment. While moving forward is a daunting task, the disease burden of schizophrenia and lack of adequate treatments are critical issues in mental health. Thorough exploration of oxytocin's potential as a therapeutic remains a worthy pursuit.
Highlights.
Studies of oxytocin in schizophrenia have yielded mixed results.
Study design and individual differences may account for this heterogeneity.
Oxytocin may improve specific deficits not captured in many studies.
Future studies should employ precise, objective outcomes to capture oxytocin effects.
Acknowledgments
Role of funding sources: This work was supported by the Career Development Award # CX000758 from the United States Department of Veterans Affairs, Office of Research and Development, Clinical Science Research and Development program, and the National Institutes of Mental Health Grant R25 MH60482. The funders did not play any role in decision to publish or preparation of the article.
Footnotes
Conflict of interest statement: The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Annals of clinical psychiatry: official journal of …. 2016 [PubMed] [Google Scholar]
- Adolphs R. The Social Brain: Neural Basis of Social Knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Alvares GA, Quintana DS, Whitehouse AJO. Beyond the hype and hope: Critical considerations for intranasal oxytocin research in autism spectrum disorder. Autism Res. 2016 doi: 10.1002/aur.1692. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Andreasen NC. The Scales of Negative (SANS) and Positive (SAPS) Symptoms. Iowa City: University of Iowa; 1984. [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic Dose Equivalents and Dose-Years: A Standardized Method for Comparing Exposure to Different Drugs. Biological Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, Iwashiro N, Natsubori T, Inoue H, Suga M, Takao H, Sasaki H, Gonoi W, Kunimatsu A, Kasai K, Yamasue H. Oxytocin improves behavioural and neural deficits in inferring others' social emotions in autism. Brain. 2014;137:3073–3086. doi: 10.1093/brain/awu231. [DOI] [PubMed] [Google Scholar]
- Atzil S, Touroutoglou A, Rudy T, Salcedo S, Feldman R, Hooker JM, Dickerson BC, Catana C, Barrett LF. Dopamine in the medial amygdala network mediates human bonding. Proc Natl Acad Sci USA. 2017;114:2361–2366. doi: 10.1073/pnas.1612233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2012;42:259–266. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van I Jzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258–14. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatric Genetics. 2014;24:45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Riem MME, Tops M, Alink LRA. Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Soc Cogn Affect Neurosci. 2012;7:951–957. doi: 10.1093/scan/nsr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic Intranasal Oxytocin Causes Long-Term Impairments in Partner Preference Formation in Male Prairie Voles. Biological Psychiatry. 2013;74:180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, O'Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. J Autism Dev Disord. 1999;29:407–418. doi: 10.1023/a:1023035012436. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test Revised Version: A Study with Normal Adults, and Adults with Asperger Syndrome or High-functioning Autism. Journal of Child Psychology and Psychiatry. 2001;42:241–251. doi: 10.1017/S0021963001006643. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz CF, Ganella EP, Labuschagne I, Bousman C, Pantelis C. Effects of oxytocin and genetic variants on brain and behaviour: Implications for treatment in schizophrenia. Schizophrenia Research. 2015;168:614–627. doi: 10.1016/j.schres.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. (Regul) 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bell M, Milstein R, Beam-Goulet J, Lysaker P, Cicchetti D. The Positive and Negative Syndrome Scale and the Brief Psychiatric Rating Scale: Reliability, Comparability, and Predictive Validity. The Journal of Nervous and Mental Disease. 1992;180:723. doi: 10.1097/00005053-199211000-00007. [DOI] [PubMed] [Google Scholar]
- Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R. Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides. 1995;28:251–255. doi: 10.1016/0143-4179(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Bethlehem RAI, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophrenia Bulletin. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Hamandi A, Mowafi M, O'Farrell D, Ozaltin E, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C, Weiss J. The Global Economic Burden of Noncommunicable Diseases. PGDA Working Papers 2012 [Google Scholar]
- Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophrenia Research. 2013;144:31–36. doi: 10.1016/j.schres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthé RM, van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front Neuroendocrinol. 2012;33:17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Brambilla M, Cotelli M, Manenti R, Dagani J, Sisti D, Rocchi M, Balestrieri M, Pini S, Raimondi S, Saviotti FM, Scocco P, de Girolamo G. Oxytocin to modulate emotional processing in schizophrenia: A randomized, double-blind, cross-over clinical trial. Eur Neuropsychopharmacol. 2016 doi: 10.1016/j.euroneuro.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Bruins J, Hijman R, Van Ree JM. Effect of a single dose of des-glycinamide-[Arg8]vasopressin or oxytocin on cognitive processes in young healthy subjects. Peptides. 1992;13:461–468. doi: 10.1016/0196-9781(92)90075-E. [DOI] [PubMed] [Google Scholar]
- Brüne M. Theory of mind and the role of IQ in chronic disorganized schizophrenia. Schizophrenia Research. 2003;60:57–64. doi: 10.1016/S0920-9964(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Brüne M, Abdel-Hamid M, Sonntag C. Linking social cognition with social interaction: non-verbal expressivity, social competence and “mentalising” in patients with schizophrenia spectrum disorders. Behav Brain …. 2009 doi: 10.1186/1744-9081-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Bauer EP. Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience. 2013;247:253–272. doi: 10.1016/j.neuroscience.2013.05.050. [DOI] [PubMed] [Google Scholar]
- Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, Moore L, Alvares GA, Redoblado Hodge MA, Guastella AJ. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophrenia Bulletin. 2015;41:483–493. doi: 10.1093/schbul/sbu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK. Neurobiology of sociability. Adv Exp Med Biol. 2012;739:187–205. doi: 10.1007/978-1-4614-1704-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Stephens SL, Young WS. Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14:190–196. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- Campbell A, Ruffman T, Murray JE, Glue P. Oxytocin improves emotion recognition for older males. Neurobiology of Aging. 2014;35:2246–2248. doi: 10.1016/j.neurobiolaging.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology. 2013;38:399–407. doi: 10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Linnen AM, Joober R, Ellenbogen MA. Coping style moderates the effect of intranasal oxytocin on the mood response to interpersonal stress. Exp Clin Psychopharmacol. 2012;20:84–91. doi: 10.1037/a0025763. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Koenig JI. The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology. 2008;33:2061–2079. doi: 10.1038/sj.npp.1301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, Sumiyoshi RD, Jackson LP, Moss JK, Strehlow MC, Cheshier SH, Partap S, Hardan AY, Parker KJ. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry. 2014;20:1085–1090. doi: 10.1038/mp.2014.132. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci USA. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Leonzino M, Braida D, Sala M. Learning About Oxytocin: Pharmacologic and Behavioral Issues. Biological Psychiatry. 2014;76:360–366. doi: 10.1016/j.biopsych.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Chong HY, Teoh SL, Wu DBC, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357–373. doi: 10.2147/NDT.S96649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Hormones and Behavior. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramidaro A, Bolte S, Schlitt S, Hainz D, Poustka F, Weber B, Bara BG, Freitag C, Walter H. Schizophrenia and Autism as Contrasting Minds: Neural Evidence for the Hypo-Hyper-Intentionality Hypothesis. Schizophrenia Bulletin. 2015;41:171–179. doi: 10.1093/schbul/sbu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. J Cogn Neurosci. 2008;20:941–951. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs DR, Penn DL, Wicher M, Waldheter E. The Ambiguous Intentions Hostility Questionnaire (AIHQ): a new measure for evaluating hostile social-cognitive biases in paranoia. Cogn Neuropsychiatry. 2007;12:128–143. doi: 10.1080/13546800600787854. [DOI] [PubMed] [Google Scholar]
- Conlisk J. Professor Zak's empirical studies on trust and oxytocin. Journal of Economic Behavior & Organization. 2011;78:160–166. doi: 10.1016/j.jebo.2011.01.002. [DOI] [Google Scholar]
- Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: Investigating “theory of mind” in people with schizophrenia. Schizophrenia Research. 1995;17:5–13. doi: 10.1016/0920-9964(95)00024-G. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Archer D. Interperting the expressive behavior of others: The Interpersonal Perception Task. Journal of Nonverbal Behavior. 1989;13:225–245. doi: 10.1007/BF00990295. [DOI] [Google Scholar]
- Dagani J, Sisti D, Abelli M, Di Paolo L, Pini S, Raimondi S, Rocchi MB, Saviotti FM, Scocco P, Totaro S, Balestrieri M, de Girolamo G. Do we need oxytocin to treat schizophrenia? A randomized clinical trial. Schizophrenia Research. 2016;172:158–164. doi: 10.1016/j.schres.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS ONE. 2014;9:e103677. doi: 10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Kemp AH, Flynn G, Harris AWF, Liddell BJ, Whitford TJ, Peduto A, Gordon E, Williams LM. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophrenia Research. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Davis MC, Green MF, Lee J, Horan WP, Senturk D, Clarke AD, Marder SR. Oxytocin-augmented social cognitive skills training in schizophrenia. Neuropsychopharmacology. 2014a;39:2070–2077. doi: 10.1038/npp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Horan WP, Nurmi EL, Rizzo S, Li W, Sugar CA, Green MF. Associations between oxytocin receptor genotypes and social cognitive performance in individuals with schizophrenia. Schizophrenia Research. 2014b;159:353–357. doi: 10.1016/j.schres.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, Marder SR. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophrenia Research. 2013;147:393–397. doi: 10.1016/j.schres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Davis MH. Interpersonal reactivity index 1980 [Google Scholar]
- De Dreu CKW, Kret ME. Oxytocin Conditions Intergroup Relations Through Upregulated In-Group Empathy, Cooperation, Conformity, and Defense. Biological Psychiatry. 2016;79:165–173. doi: 10.1016/j.biopsych.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. The effect of oxytocin on cooperation in a prisoner's dilemma depends on the social context and a person's social value orientation. Soc Cogn Affect Neurosci. 2014;9:802–809. doi: 10.1093/scan/nst040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis Cvlt Research Edition Adult Version Manual 2000 [Google Scholar]
- Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychol Med. 2013;43:553–569. doi: 10.1017/S0033291712001432. [DOI] [PubMed] [Google Scholar]
- Derntl B, Habel U, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E. General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neurosci. 2009;10 doi: 10.1186/1471-2202-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PR, Lawson KA, Barner JC, Rascati KL. Identifying Patient Characteristics Associated with High Schizophrenia-Related Direct Medical Costs in Community-Dwelling Patients. J Manag Care Pharm. 2013;19:468–477. doi: 10.18553/jmcp.2013.19.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, Gillath O, Pressman SD, Black LL, Bartz JA, Moskovitz J, Stetler DA. When the Love Hormone Leads to Violence: Oxytocin Increases Intimate Partner Violence Inclinations Among High Trait Aggressive People. Social Psychological and Personality Science. 2014;5:691–697. doi: 10.1177/1948550613516876. [DOI] [Google Scholar]
- Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective—a review. Drug Deliv and Transl Res. 2013;3:42–62. doi: 10.1007/s13346-012-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, Cohen RA. Oxytocin's effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology. 2016;69:50–59. doi: 10.1016/j.psyneuen.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Horta M, Lin T, Feifel D, Fischer H, Cohen RA. Oxytocin modulates meta-mood as a function of age and sex. Frontiers in Aging Neuroscience. 2015a;7:177. doi: 10.3389/fnagi.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Kamin H, Diaz V, Cohen RA, Macdonald K. Hormones as “difference makers” in cognitive and socioemotional aging processes. Front Psychol. 2015b;5:505. doi: 10.3389/fpsyg.2014.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Maura GM, Macdonald K, Westberg L, Fischer H. Oxytocin and socioemotional aging: Current knowledge and future trends. Front Hum Neurosci. 2013;7:487. doi: 10.3389/fnhum.2013.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld SL, Smith E, McGregor IS, Steinbeck K, Taffe J, Rice LJ, Horstead SK, Rogers N, Hodge MA, Guastella AJ. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet A. 2014;164A:2232–2239. doi: 10.1002/ajmg.a.36653. [DOI] [PubMed] [Google Scholar]
- Ekman P. Emotions Revealed. Second. Macmillan; 2007. [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect 1976 [Google Scholar]
- Feifel D, Macdonald K, Cobb P, Minassian A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophrenia Research. 2012a doi: 10.1016/j.schres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology (Berl) 1999;141:93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Belcher AM. The effects of oxytocin and its analog, carbetocin, on genetic deficits in sensorimotor gating. Eur Neuropsychopharmacol. 2012b;22:374–378. doi: 10.1016/j.euroneuro.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Macdonald K. A Review of Oxytocin's Effects on the Positive, Negative, and Cognitive Domains of Schizophrenia. Biological Psychiatry. 2015;79:1–12. doi: 10.1016/j.biopsych.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AKJ, Viechtbauer W, Dominguez MDG, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Brüne M, Ebert A, Shefet D, Levkovitz Y, Shamay-Tsoory SG. Improving social perception in schizophrenia: The role of oxytocin. Schizophrenia Research. 2013a;146:357–362. doi: 10.1016/j.schres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Levkovitz Y, Shamay-Tsoory SG. Oxytocin facilitates accurate perception of competition in men and kinship in women. Soc Cogn Affect Neurosci. 2013b;8:313–317. doi: 10.1093/scan/nsr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GGC, Roberts JA. Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology. 2016;66:185–194. doi: 10.1016/j.psyneuen.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Smith AL, Goodman MM, Bales KL. Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Social Neuroscience. 2017;12:113–123. doi: 10.1080/17470919.2016.1156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. J Neuroendocrinol. 2016 doi: 10.1111/jne.12382. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. Schizophrenia and theory of mind. Psychol Med. 2004;34:385–389. doi: 10.1017/S0033291703001326. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophrenia Bulletin. 2015;41:892–899. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of Oxytocin, Vasopressin, and Sex Hormones in the Regulation of Social Recognition. Behavioral Neuroscience. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- Gao S, Becker B, Luo L, Geng Y, Zhao W, Yin Y, Hu J, Gao Z, Gong Q, Hurlemann R, Yao D, Kendrick KM. Oxytocin, the peptide that bonds the sexes also divides them. Proc Natl Acad Sci USA. 2016 doi: 10.1073/pnas.1602620113. 201602620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophrenia Research. 2014;156:261–265. doi: 10.1016/j.schres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Glovinsky D, Kalogeras KT, Kirch DG, Suddath R, Wyatt RJ. Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophrenia Research. 1994;11:273–276. doi: 10.1016/0920-9964(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Gold R, Butler P, Revheim N, Leitman DI, Hansen JA, Gur RC, Kantrowitz JT, Laukka P, Justin PN, Silipo GS, Javitt DC. Auditory Emotion Recognition Impairments in Schizophrenia: Relationship to Acoustic Features and Cognition. Am J Psychiatry. 2012;169:424–432. doi: 10.1176/appi.ajp.2011.11081230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M, Marlow-O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophrenia Research. 2008;98:247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB. The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Research Reviews. 2009;61:210–220. doi: 10.1016/j.brainresrev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl) 2011;216:101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- Green MF, Harvey PD. Cognition in schizophrenia: Past, present, and future. Schizophrenia Research: Cognition. 2014;1:e1–e9. doi: 10.1016/j.scog.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–631. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Swerdlow NR, Gur RE, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RC, Lazzeroni LC, Nuechterlein KH, Olincy A, Radant AD, Ray A, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Light GA, Braff DL. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. American Journal of Psychiatry. 2013;170:521–532. doi: 10.1176/appi.ajp.2012.12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove TB, Burghardt KJ, Kraal AZ, Dougherty RJ, Taylor SF, Ellingrod VL. Oxytocin Receptor (OXTR) Methylation and Cognition in Psychotic Disorders. Mol Neuropsychiatry. 2016;2:151–160. doi: 10.1159/000448173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Graustella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Hormones and Behavior. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB. Oxytocin Treatment, Circuitry, and Autism: A Critical Review of the Literature Placing Oxytocin Into the Autism Context. Biological Psychiatry. 2016;79:234–242. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38:612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Ward PB, Hickie IB, Shahrestani S, Hodge MAR, Scott EM, Langdon R. A single dose of oxytocin nasal spray improves higher-order social cognition in schizophrenia. Schizophrenia Research. 2015;168:628–633. doi: 10.1016/j.schres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Gumley A, Braehler C, Macbeth A. A meta-analysis and theoretical critique of oxytocin and psychosis: prospects for attachment and compassion in promoting recovery. Br J Clin Psychol. 2014;53:42–61. doi: 10.1111/bjc.12041. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler CG, Ragland JD, Siegel SJ, Lesko K, Bilker WB, Gur RC. Flat affect in schizophrenia: Relation to emotion processing and neurocognitive measures. Schizophrenia Bulletin. 2006;32:279–287. doi: 10.1093/schbul/sbj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Filkowski MM, Cochran RN, Denison L, Ishak A, Nishitani S, Smith AK. Epigenetic modification of OXT and human sociability. Proc Natl Acad Sci USA. 2016;113:201602809–E3823. doi: 10.1073/pnas.1602809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28:17–54. doi: 10.1016/S0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, Kappenman ES, Luck SJ, Gold JM. Failure of Schizophrenia Patients to Overcome Salient Distractors During Working Memory Encoding. Biological Psychiatry. 2010;68:603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Kahn LS. Hormonal aspects of schizophrenias: an overview. Psychoneuroendocrinology. 2003;28:1–16. doi: 10.1016/S0306-4530(02)00124-5. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, McCarthy BE, Parker KJ, REISS AL. Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome. Psychoneuroendocrinology. 2012;37:509–518. doi: 10.1016/j.psyneuen.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L, Siegert RJ, McClure J. Theory of mind in schizophrenia: a critical review. Cogn Neuropsychiatry. 2005;10:249–286. doi: 10.1080/13546800444000056. [DOI] [PubMed] [Google Scholar]
- Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C, Oepen G. One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry. 1994;151:1409–1416. doi: 10.1176/ajp.151.10.1409. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, HELLHAMMER D. Selective amnesic effects of oxytocin on human memory. Physiol Behav. 2004;83:31–38. doi: 10.1016/S0031-9384(04)00346-4. [DOI] [PubMed] [Google Scholar]
- Herzmann G, Young B, Bird CW, Curran T. Oxytocin can impair memory for social and non-social visual objects: A within-subject investigation of oxytocin's effects on human memory. Brain Res. 2012;1451:65–73. doi: 10.1016/j.brainres.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis CP. The significance of age of onset in psychosis: Is adolescent-onset schizophrenia at an extreme of phenotypic severity and familial liability? Schizophrenia Research. 2000;41:62. doi: 10.1016/S0920-9964(00)90436-5. [DOI] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophrenia Research. 2006a;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Titone D, Long LS, Goff DC, Cather C, Rauch SL, Judge A, Kuperberg GR. The misattribution of salience in delusional patients with schizophrenia. Schizophrenia Research. 2006b;83:247–256. doi: 10.1016/j.schres.2005.12.858. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophrenia Research. 2011;132:140–145. doi: 10.1016/j.schres.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta de Macedo LR, Zuardi AW, Machado-de-Sousa JP, Chagas MHN, Hallak JEC. Oxytocin does not improve performance of patients with schizophrenia and healthy volunteers in a facial emotion matching task. Psychiatry Res. 2014;220:125–128. doi: 10.1016/j.psychres.2014.07.082. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni ML, Papaleo F. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffmeijer R, van IJzendoorn MH, Bakermans-Kranenburg MJ. Ageing and oxytocin: a call for extending human oxytocin research to ageing populations--a mini-review. GER. 2013;59:32–39. doi: 10.1159/000341333. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Revolution stalled. Sci Transl Med. 2012;4:155cm11–155cm11. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Insel TR. Translating Oxytocin Neuroscience to the Clinic: A National Institute of Mental Health Perspective. Biological Psychiatry. 2016;79:153–154. doi: 10.1016/j.biopsych.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Löckenhoff CE, Lane RD, Wright R, Sechrest L, Riedel R, Costa PT. Age differences in recognition of emotion in lexical stimuli and facial expressions. Psychol Aging. 2007;22:147–159. doi: 10.1037/0882-7974.22.1.147. [DOI] [PubMed] [Google Scholar]
- Janezic EM, Uppalapati S, Nagl S, Contreras M, French ED, Fellous JM. Beneficial effects of chronic oxytocin administration and social co-housing in a rodent model of post-traumatic stress disorder. Behav Pharmacol. 2016;1 doi: 10.1097/FBP.0000000000000270. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen E, Juola P, Hirvonen N, McGrath JJ, Saha S, Isohanni M, Veijola J, Miettunen J. A systematic review and meta-analysis of recovery in schizophrenia. Schizophrenia Bulletin. 2013;39:1296–1306. doi: 10.1093/schbul/sbs130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Jang JH, Byun MS, An SK, Kwon JS. Structural brain alterations in individuals at ultra-high risk for psychosis: a review of magnetic resonance imaging studies and future directions. J Korean Med Sci. 2010;25:1700–1709. doi: 10.3346/jkms.2010.25.12.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol. 2013;25:668–673. doi: 10.1111/jne.12038. [DOI] [PubMed] [Google Scholar]
- Kane JM. Tools to assess negative symptoms in schizophrenia. J Clin Psychiatry. 2013;74:e12. doi: 10.4088/JCP.12045tx2c. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kiss I, Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Social Neuroscience. 2009;4:287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kim J. Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophrenia Research. 2004;68:173–187. doi: 10.1016/S0920-9964(03)00150-6. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B. Assessing the Efficacy of Treatments for the Deficit Syndrome of Schizophrenia. Neuropsychopharmacology. 2000;22:303–310. doi: 10.1016/S0893-133X(99)00122-0. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. Presented at the Schizophrenia bulletin. Oxford University Press; 2006. The NIMH-MATRICS consensus statement on negative symptoms; pp. 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The brief negative symptom scale: psychometric properties. Schizophrenia Bulletin. 2011;37:300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Gur RE, Gur RC. Recognition of facial emotions in neuropsychiatric disorders. CNS Spectr. 2004;9:267–274. doi: 10.1017/s1092852900009202. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final Development and Validation. Am J Psychiatry. 2013;170:165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: Insights from affective science. Schizophrenia Bulletin. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Sloan DM. The Facial Expression Coding System (FACES): development, validation, and utility. Psychol Assess. 2007;19:210–224. doi: 10.1037/1040-3590.19.2.210. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin Attenuates Amygdala Reactivity to Fear in Generalized Social Anxiety Disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A, Luminet O, Nave G, Mikolajczak M. Is there a publication bias in behavioral intranasal oxytocin research on humans? Opening the file drawer of one lab. J Neuroendocrinol. 2016 doi: 10.1111/jne.12384. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Langdon R, Michie PT, Ward PB, McConaghy N, Catts SV, Coltheart M. Defective Self and/or Other Mentalising in Schizophrenia: A Cognitive Neuropsychological Approach. Cogn Neuropsychiatry. 1997;2:167–193. doi: 10.1080/135468097396324. [DOI] [PubMed] [Google Scholar]
- Larrazolo-Lopez A, Kendrick KM, Aburto-Arciniega M, Arriaga-Avila V, Morimoto S, Frias M, Guevara-Guzman R. Vaginocervical stimulation enhances social recognition memory in rats via oxytocin release in the olfactory bulb. Neuroscience. 2008;152:585–593. doi: 10.1016/j.neuroscience.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Lee MR, Wehring HJ, McMahon RP, Linthicum J, Cascella N, Liu F, Bellack A, Buchanan RW, Strauss GP, Contoreggi C, Kelly DL. Effects of adjunctive intranasal oxytocin on olfactory identification and clinical symptoms in schizophrenia: results from a randomized double blind placebo controlled pilot study. Schizophrenia Research. 2013;145:110–115. doi: 10.1016/j.schres.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux C, Gobeil MH, Lefèbvre AA, Maziade M, Roy MA. The Five-Factor Structure of the PANSS: A Critical Review of its Consistency Across Studies. Clinical Schizophrenia & Related Psychoses. 2009;3:103–110. doi: 10.3371/CSRP.3.2.5. [DOI] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biological Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Leknes S, Wessberg J, Ellingsen DM, Chelnokova O, Olausson H, Laeng B. Oxytocin enhances pupil dilation and sensitivity to “hidden” emotional expressions. Soc Cogn Affect Neurosci. 2013;8:741–749. doi: 10.1093/scan/nss062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Levine SZ, Rabinowitz J, Rizopoulos D. Recommendations to improve the positive and negative syndrome scale (PANSS) based on item response theory. Psychiatry Res. 2011;188:446–452. doi: 10.1016/j.psychres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Li H, Chan RCK, McAlonan GM, Gong QY. Facial Emotion Processing in Schizophrenia: A Meta-analysis of Functional Neuroimaging Data. Schizophrenia Bulletin. 2010;36:1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zebrowitz LA, Zhang Y. Neural activation in the “reward circuit” shows a nonlinear response to facial attractiveness. Social Neuroscience. 2010;5:320–334. doi: 10.1080/17470911003619916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/S0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Luminet O, Grynberg D, Ruzette N, Mikolajczak M. Personality-dependent effects of oxytocin: greater social benefits for high alexithymia scorers. Biol Psychol. 2011;87:401–406. doi: 10.1016/j.biopsycho.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci. 2013;7:35. doi: 10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K, Feifel D. Oxytocin in schizophrenia: a review of evidence for its therapeutic effects. Acta Neuropsychiatrica. 2012;24:130–146. doi: 10.1111/j.1601-5215.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- Mancuso F, Horan WP, Kern RS, Green MF. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophrenia Research. 2011;125:143–151. doi: 10.1016/j.schres.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and Vasopressin Agonists and Antagonists as Research Tools and Potential Therapeutics. J Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SC, Olfson M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophrenia Bulletin. 2008;34:173–180. doi: 10.1093/schbul/sbm061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Kirkpatrick B. Defining and measuring negative symptoms of schizophrenia in clinical trials. Eur Neuropsychopharmacol. 2014;24:737–743. doi: 10.1016/j.euroneuro.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Marjoram D, Gardner C, Burns J, Miller P, Lawrie SM, Johnstone EC. Symptomatology and social inference: a theory of mind study of schizophrenia and psychotic affective disorder. Cogn Neuropsychiatry. 2005;10:347–359. doi: 10.1080/13546800444000092. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3:97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- McDonald S, Bornhofen C, Shum D, Long E, Saunders C, Neulinger K. Reliability and validity of The Awareness of Social Inference Test (TASIT): a clinical test of social perception. Disabil Rehabil. 2006;28:1529–1542. doi: 10.1080/09638280600646185. [DOI] [PubMed] [Google Scholar]
- McDonald S, Flanagan S, Rollins J, Kinch J. TASIT: A new clinical tool for assessing social perception after traumatic brain injury. J Head Trauma Rehabil. 2003;18:219–238. doi: 10.1097/00001199-200305000-00001. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Fenton WS. The Positive-Negative Distinction in Schizophrenia: Review of Natural History Validators. Arch Gen Psychiatry. 1992;49:63–72. doi: 10.1001/archpsyc.1992.01820010063008. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Riecher-Roessler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, Koutsouleris N, Yung AR, Stone JM, Phillips LJ, McGorry PD, Valli I, Velakoulis D, Woolley J, Pantelis C, McGuire P. Neuroanatomical Abnormalities That Predate the Onset of Psychosis A Multicenter Study. Arch Gen Psychiatry. 2011;68:489–495. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, Guillon G, Lafitte D, Desarmenien MG, Tauber M, Muscatelli F. An Early Postnatal Oxytocin Treatment Prevents Social and Learning Deficits in Adult Mice Deficient for Magel2, a Gene Involved in Prader-Willi Syndrome and Autism. Biological Psychiatry. 2015;78:85–94. doi: 10.1016/j.biopsych.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Michalopoulou PG, Averbeck BB, Kalpakidou AK, Evans S, Bobin T, Kapur S, Shergill SS. The effects of a single dose of oxytocin on working memory in schizophrenia. Schizophrenia Research. 2015;162:62–63. doi: 10.1016/j.schres.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey BJ, Heffernan J, Heisel C, Pecina M, Hsu DT, Zubieta JK, Love TM. Oxytocin modulates hemodynamic responses to monetary incentives in humans. Psychopharmacology (Berl) 2016;233:3905–3919. doi: 10.1007/s00213-016-4423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. Is pharma running out of brainy ideas? Science. 2010;329:502–504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, Gur RE, Balderston CC, Roalf DR, Gur RC, Kohler CG, Kanes SJ, Siegel SJ, Turetsky BI. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28:1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn CG, Borgmann-Winter KE, Kohler CG, Gur RE, Turetsky BI. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophrenia Bulletin. 2014;40:50–59. doi: 10.1093/schbul/sbt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SMR, Tajdini M, Ghaleiha A, Akhondzadeh S. Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia: an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs. 2013a;27:57–65. doi: 10.1007/s40263-012-0022-1. [DOI] [PubMed] [Google Scholar]
- Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SMR, Tajdini M, Ghaleiha A, Akhondzadeh S. Intranasal Oxytocin as an Adjunct to Risperidone in Patients with Schizophrenia. CNS Drugs. 2013b;27:57–65. doi: 10.1007/s40263-012-0022-1. [DOI] [PubMed] [Google Scholar]
- Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. 2014a;45:49–57. doi: 10.1016/j.psyneuen.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. 2014b;45:49–57. doi: 10.1016/j.psyneuen.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Brockmann EM, Bayerl M, Rujescu D, Müller DJ, Gallinat J. Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: a case-control study. World J Biol Psychiatry. 2013;14:500–508. doi: 10.3109/15622975.2012.677547. [DOI] [PubMed] [Google Scholar]
- Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Curr Opin Psychiatry. 2009;22:140–146. doi: 10.1097/YCO.0b013e328324f895. [DOI] [PubMed] [Google Scholar]
- Nave G, Camerer C, McCullough M. Does Oxytocin Increase Trust in Humans? A Critical Review of Research. Perspect Psychol Sci. 2015;10:772–789. doi: 10.1177/1745691615600138. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in Neurosciences. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Nicotra E, Casu G, Piras S, Marchese G. On the use of the Positive and Negative Syndrome Scale in randomized clinical trials. Schizophrenia Research. 2015;165:181–187. doi: 10.1016/j.schres.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Duke MP. Individual differences in the nonverbal communication of affect: The diagnostic analysis of nonverbal accuracy scale. Journal of Nonverbal Behavior. 1994;18:9–35. doi: 10.1007/BF02169077. [DOI] [Google Scholar]
- Obermeier M, Mayr A, Schennach-Wolff R, Seemüller F, Möller HJ, Riedel M. Should the PANSS be rescaled? Schizophrenia Bulletin. 2010;36:455–460. doi: 10.1093/schbul/sbp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi Y, Weng S, Kossowsky J, Gerger H, Sung M. Oxytocin and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry. 2016 doi: 10.1055/s-0042-109400. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. doi: 10.2466/PR0.10.3.799-812. [DOI] [Google Scholar]
- Oya K, Matsuda Y, Matsunaga S, Kishi T, Iwata N. Efficacy and safety of oxytocin augmentation therapy for schizophrenia: an updated systematic review and meta-analysis of randomized, placebo-controlled trials. European Archives of Psychiatry and Clinical Neurosciences. 2015;266:439–450. doi: 10.1007/s00406-015-0634-9. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. The Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yuecel M, Bora E, Fornito A, Testa R, Brewer WJ, Velakoulis D, Wood SJ. Neurobiological Markers of Illness Onset in Psychosis and Schizophrenia: The Search for a Moving Target. Neuropsychology Review. 2009;19:385–398. doi: 10.1007/s11065-009-9114-1. [DOI] [PubMed] [Google Scholar]
- Parr LA, Brooks JM, Jonesteller T, Moss S, Jordano JO, Heitz TR. Effects of chronic oxytocin on attention to dynamic facial expressions in infant macaques. Psychoneuroendocrinology. 2016;74:149–157. doi: 10.1016/j.psyneuen.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz Garcia-Portilla M, Garcia-Alvarez L, Saiz PA, Al-Halabi S, Teresa Bobes-Bascaran M, Teresa Bascaran M, Muniz J, Bobes J. Psychometric evaluation of the negative syndrome of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2015;265:559–566. doi: 10.1007/s00406-015-0595-z. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia Research. 2011a;132:50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia Research. 2011b;132:50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophrenia Bulletin. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, Golshani P, Geschwind DH. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8–271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. doi: 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160:815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J, van Ree JM. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology (Berl) 1992;106:71–74. doi: 10.1007/BF02253591. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Alvares GA, Hickie IB, Guastella AJ. Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neuroscience & Biobehavioral Reviews. 2015a;49:182–192. doi: 10.1016/j.neubiorev.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Westlye LT, Alnæs D, Rustan ØG, Kaufmann T, Smerud KT, Mahmoud RA, Djupesland PG, Andreassen OA. Low dose intranasal oxytocin delivered with Breath Powered device dampens amygdala response to emotional stimuli: A peripheral effect-controlled within-subjects randomized dose-response fMRI trial. Psychoneuroendocrinology. 2016;69:1–43. doi: 10.1016/j.psyneuen.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Westlye LT, Rustan ØG, Tesli N, Poppy CL, Smevik H, Tesli M, Røine M, Mahmoud RA, Smerud KT, Djupesland PG, Andreassen OA. Low-dose oxytocin delivered intranasally with Breath Powered device affects social-cognitive behavior: a randomized four-way crossover trial with nasal cavity dimension assessment. Transl Psychiatry. 2015b;5:e602. doi: 10.1038/tp.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Woolley JD. Intranasal Oxytocin Mechanisms Can Be Better Understood, but Its Effects on Social Cognition and Behavior Are Not to Be Sniffed At. Biological Psychiatry. 2015;79:1–2. doi: 10.1016/j.biopsych.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophrenia Research. 2012;137:147–150. doi: 10.1016/j.schres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Green MF. Current Topics in Behavioral Neurosciences. Springer Berlin Heidelberg; Berlin, Heidelberg: 2015. Motivational Deficits and Negative Symptoms in Schizophrenia: Concepts and Assessments; pp. 1–17. [DOI] [PubMed] [Google Scholar]
- Reske M, Habel U, Kellermann T, Backes V, Shah NJ, Wilmsdorff von M, Gaebel W, Zilles K, Schneider F. Differential brain activation during facial emotion discrimination in first-episode schizophrenia. J Psychiatr Res. 2009;43:592–599. doi: 10.1016/j.jpsychires.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Rosenfeld AJ, Lieberman JA, Jarskog LF. Oxytocin, Dopamine, and the Amygdala: A Neurofunctional Model of Social Cognitive Deficits in Schizophrenia. Schizophrenia Bulletin. 2010;37:sbq015–1087. doi: 10.1093/schbul/sbq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Archer D, Hall JA, DiMatteo MR, Rogers PL. Nonverbal Behavior. Elsevier; 1979. Measuring Sensitivity to Nonverbal Communication: The Pons Test; pp. 67–98. [DOI] [Google Scholar]
- Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Drogos LL, Hill SK, Ruocco AC, Keedy SK, Reilly JL, Keshavan MS, Pearlson GD, Tamminga CA, Gershon ES, Sweeney JA. Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophrenia Bulletin. 2014;40:1374–1384. doi: 10.1093/schbul/sbu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophrenia Research. 2011;130:266–270. doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Connelly JJ, Reilly JL, Carter CS, Drogos LL, Pournajafi-Nazarloo H, Ruocco AC, Keedy SK, Matthew I, Tandon N, Pearlson GD, Clementz BA, Tamminga CA, Gershon ES, Keshavan MS, Bishop JR, Sweeney JA. Sex and Diagnosis-Specific Associations Between DNA Methylation of the Oxytocin Receptor Gene With Emotion Processing and Temporal-Limbic and Prefrontal Brain Volumes in Psychotic Disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:141–151. doi: 10.1016/j.bpsc.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: implications for neuropsychological models of aging. Neuroscience & Biobehavioral Reviews. 2008;32:863–881. doi: 10.1016/j.neubiorev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Carstensen LL. Socioemotional functioning and the aging brain. The handbook of social … 2011 [Google Scholar]
- Sarnyai Z, Kovács GL. Oxytocin in learning and addiction: From early discoveries to the present. Pharmacol Biochem Behav. 2014;119:3–9. doi: 10.1016/j.pbb.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Sasayama D, Hattori K, Teraishi T, Hori H, Ota M, Yoshida S, Arima K, Higuchi T, Amano N, Kunugi H. Negative correlation between cerebrospinal fluid oxytocin levels and negative symptoms of male patients with schizophrenia. Schizophrenia Research. 2012;139:201–206. doi: 10.1016/j.schres.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in Domains of Social Cognition in Schizophrenia: A Meta-Analysis of the Empirical Evidence. Schizophrenia Bulletin. 2013;39:979–992. doi: 10.1093/schbul/sbs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Marder SR, Green MF. Social cognition in schizophrenia: Relationships with neurocognition and negative symptoms. Schizophrenia Research. 2007;90:316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A. The Social Salience Hypothesis of Oxytocin. Biological Psychiatry. 2016;79:194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal Administration of Oxytocin Increases Envy and Schadenfreude (Gloating) Biological Psychiatry. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Feifel D. Potential of Oxytocin in the Treatment of Schizophrenia. CNS Drugs. 2016;30:193–208. doi: 10.1007/s40263-016-0315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NY, Park HY, Jung WH, Park JW, Yun JY, Jang JH, Kim SN, Han HJ, Kim SY, Kang DH, Kwon JS. Effects of Oxytocin on Neural Response to Facial Expressions in Patients with Schizophrenia. Neuropsychopharmacology. 2015;40:1919–1927. doi: 10.1038/npp.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirzen-Zelenskaya A, Gonzalez-Iglesias AE, de Monvel JB, Bertram R, Freeman ME, Gerber U, Egli M. Prolactin Induces a Hyperpolarising Current in Rat Paraventricular Oxytocinergic Neurones. J Neuroendocrinol. 2011;23:883–893. doi: 10.1111/j.1365-2826.2011.02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Genetic influences on social cognition. Pediatr Res. 2011;69:85R–91R. doi: 10.1203/PDR.0b013e318212f562. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. (Regul) 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Souza RP, Ismail P, Meltzer HY, Kennedy JL. Variants in the oxytocin gene and risk for schizophrenia. Schizophrenia Research. 2010;121:279–280. doi: 10.1016/j.schres.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol. 2013;16:255–260. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ. An Initial Evaluation of the Functions of Human Olfaction. Chem Senses. 2010;35:3–20. doi: 10.1093/chemse/bjp083. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A Psychometric Comparison of the Clinical Assessment Interview for Negative Symptoms and the Brief Negative Symptom Scale. Schizophrenia Bulletin. 2016;42:1384–1394. doi: 10.1093/schbul/sbw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, Catalano LT, Culbreth AJ, Carpenter WT, Kirkpatrick B. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophrenia Research. 2012;142:88–92. doi: 10.1016/j.schres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Koenig JI, Gold JM, Ossenfort KL, Buchanan RW. Plasma oxytocin levels predict olfactory identification and negative symptoms in individuals with schizophrenia. Schizophrenia Research. 2015a;162:57–61. doi: 10.1016/j.schres.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Koenig JI, Sullivan SK, Gold JM, Buchanan RW. Endogenous oxytocin levels are associated with the perception of emotion in dynamic body expressions in schizophrenia. Schizophrenia Research. 2015b;162:52–56. doi: 10.1016/j.schres.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32:426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Striepens N, Matusch A, Kendrick KM, Mihov Y, Elmenhorst D, Becker B, Lang M, Coenen HH, Maier W, Hurlemann R, Bauer A. Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology. 2014;39:74–87. doi: 10.1016/j.psyneuen.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism Spectrum Disorders and Schizophrenia: Meta-Analysis of the Neural Correlates of Social Cognition. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA. Oxytocin and social salience: a call for gene-environment interaction research. Front Neurosci. 2013;7:199. doi: 10.3389/fnins.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, Meyer ML, Dutcher JM, Castle E, Irwin MR, Lieberman MD, Eisenberger I. Oxytocin, but not vasopressin, impairs social cognitive ability among individuals with higher levels of social anxiety: A randomized controlled trial. Soc Cogn Affect Neurosci. 2016:nsw041. doi: 10.1093/scan/nsw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T, Tanizawa O, Otsuki Y, Sugita N, Haruta M, Yamaji K. Oxytocin in the cerebrospinal fluid and plasma of pregnant and nonpregnant subjects. Horm Metab Res. 1985;17:308–310. doi: 10.1055/s-2007-1013526. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Soulsby B, Kawasaki Y, McGorry PD, Suzuki M, Velakoulis D, Pantelis C. An MRI study of the superior temporal subregions in first-episode patients with various psychotic disorders. Schizophrenia Research. 2009;113:158–166. doi: 10.1016/j.schres.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Tauber M, Mantoulan C, Copet P, Jauregui J, Demeer G, Diene G, Rogé B, Laurier V, Ehlinger V, Arnaud C, Molinas C, Thuilleaux D. Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader-Willi syndrome: a randomised placebo-controlled trial in 24 patients. Orphanet J Rare Dis. 2011;6:47. doi: 10.1186/1750-1172-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-Analysis of Functional Neuroimaging Studies of Emotion Perception and Experience in Schizophrenia. Biological Psychiatry. 2012;71:136–145. doi: 10.1016/j.biopsych.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Hormones and Behavior. 2009;56:128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Hahn CG, Borgmann-Winter K, Moberg PJ. Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophrenia Bulletin. 2009;35:1117–1131. doi: 10.1093/schbul/sbp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl) 1992;109:473–476. doi: 10.1007/BF02247726. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Bhandari R, van der Veen R, Grewen KM, Bakermans-Kranenburg MJ. Elevated Salivary Levels of Oxytocin Persist More than 7 h after Intranasal Administration. Front Neurosci. 2012;6:174. doi: 10.3389/fnins.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn MH, Huffmeijer R, Alink LRA, Bakermans-Kranenburg MJ, Tops M. The Impact of Oxytocin Administration on Charitable Donating is Moderated by Experiences of Parental Love-Withdrawal. Front Psychol. 2011;2:258. doi: 10.3389/fpsyg.2011.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker DW, Ludwig M. Vasopressin, oxytocin, and social odor recognition. Hormones and Behavior. 2012;61:259–265. doi: 10.1016/j.yhbeh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Walss-Bass C, Fernandes JM, Roberts DL, Service H, Velligan D. Differential correlations between plasma oxytocin and social cognitive capacity and bias in schizophrenia. Schizophrenia Research. 2013;147:387–392. doi: 10.1016/j.schres.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Walter H, Ciaramidaro A, Adenzato M, Vasic N, Ardito RB, Erk S, Bara BG. Dysfunction of the social brain in schizophrenia is modulated by intention type: An fMRI study. Soc Cogn Affect Neurosci. 2009;4:166–176. doi: 10.1093/scan/nsn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Waldman ID, Young LJ. Statistical and Methodological Considerations for the Interpretation of Intranasal Oxytocin Studies. Biological Psychiatry. 2015;79:251–257. doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Kaneko N, Nunokawa A, Shibuya M, Egawa J, Someya T. Oxytocin receptor (OXTR) gene and risk of schizophrenia: Case-control and family-based analyses and meta-analysis in a Japanese population. Psychiatry and Clinical Neurosciences. 2012;66:622–622. doi: 10.1111/j.1440-1819.2012.02396.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale (WAIS-IIIuk) 1997 [Google Scholar]
- Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, McGuire P, Shergill SS, Fusar-Poli P. Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci. 2015a;40:E1–22. doi: 10.1503/jpn.130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, McGuire P, Shergill SS, Fusar-Poli P. Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci. 2015b;40:E1–22. doi: 10.1503/jpn.130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Burkner PC. Effects of intranasal oxytocin on symptoms of schizophrenia: A multivariate Bayesian meta-analysis. Psychoneuroendocrinology. 2016 doi: 10.1016/j.psyneuen.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Fussell C, Scherer S, Biagianti B, Fulford D, Mathalon DH, Vinogradov S. Intranasal oxytocin increases facial expressivity, but not ratings of trustworthiness, in patients with schizophrenia and healthy controls. Psychol Med. 2017:1–12. doi: 10.1017/S0033291716003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Fussell C, scherer S, Mathalon DH, Vinogradov S. Intranasal oxytocin increases facial expressivity, but not ratings of trustworthiness, in patients with schizophrenia and healthy controls. Psychol Med. doi: 10.1017/S0033291716003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Lam O, Lai W, O'Donovan A, Rankin KP, Mathalon DH, Vinogradov S. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology. 2014;47:116–125. doi: 10.1016/j.psyneuen.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Lam O, Chuang B, Ford JM, Mathalon DH, Vinogradov S. Oxytocin administration selectively improves olfactory detection thresholds for lyral in patients with schizophrenia. Psychoneuroendocrinology. 2015;53:217–222. doi: 10.1016/j.psyneuen.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The Global Burden of Disease. World Health Organization; 2008. [Google Scholar]
- Wu EQ, Birnbaum H, Kessler R, Beaulieu N, Daher M, Aggarwal J, Ascher-Svanum H, Shi L. Direct health care costs of schizophrenia in the United States: 2002. Value in Health. 2005;8:401–401. [Google Scholar]
- Young AW, Perrett D, Calder AJ, Sprengelmeyer R. Facial expressions of emotion: Stimuli and tests (FEEST) Bury St. Edmunds; Thames …: 2002. [Google Scholar]
- Young JW, Geyer MA. Developing treatments for cognitive deficits in schizophrenia: The challenge of translation. J Psychopharmacol (Oxford) 2015;29:178–196. doi: 10.1177/0269881114555252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]


