To the Editor
Follicular helper T cells (Tfh cells) are a CD4 T cell subset specialized in providing help for the development and maintenance of B cell responses1. Tfh cells are essential for germinal center formation and the development of effective humoral immunity2. In humans, circulating resting memory Tfh cells are minimally defined as CD3+CD4+CD45RO+CXCR5+3. Follicular regulatory T cells (Tfr), a T cell subset believed to originate from thymic-derived FOXP3+ T cell precursors, have been shown to regulate and suppress germinal center reactions4. Several reports suggest that Tfh cells likely play a role in mediating allergic disease5, 6, however, there is a lack of human studies of Tfh cell biology in the context of allergy 7, 8 and allergen-specific immunotherapy (AIT). Given the critical role of Tfh and Tfr cells in regulation of IgE production in the context of allergic disease in murine models6, we hypothesized that AIT modulates Tfh and/or Tfr cells.
A total of 70 subjects were recruited for this study, including 25 Timothy grass allergic patients, 32 patients who received subcutaneous shots of AIT and were in treatment maintenance at the time of blood draw and 13 non-allergic healthy controls identified as having negative skin prick tests to a panel of 32 allergen extracts and no clinical history of allergy (Table E1, online repository).
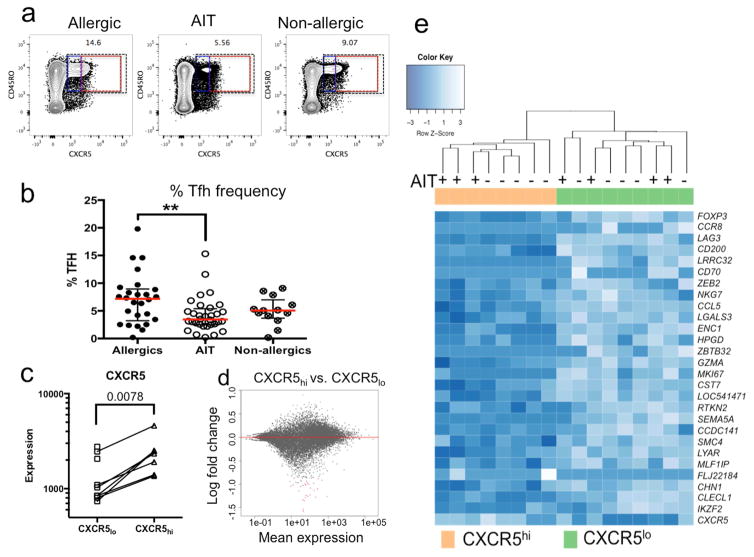
The effect of AIT on Tfh cell frequency was assessed by flow cytometry, quantifying the total peripheral memory Tfh cell population (defined as CD3+CD4+CD45RO+CXCR5+) (Figure 1a). We found a significant reduction of Tfh cells in AIT-treated patients compared to untreated allergic donors (median 6.4% in allergics, 3.5% in AIT, p=0.008, Figure 1b). Reduction of Tfh cells after AIT was also seen compared to non-allergic controls (median 5.1%) but this difference did not reach statistical significance (p=0.13, Figure 1b). To verify our manual gating analysis results, we additionally analyzed the data using a FLOCK-based automated gating strategy, which produced similar results (Figure E1).
Figure 1.
AIT-treated patients exhibit reduced Tfh frequencies compared to untreated allergic and non-allergic controls. a: Representative Tfh cell staining in an allergic, AIT-treated and non-allergic patient. Dashed boxes indicate the total Tfh population (black), CXCR5lo (blue) and CXCR5hi (red) populations. b: Tfh cell percentage in an allergic (n=25), AIT-treated (n=32) and non-allergic (n=13) patients. c: Quantification of CXCR5 gene expression in CXCR5hi and CXCR5lo sorted cells. d: MA plots comparing fold changes of gene expression between CXCR5hi and CXCR5lo cells vs. average gene expression. Genes with significant adjusted p-values are colored in red e: Heatmap of differentially expressed genes in CXCR5hi vs CXCR5lo samples from allergic and AIT-treated individuals.
Given that the reduction of Tfh cells in AIT patients is most prominent in the CXCR5hi subset, we characterized gene expression in CXCR5hi vs CXCR5lo cells in five allergic donors with a predominant CXCR5hi Tfh population (Figure 1a, red box) and five AIT-treated donors with a predominantly CXCR5lo Tfh population (Figure 1a, blue box). CXCR5hi and CXCR5lo cells were sorted and high quality RNA (RIN > 7.5) was obtained for subsequent transcriptomic analysis. As expected, transcription of CXCR5 was significantly reduced in CXCR5lo cells compared to CXCR5hi (Figure 1c). Paired comparisons of CXCR5hi vs. CXCR5lo samples identified 26 genes with significantly different expression levels (multiple hypothesis adjusted p-value <0.05, Figure 2d). Examination of the 26 differentially expressed genes revealed that several had known immunological functions, including regulatory activity (FOXP3, CCR8, LAG3, CD200, LRRC32 and CD70), inflammation (CCL5, LGALS3 and ENC1), cytotoxicity (GZMA) and proliferation (MKI67). Unsupervised hierarchical clustering of samples based on the expression pattern of the 26 genes resulted in separation of CXCR5hi from CXCR5lo cells independent of the AIT status of the donor from which the samples were derived (Figure 2e). Thus the differences observed are intrinsic properties of CXCR5hi vs CXCR5lo cells and are independent of AIT treatment status.
Figure 2.
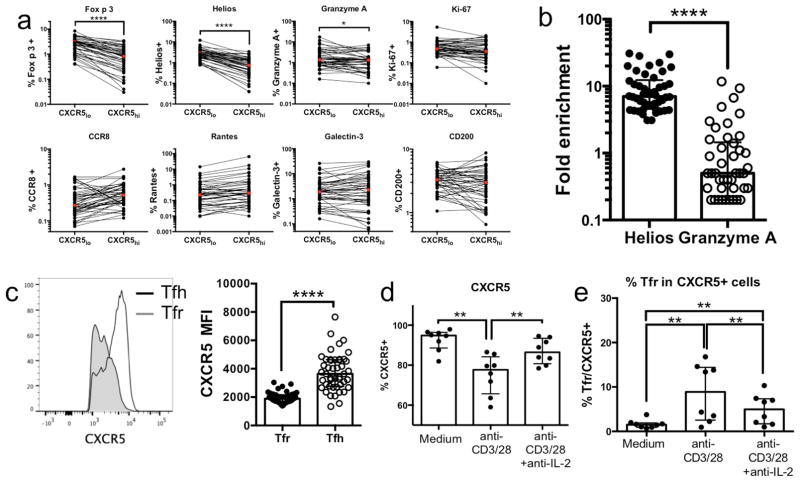
Protein expression profiles of selected markers in CXCR5hi vs CXCR5lo cells and the effect of TCR stimulation on CXCR5 expression. a: Quantification of protein expression of markers shown to be differentially expressed in CXCR5hi vs CXCR5lo cells as determined by RNAseq. b: Fold change of Helios and Granzyme A expression in CXCR5lo FOXP3+ vs CXCR5lo FOXP3− cells. c: Median fluorescent intensity (MFI) for CXCR5 expression in Tfr and Tfh cells. d: CXCR5 expression in sorted Tfh cells after 5 days of culture in medium alone or in the presence of anti-CD3/28 beads with or without anti-IL-2. e: Percent of Tfr cells (Foxp3+Helios+) in total CXCR5+ cells after culture in medium or with anti-CD3/28 beads with or without anti-IL-2. Statistical analysis was done by Wilcoxon matched-pairs signed rank test (one-sided). (n=46), *-p<0.05; ****-p<0.0001
To determine how the identified differences in mRNA levels between CXCR5hi vs CXCR5lo cell populations impacted protein expression, we performed FACS analyses for proteins from seven out of the 26 differentially expressed genes, based on antibody availability (Figure 2a). In addition, we also included antibodies against Helios because transcription of this gene was borderline significant in our analysis (p adj=0.16, Table E2) and Helios has been shown to be important for regulatory T cells function9. Significantly higher expression in CXCR5lo vs CXCR5hi cells was detected for FOXP3, Helios and Granzyme A. Comparing the frequency of Helios and Granzyme A expression in CXCR5lo FOXP3+ vs CXCR5lo FOXP3− cells revealed an enrichment of FOXP3+Helios+ cells, which was significantly higher than FOXP3+Granzyme A+ cells (median 7.0 and 0.5, respectively, p<0.0001) (Figure 2b), suggesting that Helios and FOXP3 are frequently co-expressed on a single cell level, whereas Granzyme A is expressed by a different subpopulation within the CXCR5lo cells. Given the upregulation of Foxp3 and Helios in CXCR5lo cells, we analyzed the median fluorescence intensity of CXCR5 in classical Tfh (CXCR5+FOXP3−Helios) vs Tfr (CXCR5+FOXP3+Helios+) cells (Figure 2c). A pronounced difference in CXCR5 expression (p>0.0001) was observed in the Tfr subset compared to Tfh cells, indicating that Tfr cells fall within the CXCR5lo fraction of Tfh cells in our gating strategy. Thus, the increase in CXCR5lo cells observed in AIT-treated patients may be associated with a relative increase of Tfr cells. To assess the functionality of the stimulated Tfr cells, we performed intracellular cytokine staining which revealed that IL-10 production is more than 6 fold higher in Tfr cells compared to Tfh, a fold difference higher than any other cytokine measured (Figure E2).
Finally, we wanted to determine if the observed changes in CXCR5+ cells in the AIT cohort are associated with an induction of the Tfr phenotype (FOXP3+Helios+), and if they can be induced directly by TCR stimulation, which is associated with IL-2 production. Purified Tfh cells (CD45RO+CXCR5+) from eight donors were cultured for 5 days in medium alone or in the presence of TCR stimulation (anti-CD3/38 beads) with or without anti-IL-2. Flow cytometric analysis revealed a significant reduction of CXCR5 expression in the presence of anti-CD3/28 stimulation (median 77.7%) compared to culture in medium alone (median 94.9%), which was largely rescued in the presence of anti-IL-2 (median 86.4%) (Figure 2d). Tfr cells (CXCR5+FOXP3+Helios+) were significantly increased in the presence of anti-CD3/28 stimulation (median 8.9%) compared to medium alone (median 1.5%). Addition of anti-IL-2 partially reversed Tfr induction (median 5.0%) after TCR stimulation (Figure 2e). Based on these data, we speculate that TCR stimulation and/or the presence of IL-2 during in vitro culture may have agonistic effects on the development/survival of Tfr cells over CXCR5hi Tfh cells.
In the context of AIT immunotherapy, we hypothesize that repeated administration of allergen extracts elicits IL-2 production from allergen-specific T cell responses, which globally impairs CXCR5 expression in memory Tfh cells and induces/retains Tfr cell populations. This could be an important mechanism contributing to the induction of tolerance during AIT.
Materials and methods
Study participants
Patients were recruited following Institutional Review Board approval (La Jolla Institute for Allergy and Immunology, La Jolla, CA) (Federal Wide Assurance no. 00000032). All patients enrolled in this study provided written consent. For the allergic cohort Timothy grass (TG)-allergic donors with skin prick test wheal of ≥3 mm in diameter to TG and a clinical history consistent with seasonal grass pollen allergy were recruited (n=25). The second cohort consisted of TG-allergic subjects who receive subcutaneous shots of allergen-specific immunotherapy (AIT) for a minimum of 6 months and were in treatment maintenance at the time of the blood draw (n=32). Allergen extracts used were from Alk America. Select allergens were from Greer (primarily molds) and Hollister-Stier (AP dog). The patients received one shot per month. Due to concerns about increased risk for anaphylaxis after a large volume blood draw, patients were recalled on another day outside the shot day for blood sample collection. Blood was drawn within a month of the last allergy shot. Non-allergic, healthy control donors were identified as having negative skin prick tests to a panel of 32 allergen extracts and no clinical history of allergy (n=13). A complete summary of all donors recruited for this study is provided in supplemental table 1.
Flow cytometry
To assess Tfh cell frequency, PBMCs from all 3 donor cohorts were thawed, washed and stained with an antibody cocktail for CD4 (eBioscience, San Diego, CA, clone RPA-T4), CD3 (BD, Franklin Lakes, NJ, clone UCHT1), CD45RO (eBioscience, clone UCHL1), CXCR5 (BD, clone RF8B2), and CD19 (BD, clone HIB19), CD14 (BD, clone M5E2), CD8 (BD, clone RPA-T8) and live/dead aqua fixable viability dye for exclusion. Cells were stained for 20 minutes at room temperature, washed and analyzed by flow cytometry. Frequency and CXCR5 median fluorescence intensity of Tfh cells was determined by gating on the CD4+CD3+CD45RO+CXCR5+ population.
Expression of markers of interest identified by RNA-Seq analysis was assessed by surface staining PBMC ex vivo with the panel described above, except CXCR5 (Biolegend, clone J252D4), which performed better during fixation, in addition to CCR8 (R&D, clone 191704) (panel 1) and CD200 (Biolegend, clone OX-104) (panel 3). Subsequently cells were treated with FOXP3 Fixation/Permeabilization Kit (eBioscience) and intracellular staining was performed for FOXP3 (ebioscience, clone 236A/E7), Helios (eBioscience, clone 22F6) and Ki-67 (Biolegend, clone Ki-67) (Panel 1); FOXP3 and RANTES (Biolegend, clone VL1) (Panel 2) and FOXP3, Galectin-3 (Biolegend, clone M3/38) and Granzyme A (Biolgend, clone CB9) (Panel 3). All data acquisition was performed using a BD LSR II flow cytometer and data was analyzed using FlowJo software (TreeStar, Ashland, OR). All data acquisition was performed blinded.
Tfh cell sorting for RNA-Seq
PBMCs from five allergic and 5 AIT-treated patients were stained with the antibody cocktail for CD4, CD3, CD45RO, CXCR5, and CD19, CD14, CD8 and live/dead aqua for exclusion as described above. Tfh cells were gated as CD4+CD3+CD45RO+CXCR5+ and directly sorted into 750 μl of Trizol LS (Invitrogen). Data acquisition and cell sorting was performed using a FACSAria II flow cytometer (BD, Two out of five AIT-treated donors had insufficient numbers of CXCR5hi cells for RNA-Seq analysis. One of the AIT CXCR5 samples was excluded from analysis due to low quality mRNA (RIN < 7.5). RNA-Seq analysis was performed in CXCR5lo samples collected from 5 allergic and 4 AIT-derived samples and CXCR5hi samples from 5 allergic and 3 AIT-derived samples, leaving two CXCR5lo samples and one CXCR5hi sample without corresponding match.
Microscaled RNA sequencing
Total RNA was purified as described previously30 and is described in detail in the supplemental material.
RNA sequencing analysis
RNA sequence analysis was performed as described previously30 and is described in detail in the supplemental material.
Tfh/Tfr marker expression following TCR stimulation/rIL-2 culture
CD4+ T cells were isolated from previously frozen PBMC by negative selection using CD4+ T cell isolation kit II per the manufacturers instructions (Miltenyi, San Diego, CA). The isolated CD4 cell population was stained as described above. Tfh cells (CD4+CD3+CD45RO+CXCR5+) were isolated using a BD FACSAria II cell sorter. After sorting, cells were plated in a round-bottom 96-well plate at 2 × 105/well in 200 μl serum-free AIM-V medium (Life Technologies) with human rIL-7 (4 ng/ml). Tfh cells were incubated in medium alone, with TCR stimulation (Dynabeads, human Tactivator CD3/28, Life technologies) or with human rIL-2 (125 μg/ml), all in the presence or absence of anti-human IL-2 (50 μg/ml). After 5 days of incubation, cells were harvested, washed and surface stained with antibodies for CD4 (eBioscience, San Diego, CA, clone RPA-T4), CD3 (BD, Franklin Lakes, NJ, clone UCHT1), CD45RO (eBioscience, clone UCHL1), CXCR5 (Biolegend, clone J252D4). For exclusion, stains for CD19 (BD, clone HIB19), CD14 (BD, clone M5E2), CD8 (BD, clone RPA-T8) were also performed. Intracellular stains for FOXP3 and Helios were performed as described above.
Statistical analysis
A one- or two-tailed Mann–Whitney U test was used for statistical analysis as indicated in the figure legends. For paired sample comparison, Wilcoxon signed-rank test was used. Differences with a p value <0.05 were considered significant.
Supplementary Material
Acknowledgments
Acknowledgement of funding:
This work was supported by US National Institute of Health contract HHSN272200700048C and 1U19AI100275-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 2.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–10. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–69. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–82. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, et al. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity. 2015;43:318–30. doi: 10.1016/j.immuni.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemeny DM. The role of the T follicular helper cells in allergic disease. Cell Mol Immunol. 2012;9:386–9. doi: 10.1038/cmi.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varricchi G, Harker J, Borriello F, Marone Hon G, Durham SR, Shamji MH. T Follicular Helper (Tfh) Cells in Normal Immune Responses and in Allergic Disorders. Allergy. 2016;71:1086–94. doi: 10.1111/all.12878. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–9. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.