Abstract
Extensive debates continue regarding marijuana (Cannabis spp), the most commonly used illicit substance in many countries worldwide. There has been an exponential increase of cannabis studies over the past two decades but the drug’s long-term effects still lack in-depth scientific data. The epigenome is a critical molecular machinery with the capacity to maintain persistent alterations of gene expression and behaviors induced by cannabinoids that have been observed across the individual’s lifespan and even into the subsequent generation. Though mechanistic investigations regarding the consequences of developmental cannabis exposure remain sparse, human and animal studies have begun to reveal specific epigenetic disruptions in the brain and the periphery. In this article, we focus attention on long-term disturbances in epigenetic regulation in relation to prenatal, adolescent and parental germline cannabinoid exposure. Expanding knowledge about the protracted molecular memory could help to identify novel targets to develop preventive strategies and treatments for behaviors relevant to neuropsychiatric risks associated with developmental cannabis exposure.
Keywords: cannabinoid, prenatal development, adolescence, multigenerational inheritance, epigenetics, DNA methylation, chromatin, transcription, synaptic plasticity
1. Introduction
The reduced perception regarding risks associated with marijuana (Cannabis sativa, Cannabis indica), as well as the growing industry evolving around recreational and medical cannabis, has lead to its increased use particularly among young people (SAMSHA, 2016). It is the first time in the United States’ history that adolescents smoke marijuana more than cigarettes, an increasing tendency since 2010 (Johnston et al., 2012; SAMSHA, 2016). Cannabis has low/moderate addictive properties (Gable, 2006) with only approximately 10–16% of users developing dependence (Anthony, 2006) yet, due to its prevalence today, millions of people in the United States and worldwide meet the clinical diagnosis for cannabis use disorder. This number far exceeds that of all other illicit drugs combined, even taking into consideration marijuana’s recent non-illicit status in several countries and states. While the great efforts taken to educate the public about the health risk of cigarettes have been successful, the pressure for marijuana legalization has contributed to teenagers being under the belief that marijuana is safe (SAMSHA, 2016).
In addition to recreational marijuana, ‘medical cannabis’ and cannabinoids are now being explored as potential therapies to treat various diseases and clinical symptoms. The health conditions studied thus far have been broad including chronic pain, spasticity due to multiple sclerosis, nausea and vomiting due to chemotherapy, depression, anxiety disorder, sleep disorder, psychosis and intraocular pressure associated with glaucoma to name a few. In a recent systematic study of randomized clinical trials (Whiting et al., 2015) only moderate to low quality evidence supported the beneficial clinical effects of medical cannabinoids. Another comprehensive review regarding the therapeutic and recreational use of cannabis and cannabinoids (National Academies of Sciences, 2017) suggest that the evidence is strong for the treatment of chronic pain and spasticity. Consistent conclusions in regard to mental health risk were the substantial evidence for a statistical association between cannabis and the development of schizophrenia or other psychoses (highest risk among the most frequent users) and the fact that initiating cannabis use at an earlier age is a risk factor for the development of cannabis abuse (National Academies of Sciences, 2017).
A growing body of literature has shown that the developing brain is especially sensitive to drugs compared with the adult brain (Anker and Carroll, 2010; Curran et al., 2016; Shahbazi et al., 2008; Zakharova et al., 2009) which is of important concern given that marijuana is the most commonly abused drug by two vulnerable populations — adolescents and pregnant women. Despite the perceived low health risk of cannabis use by the general public, there is now growing clinical awareness about the spectrum of behavioral and neurobiological disturbances associated with cannabis exposure such as psychosis, anxiety, depression, cognitive deficits, social impairments and subsequent drug addiction (Alegria et al., 2010; Bassir Nia et al., 2016; Charilaou et al., 2017; Crean et al., 2011; Feingold et al., 2017; Jutras-Aswad et al., 2009; Kedzior and Laeber, 2014; Leweke and Koethe, 2008; Malone et al., 2010; Morris et al., 2011; Sexton et al., 2016). These types of studies in recent years have begun to shift the perception of marijuana use being without any harm and emphasize the importance for more in-depth scientific investigations to address the potential long-term impact of cannabis use.
The impact of cannabinoid exposure on neurodevelopment is a central question since the brain undergoes rapid growth not only in the prenatal period but also during postnatal life, until early- to mid-adolescence. However, as with most human studies, data generated to date are clearly equivocal most likely due to multiple factors such as the dose and strain of cannabis and cannabinoids used, ratio of the cannabinoids and other entourage chemicals in the plant that is consumed, developmental time of exposure and genetic vulnerability that may modulate the risk for both the adverse effects and therapeutic potential. As such, scientific and medical questions are crucial to be asked about the long-term consequences of cannabis exposure on brain function and behavior. Controlled animal studies provide the potential to explore the behavioral and molecular consequences of cannabinoid exposure but also have evident challenges in recapitulating human usage of the drug such as dose range and route of administration. Nevertheless, information can be gleaned from existing human and animal studies that set the foundation for designing future studies to gain deeper neurobiological insights. In this article, we provide an overview of the current scientific data regarding vulnerabilities of the developing brain to cannabinoid exposure during particularly sensitive windows of development and its epigenetic legacy later in life.
2. Developmental “nature-nurture” interactions and cannabis use
Today, both scientists and clinicians recognize the importance of the prenatal and adolescent developmental periods in chronic and psychiatric disease. Elucidating associations between genotype, environment and phenotype has resulted in an impressive collection of data in relation to many substance use and neurodevelopmental disorders (Boivin et al., 2015; Brander et al., 2016; Enoch, 2012; Isles, 2015; Lv et al., 2013; Vrieze et al., 2012). Although the evidence is not absolute, an extensive body of literature has begun to specifically link marijuana use by pregnant women and during adolescence with adult mental health disturbances later in life (Barthelemy et al., 2016; Chadwick et al., 2013; Jutras-Aswad et al., 2009; Morris et al., 2011; Rubino and Parolaro, 2016). Cannabis exposure during these critical windows of development is thus expected to adversely affect neurobiological systems, resulting in long-lasting alterations in molecular mechanisms affecting neurocircuitry (Fig. 1). Determining how molecular mechanisms contribute to marijuana’s acute effects has been a central question in cannabis research during the last decade. However, much less is known about gene-environment interactions as they relate to the etiology of the complex neuropsychiatric phenotypes relevant to cannabis exposure.
Figure 1. Cannabis exposure during sensitive periods of development can impact epigenetic mechanisms, leading to persistent gene regulation and behavioral alterations.
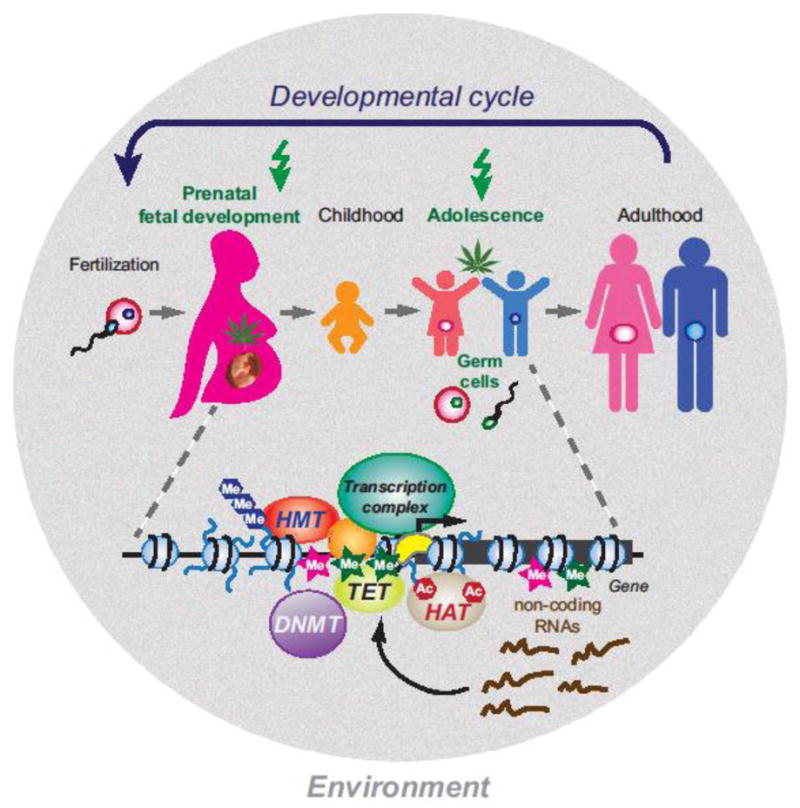
The two most likely populations to use cannabis are pregnant women and adolescents (indicated by green arrow on top). These developmental phases also correspond to periods when the brain is most vulnerable to the influence of external cannabinoids via interfering with epigenetic mechanisms (shown below the schematic of the developmental cycle). Several epigenetic mechanisms that are relevant to the effects of cannabinoids can interfere with normal gene expression via interacting with DNA elements (e.g. promoters) and transcription factors (proteins that bind to the DNA) to regulate mRNA transcript levels from a gene. Specific regulatory mechanisms include DNA methylation (Me), positioning and post-translational modifications of nucleosomes (small blue balls), recruitment of the transcription complex (sequence-specific and basal transcription factors, RNA polymerase II), and non-coding RNAs. DNA methyltranserases (DNMT) generate 5-methylcytosine (pink stars) at CpG sites. Ten-eleven translocation (TET) proteins mediate the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine (green stars), leading to demethylation of the DNA. Modifications of nucleosomal histone tails such as methylation (Me) and acetylation (Ac) are mediated by histone methyltransferases (HMT) and histone acetyltransferases (HAT), respectively. Small RNAs are produced from specific genes and either influence the transcription process or target protein-coding messenger RNAs for degradation. Germ cells (sperm, oocyte) are also sensitive to cannabinoids but the exact underlying epigenetic mechanisms remain to be determined.
What began as investigations focused on the intersection between genetics and developmental biology by scientists such as Conrad H. Waddington and Ernst Hadorn during the mid-twentieth century has evolved into the field we currently refer to as epigenetics. The term epigenetics, which was coined by Waddington in 1942, was derived from the Greek word “epigenesis” which originally described the influence of genetic processes on development (Van Speybroeck, 2002). This was subsequently expanded into broad studies on the molecular basis of Waddington’s observations regarding how environmental insults interact with the genetic material to cause certain phenotypic characteristics. Since then, a great number of research efforts have been focused on unraveling the epigenetic mechanisms related to gene-environment relationships in the context of substance use disorders. Below we provide an overview on behavioral and molecular brain alterations documented in adults exposed to cannabis during adolescence, in the offspring of women with cannabis use during pregnancy, and in subsequent generations conceived by individuals with cannabis-exposed germ cells. We propose and discuss a model for the development of cannabis-related abnormalities shown in Fig. 1 in the context of epigenetic molecular mechanisms.
3. Epigenetic regulatory mechanisms
According to the original definition, “an epigenetic trait is a stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence” (as proposed by Conrad Waddington); this view also implies heritability resulting in a phenotype (Baedke, 2013; Van Speybroeck et al., 2002). The epigenome provides the cellular context for environmental effects, including cannabis exposure during prenatal and early postnatal periods (Szutorisz and Hurd, 2016), therefore it is the most relevant biological target for the propagation of persistent abnormalities and aberrant neuronal processing (Fig. 1).
Generally, the interaction between genomic DNA elements (specific sequences with regulatory function), epigenetic modifiers, and transcription factors determines the expression state of genes. This network of processes is tightly coordinated in space and time, in the specification of different cell, tissue and organ types, and throughout the lifespan of the individual (Dambacher et al., 2013; Dillon, 2012) (Weake and Workman, 2010). In molecular biology, “epigenetic” typically has been used to refer to mechanisms that modulate gene expression without altering the genetic code. There are various epigenetic mechanisms including DNA methylation, nucleosomal structure and positioning, post-translational modifications of nucleosomal histones, histone replacement, and small RNA molecules that help to establish the molecular platform that maintains protracted effects on gene expression and ultimately behavior (Baubec and Schubeler, 2014; Dambacher et al., 2013; Dillon, 2012; Weake and Workman, 2010). In a biological mechanistic context, the complex interaction between genomic DNA elements (specific sequences with regulatory function), epigenetic modifiers and transcription factors determines the expression state of genes. This network of processes is tightly coordinated within cellular compartments, during the specification of different cell, tissue and organ types, and throughout the development and lifespan of the individual (Dambacher et al., 2013; Dillon, 2012; Weake and Workman, 2010).
Some of the most important ontogenetic regulatory decisions take place in early pre- and postnatal development and thus have critical implications in the influence of drug exposure during specifically sensitive periods. Epigenetic modifications that can regulate gene expression levels include DNA methylation, chromatin structure and remodeling, post-translational modifications of nucleosomal histones, histone replacement, and small RNA molecules that can influence protein production and transcription (Fig. 1). Mechanistic implications of several specific epigenetic processes that have thus far been linked to the effects of cannabis are described below.
3.1. DNA methylation
The role of DNA methylation in the regulation of gene expression is complex and highly dependent on genomic location, developmental stage, cell type, or disease state. Historically, CpG methylation (referring to cytosine and guanine in DNA sequence where “p” indicates that the “C” and “G” bases are connected by a phosphodiester bond) in promoter regions and transcriptional regulatory sequences has often been associated with gene repression, whereas methylation within the gene body is less understood (Baubec and Schubeler, 2014; Kato and Iwamoto, 2014). Accumulating evidence now also indicates that DNA methylation in brain is dynamic and its distribution changes throughout neuronal maturation and aging, in neurodevelopmental disorders, including addiction to drugs (Cheng et al., 2015; Feng et al., 2015). Mechanistically, DNA methylation (5-methylcytosine, 5mC) is generated by DNA methyltranserases (DNMTs). The oxidation of 5mC to 5-hydroxymethylcytosine (5hmC) by ten-eleven translocation (TET) proteins can prevent access to DNMTs and thereby can maintain an unmethylated state of the promoter, facilitating transcriptional activation (Branco et al., 2012). Interestingly, DNA methylation marks at specific gene loci have been shown to even persist during the maturation of germ cells (Szyf, 2013, 2015) and thus are interesting candidates for the transmission of cannabis effects from parent to child and possibly throughout multiple generations.
3.2. Histone modifications
On the protein level, the main epigenetic mechanism that has been implicated in neurobiological disturbances is posttranslational modifications of nucleosomal histones, which with the DNA that encircle them comprise the structural and regulatory unit of chromatin. Histones are subject to a variety of chemical modifications including but not limited to, lysine acetylation, lysine and arginine methylation, serine and threonine phosphorylation, and lysine ubiquitination and sumoylation (Bhaumik et al., 2007). These modifications occur primarily within the histone amino-terminal tails protruding from the surface of the nucleosome as well as on the globular core region, and have been shown to influence both the accessibility of genomic regions and the binding of transcription factors to the DNA (Cosgrove et al., 2004). Changes in acetylation and phosphorylation in response to drug exposure are often transient and associated with the quick activation of genes rather than the maintenance of an altered transcription state (Ciccarelli and Giustetto, 2014). However, histone lysine methylation is known to maintain stable gene expression alterations over long periods of time, and it is also the nucleosomal modification that has been associated with the persistent effects of marijuana and different cannabinoids in neurons and other cell types (Aguado et al., 2007; DiNieri et al., 2011; Tomasiewicz et al., 2012; Yang et al., 2014).
3.3. Non-coding (nc) RNAs
These functional RNA molecules are transcribed from DNA but are not translated into proteins. Many ncRNAs regulate gene expression at the transcriptional and post-transcriptional level. The ncRNAs that are known to be involved in epigenetic processes can be divided into two main groups — short ncRNAs (<30 nucleotides) and long ncRNAs (>200 nucleotides). The three major classes of short ncRNAs are microRNAs (miRNAs), short interfering RNAs (siRNAs), and piwi-interacting RNAs (piRNAs) (Chandra et al., 2015; Hegde et al., 2013; Jackson et al., 2014; Molina et al., 2011). While the exact genomic targets of specific cannabinoid-affected miRNAs remain to be characterized, these observations are mechanisticall intriguing given the variety of tissue-specific cellular and developmental processes that are influenced by miRNAs. Small RNAs have also received significant attention as regulators of multigenerational inheritance in a variety of organisms (Houri-Zeevi and Rechavi, 2017).
4. Cannabis and its neurobiological targets
4.1. Main chemicals in cannabis
The cannabis plant contains over 500 herbal compounds and cannabinoids constitute at least 100 of these (ElSohly et al., 2017). Cannabinoids interact with the endogenous systems of the body contributing to the user’s high (acute effect) and are also the molecular instigators of the long-term consequences of marijuana exposure. They are also expected to account for the ability of ‘medical cannabis’ to alleviate a variety of physiological and neuropsychiatric symptoms. Of the numerous cannabinoids, the two most extensively researched compounds are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC is known for its psychoactive properties (Martin-Santos et al., 2012) and CBD is a non-psychoactive cannabinoid shown to have anti-inflammatory effects, to protect neurons from injury or degeneration, to reduce anxiety, to attenuate drug craving in certain people, and to have antipsychotic properties (Hampson et al., 1998; Hurd et al., 2015; Leweke et al., 2012; Zuardi et al., 1982). Most strains of marijuana that has been cultivated and sold on the market over the last decade contain increasingly higher levels of THC and lower levels of CBD (Anker and Carroll, 2010; ElSohly et al., 2016; Guimaraes et al., 1994; Swift et al., 2013; Zuardi et al., 2006). Some studies indicate that, on average, variants of the Cannabis sativa species contain higher levels of THC to CBD and are commonly used for the characteristic ‘high’, whereas Cannabis indica has higher levels of CBD compared to THC and considered beneficial for its sedative, anxiolytic and analgesic properties (Hazekamp and Fischedick, 2012; Pearce et al., 2014).
4.2. Endocannabinoid (eCB) system
In the early 1990s, the neurobiological link between cannabis and its acute psychoactive effects were uncovered through identification of the eCB system. The brain creates its own set of cannabinoids that consist of lipid ligands and cannabinoid receptors, which mediate the actions of THC. The eCB system is responsible for the regulation of many important functions, such as appetite, sleep, emotion, memory and movement (Hillard, 2015; Kruk-Slomka et al., 2016; Moreira and Lutz, 2008; Prospero-Garcia et al., 2016; Tasker et al., 2015). The eCB system modulates synaptic function by on-demand synthesis and release of the ligands from the postsynaptic cell and the subsequent activation of cannabinoid receptors on the presynaptic neurons that attenuate excitatory and inhibitory neurotransmitter release within discrete neuronal circuits.
During development, CBRs play a central role in hardwiring the developing brain and contribute postnatally to the regulation of synaptic plasticity (Berghuis et al., 2007; Tortoriello et al., 2014). Two major types of cannabinoid receptors have been characterized in mammals: cannabinoid 1 receptors (CB1Rs) and cannabinoid 2 receptors (CB2Rs). CB1Rs are the most-abundant G protein-coupled receptors that are expressed in the adult brain, and they show particularly dense distribution in regions that are involved in reward processing and cognitive functions, such as the ventral pallidum, caudate putamen, nucleus accumbens (NAc), ventral tegmental area, amygdala, cingulate cortex, prefrontal cortex, and hypothalamus (Glass et al., 1997; Wang et al., 2003). CB1Rs directly inhibit the release of GABA, glutamate and acetylcholine, which produce widespread effects on neural signalling across many neurotransmitter systems (Lopez-Moreno et al., 2008). CB2Rs are expressed mainly in immune cells and the gut, although recent evidence suggests that they are also present in subsets of neurons, glia and endothelial cells of the brain (Atwood and Mackie, 2010).
The currently best-known eCB ligands are N-arachidonylethanolamide (anandamide (AEA)) and 2-arachidonoylglycerol (2-AG), which are synthesized upon induction by cleavage of membrane-bound precursors and immediately released through Ca2+-dependent mechanisms (Parsons and Hurd, 2015). AEA is derived from the phospholipid precursor N-arachidonoyl-phosphatidylethanolamine (NAPE) and, although the exact mechanisms for AEA formation are not known, the N-acyl-phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) enzyme is likely to have a role in this process. 2-AG derives primarily from the hydrolytic metabolism of 1,2-diacylglycerol (DAG) by the sn-1-selective DAG lipases (DAGLs) DAGLα and DAGLβ. Once released into the extracellular space, eCBs are vulnerable to glial cell inactivation. AEA and 2-AG both exert agonist activity at CB1R and CB2R. AEA binds with slightly higher affinity to CB1R than to CB2R and, similar to THC, it exhibits low agonist activity at both receptors. 2-AG binds with essentially equal affinity at CB1R and CB2R and exhibits greater agonist efficacy than AEA.
The normal epigenetic control of the eCB system has recently been reviewed in (D’Addario et al., 2013). Various lines of evidence strongly suggest that the eCB signaling cascades mediated via CBRs regulate cellular functions in different tissues via epigenetic alterations in DNA methylation (Paradisi et al., 2008), histone methylation (Aguado et al., 2007), and miRNAs (Jackson et al., 2014). These data highlight the role of the eCB system in regulating cellular functions through epigenetic modifications and suggest that modulation of these mechanisms with cannabis use may have long-lasting neurobiological and functional impact.
5. Critical windows of development relevant to cannabinoid exposure
The study of epigenetics in relation to drugs of abuse has been a rapidly emerging field during the past several years, yielding important mechanistic revelations about different addictions and relevant neuropsychiatric disorders (Robison and Nestler, 2011; Sweatt, 2013). However, experimental data regarding potential epigenetic effects associated with cannabis exposure are still sparse in spite of the relatively easy accessibility and frequent use and abuse of this drug (Szutorisz and Hurd, 2016). Of the few published studies (most mainly focused on THC or synthetic cannabinoids), various neuropsychiatric phenotypes and epigenetic alterations that have been reported in association with developmental cannabinoid exposure as summarized in Table 1.
Table 1.
Cannabinoid exposure at sensitive periods of development associated with long-term behavioral and epigenetic disturbances.
| Developmental period of exposure | Substance | Phenotypic disturbance later in life | Alteration observed in body | Epigenetic modification | Reference |
|---|---|---|---|---|---|
| In utero and adolescence | Cigarettes, alcohol (prenatal), Cannabis (adolescence) | Increased substance use in adolescence | Human gestational and adolescent blood | DNA methylation | (Cecil et al., 2016) |
| In utero | THC | Increased heroin seeking in adulthood | Adult rat brain (NAc) | Histone H3 methylation: H3K4me3, H3K9me2 | (DiNieri et al., 2011; Spano et al., 2007) |
| Adolescence | THC | Increased heroin seeking in adulthood | Adult rat brain (NAc) | Histone H3 methylation: H3K9me2, H3K9me3 | (Tomasie wicz et al., 2012) |
| Adolescence | Synthetic cannabinoid WIN55212.2 | Memory impairment | Adult mouse brain (hippocampus) | DNA methylation | (Tomas-Roig et al., 2016) |
| Adulthood (at time of study) | Cannabis | Schizophrenia in adulthood | Human peripheral blood | DNA methylation | (Liu et al., 2014) |
| Adolescent parental germline | THC | Increased heroin seeking in adulthood | Adult rat brain (NAc) | DNA methylation | (Szutorisz et al., 2016; Watson et al., 2015) |
5.1. Long-term effects of gestational cannabis exposure in the offspring
Various studies have evaluated the behavioral effects in the progeny of women who smoked cannabis when pregnant. Multiple review articles have previously addressed the phenotypic effects in humans (for example (Fried et al., 2003; Goldschmidt et al., 2008; Jutras-Aswad et al., 2009; Morris et al., 2011; Tomas-Roig et al., 2016). A number of preclinical animal studies have also demonstrated prenatal THC exposure on offspring behaviors and some suggested disturbances in gene expression (Campolongo et al., 2007; Rubio et al., 1998; Singh et al., 2006; Spano et al., 2007; Vela et al., 1998). Here we focus on findings linking cannabinoid exposure with epigenetic changes that are likely to cause dysregulation in the expression of genes functionally relevant to offspring neuropsychiatric phenotypes (Table 1).
Numerous investigations on the developmental effects of THC directly described molecular alterations highly relevant to addiction disorders. These studies focused in large part on the NAc, a critical neuroanatomical substrate underlying the pathophysiology of addiction (Everitt and Robbins, 2013; Girault, 2012; Koob and Volkow, 2010). Of the multiple epigenetic mechanisms, the regulation of histone modification is unique because they can have either positive or negative effects on gene transcription. Indeed, our previous studies revealed disturbances in the histone modification profile in the NAc of adult rats with prenatal THC exposure (DiNieri et al., 2011). This study identified decreased levels of the trimethylation of lysine 4 on histone H3 (H3K4me3), a transcriptionally permissive mark, increased levels of dimethylation of lysine 9 on histone H3 (H3K9me2), a repressive mark, as well as decreased RNA polymerase II association with the promoter and coding regions of the gene in the NAc. These THC-related chromatin modifications were linked to significant disturbances in the mRNA expression of the dopaminergic D2 receptor (Drd2) gene in both rats and humans that persisted into adulthood, emphasizing the enduring consequences of THC/cannabis exposure during gestational development.
More recently, Cecil et al. (Cecil et al., 2016) conducted the first genome-wide, relatively large human study to examine associations between DNA methylation alterations in blood from birth to early childhood and tobacco, cannabis and alcohol use later in adolescence in subjects from the Avon Longitudinal Study of Parents and Children (ALSPAC). They found that, at birth, variation in DNA methylation in gestational and adolescent blood across a tightly interconnected genetic network was associated with greater levels of substance use during adolescence, as well as an earlier age of onset amongst users. Affected genes included PACSIN1, NEUROD4 and NTRK2, which are implicated in neurodevelopmental processes. Although this study was not specific to the effects of cannabis exposure but also included cigarette and alcohol use, it provides valuable information on the relationship between gestational drug exposure, DNA methylation alterations detectable in the periphery, and substance abuse risk, including cannabis, later in life. Together, these findings highlight gestation as a sensitive window of biological vulnerability and provide evidence for abnormal epigenetic signatures of prenatal drug exposure and substance abuse vulnerability in postnatal life.
5.2. Consequences of adolescent cannabinoid exposure later in life
Marijuana use by teenagers and young adults often predates the abuse of harder drugs (known as the classic “gateway” concept), but the neurobiological underpinnings of such vulnerability are just beginning to be unraveled (Table 1). Converging evidence obtained from animal and human brain studies has shown that, similarly to Drd2, early cannabis exposure selectively alters the expression of opioid neuropeptide proenkephalin (Penk) in the mesocorticolimbic system, disturbances that persist into adulthood and modulate drug-seeking behavior later in life (Jutras-Aswad et al., 2012). Several years ago we reported a direct causal link between the upregulation of the Penk gene in the NAc due to THC exposure during adolescence and enhanced behavioral susceptibility to heroin seeking in adulthood (Tomasiewicz et al., 2012). On the chromatin level, persistent changes in repressive H3K9me2 and H3K9me3 were observed at the Penk locus in the NAc of adult rats following adolescent THC exposure, in line with enduring upregulation of Penk mRNA expression. This epigenetic effect represents a profound pathologic departure from the distinct developmental pattern of histone H3 methylation that normally occurs at Penk in the NAc across the transition from adolescence to adulthood. The chromatin landscape is highly complex, but trimethylation of H3K9 (a transcriptionally repressive mark) may account for the developmental transcriptional instability of NAc Penk due to adolescent THC exposure, allowing the Penk gene to be “primed” to respond to environmental cues later in life.
In the last decade not only marijuana but also synthetic cannabinoids have become increasingly popular among young people (Tournebize et al., 2016). While short-term effects on cognition and psychosis are normally observed in those individuals (Bassir Nia et al., 2016; Cohen et al., 2017; Murray et al., 2016; Spaderna et al., 2013), questions remain as to whether there may be enduring effects of adolescent synthetic cannabinoid use on adult brain and behavior. Preclinical studies have started to address this issue primarily with the use of the synthetic cannabinoid receptor agonist WIN55212.2, which acts similarly to THC but with much greater efficacy at the cannabinoid receptors. In a recent study, adult rats with a history of adolescent WIN55212.2 (self-administered or experimenter-administered) failed to show any long-term cognitive dysfunction as measured using working memory and spatial recognition tasks (Kirschmann et al., 2017). Although prefrontal GABAergic and glutamatergic signaling was altered in the prefrontal cortex of these animals, epigenetic alterations were not examined and remains to be investigated (Kirschmann et al., 2017). Another study (Tomas-Roig et al., 2016) that investigated the chronic administration of WIN55212.2 during adolescence in young adult mice did begin to address epigenetic consequences. Animals that received the drug during adolescence showed spatial memory disturbances in the Morris water maze, as well as a dose-dependent memory impairment in fear conditioning. Moreover, adolescent WIN55212.2 exposure increased adult hippocampal eCB levels and promoted DNA hypermethylation at the intragenic region of the intracellular signaling modulator Rgs7, which was accompanied by a lower rate of mRNA transcription of the gene, suggesting a potential causal relationship. RGS proteins are important regulators of striatal G protein-coupled receptors signaling (Ostrovskaya et al., 2014; Sjogren, 2011; Xie and Martemyanov, 2011). Although the concrete mechanisms underlying the behavioral observations remain to be elucidated, the study does demonstrate that exposure to a synthetic cannabinoid during adolescence could lead to epigenetic gene regulation abnormalities in adulthood. Nevertheless, the equivocal behavioral findings in the few papers published to date emphasize the need for more research focused on the effects of synthetic cannabinoids.
Of the different components of the eCB system, several investigations have focused on the epigenetic regulation of the Cnr1 gene, which encodes the CB1R. Specific genomic elements of the Cnr1 locus have been shown to interact with transcription factors, some of which are implicated in methylation of CpG sites in the DNA and histone posttranslational modifications (Lee et al., 2013; Mukhopadhyay et al., 2010; Nagre et al., 2015). CB1R expression has been reported to increase in peripheral blood lymphocytes of human schizophrenic patients with cannabis abuse and is inversely correlated to methylation of the CNR1 gene promoter (Liu et al., 2014). Interestingly, CNR1 mRNA expression levels and promoter DNA methylation detected in the blood was reported to relate to the intensity of cannabis craving as well as to the severity of nicotine, cannabis and alcohol consumption, suggesting a relevance of CNR1 epigenetic status to brain function and behavior.
In summary, these findings clearly show that cannabinoid exposure during adolescence and young adulthood can imprint on the epigenetic landscape of postnatal development and augment behavioral responses via the dysregulation of genes that have important neurobiological functions related to addiction risk.
5.3. Effects of germline cannabinoid exposure through multiple generations
A less obvious and still significantly unexplored question regarding the long-term consequences of prenatal cannabis exposure is whether there could be potential impact on subsequent generations. In recent years, findings in various disease states have demonstrated epigenetic aberrations that influence developmental risk and can be inherited through the germline from parent to child (Bohacek and Mansuy, 2013; Szyf, 2015). Several cases of parent-child transmission regarding drugs of abuse have been published, describing both behavioral phenotypes and molecular disturbances in the offspring of parents that were exposed to drugs before mating and conception (reviewed in (Vassoler and Sadri-Vakili, 2014)). Such studies have provided compelling evidence for stably heritable phenotypes resulting from epigenetic changes as originally described in the classic model of epigenetics (see section 3).
Though it is still a provocative concept, we have previously demonstrated that exposure of male and female adolescent rats before mating (“germline exposure”) leads to behavioral and molecular abnormalities in their unexposed offspring (Szutorisz et al., 2014). These studies revealed that adult progeny, themselves unexposed to THC, displayed increased work effort to self-administer heroin, demonstrating a cross-generational “gateway” most likely established in the parental germline before conception (Table 1). Importantly, acute effects of the drug are ruled out in these experiments since THC was no longer present in the body at the time of parental mating. Furthermore, all offspring were raised by surrogate dams never exposed to THC, ensuring that any cross-generational effects in the offspring were not due to drug-related abnormalities in maternal care early in life. Other investigators have also described cross-generational behavioral alterations using synthetic cannabinoid agonists. For example, adolescent female rats treated with WIN55212.2 before mating and pregnancy had progeny that exhibited increased morphine sensitivity (Byrnes et al., 2012; Vassoler et al., 2013). Neurobiologically, parental THC exposure has been associated with changes in the mRNA expression of cannabinoid, dopamine, and glutamatergic receptor genes in the striatum and altered synaptic plasticity in neurophysiological measures. Both sexes showed pronounced glutamatergic disturbances in the dorsal striatum in adulthood though stronger in females (Szutorisz et al., 2016).
Using the same paradigm as above, robust DNA methylation disturbances were detected in the NAc of adult rats with parental germline THC exposure in an epigenome-scale investigation (Watson et al., 2015). A key observation was the identification of DNA methylation alterations within an interaction network centered around the Dlg4 gene, encoding Psd-95, a membrane associated guanylate kinase scaffolding protein located in neural postsynaptic densities, involved in the regulation of dopamine-glutamate interactions (de Bartolomeis and Tomasetti, 2012). Previously, epigenetic dysregulation of Dlg4 has been linked to abnormal glutamatergic transmission involved in morphine conditioning (Wang et al., 2014), consistent with the earlier observations of increased heroin self-administration in adult offspring with germline THC exposure (Szutorisz et al., 2014). Many genes containing abnormal methylation are well-known regulators of neurotransmission and synaptic plasticity, including glutamate and kainite receptors, G-protein-coupled receptors, pre- and postsynaptic ion channels and scaffolding proteins, that have been speculated as susceptibility genes for psychiatric conditions such as schizophrenia, depression, autism and obsessive-compulsive disorder (Spiers et al., 2015; Wilson and Sengoku, 2013). Intriguingly, the dynamic control of DNA methylation and demethylation has recently been strongly implicated in the regulation of synaptic plasticity and drug addiction (Feng et al., 2015; Lunnon et al., 2016; Sweatt, 2013, 2016).
Multigenerational epigenetic effects occur when exposure to environmental stimuli triggers epigenetic alterations that are transmitted to the subsequent generation. Three different routes of multigenerational transmission have been described: fetal programming (e.g. maternal stress), behavioral/social transfer (e.g. interaction between parents and offspring), and germline transmission (Bohacek et al., 2013; Cowley and Oakey, 2012). In germline epigenetic inheritance, germ cells undergo meiosis to produce the gametes that could be vulnerable to alterations by parental THC exposure. In this context, it is important that the eCB system plays important roles not only in the development of a variety of somatic cells and physiological systems, but also in reproduction. It is known that both male and female reproductive tissues express cannabinoid receptors and eCBs and that in males, THC can disrupt the normal development of sperm cells (Banerjee et al., 2011; Bari et al., 2011). As an epigenetic correlate, studies on the impact of cannabinoids on male fertility have been conducted in Cnr1 null mutant mice that displayed higher histone retention in germ cells compared to wild type mice (Chioccarelli et al., 2010). In that study, CB1R expression was demonstrated to be necessary for spermiogenesis by controlling chromatin folding in sperm via the regulation of histone displacement. Marijuana-using women are also known to produce poor quality oocytes, associated with lower pregnancy rates (Klonoff-Cohen et al., 2006). Future studies are required to systematically address assess how possible epigenetic processes such as DNA methylation or chromatin regulation are disrupted by cannabinoids and are involved in the transmission of effects from parent to offspring and, potentially, throughout multiple generations.
6. Conclusions
The relationship between cannabis use and neuropsychiatric vulnerability is clearly complex, but the limited data accrued to date in this fast growing field already documents that early exposure during one’s lifetime leaves a long-term epigenetic memory mark which sets a legacy even onto future generations. As more studies are conducted, there are several aspects that must be considered in experimental design in order to gain greater in-depth insight and rigorous assessments to advance knowledge. These include fundamental knowledge regarding dose that better approximate human usage, timing or exposure, reversibility of behavior, and molecular events induced by developmental cannabis/cannabinoid use. Moreover, it will be important to investigate how different cannabinoids or other components of the cannabis plant and even their interactions (e.g. THC and CBD) influence behavioral, physiological and epigenetic effects in long-term users.
Due to technological advances, large genome-wide datasets are now available which makes it possible to perform complex computational analyses to help determine overlapping (e.g., transcriptome and epigenome) biological patterns that can guide the discovery of novel regulatory mechanisms. Another important variable that has not been well explored but acknowledged to be important in developmental questions regarding cannabis is potential sex-specific effects that have significant implications for disease vulnerability and treatment response.
Additionally, most information garnered to date about neurochemical and molecular mechanisms in brain are derived from homogenate approaches that are informative but limit cell-type specific knowledge. Direct insight about the epigenome and transcriptome within specific neural circuits such as the discrete striatal output pathways and prefrontal cortical circuits relevant to goal-directed behavior and decision-making would help to unravel the dynamic cellular mechanisms across development, linked to cannabis-related psychiatric disorders. One challenge of the cell-specific nature of epigenetic modifications is being able to track alterations across time in the brain within the same individual. As such, assessment of peripheral epigenetic marks in association with developmental cannabis exposure could provide an important opportunity to track the epigenetic trajectory across time in relationship to clinical outcomes. The ability to identify specific peripheral biomarkers in humans would also have significant translational value that could be integrated with animal models to allow mechanistic evaluation.
Overall, expanding knowledge about the protracted neurobiological signature of epigenetic memory associated with marijuana and various cannabinoids will identify novel targets to develop preventive strategies and treatments for behaviors relevant to neuropsychiatric risks due developmental cannabis exposure.
HIGHLIGHTS.
Long-term developmental effects of cannabis largely lack in-depth scientific data.
The epigenome underlies molecular and behavioral effects of cannabinoids.
We discuss epigenetic dysregulation by prenatal, adolescent and germline cannabis.
Expanding epigenetic knowledge will provide targets for treatment interventions.
Acknowledgments
This work was supported by grants from NIH/NIDA DA030359 and DA033660.
Footnotes
Financial Disclosure. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguado T, Carracedo A, Julien B, Velasco G, Milman G, Mechoulam R, Alvarez L, Guzman M, Galve-Roperh I. Cannabinoids induce glioma stem-like cell differentiation and inhibit gliomagenesis. The Journal of biological chemistry. 2007;282:6854–6862. doi: 10.1074/jbc.M608900200. [DOI] [PubMed] [Google Scholar]
- Alegria AA, Hasin DS, Nunes EV, Liu SM, Davies C, Grant BF, Blanco C. Comorbidity of generalized anxiety disorder and substance use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry. 2010;71:1187–1195. doi: 10.4088/JCP.09m05328gry. quiz 1252–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology. 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony J. The Epidemiology of Cannabis Dependence. Cambridge University Press; New York: 2006. Cannabis Dependence: Its Nature, Consequences and Treatment. [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. British journal of pharmacology. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baedke J. The epigenetic landscape in the course of time: Conrad Hal Waddington’s methodological impact on the life sciences. Studies in history and philosophy of biological and biomedical sciences. 2013;44:756–773. doi: 10.1016/j.shpsc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth defects research Part B, Developmental and reproductive toxicology. 2011;92:195–205. doi: 10.1002/bdrb.20295. [DOI] [PubMed] [Google Scholar]
- Bari M, Battista N, Pirazzi V, Maccarrone M. The manifold actions of endocannabinoids on female and male reproductive events. Frontiers in bioscience. 2011;16:498–516. doi: 10.2741/3701. [DOI] [PubMed] [Google Scholar]
- Barthelemy OJ, Richardson MA, Cabral HJ, Frank DA. Prenatal, perinatal, and adolescent exposure to marijuana: Relationships with aggressive behavior. Neurotoxicol Teratol. 2016;58:60–77. doi: 10.1016/j.ntt.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Bassir Nia A, Medrano B, Perkel C, Galynker I, Hurd YL. Psychiatric comorbidity associated with synthetic cannabinoid use compared to cannabis. Journal of psychopharmacology. 2016;30:1321–1330. doi: 10.1177/0269881116658990. [DOI] [PubMed] [Google Scholar]
- Baubec T, Schubeler D. Genomic patterns and context specific interpretation of DNA methylation. Curr Opin Genet Dev. 2014;25:85–92. doi: 10.1016/j.gde.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Gapp K, Saab BJ, Mansuy IM. Transgenerational epigenetic effects on brain functions. Biological psychiatry. 2013;73:313–320. doi: 10.1016/j.biopsych.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Mansuy IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:220–236. doi: 10.1038/npp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Kakooza AM, Warf BC, Davidson LL, Grigorenko EL. Reducing neurodevelopmental disorders and disability through research and interventions. Nature. 2015;527:S155–160. doi: 10.1038/nature16029. [DOI] [PubMed] [Google Scholar]
- Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nature reviews Genetics. 2012;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- Brander G, Perez-Vigil A, Larsson H, Mataix-Cols D. Systematic review of environmental risk factors for Obsessive-Compulsive Disorder: A proposed roadmap from association to causation. Neuroscience and biobehavioral reviews. 2016;65:36–62. doi: 10.1016/j.neubiorev.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Schenk ME, Byrnes EM. Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. Journal of psychopharmacology. 2012;26:1348–1354. doi: 10.1177/0269881112443745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Trezza V, Cassano T, Gaetani S, Morgese MG, Ubaldi M, Soverchia L, Antonelli T, Ferraro L, Massi M, Ciccocioppo R, Cuomo V. Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addiction biology. 2007;12:485–495. doi: 10.1111/j.1369-1600.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- Cecil CA, Walton E, Smith RG, Viding E, McCrory EJ, Relton CL, Suderman M, Pingault JB, McArdle W, Gaunt TR, Mill J, Barker ED. DNA methylation and substance-use risk: a prospective, genome-wide study spanning gestation to adolescence. Translational psychiatry. 2016;6:e976. doi: 10.1038/tp.2016.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick B, Miller ML, Hurd YL. Cannabis Use during Adolescent Development: Susceptibility to Psychiatric Illness. Frontiers in psychiatry. 2013;4:129. doi: 10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra LC, Kumar V, Torben W, Vande Stouwe C, Winsauer P, Amedee A, Molina PE, Mohan M. Chronic administration of Delta9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques. J Virol. 2015;89:1168–1181. doi: 10.1128/JVI.01754-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charilaou P, Agnihotri K, Garcia P, Badheka A, Frenia D, Yegneswaran B. Trends of Cannabis Use Disorder in the Inpatient: 2002 to 2011. The American journal of medicine. 2017 doi: 10.1016/j.amjmed.2016.12.035. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Bernstein A, Chen D, Jin P. 5-Hydroxymethylcytosine: A new player in brain disorders? Experimental neurology. 2015;268:3–9. doi: 10.1016/j.expneurol.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chioccarelli T, Cacciola G, Altucci L, Lewis SE, Simon L, Ricci G, Ledent C, Meccariello R, Fasano S, Pierantoni R, Cobellis G. Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology. 2010;151:5017–5029. doi: 10.1210/en.2010-0133. [DOI] [PubMed] [Google Scholar]
- Ciccarelli A, Giustetto M. Role of ERK signaling in activity-dependent modifications of histone proteins. Neuropharmacology. 2014;80:34–44. doi: 10.1016/j.neuropharm.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Cohen K, Kapitany-Foveny M, Mama Y, Arieli M, Rosca P, Demetrovics Z, Weinstein A. The effects of synthetic cannabinoids on executive function. Psychopharmacology. 2017;234:1121–1134. doi: 10.1007/s00213-017-4546-4. [DOI] [PubMed] [Google Scholar]
- Cosgrove MS, Boeke JD, Wolberger C. Regulated nucleosome mobility and the histone code. Nat Struct Mol Biol. 2004;11:1037–1043. doi: 10.1038/nsmb851. [DOI] [PubMed] [Google Scholar]
- Cowley M, Oakey RJ. Resetting for the next generation. Molecular cell. 2012;48:819–821. doi: 10.1016/j.molcel.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of addiction medicine. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH. Keep off the grass? Cannabis, cognition and addiction. Nature reviews Neuroscience. 2016;17:293–306. doi: 10.1038/nrn.2016.28. [DOI] [PubMed] [Google Scholar]
- D’Addario C, Di Francesco A, Pucci M, Finazzi Agro A, Maccarrone M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013;280:1905–1917. doi: 10.1111/febs.12125. [DOI] [PubMed] [Google Scholar]
- Dambacher S, de Almeida GP, Schotta G. Dynamic changes of the epigenetic landscape during cellular differentiation. Epigenomics. 2013;5:701–713. doi: 10.2217/epi.13.67. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A, Tomasetti C. Calcium-dependent networks in dopamine-glutamate interaction: the role of postsynaptic scaffolding proteins. Molecular neurobiology. 2012;46:275–296. doi: 10.1007/s12035-012-8293-6. [DOI] [PubMed] [Google Scholar]
- Dillon N. Factor mediated gene priming in pluripotent stem cells sets the stage for lineage specification. BioEssays: news and reviews in molecular, cellular and developmental biology. 2012;34:194–204. doi: 10.1002/bies.201100137. [DOI] [PubMed] [Google Scholar]
- DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, Dow-Edwards D, Hurd YL. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biological psychiatry. 2011;70:763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biological psychiatry. 2016;79:613–619. doi: 10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of Cannabis sativa L. Progress in the chemistry of organic natural products. 2017;103:1–36. doi: 10.1007/978-3-319-45541-9_1. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The influence of gene-environment interactions on the development of alcoholism and drug dependence. Current psychiatry reports. 2012;14:150–158. doi: 10.1007/s11920-011-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neuroscience and biobehavioral reviews. 2013 doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Feingold D, Rehm J, Lev-Ran S. Cannabis use and the course and outcome of major depressive disorder: A population based longitudinal study. Psychiatry research. 2017;251:225–234. doi: 10.1016/j.psychres.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Feng J, Shao N, Szulwach KE, Vialou V, Huynh J, Zhong C, Le T, Ferguson D, Cahill ME, Li Y, Koo JW, Ribeiro E, Labonte B, Laitman BM, Estey D, Stockman V, Kennedy P, Courousse T, Mensah I, Turecki G, Faull KF, Ming GL, Song H, Fan G, Casaccia P, Shen L, Jin P, Nestler EJ. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nature neuroscience. 2015;18:536–544. doi: 10.1038/nn.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25:427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Gable RS. Drugs and Society: US Public Policy. Lanham, MD: Rowman & Littlefield Publishers; 2006. [Google Scholar]
- Girault JA. Integrating neurotransmission in striatal medium spiny neurons. Advances in experimental medicine and biology. 2012;970:407–429. doi: 10.1007/978-3-7091-0932-8_18. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:254–263. doi: 10.1097/CHI.0b013e318160b3f0. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, de Aguiar JC, Mechoulam R, Breuer A. Anxiolytic effect of cannabidiol derivatives in the elevated plus-maze. General pharmacology. 1994;25:161–164. doi: 10.1016/0306-3623(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazekamp A, Fischedick JT. Cannabis - from cultivar to chemovar. Drug testing and analysis. 2012;4:660–667. doi: 10.1002/dta.407. [DOI] [PubMed] [Google Scholar]
- Hegde VL, Tomar S, Jackson A, Rao R, Yang X, Singh UP, Singh NP, Nagarkatti PS, Nagarkatti M. Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Delta9-tetrahydrocannabinol in vivo: regulation of CCAAT/enhancer-binding protein alpha by microRNA-690. The Journal of biological chemistry. 2013;288:36810–36826. doi: 10.1074/jbc.M113.503037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ. The Endocannabinoid Signaling System in the CNS: A Primer. International review of neurobiology. 2015;125:1–47. doi: 10.1016/bs.irn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houri-Zeevi L, Rechavi O. A Matter of Time: Small RNAs Regulate the Duration of Epigenetic Inheritance. Trends in genetics: TIG. 2017;33:46–57. doi: 10.1016/j.tig.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Yoon M, Manini AF, Hernandez S, Olmedo R, Ostman M, Jutras-Aswad D. Early Phase in the Development of Cannabidiol as a Treatment for Addiction: Opioid Relapse Takes Initial Center Stage. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2015;12:807–815. doi: 10.1007/s13311-015-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles AR. Neural and behavioral epigenetics; what it is, and what is hype. Genes, brain, and behavior. 2015;14:64–72. doi: 10.1111/gbb.12184. [DOI] [PubMed] [Google Scholar]
- Iyengar BR, Choudhary A, Sarangdhar MA, Venkatesh KV, Gadgil CJ, Pillai B. Non-coding RNA interact to regulate neuronal development and function. Front Cell Neurosci. 2014;8:47. doi: 10.3389/fncel.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PloS one. 2014;9:e93954. doi: 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Institute for Social Research, The University of Michigan; Ann Arbor: 2012. [Google Scholar]
- Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. European archives of psychiatry and clinical neuroscience. 2009;259:395–412. doi: 10.1007/s00406-009-0027-z. [DOI] [PubMed] [Google Scholar]
- Jutras-Aswad D, Jacobs MM, Yiannoulos G, Roussos P, Bitsios P, Nomura Y, Liu X, Hurd YL. Cannabis-dependence risk relates to synergism between neuroticism and proenkephalin SNPs associated with amygdala gene expression: case-control study. PloS one. 2012;7:e39243. doi: 10.1371/journal.pone.0039243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Iwamoto K. Comprehensive DNA methylation and hydroxymethylation analysis in the human brain and its implication in mental disorders. Neuropharmacology. 2014;80:133–139. doi: 10.1016/j.neuropharm.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population--a meta-analysis of 31 studies. BMC psychiatry. 2014;14:136. doi: 10.1186/1471-244X-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann EK, Pollock MW, Nagarajan V, Torregrossa MM. Effects of Adolescent Cannabinoid Self-Administration in Rats on Addiction-Related Behaviors and Working Memory. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2017;42:989–1000. doi: 10.1038/npp.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonoff-Cohen HS, Natarajan L, Chen RV. A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. American journal of obstetrics and gynecology. 2006;194:369–376. doi: 10.1016/j.ajog.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk-Slomka M, Dzik A, Budzynska B, Biala G. Endocannabinoid System: the Direct and Indirect Involvement in the Memory and Learning Processes-a Short Review. Molecular neurobiology. 2016 doi: 10.1007/s12035-016-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Asgar J, Zhang Y, Chung MK, Ro JY. The role of androgen receptor in transcriptional modulation of cannabinoid receptor type 1 gene in rat trigeminal ganglia. Neuroscience. 2013;254:395–403. doi: 10.1016/j.neuroscience.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addiction biology. 2008;13:264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkotter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Translational psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen J, Ehrlich S, Walton E, White T, Perrone-Bizzozero N, Bustillo J, Turner JA, Calhoun VD. Methylation patterns in whole blood correlate with symptoms in schizophrenia patients. Schizophr Bull. 2014;40:769–776. doi: 10.1093/schbul/sbt080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Gonzalez-Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addiction biology. 2008;13:160–187. doi: 10.1111/j.1369-1600.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Lunnon K, Hannon E, Smith RG, Dempster E, Wong C, Burrage J, Troakes C, Al-Sarraj S, Kepa A, Schalkwyk L, Mill J. Variation in 5-hydroxymethylcytosine across human cortex and cerebellum. Genome biology. 2016;17:27. doi: 10.1186/s13059-016-0871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Xin Y, Zhou W, Qiu Z. The epigenetic switches for neural development and psychiatric disorders. Journal of genetics and genomics = Yi chuan xue bao. 2013;40:339–346. doi: 10.1016/j.jgg.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. British journal of pharmacology. 2010;160:511–522. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farre M, Zuardi AW, McGuire PK. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Current pharmaceutical design. 2012;18:4966–4979. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- Molina PE, Amedee A, LeCapitaine NJ, Zabaleta J, Mohan M, Winsauer P, Vande Stouwe C. Cannabinoid neuroimmune modulation of SIV disease. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2011;6:516–527. doi: 10.1007/s11481-011-9301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addiction biology. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Morris CV, DiNieri JA, Szutorisz H, Hurd YL. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. The European journal of neuroscience. 2011;34:1574–1583. doi: 10.1111/j.1460-9568.2011.07884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay B, Liu J, Osei-Hyiaman D, Godlewski G, Mukhopadhyay P, Wang L, Jeong WI, Gao B, Duester G, Mackie K, Kojima S, Kunos G. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-gamma. The Journal of biological chemistry. 2010;285:19002–19011. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Quigley H, Quattrone D, Englund A, Di Forti M. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World psychiatry: official journal of the World Psychiatric Association. 2016;15:195–204. doi: 10.1002/wps.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagre NN, Subbanna S, Shivakumar M, Psychoyos D, Basavarajappa BS. CB1-receptor knockout neonatal mice are protected against ethanol-induced impairments of DNMT1, DNMT3A, and DNA methylation. Journal of neurochemistry. 2015;132:429–442. doi: 10.1111/jnc.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, E., and Medicine. The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- Ostrovskaya O, Xie K, Masuho I, Fajardo-Serrano A, Lujan R, Wickman K, Martemyanov KA. RGS7/Gbeta5/R7BP complex regulates synaptic plasticity and memory by modulating hippocampal GABABR-GIRK signaling. eLife. 2014;3:e02053. doi: 10.7554/eLife.02053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi A, Pasquariello N, Barcaroli D, Maccarrone M. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. The Journal of biological chemistry. 2008;283:6005–6012. doi: 10.1074/jbc.M707964200. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nature reviews Neuroscience. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce DD, Mitsouras K, Irizarry KJ. Discriminating the effects of Cannabis sativa and Cannabis indica: a web survey of medical cannabis users. Journal of alternative and complementary medicine. 2014;20:787–791. doi: 10.1089/acm.2013.0190. [DOI] [PubMed] [Google Scholar]
- Prospero-Garcia O, Amancio-Belmont O, Becerril Melendez AL, Ruiz-Contreras AE, Mendez-Diaz M. Endocannabinoids and sleep. Neuroscience and biobehavioral reviews. 2016;71:671–679. doi: 10.1016/j.neubiorev.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature reviews Neuroscience. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. The Impact of Exposure to Cannabinoids in Adolescence: Insights From Animal Models. Biological psychiatry. 2016;79:578–585. doi: 10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Rubio P, Rodriguez de Fonseca F, Martin-Calderon JL, Del Arco I, Bartolome S, Villanua MA, Navarro M. Maternal exposure to low doses of delta9-tetrahydrocannabinol facilitates morphine-induced place conditioning in adult male offspring. Pharmacology, biochemistry, and behavior. 1998;61:229–238. doi: 10.1016/s0091-3057(98)00099-9. [DOI] [PubMed] [Google Scholar]
- SAMSHA, 2016. Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug Use and Health: Detailed Tables. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 1 Choke Cherry Road, Rockville, MD 20857: 2016. (HHS Publication No. SMA 15-4927, NSDUH Series H-50) [Google Scholar]
- Sexton M, Cuttler C, Finnell JS, Mischley LK. A cross-sectional survey of medical cannabis users: Patterns of use and perceived efficacy. Cannabis and Cannabinoid Research. 2016;1:131–138. doi: 10.1089/can.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology. 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Singh ME, McGregor IS, Mallet PE. Perinatal exposure to delta(9)-tetrahydrocannabinol alters heroin-induced place conditioning and fos-immunoreactivity. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:58–69. doi: 10.1038/sj.npp.1300770. [DOI] [PubMed] [Google Scholar]
- Sjogren B. Regulator of G protein signaling proteins as drug targets: current state and future possibilities. Advances in pharmacology. 2011;62:315–347. doi: 10.1016/B978-0-12-385952-5.00002-6. [DOI] [PubMed] [Google Scholar]
- Spaderna M, Addy PH, D’Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology. 2013;228:525–540. doi: 10.1007/s00213-013-3188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biological psychiatry. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Spiers H, Hannon E, Schalkwyk LC, Smith R, Wong CC, O’Donovan MC, Bray NJ, Mill J. Methylomic trajectories across human fetal brain development. Genome research. 2015;25:338–352. doi: 10.1101/gr.180273.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. The emerging field of neuroepigenetics. Neuron. 2013;80:624–632. doi: 10.1016/j.neuron.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Dynamic DNA Methylation Controls Glutamate Receptor Trafficking and Synaptic Scaling. Journal of neurochemistry. 2016 doi: 10.1111/jnc.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift W, Wong A, Li KM, Arnold JC, McGregor IS. Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PloS one. 2013;8:e70052. doi: 10.1371/journal.pone.0070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, Ren Y, Miller ML, Blitzer RD, Hurd YL. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014;39:1315–1323. doi: 10.1038/npp.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, Egervari G, Sperry J, Carter JM, Hurd YL. Cross-generational THC exposure alters the developmental sensitivity of ventral and dorsal striatal gene expression in male and female offspring. Neurotoxicol Teratol. 2016 doi: 10.1016/j.ntt.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, Hurd YL. Epigenetic Effects of Cannabis Exposure. Biological psychiatry. 2016;79:586–594. doi: 10.1016/j.biopsych.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. The genome- and system-wide response of DNA methylation to early life adversity and its implication on mental health. Can J Psychiatry. 2013;58:697–704. doi: 10.1177/070674371305801208. [DOI] [PubMed] [Google Scholar]
- Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol Med. 2015;21:134–144. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Chen C, Fisher MO, Fu X, Rainville JR, Weiss GL. Endocannabinoid Regulation of Neuroendocrine Systems. International review of neurobiology. 2015;125:163–201. doi: 10.1016/bs.irn.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Tomas-Roig J, Benito E, Agis-Balboa RC, Piscitelli F, Hoyer-Fender S, Di Marzo V, Havemann-Reinecke U. Chronic exposure to cannabinoids during adolescence causes long-lasting behavioral deficits in adult mice. Addiction biology. 2016 doi: 10.1111/adb.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biological psychiatry. 2012;72:803–810. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoriello G, Morris CV, Alpar A, Fuzik J, Shirran SL, Calvigioni D, Keimpema E, Botting CH, Reinecke K, Herdegen T, Courtney M, Hurd YL, Harkany T. Miswiring the brain: Delta9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. The EMBO journal. 2014;33:668–685. doi: 10.1002/embj.201386035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Substance abuse. 2016:1–23. doi: 10.1080/08897077.2016.1219438. [DOI] [PubMed] [Google Scholar]
- Van Speybroeck L. From epigenesis to epigenetics: the case of C. H. Waddington. Annals of the New York Academy of Sciences. 2002;981:61–81. [PubMed] [Google Scholar]
- Van Speybroeck V, Reyniers MF, Marin GB, Waroquier M. The kinetics of cyclization reactions on polyaromatics from first principles. Chemphyschem: a European journal of chemical physics and physical chemistry. 2002;3:863–870. doi: 10.1002/1439-7641(20021018)3:10<863::AID-CPHC863>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Johnson NL, Byrnes EM. Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. Journal of psychopharmacology. 2013;27:1015–1022. doi: 10.1177/0269881113503504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2014;264:198–206. doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela G, Martin S, Garcia-Gil L, Crespo JA, Ruiz-Gayo M, Fernandez-Ruiz JJ, Garcia-Lecumberri C, Pelaprat D, Fuentes JA, Ramos JA, Ambrosio E. Maternal exposure to delta9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain research. 1998;807:101–109. doi: 10.1016/s0006-8993(98)00766-5. [DOI] [PubMed] [Google Scholar]
- Vrieze SI, McGue M, Iacono WG. The interplay of genes and adolescent development in substance use disorders: leveraging findings from GWAS meta-analyses to test developmental hypotheses about nicotine consumption. Human genetics. 2012;131:791–801. doi: 10.1007/s00439-012-1167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Keller E, Hurd YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. 2003;118:681–694. doi: 10.1016/s0306-4522(03)00020-4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yan P, Hui T, Zhang J. Epigenetic upregulation of PSD-95 contributes to the rewarding behavior by morphine conditioning. European journal of pharmacology. 2014;732:123–129. doi: 10.1016/j.ejphar.2014.03.040. [DOI] [PubMed] [Google Scholar]
- Watson CT, Szutorisz H, Garg P, Martin Q, Landry JA, Sharp AJ, Hurd YL. Genome-Wide DNA Methylation Profiling Reveals Epigenetic Changes in the Rat Nucleus Accumbens Associated With Cross-Generational Effects of Adolescent THC Exposure. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40:2993–3005. doi: 10.1038/npp.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weake VM, Workman JL. Inducible gene expression: diverse regulatory mechanisms. Nature reviews Genetics. 2010;11:426–437. doi: 10.1038/nrg2781. [DOI] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. Jama. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Sengoku T. Developmental regulation of neuronal genes by DNA methylation: environmental influences. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2013;31:448–451. doi: 10.1016/j.ijdevneu.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Martemyanov KA. Control of striatal signaling by g protein regulators. Frontiers in neuroanatomy. 2011;5:49. doi: 10.3389/fnana.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hegde VL, Rao R, Zhang J, Nagarkatti PS, Nagarkatti M. Histone modifications are associated with Delta9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. The Journal of biological chemistry. 2014;289:18707–18718. doi: 10.1074/jbc.M113.545210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behavioural brain research. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimaraes FS. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2006;39:421–429. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology. 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]


