Abstract
Inflammation and angiogenesis drive the development and progression of multiple devastating diseases such as atherosclerosis, cancer, rheumatoid arthritis, and inflammatory bowel disease. Though these diseases have very different phenotypic consequences, they possess several common pathophysiological features in which monocyte recruitment, macrophage polarization, and enhanced vascular permeability play critical roles. Thus, developing rational targeting strategies tailored to the different stages of the journey of monocytes, from bone marrow to local lesions, and their extravasation from the vasculature in diseased tissues will advance nanomedicine. The integration of in vivo imaging uniquely allows studying nanoparticle kinetics, accumulation, clearance, and biological activity, at levels ranging from subcellular to an entire organism, and will shed light on the fate of intravenously administered nanomedicines. We anticipate that convergence of nanomedicines, biomedical engineering, and life sciences will help to advance clinically relevant therapeutics and diagnostic agents for patients with chronic inflammatory diseases.
Keywords: Monocytes, Macrophages, Chronic inflammation, Immunomodulation, Atherosclerosis, Cancer, Angiogenesis, Nanomedicine, Molecular imaging, Targeted drug delivery
Graphical abstract
1. Introduction
Inflammation is a key underlying process in several disorders, including cancer, cardiovascular diseases, rheumatoid arthritis, and inflammatory bowel disease (IBD). The aforementioned maladies are leading causes of morbidity and mortality worldwide and impose a substantial socioeconomic burden[1,2]. Although new therapeutics are being developed, the incidence of complications remains high under the current standard of care[3,4]. This is in part because conventional therapies are not specific to the diseased tissue, and therefore cause considerable off-target adverse effects[5]. Equally important, disease heterogeneity is not taken into account. Current diagnostic approaches do not allow identification of those patients who would benefit the most from a given therapy or those who would likely suffer from complications[6–8]. Additionally, therapeutic strategies are often disease-specific, disregarding the common denominator: inflammation. Seemingly diverse, the abovementioned diseases possess common pathophysiological features driven –or aggravated– by inflammation, including leukocytosis, tissue remodeling, local cell proliferation and angiogenesis[9–11].
Extensive research of different pathophysiological processes has unveiled the role of multiple immune cells in diseased tissues that drive the development and progression of inflammatory disorders. One of the most effector cells in inflammatory lesions is a monocyte-derived macrophage, which is a key component of innate immunity[12–16]. Monocytes and macrophages not only contribute to the local inflammatory milieu of chronic inflammatory diseases but also modulate endothelial permeability and recruitment of supplementary immune cells, thereby driving disease progression[17,18]. Elucidating and understanding macrophage dynamics over the course of inflammatory disease progression offer unique opportunities for designing more specific and efficacious diagnostic and therapeutic agents. For example, our increased knowledge of the inflammatory process has yielded powerful immunotherapies[19], including anti-tumor necrosis factor alpha (TNF-α) antibodies[20], interleukin 6 (IL-6) inhibitors[21], and bifunctional antibodies[22], which modulate the immune response to achieve remission of chronic inflammation.
Simultaneously, nanomedicine –defined as “the application of nanotechnology for treatment, diagnosis, monitoring, and control of biological systems” [23]– has experienced unprecedented growth in the past decade. In addition to improving the therapeutic index of drugs, monocyte/macrophage-targeted nanomedicine can be implemented as a cell-specific drug delivery strategy to pharmacologically modulate inflammation. Such ‘immunomodulatory nanoplatforms’ can also be adopted for diagnostic and imaging purposes, not only at the level of local inflammatory lesions and organs involved in monocytosis but also to visualize macrophage dynamics systemically[24,25].
This review discusses the interplay between advances in nanomedicine and our understanding of chronic inflammatory disorders. This vantage point allows exploiting the commonalities of monocyte/macrophage dynamics across diseases for targeted therapy and diagnosis. We will highlight how nanomedicine can be applied at the inflammatory process’ different stages, in progressing disease. In addition, we will share our perspective on inflammation-oriented nanomedicine design and how it will facilitate developing improved therapies and diagnostic tools for patients. Although this review focuses on monocytes and macrophages as key effector cells in chronic inflammation and most prone to take up nanomedicines, other immune cells, such as T and B cells, have vital roles in complex paracrine signaling between macrophages and other cells in the inflammatory lesions, discussed in detail in other reviews [26–29].
2. Inflammation and angiogenesis in disease
Inflammation is a vital protective process in host defense against infections and injuries, which ultimately –through a resolution process– restores homeostatic conditions in the affected tissue [30]. However, persistent unresolved inflammation can contribute to the initiation and progression of several chronic diseases, including cancer[27], atherosclerosis[31], rheumatoid arthritis[29], and IBD [32]. The inflammatory response consists of interplay between innate and adaptive immunity[33,34]. This cross-talk modifies the function of other cells, such as epithelial cells (e.g. endothelial cells) and mesenchymal cells (e.g. smooth muscle cells) over time, leading to different disease stages[35,36]. Macrophages are key players that intercommunicate with adaptive immune T and B cells, and also directly affect the inflammatory milieu through secretion of inflammatory mediators[10,37]. Inflammatory macrophages differentiate from lesion infiltrating monocytes and express a set of pattern recognition receptors (e.g. Toll-like receptors and scavenger receptors) that can sense pathogen-associated molecular patterns (PAMPs) and endogenous tissue damage-associated molecular patterns (DAMPs)[38]. Engagement of these receptors results in activation of nuclear factor-kappa B (NF-κB), and production of proinflammatory cytokines, reactive oxygen and nitrogen species, and other mediators that can intensify the inflammatory response[39,40]. Moreover, due to elevated metabolic activity, inflamed tissues are notoriously hypoxic, activating macrophages, fibroblasts, and endothelial cells to produce hypoxia-inducible factors (HIFs) [41–43] and vascular endothelial growth factor (VEGF). Note that VEGF was originally denoted vascular permeability factor (VPF). Chronic exposure to HIF 1 and 2, VEGF, and inflammatory cytokines results in degradation of endothelial cell adherens junctions, dissolution of the basement membrane, and dramatic destabilization of existing microvessels[44–46]. Moreover, these factors facilitate endothelial cells’ proliferation and migration, inducing the growth of new blood vessels from existing ones, a process known as angiogenesis.. Angiogenesis and chronic inflammation, long treated as two distinct processes, are thus codependent [47–49]. In the next sections, we will provide an overview of the involvement of monocytes and macrophages in the pathogenesis of several chronic inflammatory diseases, and discuss the common aberrant endothelial function and pathological angiogenesis, which are affected by the local inflammatory milieu.
2.1 Cancer
Cancer is a multifaceted malady in which cells are no longer completely responsive to the signals that regulate cellular differentiation, growth, proliferation, and death. As a result, these cancerous cells accumulate within the tissue, causing local damage and inflammation[27]. Rudolf Virchow established the first link between cancer and inflammation in the 19th century when he observed the infiltration of leukocytes in neoplastic tissues[50]. In the last twenty years, our understanding of carcinogenesis and the tumor microenvironment has supported Virchow’s hypothesis, substantiating the role of inflammation in the initiation, promotion, and metastatic progression of cancer[51,52]. Moreover, increasing epidemiological evidence suggests that several chronic inflammatory diseases (e.g. IBD and hepatitis), associated or not with infectious agents, predispose individuals to several types of cancer[51,53]. Tumor-associated macrophages (TAMs) are the most abundant immune cells in the tumor microenvironment[54]. They are derived from circulating inflammatory monocytes, which are preferentially attracted to lesions by tumor-derived chemotactic factors, like C-C motif chemokine ligand 2 (CCL-2; also known as monocyte chemoattractant protein 1, MCP-1)[55]. TAM density is positively correlated with the levels of tumor-derived CCL-2, and usually associated with poor prognosis in various types of cancer[56–58]. The protumorigenic activities of TAMs can be appreciated in cancer initiation, tumor cell invasion and migration, and metastasis [59]. Inflammatory macrophages are key producers of reactive nitrogen and oxygen species that can generate oncogenic mutations and lead to tumor initiation[60]. These reactive species also cause activation of epithelial cells and the consequent recruitment of more monocytes[61]. In addition to the initiation phase, most invasive tumors demonstrate collective intravasation, in which groups of tumor cells invades the blood vessels while maintaining cell-cell contacts [62,63]. Tumor cell intravasation is shown to occur upon physical contact with macrophages, which induces RhoA GTPase activity in tumor cells, regulates actin cytoskeleton, and triggers the formation of invadopodia [64,65]. The combined effect of improved RhoA GTPase activity and proteolytic enzymes (e.g. MMPs), secreted by TAMs and cancer-associated fibroblasts (CAFs), enables tumor cells to degrade and break through matrix barriers in the course of tumor cell transendothelial migration [66–68]. Moreover, the paracrine signaling between tumor cells and stromal cells (e.g. CAFs and TAMs), through epidermal growth factor (EGF) and colony stimulating factor-1 (CSF-1), can activate tumor cell growth, and enhance migration and extravasation [69,70].. In most tumors, malignant switch and tumor propagation require an adequate supply of oxygen and nutrients, and an effective way to remove waste products, which is achieved through the development of a dense vessel network that connects the tumor to host circulation. Tumor vasculature development, also known as the angiogenic switch, is profoundly dependent on TAMs and other stromal cells (e.g. endothelial cells, smooth muscle cells, fibroblasts), which secrete several angiogenic growth factors and proteinases, including VEGFs [71,72] and matrix metalloproteinases[73]. The developed tumor vasculature is usually chaotic and leaky (i.e. with large inter-endothelial cell fenestrations), facilitating tumor cell intravasation and metastatic dissemination [74]. These migrating cells establish themselves at metastatic niches where they recruit monocytes and macrophages, mainly via CCL-2. Metastasis-associated macrophages (MAMs) secrete another chemokine, CCL-3, which affects MAM retention and interaction with tumor cells, enhancing metastatic seeding and growth[57].
2.2 Atherosclerotic cardiovascular diseases
Atherosclerosis is a chronic inflammatory disease of the large- and medium-sized arteries[37]. Although atherosclerotic cardiovascular diseases are as old as ancient Egyptian mummies[75], they remain the leading cause of mortality worldwide, accounting for most deaths among non-communicable diseases. According to the World Health Organization, 17.7 million deaths per year, an estimated 31% of all deaths worldwide, can be attributed to atherosclerotic diseases[76]. Due to recent advances in immunology, the inflammatory component of atherosclerosis is now more appreciated, and the disease is no longer thought to be only due to aberrant lipid deposition. Inflammation plays a prominent role at different stages of atherosclerosis and contributes to its complications[40,77]. At early stages, risk factors such as smoking, hypertension, and elevated levels of apolipoprotein B-containing lipoproteins can induce, partially via activating NF-κB, focal expression of endothelial adhesion molecules, including vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecules (ICAMs)[78,79]. The focal expression of these adhesion molecules in areas that are prone to develop atherosclerotic lesions (e.g. aortic root and arches) facilitates the binding and adherence of circulating immune cells, such as monocytes. Once adhered, monocytes transmigrate to the subendothelial space and mature into resident macrophages under the influence of a complex mix of vascular wall-derived chemokines, such as macrophage colony-stimulating factor (M-CSF)[80]. The accumulation of lipoproteins, calcium, and immune cells within the vessel wall leads to the development of focal lesions, known as atherosclerotic plaques. This buildup is mainly affected by plaque-resident macrophages, which secrete apoB-lipoprotein binding proteoglycans increasing apoB retention in the subendothelial space[81]. Moreover, macrophages produce reactive oxygen and nitrogen species inducing lipoprotein modifications, mainly through peroxidation. These modifications fuel further inflammatory processes that result in recruitment of more monocytes into the arterial intima[82]. Additionally, macrophages engulf the native and modified lipoproteins leading to the formation of foam cells, which further amplify lipoprotein oxidation, uptake, retention, and modification through expression of scavenger receptors, such as type A scavenger receptor (SRA) and a member of the type B family, namely CD36[83,84]. These receptors can also cooperate with toll-like receptors and promote inflammasome activation and secretion of proinflammatory cytokines in response to modified lipoproteins [85,86]. Moreover, macrophages secrete proteolytic enzymes, including MMPs, which may contribute to atherosclerotic plaque remodeling and instability[87]. Similar to tumor angiogenesis, atherosclerotic plaques also undergo neovascularization under the influence of macrophage-derived angiogenic factors[88]. Neovascularization contributes to advanced plaques instability by facilitating the infiltration of additional inflammatory immune cells and/or acting as a source of intraplaque hemorrhage[89,90]. Furthermore, perturbed lipid efflux may increase macrophage cell death in advanced stages, aggravating the inflammatory burden and decreasing plaque stability[91].
2.3 Rheumatoid arthritis
Rheumatoid arthritis (RA) is an immune-mediated, chronic inflammatory disease that primarily affects synovial membranes, cartilages, and bones[29]. The prevalence of the disease is around 1% globally and usually associated with progressive disability, severe morbidity and systemic complications, such as accelerated atherosclerosis[92]. The cause of RA is unknown[93]. However, the increasing understanding of RA pathophysiology underscores the role of immune cells in the initiation and progression of the disease[94]. The cross-talk between immune cells and skeletal systems, the two key components of RA osteoimmunology, results in the production of autoantibodies, infiltration of immune cells into the affected joints, and ultimately joint destruction[95]. Macrophages are key effectors in these processes, as evidenced by their abundance in the synovial lining and the strong correlation between macrophage number and the extent of joint destruction[96,97]. The crosstalk between macrophages and T cells plays a crucial role in disease initiation. Macrophages activate naïve T cells, likely by acting as antigen presenting cells and/or through T cell-monocyte/macrophage interaction[98,99]. Once activated, naïve T cells enter a proliferative state and secrete cytokines, including interferon-gamma (IFN-γ) and IL-17, which further skew macrophages towards an inflammatory state[100]. Immunohistological studies of isolated synovium have shown that activated macrophages are the main producers of proinflammatory cytokines, including IL-1, IL-6, and TNF-α[101,102]. These cytokines trigger synovial fibroblast activation, leading to hypertrophied synovium (also called pannus) which is a tumor-like tissue that invades and destroys the local articular tissue[102,103]. Furthermore, macrophage-derived granulocyte-macrophage colony stimulating factor (GM-CSF) and other cytokines stimulate the maturation of innate immune cells, their efflux from bone marrow, and their migration into the synovium[104]. In addition to the inflammatory component, RA progression is usually associated with pathophysiological angiogenesis. The infiltration of immune cells and the highly inflammatory milieu results in local hypoxia, secretion of HIFs and growth factors (mainly by macrophages and fibroblasts), increased vascular permeability, and formation of new blood vessels to supply nutrients and oxygen [105,106]. Such a complex microenvironment of proinflammatory cytokines and immune cells stimulate osteoclasts –multinucleated cells of the monocytic lineage– to degrade bone matrix and solubilize bone calcium, leading to inflammation-driven bone erosion in advanced stages[107,108].
2.4 Inflammatory bowel disease
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, relapsing inflammatory diseases of the gastrointestinal (GI) tract[109]. In these diseases, inflammation impairs the ability of the affected GI to function properly, resulting in persistent diarrhea, abdominal pain, cramping, and rectal bleeding. As most chronic inflammatory diseases, genetic predispositions, environmental triggers, and immune cells contribute to IBD pathogenesis and progression[109,110]. Under normal physiological conditions, the main function of intestinal immune cells is to maintain the integrity of the epithelial barrier along the GI tract against external stimuli –e.g. food and the microflora– without eliciting a strong inflammatory reaction[111]. Intestinal macrophages, strategically located in the intestinal lamina propria, represent the largest macrophage population in the body[112]. During an IBD flare, this tolerance is lost either by a change in the microflora or a defect in the immune response to the existing flora[113,114]. Such a change leads to imbalance in the number of T cells and recruitment of blood monocyte and their differentiation into activated macrophages, which are phenotypically different from resident macrophages[115]. The activated macrophages secrete proinflammatory cytokines, including IL-23 and TNF-α [116,117]. In addition to their inflammatory actions, these cytokines modulates epithelial cell growth, T cell activity, intestinal and vascular permeability, and the production of reactive oxygen species[118,119]. The active phase of IBD is usually accompanied by increased microvascular density and permeability, which facilitate the infiltration of more proinflammatory immune cells[120]. Ultimately, these inflammation-driven changes lead to barrier dysfunction, tissue damage, and fibrosis[121,122].
3. Applying nanomedicine in inflammation dynamics
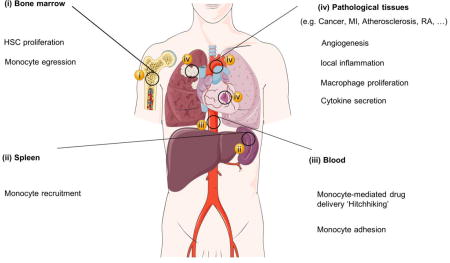
There are strong associations and comorbidities between different types of chronic inflammatory disorders[123–125]. Also, striking similarities in the dynamics of the aforementioned diseases and other chronic inflammatory disorders exist. The diseases are usually initiated by a persistent local tissue insult or injury that activates the (extra) medullar hematopoiesis and attracts inflammatory monocytes to the lesions. Infiltrating monocytes and lesion-associated macrophages affect other cells in the microenvironment and induce pathological angiogenesis and local tissue remodeling. Such holistic understanding of chronic inflammation dynamics offers opportunities to develop therapeutics and diagnostic agents that can be applied to multiple diseases. Employing nanomedicines in chronic inflammatory diseases can advance, revive, and repurpose efficacious therapeutics and diagnostic agents. Nanomaterials are highly tunable and can be designed to exhibit different sizes, shapes, and surface chemistry, which can be exploited to modulate nanoparticles’ in vivo behavior (e.g. circulation kinetics, cell uptake, and tissue penetration)[126]. Moreover, nanoparticles can be used as carriers for different therapeutic cargos such as small molecule drugs (hydrophilic and hydrophobic) [127], peptides[128], and nucleic acids[129]. Nanoparticles can also be used to solubilize poorly water-soluble compounds that are intended for parental use[130]. Additionally, nanomedicine\approaches may lead to a drug’s improved therapeutic index through increasing the on-target efficacy, while reducing the off-target toxicity [131]. As diagnostic probes, nanoparticles are highly amenable and can be labeled for optical, magnetic resonance imaging (MRI), computed tomography (CT) and nuclear imaging approaches[24,132,133]. In addition, due to the high phagocytic activities of monocytes and macrophages, nanoparticles are suitable for imaging not only inflammatory lesions, but also the dynamics of inflammatory cells in disease [24,134].. Thus, applying nanomedicine at the different stages of monocyte/macrophage dynamics can foster our understanding of inflammation and can be used to modulate these processes. An overview of these processes that can be tackled by nanomedicine on a systemic level is depicted in Fig. 1. In subsequent sections, we will zoom in on the exploitation of nanomedicine and the processes that monocytes and macrophages modulate during chronic inflammation in their corresponding compartments. In this context, we will share lessons learned and challenges faced, followed by our outlook on the future.
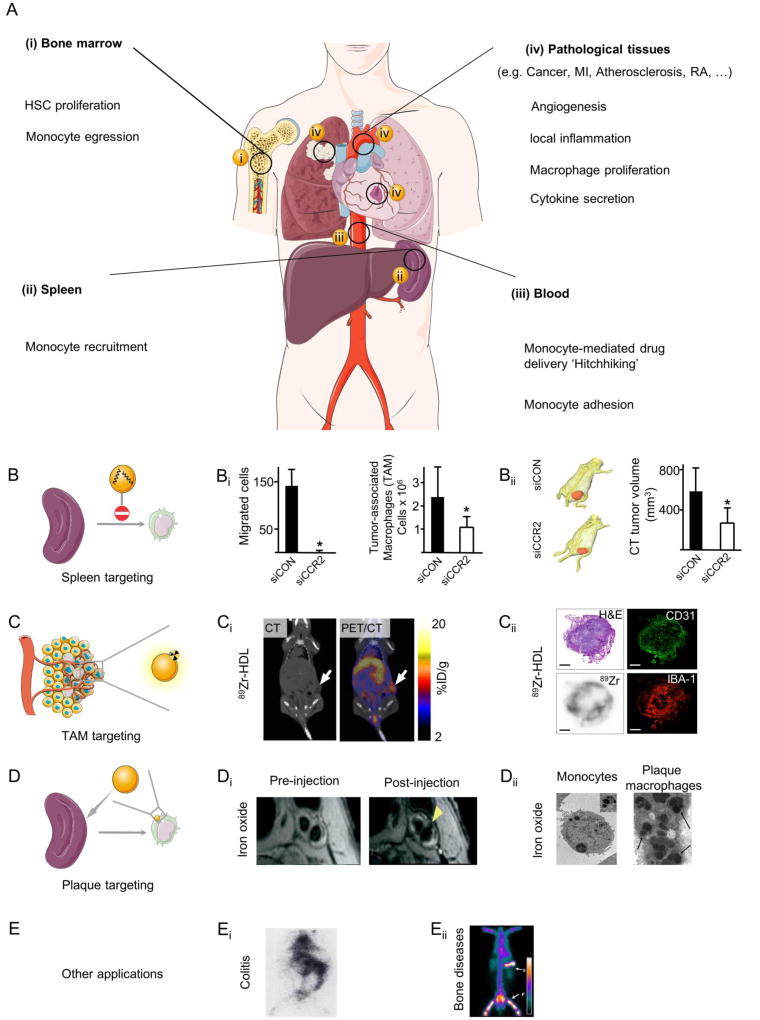
Fig. 1. Applying nanomedicine along the journey of monocytes in inflammatory disorders.
(A) Several processes that contribute to monocyte/macrophage dynamics in maladaptive inflammation and angiogenesis can be exploited for imaging and therapeutic purposes at the systems level. In A (i) and (ii), tackling hematopoietic stem cell (HSC) proliferation and monocyte migration from the bone marrow and spleen by nanomedicines can be an upstream approach to control inflammation. In A (iii), trafficking of inflammatory monocytes in the circulation and their adhesion to activated endothelial cells can be exploited for cell-mediated therapies and diagnosis. A (iv) Applying nanomedicines at the lesion level can be realized by tackling the enhanced vascular permeability, local macrophage proliferation and activity, and the secretion of proteases or cytokines. Examples of nanomedicines targeting (B) the spleen, (C) tumor-associated macrophages, (D) monocyte migration, and (E) other diseased tissues. Graphs and images in Bi and Bii are adapted, with permission, from [135]. PET/CT in Ci and histological images in Cii are reproduced, with permission, from[136]. MRI images in Di were adapted, with permission, from [137] while histological images in Dii are adapted, with permission, from [138] (left) and [139] (right). Scintigraphic images of rabbits in Ei and Eii are reproduced, with permission, from [140] and [141], respectively. MI: myocardial infarction, RA: rheumatoid arthritis, TAM: tumor-associated macrophages.
3.1 Bone marrow activation
Under homeostatic conditions, the bone marrow contains self-renewing, quiescent hematopoietic stem cells (HSCs), which maintain the blood levels of monocytes and other immune cells within normal physiological values. However, chronic inflammatory diseases are associated with elevated systemic levels of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α [142–144], and TLR agonists, like heat-shock proteins, and saturated and unsaturated fatty acids[145]. Furthermore, stress, which results in increased sympathetic activity and release of noradrenaline, is often associated with chronic inflammatory diseases [146–148]. Such biochemical changes can lead to HSC proliferation and overproduction of inflammatory monocytes, which ultimately accumulate in lesions[146,149]. The mobilization/egression of inflammatory monocytes from the bone marrow is enhanced by the CCL-2/CCR-2 interaction and the increased permeability of blood vessels in the bone marrow[150]. Moreover, bone marrow components and inflammation contribute to the local angiogenic processes. A classic example is bone marrow angiogenesis in patients with multiple myeloma[151] and breast cancer [152]. Metastatic breast cancer and myeloma plasma cells induce activation of inflammatory cells, including macrophages, to secrete angiogenic factors such as VEGF, fibroblast growth factor-2, and granulocyte macrophage-colony stimulating factor[153], which activate endothelial cells and accelerate angiogenesis in the bone marrow. Moreover, bone marrow macrophages, under the influence of fibroblast- and plasma cells- secreted factors, can acquire endothelial cell markers and transform into cells that are functionally and phenotypically similar to bone marrow endothelial cells [154]. Thus, they participate in the development of the bone marrow microvascular system (i.e. vasculogenic mimicry). Bone marrow also is the main source of endothelial progenitor cells (EPCs) and other myeloid cells which contribute to the angiogenic processes at distant inflammatory lesions[155].
Nanomedicine’s application at different stages of bone marrow activation holds promise for the diagnosis and treatment of inflammatory diseases (Fig. 2). Nanoparticle delivery of antiinflammatory drugs (e.g. corticosteroids)[156] or CCR-2 siRNA [135] can modulate the bone marrow response to circulating proinflammatory cytokines. The enhanced permeability of bone marrow blood vessels and the increased nanoparticle uptake by activated macrophages can facilitate their accumulation and retention[157–159]. Nanoparticles, including liposomes and reconstituted high-density lipoprotein (rHDL), have been shown to accumulate in the bone marrow of animals with inflammatory conditions, such as osteomyelitis[160] and atherosclerosis[161], to a greater extent than in non-diseased counterparts. Moreover, nanoparticle accumulation in the bone marrow can be further enhanced through active targeting. For example, conjugation of bisphosphonates (or alendronate) to poly(lactic-co-glycolic acid) (PLGA) polymeric nanoparticles increases their affinity to hydroxyapatite, a major mineral component of the bone[162,163]. Alternatively, incorporation of certain synthetic substances has been shown to relatively reduce nanoparticle accumulation in the liver while, concomitantly, increase their accumulation in the bone. Two of such materials are the hydrophilic non-ionic surfactant poloxamer 407 and the anionic amphiphilic lipid L-glutamic acid N-(3-carboxy-1-oxopropyl)-1,5-dihexadecyl ester which have been used as integral components of bone marrow-targeted nanospheres[164] and liposomes[141], respectively. The use of bone marrow-targeted nanomedicines can improve the therapeutic efficacy of small molecules, such as bortezomib in myeloma[162]. Tackling the hyper-proliferative state and/or the egression of HSCs and inflammatory monocytes has been shown to be an upstream approach for managing inflammatory diseases[135]. In addition, combining bone marrow-targeted nanomedicines with molecular imaging can be used to assess the effect of this therapeutic approach on bone marrow activation. For instance, the proliferation of bone marrow macrophages and HSCs can be quantified noninvasively with molecular imaging using 18F-3'-fluoro-3'-deoxythymidine (18F-FLT) positron emission tomography–computed tomography (PET/CT)[165–167]. Furthermore, their activation can be evaluated by 18F-fluorodeoxyglucose (18F-FDG) PET/CT[168].
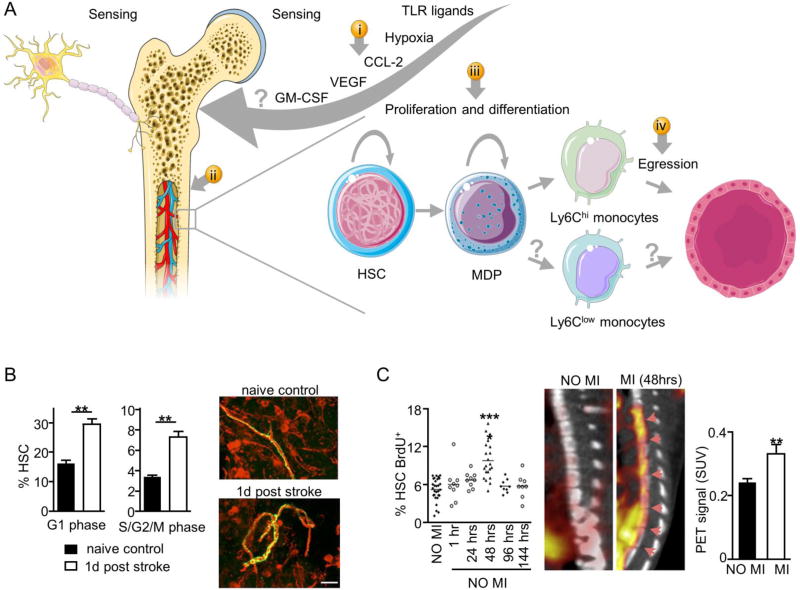
Fig. 2. Bone marrow activation.
Inflammatory disorders are characterized by elevated levels of circulating cytokines, growth factors, and damage-associated molecules. The stress and pain associated with the disease increase sympathetic nervous activity. Both biochemical and neuronal changes increase proliferation and migration of both HSCs and inflammatory monocytes (Ly6Chi) from bone marrow niches. (A) Nanomedicine can be used to target different features of bone marrow activation, including (i) circulating bone marrow activators, (ii) bone marrow permeability, (iii) HSC proliferation, and (iv) monocyte egress. Combining nanomedicines with molecular imaging at the medullar level can advance our understanding of disease progress. For example, (B) ischemic stroke increases the sympathetic nervous activity, which regulates the proliferation and cell cycle of HSCs, as shown by immunofluorescence staining of tyrosine hydroxylase rich nerve fibers of the sternal bone marrow. (C) Myocardial infarction (MI) increases HSC proliferation in the bone marrow, a process that can be quantified by BrdU staining, and imaged by 18F-FLT positron emission tomography/computed tomography (PET/CT). Panel B is modified, with permission, from[169]. Panel C is modified, with permission, from [170]. TLR: toll-like receptor, CCL-2: C-C motif chemokine 2, VEGF: vascular endothelial growth factor, GM-CSF: granulocyte-macrophage colony-stimulating factor, HSC: hematopoietic stem cell, MDP: monocyte and dendritic cell progenitor, SUV: standardized uptake value, BrdU: bromodeoxyuridine.
3.2 Monocyte mobilization and recruitment
In the course of chronic inflammation, recruitment and trafficking of inflammatory monocytes are driven by a gradient of circulating chemokines. Although bone marrow is the major source of blood monocytes, extramedullar hematopoiesis (i.e. outside the bone marrow), especially in the spleen, is also driven by inflammation[170,171] (Fig. 3). After fetal development, splenic hematopoiesis remains dormant, and the spleen acts as a reservoir seeded with HSCs mobilized from the bone marrow. However, these spleen-resident HSCs are highly responsive, and they regain their proliferative capacity upon exposure to hormonal and inflammatory cues[170,172,173]. For example, inflammatory conditions, such as atherosclerosis-triggered ischemic events, can activate splenic hematopoiesis and result in overproduction and deployment of splenic inflammatory monocytes into the circulation[172]. Hence, imaging splenic activity in such pathological conditions, for example by 18F-FDG-PET, can be useful to monitor therapeutic response and has been shown to serve as a good predictor of future complications by Tawakol and colleagues[174]. The accelerated hematopoiesis and rapid egress of inflammatory monocytes from splenic niches rely in part on CCL-2/CCR-2 interaction and angiotensin II (Ang II)-Ang type I and II receptors[14,175,176]. Applying nanomedicine to target splenic inflammatory monocytes is compelling due to the high accumulation of systemically administered nanoparticles in the spleen[177]. Leuschner et al. have used CCR-2 siRNA-loaded nanoparticles to selectively knock down the expression of CCR-2 in splenic inflammatory monocytes without affecting the patrolling, non-inflammatory subset[135]. Such a treatment led to a monocyte reduction in lesions and was shown a viable therapeutic approach in multiple conditions such as acute myocardial infarction (AMI), pancreatic islet transplantation, and cancer[135]. Similarly, irbesartan-loaded PLGA nanoparticles have been used to block Ang II type 1 receptor, inhibiting the recruitment of inflammatory monocytes and reducing infarct size in a myocardial ischemia-reperfusion (IR) injury model[178].
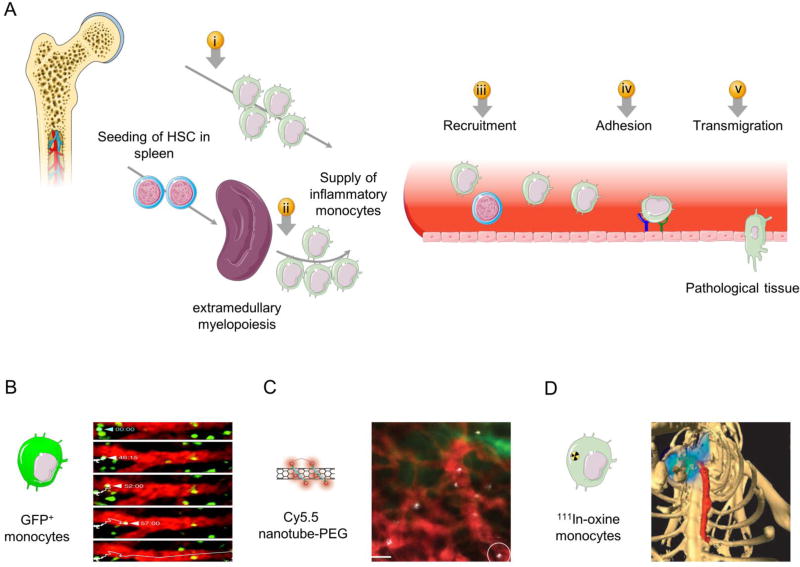
Fig. 3. Monocyte mobilization and recruitment.
(A) In response to inflammation, the spleen, in addition to the bone marrow, overproduces monocytes that enter the circulation. The inflammatory monocytes are guided by a gradient of chemokines in their journey to the inflamed lesions. The adhesion and the preferential accumulation of monocytes in lesions are driven by overexpression of certain receptors by the inflamed endothelium, including vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1). Nanomedicines can be exploited for imaging and therapeutic purposes at different stages of the monocyte journey in the circulation, starting from (i) monocyte egress from bone marrow, (ii) monocyte production and release from the spleen, (iii) monocyte trafficking in the blood stream, (iv) adhesion of monocytes to the inflamed endothelium, and (v) monocyte accumulation in the lesions. (B) Ischemic myocardial injury induces rapid deployment of splenic monocytes, which can be imaged by intravital microscopy of green fluorescent protein (GFP+) monocytes. (C) The preferential uptake of nanoparticles by Ly6Chi monocytes allows studying both monocyte and nanoparticle trafficking in the circulation using intravital microscopy. (D) Labeling monocytes with a radiotracer (e.g. [111Indium] oxyquinoline, 111In-oxine) enables noninvasive tracking and visualization of monocyte accumulation in atherosclerotic plaques and other inflammatory lesions using single photon emission/computed tomography (SPECT/CT). Panel B is adapted, with permission, from [175]. Panel C is reproduced, with permission, from[179]. Panel D is adapted, with permission, from[180].
Noninvasive monitoring of the journey of inflammatory monocytes through the circulation could provide insights into their homing, tissue adhesion, and tissue penetration characteristics. Such understanding would not only allow evaluating disease progression and response to treatment but could also help to design immunomodulatory therapeutics which have monocyte-like features to increase their tissue/lesion penetration. Several imaging techniques combined with or without nanomedicines have been used to monitor monocytes on the move. For example, intravital microscopy (IVM) can be used to detect fluorescent cells (e.g. GFP+ monocytes) in live organisms and to study their behavior in the circulation, in hematopoietic tissues, and in healthy and diseased tissues with a single-cell resolution[175,181–183]. Moreover, the exclusive uptake of certain fluorescently labeled nanoparticles, such as single-walled carbon nanotube (SWNT), by inflammatory monocytes allows studying both nanoparticle and monocyte trafficking in tumor models using IVM[179]. Alternatively, monocyte dynamics can be studied in deeper tissues with excellent spatial resolution using magnetic resonance imaging (MRI). Contrast MRI uses exogenous cell labels, such as superparamagnetic iron oxide (SPIOs) nanoparticles or Fluorine-19 (19F)-based perfluorocarbon (PFC) nanoemulsions[184]. Iron oxide nanoparticles have already been used to image monocyte dynamics in a rodent model of glioblastoma[185] and in patients with myocardial infarction[186]. Alternatively, 19F-MRI allows direct detection and imaging of labeled cells for unambiguous identification and quantification unlike iron oxide-based MRI [187]. Therefore, the detected signal can be derived directly from the injected PFC nanoemulsion engulfed by monocytes, for example after myocardial infarction[188].
Nuclear imaging with PET or single-photon-emission-computed-tomography (SPECT), which are highly sensitive and quantitative methods, could be another method to evaluate monocyte trafficking in vivo. SPECT with Technetium-99m tagged autologous monocytes –radiolabeled ex vivo and reinjected in the same patients– has been used to visualize the continuous migration of monocytes into the inflamed synovial tissue of RA patients[189]. The same approach has been used to monitor monocyte recruitment in live atherosclerotic apolipoprotein E knockout (Apoe−/−) mice for up to 7 days with Indium-111 (111In)-tagged monocytes and micro-SPECT/CT [180]. Moreover, the use of Zirconium-89 (89Zr)-feraheme nanoparticles, which are specifically taken up by blood monocytes, can enable studying monocyte dynamics by PET[190].
The homing features of circulating monocytes can be exploited to advance targeted therapeutics and diagnostic agents in inflammatory diseases. Such an approach has been realized by designing monocyte-mimicking nanoparticles as drug delivery systems to prolong their circulation times and achieve specific tissue targeting. Nanoporous silicon nanoparticles coated with leukocyte membranes possess cell-like functions and exert their targeting through receptor-ligand interaction, evading opsonization and clearance by the immune system[191]. In a similar approach, Cao et al. have prepared camouflaged liposomes with isolated macrophage membranes, which homed to lung metastasis and improved the delivery of an anti-cancer drug[192]. Furthermore, incorporating lipid and protein components extracted from leukocytes into a liposomal formulation has been shown to improve their targeting to inflamed vasculature, enabling selective and effective delivery of dexamethasone[193].
An alternative approach is hitchhiking/backpacking with monocytes, which can be achieved by designing polymeric nanoparticles that strongly attach to the surface of monocytes[194]. These cellular backpacks were shown to evade monocyte phagocytosis (due to size, disk-like shape, and flexibility) without affecting monocytes’ ability to target inflamed tissues in vivo[195,196].
The interactions between circulating inflammatory monocytes and the adhesion molecules expressed on the activated endothelium are inherently linked to lesion inflammation, and, therefore, also represent a potential therapeutic target. Knocking down multiple cell adhesion molecules using targeted delivery of siRNA-loaded nanoparticles has been shown to decrease monocyte recruitment in atherosclerotic plaques and ischemic myocardium, thereby reducing inflammation of infarcted myocardium and improving recovery after ischemia in mice [197]. Additionally, targeting adhesion molecules, like VCAM-1, P-selectin, or ICAM-1, with nanoparticles can be used for molecular imaging of the activated endothelium in atherosclerosis[198,199], cancer[200], and arthritis[201]. For example, echogenic nanoparticles can be applied for molecular ultrasound imaging of vascular markers in inflammatory lesions. Lee et al. demonstrated a positive correlation between in vivo ultrasound imaging of VEGF receptor-2 (VEGFR-2)-targeted microbubbles and the VEGFR-2 expression on endothelial cells of breast cancer tumors[202]. Furthermore, echogenic immunoliposomes targeting ICAM-1, VCAM-1, and tissue factor have been applied for atherosclerosis imaging [203].
3.3 Enhanced microvascular permeability
Local macrophage activity and lesion growth (e.g. tumors, atherosclerotic plaque, or RA pannus) drive the generation of blood vessels to meet the increased demand for oxygen and nutrients. Angiogenic neovessels in chronic inflammation are phenotypically heterogeneous and characterized by excessive branching, chaotic patterns and enhanced permeability to macromolecules and nanomedicines[45]. Therefore, applying nanomedicine to target the processes governing angiogenesis is an attractive approach to delivering therapeutic and imaging agents[204]. The formation of neovessels is intricately controlled by angiogenic factors, including VEGFs and VEGFRs (Fig. 4). Inhibition of angiogenesis using polymeric nanoparticles loaded with angiogenesis inhibitors such as the fumagillin analog TNP-470 has been shown to 1) suppress tumor growth in melanoma [205], Lewis lung carcinoma[205], and ovarian cancer mouse models[206]; 2) reduce plaque angiogenesis and inhibit advanced atherosclerosis in Apoe−/− mice[207,208]; and 3) suppress arthritis and protect from bone destruction in mice[209]. Alternatively, inhibiting the secretion of proinflammatory cytokines and proteases, which affect the proliferation and migration behavior of the preexisting endothelial cells, can be a complementary strategy to direct anti-angiogenic therapies. For example, liposomal delivery of anti-inflammatory compounds such as glucocorticoids has been shown to suppress tumor growth in mice, partially through inhibition of tumor angiogenesis[210].
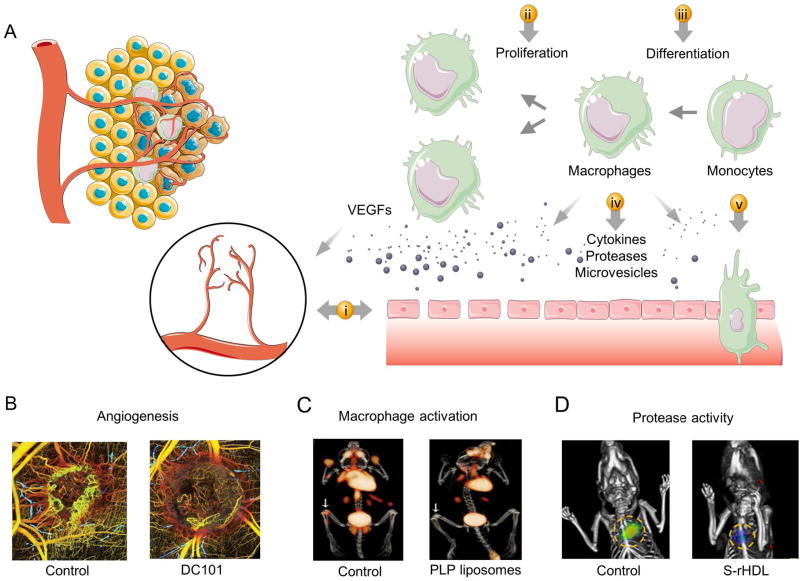
Fig. 4. Enhanced microvascular permeability and local inflammation.
(A) Inflammatory monocytes accumulate in inflamed lesions and differentiate into tissue-resident macrophages. A cascade of events ensues such as local macrophage proliferation, the release of proinflammatory cytokines, proteases and cellular vesicles, which can aggravate the inflammatory condition and recruit more inflammatory cells. In addition, the release of vascular endothelial growth factors and other cytokines induce angiogenesis and increase microvascular permeability to macromolecules. Diagnostic and therapeutic nanoparticles can be used to tackle (i) pathological angiogenesis and enhanced permeability, (ii) local cell proliferation, (iii) monocyte differentiation, (iv) cytokine and chemokine release (v) and monocyte infiltration. (B) Cancer-associated angiogenesis and response to anti-angiogenic therapies can be monitored in vivo using optical frequency domain imaging. (C) Anti-inflammatory effect of prednisolone phosphate (PLP) liposomes in rheumatoid arthritis can be noninvasively assessed by 18F-FDG PET/CT. (D) Protease activity in response to simvastatin rHDL nanoparticles can be monitored in vivo using fluorescence molecular tomography/computed tomography (FMT/CT). Panel B is adapted, with permission, from[211]. Panel C is reproduced, with permission, from[212]. Panel D is adapted, with permission, from[213].
Angiogenic neovessels are also characterized by overexpression of certain receptors like αvβ3 integrin adhesion receptors compared to quiescent vessels[214–217]. Attaching ligands (e.g. antibodies, nanobodies, and peptides) to the nanoparticles can allow better distribution in the vascular bed of the lesion and increase their internalization by target endothelial cells[218,219]. For example, αvβ3 integrin-targeted delivery of an anti-angiogenic gene has been shown to selectively increase apoptosis of tumor endothelial cells, which subsequently led to tumor cell apoptosis and regression of primary and metastatic tumors[220]. Additionally, αvβ3 integrin-targeted ligands and nanoparticles have been used to image angiogenesis in cancer[221–223], atherosclerosis[224,225], and arthritis[226], using different imaging modalities such as ultrasound, MRI, PET, and SPECT. These αvβ3-targeted imaging strategies can also be used to monitor the therapeutic response[207,208].
3.4 The proinflammatory milieu and local cell proliferation
Tackling the adhesion of circulating monocytes to the lesion vasculature can yield favorable therapeutic outcomes in chronic inflammatory diseases as mentioned before. However, once monocytes adhere, they infiltrate into the lesions and differentiate into macrophages, which possess an inflammatory phenotype and secrete large amounts of proteases, inflammatory cytokines, reactive radicals, and auto- and paracrine signaling molecules [227–229]. These “flaring” molecules drive lesion remodeling, invasiveness (and metastasis in the case of cancer), and can induce a phenotypical change in preexisting macrophages and other cells[230–232]. Thus, preventing macrophage activation and dampening local inflammation by using nanomedicines to deliver anti-inflammatory drugs can induce a favorable phenotype[233]. Glucocorticoids are potent anti-inflammatory drugs, which, however, generally come with severe side effects upon prolonged use[234]. Alternatively, glucocorticoid nanomedicines have been shown to silence local inflammation and halt the progression of several chronic inflammatorydiseases, including atherosclerosis[235], cancer[236], and RA[212,237] while minimizing side effects. Another approach is to activate inflammation-resolution pathways through delivering proresolving mediators to lesions (e.g. annexin A1 and resolvin D1). Such an approach can result not only in anti-inflammatory effects but also in tissue repair and restoration of homeostatic conditions[238]. Nanoparticle delivery of an annexin A1-mimicking peptide to atherosclerotic lesions results in a marked improvement in key advanced plaque features, including an increase in the protective collagen layer, a decrease in protease activity, suppression of oxidative stress, and reduced plaque necrosis[239].
Although monocyte recruitment is a key process driving the settlement of lesion-associated macrophages, the expansion of the macrophage population can also be driven by local proliferation[240–243]. Tackling monocyte recruitment alone may not be sufficient to reduce local inflammation, especially in advanced stages[244–246]. Consequently, restricting the expansion of the macrophage population can reduce the burden of local inflammation. For example, depletion of TAMs using clodronate liposomes slows down tumor growth in vivo and increases the efficacy of other anti-cancer drugs[247,248]. Additionally, clodronate liposomes have been used to deplete synovial macrophages, which resulted in reduced inflammation and prevented joint destruction[249,250]. Furthermore, simvastatin reconstituted HDL nanoparticles have been applied to reduce atherosclerotic plaque inflammation[213] by inhibiting local macrophage proliferation[251] in Apoe−/− mice with advanced atherosclerotic plaques. Methotrexate, a folate inhibitor with anti-proliferative activities, conjugated to dendrimer nanoparticles has been shown to reduce arthritis-induced inflammatory parameters such as ankle swelling, paw volume, cartilage damage, and bone resorption in a rat model of collagen-induced arthritis[252]. The same group has also demonstrated that methotrexate nanoparticles improve the therapeutic response in an animal model of human epithelial cancer[253]. Of note, low-dose of methotrexate is currently being studied in the Cardiovascular Inflammation Reduction Trial (CIRT) to test if it can reduce vascular inflammation and decrease rates of myocardial infarction, stroke, and cardiovascular death (Clinical Trial Identifier: NCT01594333).
The increased macrophage activity drives changes in the inflammatory milieu, which include elevated protease activity, abnormal glucose metabolism, and decreased pH [227,254,255]. Such changes can be exploited to develop “smart” probes and nanomedicines that are responsive to the changes inherent to inflammatory lesions. An attractive approach is to design nanomedicines with bioactive domains that can be activated by the increased activity of proteases like caspases, MMPs, and proteinases[256–259]. For example, Boeneman et al. have developed caspase 3-sensitive quantum dot-fluorescent protein nanoparticles[260]. Similarly, cathepsin B-sensitive fluorogenic chitosan nanoparticles have been used to discriminate metastases in vivo in three metastatic mouse models[261]. Also, Ferber et al. have adopted a nanotheranostic approach by developing two cathepsin B-sensitive N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-based systems for noninvasive imaging of breast cancer progression and drug release[262]. MMP-sensitive dual 64Cu-labeled fluorescent chitosan nanoparticles have also been used to visualize tumors in vivo by PET and near-infrared fluorescence imaging[263]. Moreover, active targeting of MMP sensitive dendrimer nanoparticles designed for dual MRI/optical imaging improves MRI-guided clinical staging, presurgical planning, and intraoperative fluorescence-guided surgery[264]. In addition to the biochemical changes, lesion-associated macrophages overexpress certain receptors such as major histocompatibility complex class II (Class II MHC) and surface integrin CD11b, which can also be exploited for imaging purposes[265]. Rashidian et al. have developed nanobodies labeled with 18F targeting MHC II+ and CD11b+ cells, which allow specific, noninvasive imaging of immune responses by PET/CT, showing higher specificity over the clinically used 18F-FDG PET/CT[266]. Alternatively, we have developed 89Zr-labeled rHDL to image TAM by PET[136] specifically.
4. Lessons, challenges, and perspectives
Chronic inflammatory diseases and associated angiogenesis share common pathophysiological features in which monocytes and macrophages are key effector cells. Understanding and exploiting monocyte dynamics and functions can lead to the development of improved therapeutic and diagnostic agents that are clinically useful in multiple diseases and disease settings. Nanomedicines can be engineered, developed, and customized in numerous ways to tackle the different features of maladaptive inflammation and angiogenesis, for therapeutic and diagnostics endeavors. Additionally, due to monocytes and macrophages’ high uptake capacity, nanomedicines can also enable us to study these cells’ dynamics in disease and decipher different pathophysiological features.. Therefore, it stands out as a promising approach to modulate monocyte/macrophage function and to also visualize and quantitate their dynamics. However, to broaden our exploitation of nanomedicine and reinvigorate clinical translation, several challenges and opportunities need to be taken into consideration:
First, the application of nanomedicine, especially for drug delivery, needs to be rigorously assessed. Traditionally, nanomedicine’s main application was improving a drug’s therapeutic index by increasing the concentration in the target tissue, while simultaneously minimizing the off-site effects. Today, the community is concerned about the small quantities of injected nanomaterial that reaches target lesions (e.g. tumor) in patients, and if nanomedicines provide real benefits [267–269]. These debates are mainly fueled by limited clinical experience with nanomedicines and undermine a rational discourse.
Second, research groups are often inclined to apply their nanomedicine in a single disease, such as cancer, overlooking the potential application in other diseases. A holistic understanding of the pathophysiological processes in chronic inflammation would allow repurposing, reviving and reapplying nanomedicines across a range of maladies without the need to create novel nanomedicines. Patient/disease-centric approaches for applying nanomedicines are more valuable than material-based approaches[270,271].
Additionally, we have witnessed an expansion in the development of new materials for nanomedicine, but these are rarely fully evaluated under biologically relevant conditions. Implementing screening procedures to evaluate new and preexisting nanomaterials with regards to their compatibility with different drugs, their biological behavior and tissue/cell specificity and their toxicity profile is the key to developing clinically relevant nanotherapeutics and diagnostic agents[272–274].
Applying nanomedicines in maladaptive inflammation and angiogenesis can be more challenging due to the inherent heterogeneity of the disease and the dynamic changes in lesion phenotype over time. Furthermore, heterogeneity and plasticity are hallmarks of monocytes and macrophages[59,275]. Growing evidence demonstrates the need to liberate our research from the dichotomous concept of macrophage activation: classical vs. alternative, i.e. M1 and M2, respectively [276,277]. These facts have implications on how nanomedicines can be best applied. Firstly, lesion heterogeneity can be manifested in the remarkable heterogeneity of the enhanced permeability and retention (EPR) effect, which impacts nanomedicine accumulation and, hence, their efficacy. Implementation of screening methods to identify patients who can benefit from nanomedicine should be a key factor for further developments [278]. Secondly, developing nanomedicines based on the M1/M2 macrophage paradigm is a shorthand approach that will probably not lead to clinically viable products. Understanding the function, dynamics, and memory of monocytes and macrophages, in their systemic and local milieu, is a rather more enabling and clinically relevant strategy.
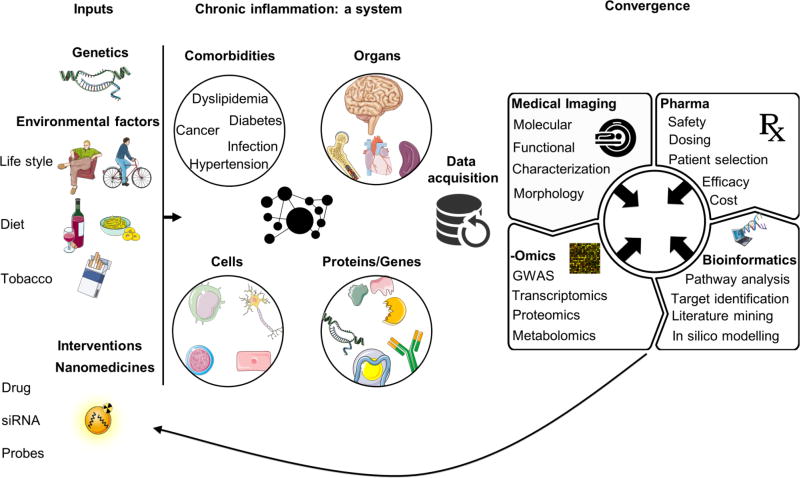
Given the impact of chronic inflammatory diseases, successful translation of nanomedicines into the clinic can be socioeconomically rewarding, yet challenging. Convergence of nanomedicine, life and physical sciences, bioinformatics, bioengineering, and pharma perspectives can help to reduce development costs, close the gap between preclinical research and clinical translation, and ultimately bring more efficacious nanomedicines to patients (Fig. 5).
Fig. 5. Considerations for applying and developing nanomedicines for chronic inflammatory diseases.
Chronic inflammatory diseases are multifactorial disorders in which genetic background and environmental factors interact and affect different dynamic systems, including genes, signaling pathways, cells, and organs. Nanomedicine should be approached in a holistic way, in which nanodrugs’ systemic interactions are investigated, and can be used to visualize and/or modulate multiple processes. Data acquisition and convergence of nanomedicine with the different biomedical fields and big data (e.g. transcriptomics, proteomics, and genomics) can not only contribute to deciphering these complex diseases but also help to predict the efficacy of nanomedicines and to develop clinically relevant products.
Acknowledgments
This work was supported by a European Framework Program 7 grant (FP7-Health 309820: Nano-Athero).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. doi:06-PLME-RA-0071R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs P, Bissonnette R, Guenther LC. Socioeconomic burden of immune-mediated inflammatory diseases--focusing on work productivity and disability. J. Rheumatol. 2011;88:55–61. doi: 10.3899/jrheum.110901. [DOI] [PubMed] [Google Scholar]
- 3.Strong K, Mathers C, Leeder S, Beaglehole R. Preventing chronic diseases: how many lives can we save? Lancet (London, England) 2005;366:1578–1582. doi: 10.1016/S0140-6736(05)67341-2. doi:S0140-6736(05)67341-2. [DOI] [PubMed] [Google Scholar]
- 4.Mitka M. New Basic Care Goals Seek to Rein In Global Rise in Cardiovascular Disease. JAMA. 2012;308:1725–1726. doi: 10.3201/eid1811.111850. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamburg MA, Collins FS. The path to personalized medicine. N. Engl. J. Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 7.Parkinson DR, Johnson BE, Sledge GW. Making personalized cancer medicine a reality: challenges and opportunities in the development of biomarkers and companion diagnostics. Clin. Cancer Res. 2012;18:619–624. doi: 10.1158/1078-0432.CCR-11-2017. [DOI] [PubMed] [Google Scholar]
- 8.Godman B, Finlayson AE, Cheema PK, Zebedin-Brandl E, Gutiérrez-Ibarluzea I, Jones J, Malmström RE, Asola E, Baumgärtel C, Bennie M, Bishop I, Bucsics A, Campbell S, Diogene E, Ferrario A, Fürst J, Garuoliene K, Gomes M, Harris K, Haycox A, Herholz H, Hviding K, Jan S, Kalaba M, Kvalheim C, Laius O, Lööv SA, Malinowska K, Martin A, McCullagh L, Nilsson F, Paterson K, Schwabe U, Selke G, Sermet C, Simoens S, Tomek D, Vlahovic-Palcevski V, Voncina L, Wladysiuk M, van Woerkom M, Wong-Rieger D, Zara C, Ali R, Gustafsson LL. Personalizing health care: feasibility and future implications. BMC Med. 2013;11:179. doi: 10.1186/1741-7015-11-179. doi:1741-7015-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Discov. 2007;6:273–286. doi: 10.1038/nrd2115. doi:nrd2115. [DOI] [PubMed] [Google Scholar]
- 10.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur. Heart J. 2015;36:482–9c. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 14.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittet MJ, Swirski FK. Monocytes link atherosclerosis and cancer. Eur. J. Immunol. 2011;41:2519–2522. doi: 10.1002/eji.201141727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbach EC, Plevy SE. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm. Bowel Dis. 2014;20:166–175. doi: 10.1097/MIB.0b013e3182a69dca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahrendorf M, Swirski FK. Immunology. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science. 2015;349:237–238. doi: 10.1126/science.aac7801. [DOI] [PubMed] [Google Scholar]
- 19.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 20.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR. TNFalpha blockade in human diseases: mechanisms and future directions. Clin. Immunol. 2008;126:121–136. doi: 10.1016/j.clim.2007.08.013. doi:S1521-6616(07)01327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R, Investigators O. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet (London, England) 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 22.Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009;1:539–547. doi: 10.4161/mabs.1.6.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BY, Rutka JT, Chan WC. Nanomedicine. N. Engl. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 24.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat. Mater. 2014;13:125–138. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 25.Mulder WJ, Jaffer FA, Fayad ZA, Nahrendorf M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci. Transl. Med. 2014;6:239sr1. doi: 10.1126/scitranslmed.3005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottesman MM, Lavi O, Hall MD, Gillet JP. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016;56:85–102. doi: 10.1146/annurev-pharmtox-010715-103111. [DOI] [PubMed] [Google Scholar]
- 27.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat. Rev. 2011;8:348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [Google Scholar]
- 29.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 2006;12(Suppl 1):S3–9. doi: 10.1097/01.mib.0000195385.19268.68. doi:00054725-200601001-00002. [DOI] [PubMed] [Google Scholar]
- 33.Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin. Immunopathol. 2009;31:5–22. doi: 10.1007/s00281-009-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. 2005;5:749–759. doi: 10.1038/nri1703. doi:nri1703. [DOI] [PubMed] [Google Scholar]
- 36.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. doi:354/6/610. [DOI] [PubMed] [Google Scholar]
- 37.Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 38.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. doi:S0092867402012011. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat. Rev. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J. Immunol. (Baltimore, Md. 1950) 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. doi:175/10/6257. [DOI] [PubMed] [Google Scholar]
- 42.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strehl C, Fangradt M, Fearon U, Gaber T, Buttgereit F, Veale DJ. Hypoxia: how does the monocyte-macrophage system respond to changes in oxygen availability? J. Leukoc. Biol. 2014;95:233–241. doi: 10.1189/jlb.1212627. [DOI] [PubMed] [Google Scholar]
- 44.Bates D, Harper S. Regulation of vascular permeability by vascular endothelial growth factors. Vascul. Pharmacol. 2002;39:225–237. doi: 10.1016/S1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 45.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claesson-Welsh L. Vascular permeability--the essentials. Ups. J. Med. Sci. 2015;120:135–43. doi: 10.3109/03009734.2015.1064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- 48.Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr. Drug targets.Inflammation Allergy. 2005;4:3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 49.Walsh DA, Pearson CI. Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. 2001;3:147–153. doi: 10.1186/ar292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England) 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. doi:S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 51.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 52.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat. Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 53.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franklin RA, Li MO. Ontogeny of Tumor-associated Macrophages and Its Implication in Cancer Regulation. Trends in Cancer. 2016;2:20–34. doi: 10.1016/j.trecan.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015;212:1043–1059. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697–28710. doi: 10.18632/oncotarget.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. doi:ncb1616. [DOI] [PubMed] [Google Scholar]
- 63.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 64.Roh-Johnson M, Bravo-Cordero J, Patsialou A, Sharma V, Guo P, Liu H, Hodgson L, Condeelis J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2013;33:4203–4212. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wyckoff JB, Wang Y, Lin EY, Li J-F, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct Visualization of Macrophage-Assisted Tumor Cell Intravasation in Mammary Tumors. Cancer Res. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 66.Friedl P, Wolf K. Tube travel: The role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 67.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guiet R, Van Goethem E, Cougoule C, Balor S, Valette A, Al Saati T, Lowell CA, Le Cabec V, Maridonneau-Parini I. The process of macrophage migration promotes matrix metalloproteinase-independent invasion by tumor cells. J. Immunol. (Baltimore, Md. 1950) 2011;187:3806–3814. doi: 10.4049/jimmunol.1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. 2004:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 70.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. doi:65/12/5278. [DOI] [PubMed] [Google Scholar]
- 71.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. doi:67/11/5064. [DOI] [PubMed] [Google Scholar]
- 72.Owen JL, Mohamadzadeh M. Macrophages and chemokines as mediators of angiogenesis. Front. Physiol. 2013;4:159. doi: 10.3389/fphys.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. doi:009.002.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCarthy N. Leaky effect. Nat. Rev. 2012;12:157. doi: 10.1038/nrc3227. [DOI] [PubMed] [Google Scholar]
- 75.Allam AH, Thompson RC, Wann LS, Miyamoto MI, Nur El-Din A-H, El-Maksoud GA, Al-Tohamy Soliman M, Badr I, El-Rahman Amer HA, Sutherland ML, Sutherland JD, Thomas GS. Atherosclerosis in ancient Egyptian mummies: the Horus study. JACC.Cardiovascular Imaging. 2011;4:315–327. doi: 10.1016/j.jcmg.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization. Noncommunicable diseases mortality and morbidity. 2015;2015 http://www.who.int/gho/ncd/mortality_morbidity/en/ [Google Scholar]
- 77.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ. J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. doi:JST.JSTAGE/circj/CJ-09-0706. [DOI] [PubMed] [Google Scholar]
- 78.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. doi:S0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 79.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 80.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, Lusis AJ, Rajavashisth TB. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am. J. Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 81.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. doi:116/16/1832. [DOI] [PubMed] [Google Scholar]
- 82.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 2009;104:210–8. doi: 10.1161/CIRCRESAHA.108.181040. 21p following 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 2000;105:1095–1108. doi: 10.1172/jci8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. doi:01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 85.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–1569. [PubMed] [Google Scholar]
- 88.Isner JM. Cancer and atherosclerosis: the broad mandate of angiogenesis. Circulation. 1999;99:1653–1655. doi: 10.1161/01.cir.99.13.1653. [DOI] [PubMed] [Google Scholar]
- 89.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. doi:113/18/2245. [DOI] [PubMed] [Google Scholar]
- 90.Hutter R, Speidl WS, Valdiviezo C, Sauter B, Corti R, Fuster V, Badimon JJ. Macrophages transmit potent proangiogenic effects of oxLDL in vitro and in vivo involving HIF-1alpha activation: a novel aspect of angiogenesis in atherosclerosis. J. Cardiovasc. Transl. Res. 2013;6:558–569. doi: 10.1007/s12265-013-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, Buchbinder R, Woolf A, March L. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 93.McInnes IB, O’Dell JR. State-of-the-art: rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:1898–1906. doi: 10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 94.Gierut A, Perlman H, Pope RM. Innate immunity and rheumatoid arthritis. Rheum. Dis. Clin. North Am. 2010;36:271–296. doi: 10.1016/j.rdc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takayanagi H. Osteoimmunology in 2014: Two-faced immunology-from osteogenesis to bone resorption. Nat. Rev. 2015;11:74–76. doi: 10.1038/nrrheum.2014.219. [DOI] [PubMed] [Google Scholar]
- 96.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 97.Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJM, Kraan MC, Baeten D, McInnes IB, Bresnihan B, Tak PP. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2005;64:834–838. doi: 10.1136/ard.2004.029751. doi:ard.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li JM, Isler P, Dayer JM, Burger D. Contact-dependent stimulation of monocytic cells and neutrophils by stimulated human T-cell clones. Immunology. 1995;84:571–576. [PMC free article] [PubMed] [Google Scholar]
- 99.Frucht DM, Aringer M, Galon J, Danning C, Brown M, Fan S, Centola M, Wu CY, Yamada N, El Gabalawy H, O’Shea JJ. Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J. Immunol. (Baltimore, Md. 1950) 2000;164:4659–4664. doi: 10.4049/jimmunol.164.9.4659. doi:ji_v164n9p4659. [DOI] [PubMed] [Google Scholar]
- 100.Roberts CA, Dickinson AK, Taams LS. The Interplay Between Monocytes/Macrophages and CD4(+) T Cell Subsets in Rheumatoid Arthritis. Front. Immunol. 2015;6:571. doi: 10.3389/fimmu.2015.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006;8:R187. doi: 10.1186/ar2099. doi:ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kinne RW, Stuhlmuller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res. Ther. 2007;9:224. doi: 10.1186/ar2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ishikawa H, Hirata S, Andoh Y, Kubo H, Nakagawa N, Nishibayashi Y, Mizuno K. An immunohistochemical and immunoelectron microscopic study of adhesion molecules in synovial pannus formation in rheumatoid arthritis. Rheumatol. Int. 1996;16:53–60. doi: 10.1007/BF01816436. [DOI] [PubMed] [Google Scholar]
- 104.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. 2007;7:429–442. doi: 10.1038/nri2094. doi:nri2094. [DOI] [PubMed] [Google Scholar]
- 105.Kasama T, Shiozawa F, Kobayashi K, Yajima N, Hanyuda M, Takeuchi HT, Mori Y, Negishi M, Ide H, Adachi M. Vascular endothelial growth factor expression by activated synovial leukocytes in rheumatoid arthritis: critical involvement of the interaction with synovial fibroblasts. Arthritis Rheum. 2001;44:2512–2524. doi: 10.1002/1529-0131(200111)44:11<2512::aid-art431>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 106.Paleolog EM. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):S81–90. doi: 10.1186/ar575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Charles JF, Hsu LY, Niemi EC, Weiss A, Aliprantis AO, Nakamura MC. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J. Clin. Invest. 2012;122:4592–4605. doi: 10.1172/JCI60920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adamopoulos IE, Mellins ED. Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat. Rev. 2015;11:189–194. doi: 10.1038/nrrheum.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. doi:nature06005. [DOI] [PubMed] [Google Scholar]
- 110.Yamamoto-Furusho JK, Podolsky DK. Innate immunity in inflammatory bowel disease. World J. Gastroenterol. 2007;13:5577–5580. doi: 10.3748/wjg.v13.i42.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 112.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 113.Neut C, Bulois P, Desreumaux P, Membre JM, Lederman E, Gambiez L, Cortot A, Quandalle P, van Kruiningen H, Colombel JF. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am. J. Gastroenterol. 2002;97:939–946. doi: 10.1111/j.1572-0241.2002.05613.x. [DOI] [PubMed] [Google Scholar]
- 114.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J. Immunol. (Baltimore, Md. 1950) 2002;168:5342–5351. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- 118.Neurath MF. Cytokines in inflammatory bowel disease. Nat. Rev. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 119.McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56:1333–1336. doi: 10.1136/gut.2006.115402. doi:gt115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Griga T, May B, Pfisterer O, Muller KM, Brasch F. Immunohistochemical localization of vascular endothelial growth factor in colonic mucosa of patients with inflammatory bowel disease. Hepatogastroenterology. 2002;49:116–123. [PubMed] [Google Scholar]
- 121.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- 123.Han C, Robinson DW, Jr, V Hackett M, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J. Rheumatol. 2006;33:2167–2172. doi: 06/13/0912. [PubMed] [Google Scholar]
- 124.Arvikar SL, Fisher MC. Inflammatory bowel disease associated arthropathy. Curr. Rev. Musculoskelet. Med. 2011;4:123–131. doi: 10.1007/s12178-011-9085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Whitlock MC, Yeboah J, Burke GL, Chen H, Klepin HD, Hundley WG. Cancer and Its Association With the Development of Coronary Artery Calcification: An Assessment From the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002533. 10.1161/JAHA.115.002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S, Balogh LP, Ballerini L, Bestetti A, Brendel C, Bosi S, Carril M, Chan WCW, Chen C, Chen X, Chen X, Cheng Z, Cui D, Du J, Dullin C, Escudero A, Feliu N, Gao M, George M, Gogotsi Y, Grünweller A, Gu Z, Halas NJ, Hampp N, Hartmann RK, Hersam MC, Hunziker P, Jian J, Jiang X, Jungebluth P, Kadhiresan P, Kataoka K, Khademhosseini A, Kopeček J, Kotov NA, Krug HF, Lee DS, Lehr C-M, Leong KW, Liang X-J, Ling Lim M, Liz-Marzán LM, Ma X, Macchiarini P, Meng H, Möhwald H, Mulvaney P, Nel AE, Nie S, Nordlander P, Okano T, Oliveira J, Park TH, Penner RM, Prato M, Puntes V, Rotello VM, Samarakoon A, Schaak RE, Shen Y, Sjöqvist S, Skirtach AG, Soliman MG, Stevens MM, Sung H-W, Tang BZ, Tietze R, Udugama BN, VanEpps JS, Weil T, Weiss PS, Willner I, Wu Y, Yang L, Yue Z, Zhang Q, Zhang Q, Zhang X-E, Zhao Y, Zhou X, Parak WJ. Diverse Applications of Nanomedicine. ACS Nano. 2017;11:2313–2381. doi: 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 128.Niu Z, Conejos-Sanchez I, Griffin BT, O’Driscoll CM, Alonso MJ. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016;106:337–354. doi: 10.1016/j.addr.2016.04.001. doi:S0169-409X(16)30100-4. [DOI] [PubMed] [Google Scholar]
- 129.Ofek P, Tiram G, Satchi-Fainaro R. Angiogenesis regulation by nanocarriers bearing RNA interference. Adv. Drug Deliv. Rev. 2017 doi: 10.1016/j.addr.2017.01.008. doi:S0169-409X(17)30027-3. [DOI] [PubMed] [Google Scholar]
- 130.Kipp JE. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 2004;284:109–122. doi: 10.1016/j.ijpharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 131.Gabizon A, Bradbury M, Prabhakar U, Zamboni W, Libutti S, Grodzinski P. Cancer nanomedicines: Closing the translational gap. Lancet. 2014;384:2175–2176. doi: 10.1016/S0140-6736(14)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ojha T, Rizzo L, Storm G, Kiessling F, Lammers T. Image-guided drug delivery: preclinical applications and clinical translation. Expert Opin. Drug Deliv. 2015;12:1203–7. doi: 10.1517/17425247.2015.1059420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chow EK, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci. Transl. Med. 2013;5:216rv4. doi: 10.1126/scitranslmed.3005872. [DOI] [PubMed] [Google Scholar]
- 134.Kiessling F, Mertens ME, Grimm J, Lammers T. Nanoparticles for Imaging: Top or Flop? Radiology. 2014;273:10–28. doi: 10.1148/radiol.14131520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pérez-Medina C, Tang J, Abdel-Atti D, Hogstad B, Merad M, Fisher EA, Fayad ZA, Lewis JS, Mulder WJM, Reiner T, Perez-Medina C, Tang J, Abdel-Atti D, Hogstad B, Merad M, Fisher EA, Fayad ZA, Lewis JS, Mulder WJM, Reiner T. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J. Nucl. Med. 2015;56:1272–1277. doi: 10.2967/jnumed.115.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, U-King-Im JM, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, Hayes PD, Varty K, Boyle JR, Gaunt ME, Zalewski A, Gillard JH. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 138.Alam SR, Stirrat C, Richards J, Mirsadraee S, Semple SI, Tse G, Henriksen P, Newby DE. Vascular and plaque imaging with ultrasmall superparamagnetic particles of iron oxide. J. Cardiovasc. Magn. Reson. 2015;17:83–84. doi: 10.1186/s12968-015-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 140.Brouwers AH, De Jong DJ, Dams ET, Oyen WJ, Boerman OC, Laverman P, Naber TH, Storm G, Corstens FH. Tc-99m-PEG-Liposomes for the evaluation of colitis in Crohn’s disease. J. Drug Target. 2000;8:225–233. doi: 10.3109/10611860008997901. [DOI] [PubMed] [Google Scholar]
- 141.Sou K, Goins B, Takeoka S, Tsuchida E, Phillips WT. Selective uptake of surface-modified phospholipid vesicles by bone marrow macrophages in vivo. Biomaterials. 2007;28:2655–2666. doi: 10.1016/j.biomaterials.2007.01.041. doi:S0142-9612(07)00127-5. [DOI] [PubMed] [Google Scholar]
- 142.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011;70(Suppl 1):i104–8. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 143.Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner J-MM, Will B, Nerlov C, Steidl U, Manz MG, Schroeder T, Passegue E, Passegué E. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 2016;18:607–618. doi: 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Strober W, Fuss IJ. Pro-Inflammatory Cytokines in the Pathogenesis of IBD. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zuany-Amorim C, Hastewell J, Walker C. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat. Rev. Discov. 2002;1:797–807. doi: 10.1038/nrd914. [DOI] [PubMed] [Google Scholar]
- 146.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–442. doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating Toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vacca A, Ribatti D. Bone marrow angiogenesis in multiple myeloma. Leukemia. 2006;20:193–199. doi: 10.1038/sj.leu.2404067. doi:2404067. [DOI] [PubMed] [Google Scholar]
- 152.Chavez-Macgregor M, Aviles-Salas A, Green D, Fuentes-Alburo A, Gomez-Ruiz C, Aguayo A. Angiogenesis in the bone marrow of patients with breast cancer. Clin. Cancer Res. 2005;11:5396–5400. doi: 10.1158/1078-0432.CCR-04-2420. doi: 11/15/5396. [DOI] [PubMed] [Google Scholar]
- 153.Ria R, Reale A, De Luisi A, Ferrucci A, Moschetta M, Vacca A. Bone marrow angiogenesis and progression in multiple myeloma. Am. J. Blood Res. 2011;1:76–89. [PMC free article] [PubMed] [Google Scholar]
- 154.Scavelli C, Nico B, Cirulli T, Ria R, Di Pietro G, Mangieri D, Bacigalupo A, Mangialardi G, Coluccia AM, Caravita T, Molica S, Ribatti D, Dammacco F, Vacca A. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27:663–674. doi: 10.1038/sj.onc.1210691. doi:1210691. [DOI] [PubMed] [Google Scholar]
- 155.Jain RK, Duda DG. Role of bone marrow-derived cells in tumor angiogenesis and treatment. Cancer Cell. 2003;3:515–516. doi: 10.1016/s1535-6108(03)00138-7. doi:S1535610803001387. [DOI] [PubMed] [Google Scholar]
- 156.Ozbakir B, Crielaard BJ, Metselaar JM, Storm G, Lammers T. Liposomal corticosteroids for the treatment of inflammatory disorders and cancer. J. Control. Release. 2014;190:624–636. doi: 10.1016/j.jconrel.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 157.Proulx ST, Kwok E, You Z, Papuga MO, Beck CA, Shealy DJ, Calvi LM, Ritchlin CT, Awad HA, Boyce BF, Xing L, Schwarz EM. Elucidating bone marrow edema and myelopoiesis in murine arthritis using contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2008;58:2019–29. doi: 10.1002/art.23546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Daldrup-Link HE, Link TM, Rummeny EJ, August C, Konemann S, Jurgens H, Heindel W. Assessing permeability alterations of the blood-bone marrow barrier due to total body irradiation: in vivo quantification with contrast enhanced magnetic resonance imaging. Bone Marrow Transplant. 2000;25:71–78. doi: 10.1038/sj.bmt.1702087. [DOI] [PubMed] [Google Scholar]
- 159.Ziadloo A, Burks SR, Gold EM, Lewis BK, Chaudhry A, Merino MJ, Frenkel V, Frank JA. Enhanced homing permeability and retention of bone marrow stromal cells by noninvasive pulsed focused ultrasound. Stem Cells. 2012;30:1216–1227. doi: 10.1002/stem.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Awasthi V, Goins B, Klipper R, Loredo R, Korvick D, Phillips WT. Imaging experimental osteomyelitis using radiolabeled liposomes. J. Nucl. Med. 1998;39:1089–1094. [PubMed] [Google Scholar]
- 161.Pérez-Medina C, Binderup T, Lobatto ME, Tang J, Calcagno C, Giesen L, Wessel CH, Witjes J, Ishino S, Baxter S, Zhao Y, Ramachandran S, Eldib M, Sánchez-Gaytán BL, Robson PM, Bini J, Granada JF, Fish KM, Stroes ESG, Duivenvoorden R, Tsimikas S, Lewis JS, Reiner T, Fuster V, Kjær A, Fisher EA, Fayad ZA, Mulder WJM. In Vivo PET Imaging of HDL in Multiple Atherosclerosis Models. JACC Cardiovasc. Imaging. 2016;9:950–961. doi: 10.1016/j.jcmg.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, Zhang S, Moschetta M, Seevaratnam D, Zhang Y, Liu J, Memarzadeh M, Wu J, Manier S, Shi J, Bertrand N, Lu ZN, Nagano K, Baron R, Sacco A, Roccaro AM, Farokhzad OC, Ghobrial IM. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10287–10292. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hengst V, Oussoren C, Kissel T, Storm G. Bone targeting potential of bisphosphonate-targeted liposomes. Preparation, characterization and hydroxyapatite binding in vitro. Int. J. Pharm. 2007;331:224–227. doi: 10.1016/j.ijpharm.2006.11.024. doi:S0378-5173(06)00981-1. [DOI] [PubMed] [Google Scholar]
- 164.Porter CJ, Moghimi SM, Illum L, Davis SS. The polyoxyethylene/polyoxypropylene block co-polymer poloxamer-407 selectively redirects intravenously injected microspheres to sinusoidal endothelial cells of rabbit bone marrow. FEBS Lett. 1992;305:62–66. doi: 10.1016/0014-5793(92)80655-z. doi: 0014-5793(92)80655-Z. [DOI] [PubMed] [Google Scholar]
- 165.Agool A, Slart RH, Thorp KK, Glaudemans AW, Cobben DC, Been LB, Burlage FR, Elsinga PH, Dierckx RA, Vellenga E, Holter JL. Effect of radiotherapy and chemotherapy on bone marrow activity: a 18F-FLT-PET study. Nucl. Med. Commun. 2011;32:17–22. doi: 10.1097/mnm.0b013e328340798c. [DOI] [PubMed] [Google Scholar]
- 166.Fuchs K, Kohlhofer U, Quintanilla-Martinez L, Lamparter D, Kotter I, Reischl G, Rocken M, Pichler BJ, Kneilling M. In vivo imaging of cell proliferation enables the detection of the extent of experimental rheumatoid arthritis by 3’-deoxy-3’-18f-fluorothymidine and small-animal PET. J. Nucl. Med. 2013;54:151–158. doi: 10.2967/jnumed.112.106740. [DOI] [PubMed] [Google Scholar]
- 167.Ye YX, Calcagno C, Binderup T, Courties G, Keliher EJ, Wojtkiewicz GR, Iwamoto Y, Tang J, Pérez-Medina C, Mani V, Ishino S, Johnbeck CB, Knigge U, Fayad ZA, Libby P, Weissleder R, Tawakol A, Dubey S, Belanger AP, Di Carli MF, Swirski FK, Kjaer A, Mulder WJM, Nahrendorf M. Imaging Macrophage and Hematopoietic Progenitor Proliferation in Atherosclerosis. Circ. Res. 2015;117:835–845. doi: 10.1161/CIRCRESAHA.115.307024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomograpic imaging. Circ. Imaging. 2014;7:454–460. doi: 10.1161/CIRCIMAGING.113.001093. [DOI] [PubMed] [Google Scholar]
- 169.Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ. Res. 2015;116:407–417. doi: 10.1161/CIRCRESAHA.116.305207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, Silberstein L, Heidt T, Sebas M, Sun Y, Wojtkiewicz G, Feruglio PF, King K, Baker JN, van der Laan AM, Borodovsky A, Fitzgerald K, Hulsmans M, Hoyer F, Iwamoto Y, Vinegoni C, Brown D, Di Carli M, Libby P, Hiebert SW, Scadden DT, Swirski FK, Weissleder R, Nahrendorf M. Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells. Cell Stem Cell. 2015;16:477–487. doi: 10.1016/j.stem.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dutta P, Nahrendorf M. Regulation and consequences of monocytosis. Immunol. Rev. 2014;262:167–178. doi: 10.1111/imr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary Hematopoiesis Generates Ly-6C(high) Monocytes that Infiltrate Atherosclerotic Lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, Kuswanto W, Rauch PJ, Chudnovskiy A, Iwamoto Y, Kohler R, Marinelli B, Gorbatov R, Wojtkiewicz G, Panizzi P, Mino-Kenudson M, Forghani R, Figueiredo JL, Chen JW, Xavier R, Swirski FK, Nahrendorf M, Weissleder R, Pittet MJ. Angiotensin II drives the production of tumor-promoting macrophages. Immunity. 2013;38:296–308. doi: 10.1016/j.immuni.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JHF, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong QA, Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A. Splenic Metabolic Activity Predicts Risk of Future Cardiovascular Events. JACC Cardiovasc. Imaging. 2015;8:121–130. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo J-LL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mellak S, Ait-Oufella H, Esposito B, Loyer X, Poirier M, Tedder TF, Tedgui A, Mallat Z, Potteaux S. Angiotensin II mobilizes spleen monocytes to promote the development of abdominal aortic aneurysm in Apoe2212;/2212; mice. Arterioscler. Thromb. Vasc. Biol. 2015;35:378–388. doi: 10.1161/ATVBAHA.114.304389. [DOI] [PubMed] [Google Scholar]
- 177.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakano Y, Matoba T, Tokutome M, Funamoto D, Katsuki S, Ikeda G, Nagaoka K, Ishikita A, Nakano K, Koga J, Sunagawa K, Egashira K. Nanoparticle-Mediated Delivery of Irbesartan Induces Cardioprotection from Myocardial Ischemia-Reperfusion Injury by Antagonizing Monocyte-Mediated Inflammation. Sci. Rep. 2016;6:29601. doi: 10.1038/srep29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smith BR, Ghosn EEB, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, Gambhir SS. Selective uptake of single walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 2014;9:481–487. doi: 10.1038/nnano.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kircher MF, Grimm J, Swirski FK, Libby P, Gerszten RE, Allport JR, Weissleder R. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008;117:388–395. doi: 10.1161/CIRCULATIONAHA.107.719765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Carlin LM, Auffray C, Geissmann F. Measuring intravascular migration of mouse Ly6C(low) monocytes in vivo using intravital microscopy. Curr. Protoc. Immunol. 2013;(Unit 14.33.1-16) doi: 10.1002/0471142735.im1433s101. Chapter 14. [DOI] [PubMed] [Google Scholar]
- 182.Rua R, McGavern DB. Elucidation of monocyte/macrophage dynamics and function by intravital imaging. J. Leukoc. Biol. 2015;98:319–332. doi: 10.1189/jlb.4RI0115-006RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McArdle S, Mikulski Z, Ley K. Live cell imaging to understand monocyte, macrophage, and dendritic cell function in atherosclerosis. J. Exp. Med. 2016;213:1117–1131. doi: 10.1084/jem.20151885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ahrens ET, Bulte JWM. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. 2013;13:755–763. doi: 10.1038/nri3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang R, Sarkar S, Korchinski DJ, Wu Y, Yong VW, Dunn JF. MRI monitoring of monocytes to detect immune stimulating treatment response in brain tumor. Neuro. Oncol. 2016 doi: 10.1093/neuonc/now180. doi:now180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Richards JM, Shaw CA, Lang NN, Williams MC, Semple SI, MacGillivray TJ, Gray C, Crawford JH, Alam SR, Atkinson AP, Forrest EK, Bienek C, Mills NL, Burdess A, Dhaliwal K, Simpson AJ, Wallace WA, Hill AT, Roddie PH, McKillop G, Connolly TA, Feuerstein GZ, Barclay GR, Turner ML, Newby DE. In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: feasibility and safety in humans. Circ. Imaging. 2012;5:509–517. doi: 10.1161/CIRCIMAGING.112.972596. [DOI] [PubMed] [Google Scholar]
- 187.Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011;24:114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bonner F, Merx MW, Klingel K, Begovatz P, Flogel U, Sager M, Temme S, Jacoby C, Salehi Ravesh M, Grapentin C, Schubert R, Bunke J, Roden M, Kelm M, Schrader J. Monocyte imaging after myocardial infarction with 19F MRI at 3 T:a pilot study in explanted porcine hearts. Eur. Heart J. Cardiovasc. Imaging. 2015;16:612–620. doi: 10.1093/ehjci/jev008. [DOI] [PubMed] [Google Scholar]
- 189.Thurlings RM, Wijbrandts CA, Bennink RJ, Dohmen SE, Voermans C, Wouters D, Izmailova ES, Gerlag DM, van Eck-Smit BLF, Tak PP. Monocyte Scintigraphy in Rheumatoid Arthritis: The Dynamics of Monocyte Migration in Immune-Mediated Inflammatory Disease. PLoS One. 2009;4:e7865. doi: 10.1371/journal.pone.0007865. doi:09-PONE-RA-09911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Normandin MD, Yuan H, Wilks MQ, Chen HH, Kinsella JM, Cho H, Guehl NJ, Absi-Halabi N, Hosseini SM, El Fakhri G, Sosnovik DE, Josephson L. Heat-Induced Radiolabeling of Nanoparticles for Monocyte Tracking by PET. Angew. Chemie (International Ed.in English) 2015;54:13002–13006. doi: 10.1002/anie.201505525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, Li Y. Liposomes Coated with Isolated Macrophage Membrane Can Target Lung Metastasis of Breast Cancer. ACS Nano. 2016;10:7738–7748. doi: 10.1021/acsnano.6b03148. [DOI] [PubMed] [Google Scholar]
- 193.Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, De Vita A, Toledano Furman NE, Wang X, Parodi A, Tasciotti E. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 2016;15:1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Polak R, Lim RM, Beppu MM, Pitombo RN, Cohen RE, Rubner MF. Liposome-Loaded Cell Backpacks. Adv. Healthc. Mater. 2015;4:2832–2841. doi: 10.1002/adhm.201500604. [DOI] [PubMed] [Google Scholar]
- 195.Anselmo AC, Kumar S, Gupta V, Pearce AM, Ragusa A, Muzykantov V, Mitragotri S. Exploiting shape, cellular-hitchhiking and antibodies to target nanoparticles to lung endothelium: Synergy between physical, chemical and biological approaches. Biomaterials. 2015;68:1–8. doi: 10.1016/j.biomaterials.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 196.Anselmo AC, Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J. Control. Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sager HB, Dutta P, Dahlman JE, Hulsmans M, Courties G, Sun Y, Heidt T, Vinegoni C, Borodovsky A, Fitzgerald K, Wojtkiewicz GR, Iwamoto Y, Tricot B, Khan OF, Kauffman KJ, Xing Y, Shaw TE, Libby P, Langer R, Weissleder R, Swirski FK, Anderson DG, Nahrendorf M. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med. 2016;8:342ra80. doi: 10.1126/scitranslmed.aaf1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur Muhlen C, Greaves DR, Neubauer S, Channon KM, Choudhury RP. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler. Thromb. Vasc. Biol. 2008;28:77–83. doi: 10.1161/ATVBAHA.107.145466. doi:ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ. Res. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. doi:01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 200.Gosk S, Moos T, Gottstein C, Bendas G. VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochim. Biophys. Acta. 2008;1778:854–863. doi: 10.1016/j.bbamem.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 201.Shao X, Zhang H, Rajian JR, Chamberland DL, Sherman PS, Quesada CA, Koch AE, Kotov NA, Wang X. 125I-labeled gold nanorods for targeted imaging of inflammation. ACS Nano. 2011;5:8967–8973. doi: 10.1021/nn203138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee DJ, Lyshchik A, Huamani J, Hallahan DE, Fleischer AC. Relationship between retention of a vascular endothelial growth factor receptor 2 (VEGFR2)-targeted ultrasonographic contrast agent and the level of VEGFR2 expression in an in vivo breast cancer model. J. Ultrasound Med. 2008;27:855–66. doi: 10.7863/jum.2008.27.6.855. [DOI] [PubMed] [Google Scholar]
- 203.Hamilton AJ, Huang S-L, Warnick D, Rabbat M, Kane B, Nagaraj A, Klegerman M, McPherson DD. Intravascular ultrasound molecular imaging of atheroma components in vivo. J. Am. Coll. Cardiol. 2004;43:453–460. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 204.Jarzyna PA, Deddens LH, Kann BH, Ramachandran S, Calcagno C, Chen W, Gianella A, Dijkhuizen RM, Griffioen AW, Fayad ZA, Mulder WJM. Tumor Angiogenesis Phenotyping by Nanoparticle-facilitated Magnetic Resonance and Near-infrared Fluorescence Molecular Imaging. Neoplasia. 2012;14:964–973. doi: 10.1593/neo.121148. doi:121148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Satchi-Fainaro R, Puder M, Davies JW, Tran HT, Sampson DA, Greene AK, Corfas G, Folkman J. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat. Med. 2004;10:255–261. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 206.Wang Y, Liu P, Duan Y, Yin X, Wang Q, Liu X, Wang X, Zhou J, Wang W, Qiu L, Di W. Specific cell targeting with APRPG conjugated PEG-PLGA nanoparticles for treating ovarian cancer. Biomaterials. 2014;35:983–992. doi: 10.1016/j.biomaterials.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 207.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, Zhang H, Wickline SA, Lanza GM. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. doi:01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 208.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC.Cardiovascular Imaging. 2008;1:624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou H, Chan HW, Wickline SA, Lanza GM, Pham CTN. α(v)Î2(3)-Targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 2009;23:2978–2985. doi: 10.1096/fj.09-129874. doi:09-129874 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Banciu M, Metselaar JM, Schiffelers RM, Storm G. Liposomal glucocorticoids as tumor-targeted anti-angiogenic nanomedicine in B16 melanoma-bearing mice. J. Steroid Biochem. Mol. Biol. 2008;111:101–110. doi: 10.1016/j.jsbmb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 211.Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009;15:1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.van der Geest T, Metselaar JM, Gerrits D, van Lent PL, Storm G, Laverman P, Boerman OC. (18)]F FDG PET/CT imaging to monitor the therapeutic effect of liposome-encapsulated prednisolone in experimental rheumatoid arthritis. J. Control. Release. 2015;209:20–26. doi: 10.1016/j.jconrel.2015.04.019. [doi] [DOI] [PubMed] [Google Scholar]
- 213.Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, Priem B, Kuan EL, Martel C, Hewing B, Sager H, Nahrendorf M, Randolph GJ, Stroes ESG, Fuster V, Fisher EA, Fayad ZA, Mulder WJM, Grundy SM, Libby P, Milonas C, Dutta P, Libby P, Ridker PM, Hansson GK, van der Wal AC, Becker AE, van der Loos CM, Das PK, Weber C, Noels H, Goldstein JL, Brown MS, Kwak B, Mulhaupt F, Myit S, Mach F, Weitz-Schmidt G, Pahan K, Sheikh FG, Namboodiri AM, Singh I, Sparrow CP, Armitage J, Bellosta S, Paoletti R, Corsini A, Bell FP, Skajaa T, Skajaa T, Osada J, Joven J, Maeda N, Quarfordt SH, Vyas KP, Kari PH, Prakash SR, Duggan DE, Rader D, Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N, Shah PK, Shah PK, Nissen SE, Tardif JC, Vaisar T, Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT, Liao JK, Jain MK, Ridker PM, Lobatto ME, Fuster V, Fayad ZA, Mulder WJM, Jonas ANA, Forte T, Carlucci G, Mazzeo P, Biordi L, Bologna M, Trogan E, Folch J, Lees M, Stanley GHS. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014;5:227–239. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Maile LA, Busby WH, Nichols TC, Bellinger DA, Merricks EP, Rowland M, Veluvolu U, Clemmons DR. A monoclonal antibody against alphaVbeta3 integrin inhibits development of atherosclerotic lesions in diabetic pigs. Sci. Transl. Med. 2010;2:18ra11. doi: 10.1126/scitranslmed.3000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dixon S, Bashutski J, Rütz M, Goldberg H, Sims S, Gramoun A, Shorey S, Manolson M, Heersche J, Zhang H, Suzich J, Mao S. The αvβ3 integrin as a therapeutic target for inhibition of bone resorption. Arthritis Res. Ther. 2004;6:23. doi:ar1358. [Google Scholar]
- 216.Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, Fiocchi C. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. doi:S0016-5085(06)00739-6. [DOI] [PubMed] [Google Scholar]
- 217.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. doi:bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. doi:66/13/6732. [DOI] [PubMed] [Google Scholar]
- 219.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. doi:0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 221.Montet X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging of integrins on tumor cells. Neoplasia. 2006;8:214–222. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mulder WJ, Castermans K, van Beijnum JR, Oude Egbrink MG, Chin PT, Fayad ZA, Lowik CW, Kaijzel EL, Que I, Storm G, Strijkers GJ, Griffioen AW, Nicolay K. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis. 2009;12:17–24. doi: 10.1007/s10456-008-9124-2. [DOI] [PubMed] [Google Scholar]
- 223.Anderson CR, Hu X, Zhang H, Tlaxca J, Decleves AE, Houghtaling R, Sharma K, Lawrence M, Ferrara KW, Rychak JJ. Ultrasound molecular imaging of tumor angiogenesis with an integrin targeted microbubble contrast agent. Invest. Radiol. 2011;46:215–224. doi: 10.1097/RLI.0b013e3182034fed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 225.Beer AJ, Pelisek J, Heider P, Saraste A, Reeps C, Metz S, Seidl S, Kessler H, Wester HJ, Eckstein HH, Schwaiger M. PET/CT imaging of integrin alphavbeta3 expression in human carotid atherosclerosis. JACC.Cardiovascular Imaging. 2014;7:178–187. doi: 10.1016/j.jcmg.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 226.Zheleznyak A, Wadas TJ, Sherman CD, Wilson JM, Kostenuik PJ, Weilbaecher KN, Anderson CJ. Integrin αvβ3 as a PET Imaging Biomarker for Osteoclast Number in Mouse Models of Negative and Positive Osteoclast Regulation. Mol. Imaging Biol. 2012;14:500–508. doi: 10.1007/s11307-011-0512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr. Opin. Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 228.Quillard T, Croce K, Jaffer FA, Weissleder R, Libby P. Molecular imaging of macrophage protease activity in cardiovascular inflammation in vivo. Thromb. Haemost. 2011;105:828–836. doi: 10.1160/TH10-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Park KY, Li G, Platt MO. Monocyte-derived macrophage assisted breast cancer cell invasion as a personalized, predictive metric to score metastatic risk. Sci. Rep. 2015;5:13855. doi: 10.1038/srep13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Verollet C, Charriere GM, Labrousse A, Cougoule C, Le Cabec V, Maridonneau-Parini I. Extracellular proteolysis in macrophage migration: losing grip for a breakthrough. Eur. J. Immunol. 2011;41:2805–2813. doi: 10.1002/eji.201141538. [DOI] [PubMed] [Google Scholar]
- 232.Caldwell AB, Cheng Z, Vargas JD, Birnbaum HA, Hoffmann A. Network dynamics determine the autocrine and paracrine signaling functions of TNF. Genes Dev. 2014;28:2120–2133. doi: 10.1101/gad.244749.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Crielaard BJ, Lammers T, Schiffelers RM, Storm G. Drug targeting systems for inflammatory disease: one for all, all for one. J. Control. Release. 2012;161:225–234. doi: 10.1016/j.jconrel.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 234.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. doi:S0303-7207(10)00210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lobatto ME, Fayad ZA, Silvera S, Vucic E, Calcagno C, Mani V, Dickson SD, Nicolay K, Banciu M, Schiffelers RM, Metselaar JM, van Bloois L, Wu H-SS, Fallon JT, Rudd JH, Fuster V, Fisher EA, Storm G, Mulder WJM, Wiener MA. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol. Pharm. 2010;7:2020–2029. doi: 10.1021/mp100309y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Deshantri AK, Kooijmans SA, Kuijpers SA, Coimbra M, Hoeppener A, Storm G, Fens MH, Schiffelers RM. Liposomal prednisolone inhibits tumor growth in a spontaneous mouse mammary carcinoma model. J. Control. Release. 2016;243:243–249. doi: 10.1016/j.jconrel.2016.10.016. doi:S0168-3659(16)30658-7. [DOI] [PubMed] [Google Scholar]
- 237.Quan L, Zhang Y, Crielaard BJ, Dusad A, Lele SM, Rijcken CJ, Metselaar JM, Kostkova H, Etrych T, Ulbrich K, Kiessling F, Mikuls TR, Hennink WE, Storm G, Lammers T, Wang D. Nanomedicines for inflammatory arthritis: head-to-head comparison of glucocorticoid-containing polymers, micelles, and liposomes. ACS Nano. 2014;8:458–466. doi: 10.1021/nn4048205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 2015;7:275ra20. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of Th2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, GarcÃ-a-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38 doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Swirski FK, Hilgendorf I, Robbins CS. From proliferation to proliferation: monocyte lineage comes full circle. Semin. Immunopathol. 2014;36:137–148. doi: 10.1007/s00281-013-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO, Lin A, Adeyanju OO, Li S, Gong C, McGrath M, Olopade OI, Esserman LJ. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res. Treat. 2011;128:703–711. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo J-LL, Gorbatov R, Sukhova GK, Gerhardt LMS, Smyth D, Zavitz CCJ, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lindau A, Härdtner C, Hergeth SP, Blanz KD, Dufner B, Hoppe N, Anto-Michel N, Kornemann J, Zou J, Gerhardt LMS, Heidt T, Willecke F, Geis S, Stachon P, Wolf D, Libby P, Swirski FK, Robbins CS, McPheat W, Hawley S, Braddock M, Gilsbach R, Hein L, von zur Mühlen C, Bode C, Zirlik A, Hilgendorf I, Hardtner C, Hergeth SP, Blanz KD, Dufner B, Hoppe N, Anto-Michel N, Kornemann J, Zou J, Gerhardt LMS, Heidt T, Willecke F, Geis S, Stachon P, Wolf D, Libby P, Swirski FK, Robbins CS, McPheat W, Hawley S, Braddock M, Gilsbach R, Hein L, von zur Muhlen C, Bode C, Zirlik A, Hilgendorf I. Atheroprotection through SYK inhibition fails in established disease when local macrophage proliferation dominates lesion progression. Basic Res. Cardiol. 2016;111 doi: 10.1007/s00395-016-0535-8. 20–016–0535–8. Epub 2016 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br. J. Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. doi:6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fritz JM, Tennis MA, Orlicky DJ, Yin H, Ju C, Redente EF, Choo KS, Staab TA, Bouchard RJ, Merrick DT, Malkinson AM, Dwyer-Nield LD. Depletion of Tumor-Associated Macrophages Slows the Growth of Chemically Induced Mouse Lung Adenocarcinomas. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00587. 10.3389/fimmu.2014.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Richards PJ, Williams AS, Goodfellow RM, Williams BD. Liposomal clodronate eliminates synovial macrophages, reduces inflammation and ameliorates joint destruction in antigen-induced arthritis. Rheumatology (Oxford) 1999;38:818–825. doi: 10.1093/rheumatology/38.9.818. [DOI] [PubMed] [Google Scholar]
- 250.Barrera P, Blom A, van Lent PL, van Bloois L, Beijnen JH, van Rooijen N, de Waal Malefijt MC, van de Putte LB, Storm G, van den Berg WB. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000;43:1951–1959. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. doi:10.1002/1529-0131(200009)43-9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 251.Tang J, Lobatto ME, Hassing L, van der Staay S, van Rijs SM, Calcagno C, Braza MS, Baxter S, Fay F, Sanchez-Gaytan BL, Duivenvoorden R, Sager HB, Astudillo YM, Leong W, Ramachandran S, Storm G, Perez-Medina C, Reiner T, Cormode DP, Strijkers GJ, Stroes ESG, Swirski FK, Nahrendorf M, Fisher EA, Fayad ZA, Mulder WJM. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015;1:e1400223. doi: 10.1126/sciadv.1400223. doi:e1400223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Thomas TP, Goonewardena SN, Majoros IJ, Kotlyar A, Cao Z, Leroueil PR, Baker JR., Jr Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 2011;63:2671–2680. doi: 10.1002/art.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR., Jr Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. doi:65/12/5317. [DOI] [PubMed] [Google Scholar]
- 254.Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B, Cherqui G, Baud L. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. Evidence for involvement of nuclear factor-kappaB activation. J. Biol. Chem. 1998;273:5086–5092. doi: 10.1074/jbc.273.9.5086. [DOI] [PubMed] [Google Scholar]
- 255.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, Weyand CM. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016;213:337–354. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Harris TJ, von Maltzahn G, Lord ME, Park JH, Agrawal A, Min DH, Sailor MJ, Bhatia SN. Protease-triggered unveiling of bioactive nanoparticles. Small. 2008;4:1307–1312. doi: 10.1002/smll.200701319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kisin-Finfer E, Ferber S, Blau R, Satchi-Fainaro R, Shabat D. Synthesis and evaluation of new NIR-fluorescent probes for cathepsin B: ICT versus FRET as a turn-ON mode-of-action. Bioorg. Med. Chem. Lett. 2014;24:2453–2458. doi: 10.1016/j.bmcl.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 258.Witney TH, Hoehne A, Reeves RE, Ilovich O, Namavari M, Shen B, Chin FT, Rao J, Gambhir SS. A Systematic Comparison of 18F-C-SNAT to Established Radiotracer Imaging Agents for the Detection of Tumor Response to Treatment. Clin. Cancer Res. 2015;21:3896–3905. doi: 10.1158/1078-0432.CCR-14-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gandhi S, Arami H, Krishnan KM. Detection of Cancer-Specific Proteases Using Magnetic Relaxation of Peptide-Conjugated Nanoparticles in Biological Environment. Nano Lett. 2016;16:3668–3674. doi: 10.1021/acs.nanolett.6b00867. [DOI] [PubMed] [Google Scholar]
- 260.Boeneman K, Mei BC, Dennis AM, Bao G, Deschamps JR, Mattoussi H, Medintz IL. Sensing caspase 3 activity with quantum dot-fluorescent protein assemblies. J. Am. Chem. Soc. 2009;131:3828–3829. doi: 10.1021/ja809721j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ryu JH, Na JH, Ko HK, You DG, Park S, Jun E, Yeom HJ, Seo DH, Park JH, Jeong SY, Kim IS, Kim BS, Kwon IC, Choi K, Kim K. Non-invasive optical imaging of cathepsin B with activatable fluorogenic nanoprobes in various metastatic models. Biomaterials. 2014;35:2302–2311. doi: 10.1016/j.biomaterials.2013.11.080. [DOI] [PubMed] [Google Scholar]
- 262.Ferber S, Baabur-Cohen H, Blau R, Epshtein Y, Kisin-Finfer E, Redy O, Shabat D, Satchi-Fainaro R. Polymeric nanotheranostics for real-time non-invasive optical imaging of breast cancer progression and drug release. Cancer Lett. 2014;352:81–89. doi: 10.1016/j.canlet.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 263.Lee S, Kang SW, Ryu JH, Na JH, Lee DE, Han SJ, Kang CM, Choe YS, Lee KC, Leary JF, Choi K, Lee KH, Kim K. Tumor-homing glycol chitosan-based optical/PET dual imaging nanoprobe for cancer diagnosis. Bioconjug. Chem. 2014;25:601–610. doi: 10.1021/bc500020g. [DOI] [PubMed] [Google Scholar]
- 264.Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat. Rev. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rashidian M, Keliher EJ, Bilate AM, Duarte JN, Wojtkiewicz GR, Jacobsen JT, Cragnolini J, Swee LK, Victora GD, Weissleder R, Ploegh HL. Noninvasive imaging of immune responses. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6146–6151. doi: 10.1073/pnas.1502609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 268.McNeil SE, Verwoert S, Charrois GJ, Allen TM. Evaluation of nanomedicines: stick to the basics. Nat. Rev. Mater. 2016;1:16073. doi: 10.1038/natrevmats.2016.73. [DOI] [Google Scholar]
- 269.Lammers T, Kiessling F, Ashford M, Hennink W, Crommelin D, Strom G. Cancer nanomedicine: is targeting our target? Nat. Rev. Mater. 2016;1:16069. doi: 10.1038/natrevmats.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat. Nanotechnol. 2008;3:242–244. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 271.van der Meel R, Lammers T, Hennink WE. Cancer nanomedicines: oversold or underappreciated? Expert Opin. Drug Deliv. 2017;14:1–5. doi: 10.1080/17425247.2017.1262346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhao Y, Fay F, Hak S, Manuel Perez-Aguilar J, Sanchez-Gaytan BL, Goode B, Duivenvoorden R, de Lange Davies C, Bjorkoy A, Weinstein H, Fayad ZA, Perez-Medina C, Mulder WJM, Bjørkøy A, Weinstein H, Fayad ZA, Pérez-Medina C, Mulder WJM. Augmenting drug-carrier compatibility improves tumour nanotherapy efficacy. Nat. Commun. 2016;7:11221. doi: 10.1038/ncomms11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tang J, Baxter S, Menon A, Alaarg A, Sanchez-Gaytan BL, Fay F, Zhao Y, Ouimet M, Braza MS, Longo VA, Abdel-Atti D, Duivenvoorden R, Calcagno C, Storm G, Tsimikas S, Moore KJ, Swirski FK, Nahrendorf M, Fisher EA, Pérez-Medina C, Fayad ZA, Reiner T, Mulder WJM. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl. Acad. Sci. U. S. A. 2016;113 doi: 10.1073/pnas.1609629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:10.12703/P6–13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ. Res. 2016;119:414–417. doi: 10.1161/CIRCRESAHA.116.309194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pérez-Medina C, Abdel-Atti D, Tang J, Zhao Y, Fayad ZA, Lewis JS, Mulder WJM, Reiner T, Perez-Medina C, Abdel-Atti D, Tang J, Zhao Y, Fayad ZA, Lewis JS, Mulder WJM, Reiner T. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat. Commun. 2016;7:11838. doi: 10.1038/ncomms11838. [DOI] [PMC free article] [PubMed] [Google Scholar]