Abstract
Aim:
The objective of the present study was to prepare a trivalent inactivated vaccine of Newcastle disease virus (NDV), H5N1, and H9N2 viruses.
Materials and Methods:
Three monovalent and a trivalent vaccines were prepared by emulsifying inactivated NDV (LaSota strain), reassortant H5N1, and H9N2 viruses with Montanide ISA 71 oil adjuvant. Parameters used for evaluation of the efficacy of the prepared vaccines in specific pathogen-free chickens were cellular immunity assays (blastogenesis, interferon gamma, interleukin 1 [IL1], and IL6), humoral immunity by hemagglutination inhibition, protection percentage, and shedding.
Results:
A single immunization with trivalent vaccine-enhanced cell-mediated immunity as well as humoral immune response with 90% protection against challenges with highly pathogenic avian influenza (HPAI) H5N1 and low pathogenic (LP) avian influenza H9N2 viruses with 100% protection after challenge with NDV.
Conclusion:
Development and evaluation of the trivalent vaccine in the study reported the success in preparation of a potent and efficacious trivalent vaccine which is a promising approach for controlling HPAI H5N1, LP H9N2, and ND viral infections.
Keywords: avian influenza, immunization, Newcastle disease virus, shedding, trivalent vaccine
Introduction
Avian influenza virus (AIV) belongs to the Orthomyxovirus family. AIV infections can cause various disease symptoms in chickens, ranging from asymptomatic infection to respiratory disease, accompanied with reduced egg production and/or severe systemic diseases with near 100% mortality rates. The severity of the disease in poultry is determined by genetic features where the infection is classified as either low pathogenic (LP) avian influenza (LPAI) or highly pathogenic avian influenza (HPAI) [1]. Evidence suggests that virus elimination in poultry is improbable in a few countries where the virus remains endemic. In these endemic countries such as Bangladesh, China, Egypt, India, Indonesia, and Vietnam, all accessible tools for prevention and control of the disease should be considered, including vaccination using suitable and quality biologicals [2]. Production of a safe and high-yield H5N1 vaccine strain is challenging for vaccine manufacturers; therefore, the reverse genetics system, which uses a high-growth backbone virus, offers a key for the generation of a high-yield, avirulent influenza vaccine strains for vaccination of poultry species [3,4]. It has been reported that as the quantity of AIV antigen in the vaccines increases, all parameters of protection improve, which is strain dependent [5].
AIV subtype H9N2 is categorized as LPAI virus (LPAIV), but it can cause serious economic losses in poultry industry including reduced egg production and decreased growth rate. Moreover, it can occasionally cross the species barrier and cause human infections, which has raised public health concerns. In February 2015, the first human case of H9N2 subtype virus infection in Egypt was reported [6]. This event compelled national and international authorities to examine the reasons behind the increase in human infections and implement control measures [6]. Coinfection of H9N2 with H5N1 was also reported in many cases in poultry in Egypt [7-10]. Although predictable, reassortment between H5N1 and H9N2 has not been yet reported [11]. H9N2 vaccination has been used to face the field outbreaks [12]. A bivalent mucosal inactivated H9N2 and Newcastle disease virus (NDV) have already been established in the country [13].
ND caused by Avian avulavirus 1 (avian paramyxovirus serotype-1) is considered as one of the most overwhelming poultry infections, owing to its worldwide distribution and economic implications. NDVs have been categorized into lentogenic, mesogenic, and velogenic strains according to disease severity in chickens [14]. Live vaccines based on the lentogenic LaSota or other lentogenic strains are routinely applied to chicken and have been proved to induce high levels of immunogenicity and protective efficacy against lethal velogenic strains [15,16]. Practically, using these, virus vaccines separately stressful for both the worker and the bird. Handling of laying birds usually results in decreased production, and sometimes, severe egg peritonitis may occur also, and the labor expense can be partly offset by the use of polyvalent vaccines. Inactivated oil-emulsion vaccines are not as badly affected by maternal immunity as live vaccines and can be used in day-old chicks. In this study, we developed a trivalent vaccine containing the inactivated NDV LaSota strain antigen, reassortant H5N1, and LPAI H9N2 virus antigens for vaccination in poultry. We also evaluated its immunogenicity and protective efficacy against lethal HPAI H5N1, virulent NDV virus infection, and LPAI H9N2 infection.
Materials and Methods
Ethical approval
Animal experiments were conducted in accordance with the guidelines laid down by the International Animal Ethics Committee and in accordance with the local laws and regulations.
Vaccine preparation
Viruses’ propagation and titration
Vaccine strains and seed viruses were propagated in specific pathogen-free (SPF) embryonated chicken egg (ECE) [17,18] for H9N2, reassortant H5N1 viruses [18], and for Lasota NDV [19]. The obtained harvest from each virus was titrated in SPF ECEs and calculated according to a method of Read and Muench [17].
Avian influenza (AI) H9N2 master seed virus
The LP (A/chicken/Egypt/114922v/2011 [H9N2]), with accession Number (JQ419502), virus was provided by the National Laboratory for Quality Control on Poultry Production, Animal Health Research Institute, Dokki, Egypt. The virus was used for preparation of the vaccine seed virus. The original titer of the virus was 109.5 egg infective dose (EID) 50/ml with hemagglutination (HA) activity of 10 Log2.
AI H5N1 master seed virus
Two reassortant AIVs (A/Chicken/Egypt/Q1995D/2010 [H5N1]) with a titer 1010 EID 50/ml and 10 Log2 HA activity and A/Duck/Egypt/M2583D/2010 (H5N1) of a titer 1011 EID 50/ml and 11 Log2 HA activity were used. These viruses were generated in the National Research Center, Giza, Egypt, and provided to the Veterinary Serum and Vaccine Research Institute, Newcastle Disease Unit, Abbasia, Cairo, Egypt.
Newcastle disease master seed virus
Lasota strain of NDV (lentogenic) was supplied by the Central Veterinary laboratories, New Haw, Weighbridge, Surry, UK. The virus was propagated in SPF chicken eggs. The allanto-amniotic fluids were harvested, dispensed in vials, lyophilized, and stored at −70°C. The original titer of the virus was 1010.5 EID 50/ml with HA activity 10 Log2.
SPF ECEs
Eggs were obtained from Nile SPF Farm, Kom Oshiem, Fayom, Egypt, and used for virus propagation, virus titration, and assurance of complete inactivation.
Inactivation of viruses
Inactivation of AI subtypes H9N2 and reassortant H5N1 and NDV viruses was carried out using formalin in a final concentration of 0.1% of the total volume. The fluid was blended using magnetic stirrer for about 20 h at 25°C. Sodium bisulfite was added as a final concentration of 2% to stop the action of formalin [20]. Samples from each inactivated virus were tested for complete inactivation in 10-day-old SPF ECE for two successive blind passages before it was considered free from residual live virus.
Antigen emulsification
Four vaccines were prepared (monovalent inactivated H9N2, H5N1, and NDV and a combined trivalent vaccine containing H9N2, H5N1, and NDV) as oil adjuvant vaccines using Montanide™ ISA 71 VG adjuvant (SEPPIC France) as per the manufacturer’s instructions.
Vaccine evaluation
Safety test
An experimental batch of the prepared vaccine was tested for its safety by inoculating double dose subcutaneously in 10 3-week-old birds, and these are observed for 2 weeks for the presence of clinical signs of disease or local lesions [18].
Sterility test
An experimental batch of the prepared vaccine candidate was tested for sterility and freedom from any fungal or bacterial contaminants by culturing on specific media [18].
Potency of prepared vaccines
A total of 250 1-day-old SPF chicks were purchased from Kom Oshiem SPF Farm, Fayoum, Egypt. The chicks were divided into five groups: Group 1 injected with monovalent H9N2, Group 2 for monovalent H5N1, Group 3 for monovalent NDV vaccine, Group 4 for trivalent vaccine (all chickens injected with 0.5 ml I/M of previously prepared vaccines), and Group 5 kept as non-vaccinated control group. Chickens housed in isolation facilities till they became 21 days of age with free access to water and feed.
Evaluation of cellular immune response
Heparinized blood samples were collected from the five groups at 3rd, 5th, 7th, 15th, and 21st days postvaccination for lymphocyte proliferation assay and at 5th, 10th, 15th, and 21st postvaccination for identification of interleukin 1 (IL1), IL6, and interferon gamma (IFN γ) genes by real-time polymerase chain reaction (PCR).
Evaluation of humoral immune response using HA inhibition (HI)
It was carried out using 4 HAU of homologous antigen (H9N2 AIV, H5N1, and NDV Lasota strain), to estimate antibody titers in sera of vaccinated and unvaccinated chickens [21].
Evaluation of vaccine protection and viral shedding
Challenge with viscerotropic velogenic NDV (VVNDV)
Ten birds were chosen randomly from trivalent vaccine-vaccinated group and control unvaccinated group were subjected to challenge test against NDV using the VVND [22], each bird received a dose of 0.5 ml I/M from the virulent VVNDV strain (106 EID 50/ml) and observed for 15 days after challenge. Birds which died within this period were collected for a detailed P.M. examination for any characteristic lesions.
Protection % against VVNDV=Number of survivals/total number of challenged birds×100
Challenge with H9N2 LPAIV
Twenty chicks from the vaccinated and non-vaccinated groups were challenged with the LPAI A/chicken/Egypt/114922v/2011 (H9N2) at 30-day postvaccination. The birds were inoculated through the intranasal route (100 µl/chick) of allantoic fluid containing 106 EID 50 of the virus. Tracheal swabs were collected at 3,5 and 7 days post challenge (DPC) to determine the virus shedding.
Challenge with HPAI H5N1
SPF chicken groups were vaccinated at 4 weeks of age. At 28-day postvaccination, all birds were challenged intranasally by local Egyptian HPAI H5N1 isolates (A\chicken\Egypt\VSVRI\2009). The challenge virus dose was 0.1 ml containing 5.5×105 EID 50. Another group of chicks were kept as control unvaccinated and challenged with the same dose of the challenge virus. Birds were observed daily for 15 DPC. Three DPC, the morbidity and mortality rates were recorded for each group till the end of the observation period to measure the protection %. Tracheal swabs were collected at 3,5 and 7 days DPC to determine the virus shedding.
Statistical analysis
Using computer software SPSS version 22.0 [23], simple one-way ANOVA was used to study lymphocyte blastogenesis assay and HI test, and Duncan’s multiple range tests were used to differentiate between significant mean [24]. The recorded data of cytokines (IL1, IL6, and IFN γ) were analyzed using two-sided Fisher’s exact test, and p<0.05 was considered as statistically significant.
Results
Sterility and safety of the prepared vaccines
All the four vaccine candidates were found to be sterile and safe in vaccinated birds, where they induced neither any bacterial or fungal growth nor any abnormal clinical signs.
Lymphocyte blastogenesis
There was a significant increase in lymphocyte proliferation at the 3rd day postvaccination in all vaccinated groups compared to the control unvaccinated group with a significant difference between monovalent NDV, trivalent vaccine, and monovalent H9N2 and H5N1 groups (Figure-1). The lymphocyte proliferation reaches to maximum at the 7th day postvaccination with no significant difference between all vaccinated groups at this age. However, at 21-day postvaccination, there was a significant increase in the lymphocyte proliferation in the trivalent vaccine compared with other monovalent groups.
Figure-1.

Lymphocyte blastogenesis assay using 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide reagent expressed by DOD means with different alphabetical (a, b, c, and d) within the column are significantly different at p<0.05 using Duncan’s multiple range test.
IL1 response of vaccinated chickens
IL1 response of vaccinated groups of chicks at interval days as measured by quantitative reverse transcription (qRT)-PCR assay showed waves of increasing and gradual decreasing values differ from group to group as shown in Figure-2. There was no significant difference between the vaccinated groups at the 5th and 10th days postvaccination. At the 15th day postvaccination, there was a significant difference in IL1 in group that received monovalent NDV vaccine compared to other groups. Meanwhile, at the 21st day postvaccination, there was a significant increase in IL1 in a group of chicks vaccinated with trivalent vaccine.
Figure-2.
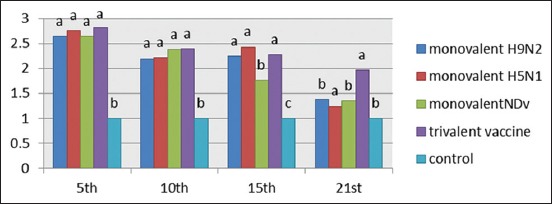
Interleukin-1 response after vaccination with 4 prepared vaccines at interval days postvaccination. The recorded data were analyzed using two-sided Fisher’s exact test, different alphabetical (a, b, c, and d) within the columns are significantly different at p<0.05 using Duncan’s multiple range test.
IL6 response of vaccinated chickens
Results of IL6 response in vaccinated chickens at interval days postvaccination showed no significant difference between the vaccinated groups at the 5th and 10th day postvaccination, while there was a significant difference between groups compared with internal control. At the 21st day postvaccination, there was no significant increase in the vaccinated groups compared to internal control.
IFN γ response of vaccinated chickens
Measuring the IFN γ response of vaccinated chicken groups by qRT-PCR assay showed increase in values at the 5th and 10th days postvaccination with no significant difference between vaccinated groups, while at the 15th day postvaccination, there was a significant increase in IFN γ in group which received the trivalent vaccine. By 21st day postvaccination, IFN γ began to decline with no significant difference between all groups and the internal control.
Evaluation of humoral immune response
Monitoring of AI subtype H9N2 humoral immune response by HI test
It was noticed that chicks vaccinated with inactivated AI (H9N2) vaccine and the trivalent AI (H9N2-H5N1)+ND vaccine showed increased mean log2 HI antibody titer (7.67 Log2 and 7.33 Log2) from the 3rd week postvaccination (WPV), respectively. The highest HI antibody titer (8.67 Log2 and 9.33 Log2) reached the 8th WPV, and then, declined gradually to reach the lowest HI antibody titer (3.67 Log2 and 4.33 Log2) at the 24th WPV for H9N2 monovalent vaccine and trivalent vaccine, respectively (Figure-3).
Figure-3.
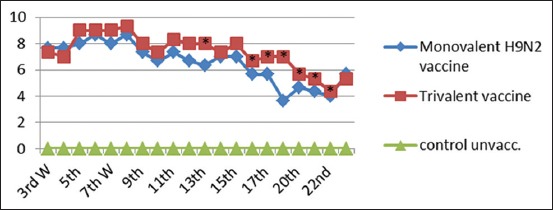
Mean hemagglutination inhibition antibody titers to H9N2 in vaccinated chickens with inactivated monovalent (H9N2) vaccine and trivalent (H5N1+H9N2+Newcastle disease virus). *Significant difference at p<0.05 using Duncan’s multiple range test.
Monitoring of AI subtype H5N1 humoral immune response by HI test
It was noticed that chicks no significant difference in groups vaccinated with inactivated monovalent AI (H5N1) and trivalent vaccines with increased mean log2 HI antibody titers (7 Log2 and 7.67 Log2) from the 3rd WPV, and then, reached the highest HI antibody titer (9 Log2) at the 8th WPV, respectively. While at the 9th, 12th, 14th, 15th, 16th, 18th, and 22nd WPV, there was a significant difference between the two vaccinated groups.
Monitoring of NDV humoral immune response by HI test
It was noticed that chicks vaccinated with inactivated Lasota NDV vaccine and trivalent AI (H9N2-H5N1)+ND vaccine showed increased mean log2 HI antibody titer (6.33 Log2 and 8.33 Log2) from the 3rd WPV with significant difference between two groups at 3rd, 7th, 8th, 10th, 12th, 13th, 17th, 18th, and 22nd WPV. The mean Log2 HI antibody titer reaches highest at the 8th WPV (9 and 10.67 Log2) for monovalent NDV and trivalent vaccines, respectively, and then began to decline gradually (4 and 4.33 Log2 for monovalent NDV and trivalent vaccine, respectively) with no significant difference.
Efficacy of the prepared vaccines (challenge and shedding)
Protective effectiveness of AI Type A H5N1 inactivated vaccine and combined vaccine against HPAI H5N1
All vaccinated chickens did not show any H5N1 symptoms post challenge while unvaccinated group showed typical HPAI H5N1 clinical and postmortem signs. The protection percent was 90% in both monovalent and trivalent vaccines (Table-1). Shedding test was carried out at 3, 5, and 7 DPC from oropharyngeal swabs revealed that both monovalent H5N1 and trivalent vaccines could reduce shedding of the H5N1 virus. Only one bird shed the virus 3 and 5 DPC. The shedding levels were 2 and 1 logs of EID 50/ml, respectively, for monovalent H5N1 with no viral shedding at 7 DPC. On group vaccinated with trivalent vaccine, only one bird shed H5N1 at 3 and 5 DPC. The shedding level was 1.5 and 1 logs of EID 50/ml, respectively, with no shedding virus at 7 DPC.
Table-1.
Protective effectiveness of AI Type A H5N1 monovalent vaccine and trivalent vaccines against HPAI H5N1.
| Chicken groups | Mean antibody titter in HI test (Log2) | Virus isolation from oropharyngeal (mean EID 50/ml) | Dead birds/total birds | Protection % | |||
|---|---|---|---|---|---|---|---|
| – | |||||||
| 1 WPC | 2 WPC | 3rd DPC | 5th DPC | 7th DPC | |||
| Monovalent H5N1 | 7 | 8 | (1/10) 2 | (1/10)=1.0 | 0/10 | 1/10 | 90 |
| Trivalent vaccine | 7.5 | 8 | (1/10) 1.5 | (1/10)=1.0 | 0/10 | 1/10 | 90 |
| Control non vaccinated | 0 | 0 | 0 | N/A | N/A | 10/10 | 0 |
WPC=Week post challenge, DPC=Days post challenge, N/A=Not applicable, EID=Egg infective dose, HI=Hemagglutination inhibition, AI=Avian influenza
The mean antibody titers at 1 and 2 weeks post challenge (WPC) were 7 and 8 Log2 in monovalent H5N1 vaccine and 7.5 and 8 Log2 in the trivalent vaccine groups, respectively.
Protective effectiveness of single H9N2 vaccine and combined vaccine against H9N2
The challenge test was carried out against LPAI H9N2 for both monovalent H9N2 and trivalent vaccines. Shedding test was carried out at 3, 5, and 7 DPC from oropharyngeal swab revealed that both monovalent H9N2 vaccine and combined trivalent vaccine were able to reduce the shedding of H9N2 virus that only one bird shed the virus 3 DPC, the shedding level was 1 EID 50/ml for monovalent H9N2 vaccine while there is no viral shedding at 5 and 7 DPC. In trivalent vaccine group, there was no viral shedding through the entire testing period. The mean HI antibody titer was 7.5 and 8 Log2 after the 1 and 2 WPC, respectively, in monovalent H5N1 vaccinated group, while in combined vaccine, the mean HI Ab titer was 7.7 and 9 Log2 at 1st and 2nd WPC, respectively. Compared to the control group, there was a viral shedding, and among the entire testing period, it was recorded as 5.5, 6, and 5 EID 50/ml at 3, 5, and 7 DPC, respectively, while there was no HI titer in the control group.
Protective effectiveness of single NDV vaccine and trivalent vaccine against challenge with virulent NDV
Challenge test was carried out against VVNDV for monovalent NDV and trivalent vaccines. All vaccinated chickens did not show any symptoms post challenge. Control non-vaccinated chickens showed typical clinical and postmortem signs of VVNDV infection. All non-vaccinated birds died after 5 DPC. This test reflects the protection percent induced by the prepared vaccine candidates as it was 100% in both monovalent NDV and trivalent vaccine. A rapid increase in the HI titer against NDV after the challenge was observed. The mean titer was 8 and 9.5 Log2 after 1st and 2nd WPC in monovalent vaccine while in trivalent vaccine the titers were 8.5 and 10.5 Log2 HI, respectively.
Discussion
Polyvalent vaccine strategies increase reactivity for many pathogens including, but not limited to, influenza [25,26] although polyvalent vaccine formulations clearly expand the breadth of a single vaccine formulation, the reactivity is still limited to the individual components. The goal of polyvalency is to increase the breadth of vaccine coverage by combining diverse components into a single vaccination.
In this study, the obtained results revealed that all the prepared forms of ND and AI subtypes H5N1 and H9N2 vaccine candidates either monovalent or polyvalent were free from foreign contaminants and safe for vaccinating chickens which showed no detectable signs of illness as the recommendation of OIE [18]. The role for cell-mediated immunity in protection against AI virus is limited. T cells are the most important cells that mediate the cellular immune response, and the T cell subpopulations with diverse functions have been identified in chickens [27]. In this study, the cellular immune response in vaccinated and control groups was estimated using the lymphocyte proliferation test as well as cytokines (IL1, IL6, and IFN γ).
Analysis of the results of lymphocyte blastogenesis test Figure-1 revealed that all the vaccinated groups demonstrate cellular immune response with a significant increase (p<0.05) compared with the control unvaccinated groups. Similar observation was previously reported by El-Bagoury et al. [28] where chicken vaccinated with inactivated NDV ISA 71 vaccine induced higher cellular immune response as estimated by lymphocyte proliferation test.
Using qPCR to characterize the expression of IL1B, IFN γ, and IL6 genes to provide insights into the role of innate immune response in protection against NDV and AIV subtypes H5N1 and H9N2 infection, results of cytokines (IL1b, IL6, and IFN γ) genes expression showed the presence of upregulation of the three genes with marked increase in IL6 gene expression in all types of prepared vaccines compared to the internal control of chickens 5-day postvaccination (Figures-2,4,5).
Figure-4.
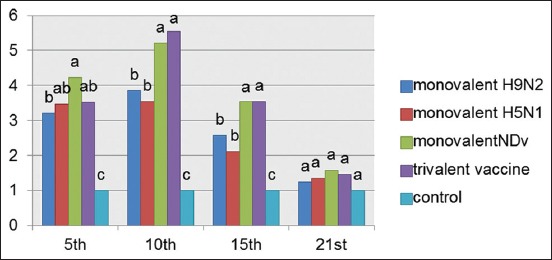
Interleukin-6 response in chicken vaccinated with four types of vaccines at interval days postvaccination. The recorded data were analyzed using two-sided Fisher exact test, different alphabetical (a, b, c, and d) within the columns are significantly, different at p<0.05 using Duncan’s multiple range test.
Figure-5.
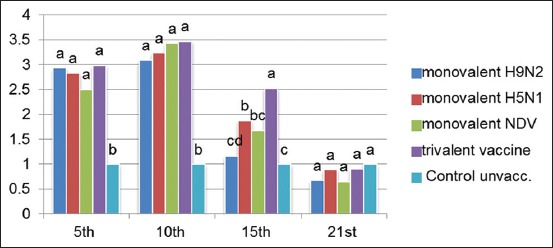
Interferon gamma response in vaccinated chickens at interval days postvaccination. The recorded data were analyzed using two-sided Fisher’s exact test, different alphabetical (a, b, c, and d) within the columns are significantly different at p<0.05 using Duncan’s multiple range test.
IL1 response of vaccinated groups of chicks at specific intervals showed waves of increasing and gradually decreasing values differing from group to group (Figure-2) where the highest value at the 5th day postvaccination and then decreased gradually. On the 21st day postvaccination, Group 4 which received combined trivalent vaccine showed superior value. IL1 production would be expected in many avian infections as a pro-inflammatory response, and infection models have also been used to determine activity following viral and bacterial infections in the chicken [29-31].
As shown in Figure-4, there was upregulation of IL6 at the 15th day but to lower extent compared to early stages, these finding and skewing of the immune response to specific humeral immune response are similar to the responses of mammals [32,33]. Similar findings of IL6 being highly upregulated in HPAI H5N1 virus-infected chicken cells were reported by Kaiser et al. [34]. Elevation of IL6 has been observed in influenza-infected humans, primates, and ferrets, which appear to correlate with symptom severity [35,36]. IL-6 is a Th2 cytokine and induces antibody production in B cells, and promotes T cell activation and differentiation [37].
Expression of IFNs and pro-inflammatory cytokines influences both viral clearance and clinical disease presentation. In this study, results showed marked upregulation for the IFN γ in all vaccinated groups compared to the non-vaccinated group as shown in Figure-5. While at the 21 days postvaccination, there was a marked decrease in IFN-γ level in all formulated vaccines. A study found strong upregulation of IFN-γ mRNA in the lung and bursa of ducks but not chicken following infection with a LPAI H7N1 virus [38]. It is possible that IFN-γ could be important in protection against virulent influenza infection in avian hosts which permits further studies.
Collectively, the results showed that values of cellular immune response at later stages came in agreement with others [39] who stated that, once the humoral immune response becomes established; there is a corresponding decrease in the cellular immune response.
The performance of adjuvant vaccines not be evaluated only by their early response but also the level and duration of humoral immune response. Those parameters were investigated for each of the prepared vaccines by monitoring antibodies in the sera collected from vaccinated groups up to 20 WPV.
Anti-H9 serological evidence of experimentally vaccinated chicks against LPAI H9N2 in both monovalent vaccine and trivalent vaccine as shown in Figure-3 increased mean log2 HI antibody titer from the 3rd WPV, respectively, then reached the highest HI antibody titer at the 8th WPV, and then declined gradually to reach the lowest HI antibody titer at the 22th WPV [40]. Reports showed that all vaccinated chickens with AI and AI+ND vaccines demonstrated high titers when tested by HI using homologous H9N2 antigen.
HI serology of the prepared monovalent H5N1 and combined trivalent vaccine against H5N1 antigen as shown in Figure-6 revealed that Chickens in different vaccinated groups showed increased mean Log2 HI antibody from the 3rd WPV, then reached the highest HI antibody titer (9 Log2) at the 8th WPV for both vaccines, and then declined gradually to reach the lowest HI antibody titer at the 22nd WPV. These findings suggested that the reassortant HPAI H5N1 viruses were avirulent and highly immunogenic [41]. It was noticed that the mean value of HI titer in the combined trivalent vaccine was significantly higher than monovalent vaccine in the 9th, 12th, 14th, 15th, 16th, 18th, and 22nd WPV (p≤0.05), these results are in accordance with the study of El Sayed et al., [42] who made a bivalent vaccine of NDV and H5N1 that give higher HI titers than the monovalent vaccines.
Figure-6.
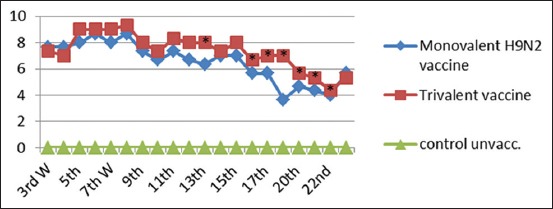
Mean anti-(H5N1) hemagglutination inhibition antibody titers in vaccinated chickens with monovalent avian influenza (AI) (H5N1) vaccine and trivalent AI (H9N2, H5N1)+Newcastle disease. *Significant difference at p<0.05 using Duncan’s multiple range tests.
Detectable ND antibodies were detected in vaccinated chickens vaccinated with inactivated lasota NDV vaccine and combined trivalent AI (H9N2, H5N1) -ND vaccine with Montanide 71 adjuvant showed increased mean log2 HI antibody titer (6.33 log2 and 8.33 log2) from the 3rd week post vaccination (WPV), then reached the highest HI antibody titer (9 log2 and 10.67 log2) at the 8th WPV respectively then declined gradually to reach the lowest HI antibody titer (4.33 log2 and 5 log2) at the 22th WPV as shown in Figure-7.
Figure-7.
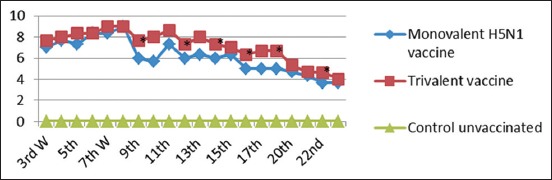
Mean Newcastle disease virus (NDV) - hemagglutination inhibition antibody titers in vaccinated chickens with monovalent NDV vaccine and trivalent avian influenza (H9N2, H5N1) + Newcastle disease. *Significant difference at p<0.05 using Duncan’s multiple range tests.
The fact that highest level of both cellular and humoral responses was conferred by the trivalent vaccine supports that there is an important factor contributes to the ability to confer immunity which is antigenic mass [5].
Challenge results showed severe clinical signs with 100% mortality in the control unvaccinated group post challenge. At the same time, 90% of the vaccinated chickens were protected from mortality and showed no clinical signs of HPAI infection for both monovalent H5N1 and trivalent vaccinated groups. Shedding test was carried out at the 3, 5, and 7 DPC from oropharyngeal swabs revealed that both monovalent H5N1 vaccine and trivalent vaccine were able to reduce the shedding of H5N1 virus. There was no viral shedding at 7 DPC. To examine the protective efficacy against H9N2, shedding level detection was carried out at 3, 5, and 7 DPC from oropharyngeal swabs revealed that both monovalent H9N2 vaccine and trivalent vaccine reduced H9N2 virus shedding. Sera from the control group were negative by HI (Table-2). This fulfills the OIE (2012) [43] requirements concerning the evaluation of vaccines by challenge test as any vaccine candidate must reduce the shed virus compared with group that receive challenge virus only (positive control group).
Table-2.
Protective effectiveness of monovalent AI subtypes H9N2 and combined vaccine against H9N2.
| Chicken group | Mean AB titter in HI test | Number of the shedding bird/total number of virus isolation from oropharyngeal (mean EID 50/ml) | Amount of dead birds/amount of birds in experiment | Protection % | |||
|---|---|---|---|---|---|---|---|
| 1 WPC | 2 WPC | 3rd DPC | 5th DPC | 7th DPC | |||
| Monovalent H9N2 | 7.5 | 8 | (1/10)=1.0 | (0/10) | 0/10 | 1/10 | 90 |
| Trivalent vaccine | 7.7 | 9 | 0/10 | 0/10 | 0/10 | 1/10 | 90 |
| Control unvaccinated | 0 | 0 | (10/10)=5.5 | 6 | 5 | 4/10 | 60 |
WPC=Week post challenge, DPC=Days post challenge, EID=Egg infective dose, HI=Hemagglutination inhibition, AI=Avian influenza
On the other hand, protection percent of the prepared vaccines against challenge with NDV was 100% in both monovalent NDV and trivalent vaccines confirming the potency and efficacy of the prepared vaccines (Table-3).
Table-3.
Protective effectiveness of single NDV vaccine and combined vaccine against challenge with virulent NDV.
| Birds | Mean Ab titer in HI test (Log2) | Amount of dead birds/amount of birds in experiment | Protection % | |
|---|---|---|---|---|
| 1 WPC | 2 WPC | |||
| Monovalent NDV vaccine | 8 | 9.5 | 0/10 | 100 |
| Trivalent vaccine | 8.5 | 10.5 | 0/10 | 100 |
| Control | 0 | 0 | 10/10 | 0 |
WPC=Weeks post challenge, NDV=Newcastle disease virus, HI=Hemagglutination inhibition
Conclusion
The prepared trivalent vaccine candidate against NDV, H5N1, and H9N2 was as efficacious as monovalent counterparts, induced high titers of H5N1-, H9N2-, and NDV-specific antibodies and reduced H5N1 and H9N2 viral shedding.
Multivalent vaccines offer a number of practical advantages over monovalent vaccines; first advantage of multivalent vaccine is the fewer vaccinations required to mount an effective protection against several diseases, second is the reduced stress for the worker and the birds.
Authors’ Contributions
This work is a part of ZMA’s PhD thesis supervised by AAEE, HAH, and MAEH. ZMA: Conducted the laboratory animal experimental work and drafted and revised the manuscript. MAMEH: Shared in design of the experimental work and followed up the practical part of the research. HAH: Set the design, supervised the work, drafted, and revised the manuscript. BMA: Analyzed the data and drafted and revised the manuscript. AAE: Conceived the study, set the design and supervised the work. All authors have revised and approved the final manuscript.
Acknowledgments
The authors would like to thank VSVRI, Cairo, Egypt, for covering all the expenses of the experiment needed to conduct the work.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Swayne D.E, Suarez D.L, Sims L.D. Influenza. In: Swayne D.E, Glisson J.R, McDougald L.R, Nair V, Nolan L.K, Suarez D.L, Nair V, editors. Diseases of Poultry. Vol. 13. Ames, Iowa: Willey-Blackwell; 2013. pp. 181–218. [Google Scholar]
- 2.FAO. Improvement of Biosecurity and Production Practices in Hatcheries and Parent Flocks. Rome. 2015. [Last accessed on 20-06-2016]. Available from: http://www.fao.org/3/a-bb033e.pdf .
- 3.Webster R.G, Webby R.J, Hoffmann E, Rodenberg J, Kumar M, Chu H.J, Seiler P, Krauss S, Songserm T. The immunogenicity and efficacy against H5N1 challenge of reverse genetics derived H5N3 influenza vaccine in ducks and chickens. Virology. 2006;351:303–311. doi: 10.1016/j.virol.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Tian G, Zeng X, Li Y, Shi J, Chen H. Protective efficacy of the H5 inactivated vaccine against different highly pathogenic H5N1 avian influenza viruses isolated in China and Vietnam. Avian Dis. 2010;54:287–289. doi: 10.1637/8707-031709-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 5.Swayne D.E, Beck J.R, Garcia M, Stone H.D. Influence of virus strain and antigen mass on the efficacy of H5 avian influenza inactivated vaccines. Avian Pathol. 1999;28:245–255. doi: 10.1080/03079459994731. [DOI] [PubMed] [Google Scholar]
- 6.Kayali G, Kandeil A, El-Shesheny R, Kayed A.S, Maatouq A.M, Cai Z, Ali M.A. Avian influenza A (H5N1) virus in Egypt. Emerg. Infect. Dis. 2016;22(3):379–388. doi: 10.3201/eid2203.150593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afifi M.A, El-Kady M.F, Zoelfakar S.A, Abdel-Moneim A.S. Serological surveillance reveals widespread influenza A H7 and H9 subtypes among chicken flocks in Egypt. Trop. Anim. Health Prod. 2013;45:687–690. doi: 10.1007/s11250-012-0243-9. [DOI] [PubMed] [Google Scholar]
- 8.Monne I, Hussein H.A, Fusaro A, Valastro V, Hamoud M.M, Khalefa R.A, Dardir S.N, Radwan M.I, Capua I, Cattolim G. H9N2 influenza a virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respir Viruses. 2013;7:240–243. doi: 10.1111/j.1750-2659.2012.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arafa A.S, Hagag N.M, Yehia N, Zanaty A.M, Naguib M.M, Nasef S.A. Effect of circulation of highly pathogenic avian influenza H5N1 subtype with low pathogenic H9N2 subtype on the spread of infections. Avian Dis. 2013;56:849–857. doi: 10.1637/10152-040812-Reg.1. [DOI] [PubMed] [Google Scholar]
- 10.Kayali G, Kandeil A, El-Shesheny R, Kayed A.S, Gomaa M.M, Maatouq A.M, Shehata M.M, Moatasim Y, Bagato O, Cai Z, Rubrum A, Mohamed A.K, Pamela P.M, Webster R.G, Webby R.J, Mohamed A.A. Active surveillance for avian influenza virus, Egypt, 2010-2012. Emerg. Infect. Dis. 2014;20:542–551. doi: 10.3201/eid2004.131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandeil A, El-Shesheny R, Maatouq A.M, Moatasim Y, Shehata M.M, Bagato O, Rubrum A, Shanmuganatham K, Webby R.J, Ali M.A, Kayali G. Genetic and antigenic evolution of H9N2 avian influenza viruses circulating in Egypt between 2011 and 2013. Arch. Virol. 2014;159(11):2861–2876. doi: 10.1007/s00705-014-2118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalil A.A, Hussein H.A, Tolba S.K, El-Sanousi A.A. Preparation and evaluation of H9N2 vaccine adjuvant with Montanide ISA 71. Glob. Vet. 2015;14(5):670–674. [Google Scholar]
- 13.El-Naggar H.M, Madkour M.S, Hussein H.A. Preparation of mucosal nanoparticles and polymer-based inactivated vaccine for Newcastle disease and H9N2 AI viruses. Vet. World. 2017;10(2):187–193. doi: 10.14202/vetworld.2017.187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller P.J, Koch G. Newcastle disease, other avian paramyxoviruses and avian metapneumovirus infections. In: Swayne D.E, Glisson J, McDougald L.R, Nolan L.K, Suarez D.L, Nair V, editors. Diseases of Poultry. Vol. 13. Hoboken, NJ: Wiley-Blackwell; 2013. pp. 89–138. [Google Scholar]
- 15.Liu X.F, Wan H.Q, Ni X.X, Wu Y.T, Liu W.B. Pathotypical and genotypical characterization of strains of Newcastle disease virus isolated from outbreaks in chicken and goose flocks in some regions of China during 1985-2001. Arch. Virol. 2003;148:1387–1403. doi: 10.1007/s00705-003-0014-z. [DOI] [PubMed] [Google Scholar]
- 16.Jeon W.J, Lee E.K, Lee Y.J, Jeong O.M, Kim Y.J, Kwon J.H, Choi K.S. Protective efficacy of commercial inactivated Newcastle disease virus vaccines in chickens against a recent Korean epizootic strain. J. Vet. Sci. 2008;9:295–300. doi: 10.4142/jvs.2008.9.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed L.J, Muench H. Simple method of estimating 50 percent end point. Amer. J. Hyg. 1938;27:793–799. [Google Scholar]
- 18.OIE. Avian Influenza OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Ch. 2.3.4. Paris, France: OIE; 2014. [Google Scholar]
- 19.Allan W.H, Cough R.E. A comparison between the haemagglutination inhibition and complement fixation tests for new castle disease. Vet. Sci. 1976;20:101–103. [PubMed] [Google Scholar]
- 20.OIE. Highly Pathogenic Avian Influenza. International Health Code. Ch. 2.7.12. Paris: OIE; 2004. [Google Scholar]
- 21.OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals:Mammals, Birds and Bees, Biological Standards Commission. 7th ed. Paris: World Organization for Animal Health; 2012. [Google Scholar]
- 22.Reda I.M, Sheble A. Isolation and Characterization of Local Viscertropic NDV Unpublished Data. 1976 [Google Scholar]
- 23.SPSS. PC. Software. Version. 22.0. Chicago, IL: SPSS Inc; 2015. [Google Scholar]
- 24.Snedecor G.W, Cochran W.G. Statistical Methods. 8th ed. Ames, IA, USA: Iowa State Uuniv; 1989. [Google Scholar]
- 25.Crevar C.J, Ross T.M. Elicitation of protective immune responses using a bivalent H5N1 VLP vaccine. Virol. J. 2008;5(131):131. doi: 10.1186/1743-422X-5-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiore A.E, Timothy M.U, Karen B, Lyn F, James A.S, Flavia Z, Analia B, Ariel P, Oscar T, Elisa C. Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP) 2010;59(RR08):1–62. [PubMed] [Google Scholar]
- 27.Sharma J.M. The avian immune system. In: Saif Y.M, Barnes H.J, Glisson J.R, Fadly A.M, McDougald L.R, Swayne D.E, editors. Disease of Poultry. Ames, Iowa: Iowa State Univ. Press; 2003. pp. 5–16. [Google Scholar]
- 28.El-Bagoury G.F, Nasr M.H.M, El-Habbaa A.S, Hala M.E.M. A trial to improve stability and immunogenicity of inactivated NDV vaccine with paraffin oil adjuvant using aluminum stearate. Benha Vet. Med. J. 2015;28(1):199–209. [Google Scholar]
- 29.Heggen C.L, Qureshi M.A, Edens F.W, Barnes H.J. Alterations in macrophage-produced cytokines and nitrite associated with poult enteritis and mortality syndrome. Avian Dis. 2000;44:59–65. [PubMed] [Google Scholar]
- 30.Kaiser P, Rothwell L, Galyov E.E, Barrow P.A, Burnside J, Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium Salmonella enteritidis and Salmonella gallinarum. Microbiology. 2000;146:3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 31.Peschke T, Bender A, Nain M, Gemsa D. Role of macrophage cytokines in influenza A virus infections. Immunobiology. 1993;189:340–355. doi: 10.1016/s0171-2985(11)80365-7. [DOI] [PubMed] [Google Scholar]
- 32.Van Reeth K. Cytokines in the pathogenesis of influenza. Vet. Microbiol. 2000;74:109–116. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 33.Karpala A.J, Bingham J, Schat K.A, Chen L.M, Donis R.O, Lowenthal J.W, Bean A.G. Highly pathogenic (H5N1) avian influenza induces an inflammatory T helper Type 1 cytokine response in the chicken. J. Interferon Cytokine Res. 2011;31(4):393–400. doi: 10.1089/jir.2010.0069. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser L, Fritz R.S, Straus S.E, Gubareva L, Hayden F.G. Symptom pathogenesis during acute influenza:Interleukin-6 and other cytokine responses. J. Med. Virol. 2001;64:262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 35.Svitek N, Rudd P.A, Obojes K, Pillet S, von Messling V. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology. 2008;376(1):53–59. doi: 10.1016/j.virol.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 36.Lienenluke B, Christ B. Impact of interleukin-6 on the glucose metabolic capacity in rat liver. Histochem. Cell Biol. 2007;128(4):371–377. doi: 10.1007/s00418-007-0327-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Wohlford-Lenane C, Zhao J, Fleming E, Lane T.E, McCray P.B, Jr, Perlman S. Intranasal treatment with poly(I•C) protects aged mice from lethal respiratory virus infections. J. Virol. 2012;86(21):11416–11424. doi: 10.1128/JVI.01410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornelissen J.B, Post J, Peeters B, Vervelde L, Rebel J.M. Differential innate responses of chickens and ducks to low-pathogenic avian influenza. Avian Pathol. 2012;41(6):519–529. doi: 10.1080/03079457.2012.732691. [DOI] [PubMed] [Google Scholar]
- 39.Cary A.R, Leonardo S, Ingrid C, Corrie C.B, Darrell R.K, David L.S, Daniel J.K, Patti J.M, Claudio L.A. Virulent Newcastle disease virus elicits a strong innate immune response in chickens. J. Gen. Virol. 2011;92:931–939. doi: 10.1099/vir.0.025486-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, Huiming Y, Hongjun X, Zengbin M, Guozhong Z.E. Efficacy of an inactivated bivalent vaccine against the prevalent strains of Newcastle disease and H9N2 avian influenza. Virol. J. 2017;14:56. doi: 10.1186/s12985-017-0723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong-Hun L, Jae-Keun P, Jung-Hoon K, Seong-Su Y, Tseren-Ochir E.O, Yo-Han J, Baik-Lin S, Lee Y.P, Seung-Yong P, In-Soo C, Chang-Seon S. Efficacy of single dose of a bivalent vaccine containing inactivated Newcastle disease virus and reasserting highly pathogenic avian influenza H5N1 virus against lethal HPAI and NDV infection in chickens. PLoS One. 2013;8(3):e58186. doi: 10.1371/journal.pone.0058186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Sayed D.A.A, Abdou A.M, Shalash S.M.M, Safaa H.M, Riad S.A. Productivity and immune response of broiler chickens vaccinated with different avian influenza vaccines at one or seven days of age. Aust. J. Basic Appl. Sci. 2011;5(10):325–334. [Google Scholar]
- 43.OIE. Disease Immediate Notification. OIE. 2012;25(3):19. [Google Scholar]


