Abstract
It has become apparent that gut microbiota is closely associated with cardiometabolic diseases (CMDs), and alteration in microbiome compositions is also linked to the host environment. Next generation sequencing (NGS) has facilitated in-depth studies on the effects of herbal medicine and functional food on gut microbiota. Both herbal medicine and functional food contain fiber, polyphenols and polysaccharides, exerting prebiotics-like activities in the prevention and treatment of CMDs. The administrations of herbal medicine and functional food lead to increased the abundance of phylum Bacteroidetes, and genus Akkermansia, Bifidobacteria, Lactobacillus, Bacteroides and Prevotella, while reducing phylum Firmicutes and Firmicutes/Bacteroidetes ratio in gut. Both herbal medicine and functional food interact with gut microbiome and alter the microbial metabolites including short-chain fatty acids (SCFAs), bile acids (BAs) and lipopolysaccharides (LPS), which are now correlated with metabolic diseases such as type 2 diabetes (T2D), obesity and non-alcoholic fatty liver disease (NAFLD). In addition, trimethylamine (TMA)-N-oxide (TMAO) is recently linked to atherosclerosis (AS) and cardiovascular disease (CVD) risks. Moreover, gut-organs axes may serve as the potential strategy for treating CMDs with the intervention of herbal medicine and functional food. In summary, a balance between herbal medicine and functional food rich in fiber, polyphenols and polysaccharides plays a vital role in modulating gut microbiota (phylum Bacteroidetes, Firmicutes and Firmicutes/Bacteroidetes ratio, and genus Akkermansia, Bifidobacteria, Lactobacillus, Bacteroides and Prevotella) through SCFAs, BAs, LPS and TMAO signaling regarding CMDs. Targeting gut-organs axes may serve as a new therapeutic strategy for CMDs by herbal medicine and functional food in the future. This review aims to summarize the balance between herbal medicine and functional food utilized for the prevention and treatment of CMDs through modulating gut microbiota.
Keywords: herbal medicine, functional food, cardiovascular disease, metabolic disease, intestinal microbiota
Introduction
The Human Microbiome Project funded by National Institutes of Health (NIH) (Qin et al., 2010) and Metagenomics of the Human Intestinal Tract (MetaHIT) consortium funded by European Commission (Turnbaugh et al., 2007) have promoted better understanding of the functional properties and healthy composition of gut microbiota. Various microbial communities and their genes (the microbiome) are present in human body, influencing human health and diseases (Human Microbiome Project, 2012). The human gut microbiota contains a diverse array of microorganisms, including bacteria, archaea and fungi that colonize the surfaces of the gastrointestinal (GI) tract; bacteriophage are also in high abundance in GI tract (Savage, 1977). Six bacterial phyla dominate the gut microbiota of healthy adult subjects: Firmicutes, Proteobacteria, Bacteroidetes, Fusobacteria, Actinobacteria, and Verrucomicrobia. The intestine hosts >1014 microorganisms with critical physiological roles, and the microbial compositions differ along the digestive tract (Aron-Wisnewsky and Clement, 2016). The large intestine, particularly the colon, harbors a complex and dynamic microbial ecosystem with high densities of living bacteria. These bacteria achieve concentrations of approximately 1011–1012 cells/g of luminal contents (Simon and Gorbach, 1984; Guarner and Malagelada, 2003).
A multitude of literature supports the role of gut microbiota in the development and progression of cardiometabolic diseases (CMDs). CMDs have become a worldwide epidemic, with dramatically increasing prevalence of cardiovascular disease (CVD), obesity, type 2 diabetes (T2D), non-alcoholic fatty liver disease (NAFLD), atherosclerosis (AS), hypertension, and dyslipidemia (Hansen et al., 2015; Aron-Wisnewsky and Clement, 2016; Meyer and Bennett, 2016; Woting and Blaut, 2016; Micha et al., 2017). In the search for novel therapeutic leads, the association of gut microbiota and microbial metabolites with the development of CMDs holds the potential in future drug discovery (Koopen et al., 2016).
Disruption of microbial ecosystems during crucial developmental periods could affect body physiology or cause undesired negative effects. For instance, the overuse of antibiotics in early life is associated with obesity in both humans and rodents (Cho et al., 2012). Herbal medicine such as traditional Chinese medicine (TCM) can be used as an alternative strategy to modulate microbiota and for modern drug discovery. Moreover, certain food components provided benefits beyond basic nutrition, leading to the concept of functional food and nutraceuticals. Functional food offer benefits beyond basic nutrition when consumed regularly as part of a diet. Herbal medicine and functional food produce a large diversity of secondary metabolites which display a broad array of biological and pharmacological properties (Wink, 2015) and are widely accepted as high-efficiency and low toxicity “medicinal diets” which are capable of avoiding certain side-effects. In this review article, recently discovered mechanisms of herbal medicine and functional food are summarized and their contributions to prevention and treatment of CMDs through modulating microbiota are also outlined.
Gut microbiota in CMDs
Gut microbiota in cardiovascular diseases
In recent years, an increasing number of reseachers begin to pay their attention to the mutual effects on intestinal flora and CVDs, resulting from the new findings of gut microbe–derived metabolite trimethylamine (TMA)-N-oxide (TMAO). Gut microbiota has an intimate relationship with CVDs, including thrombosis, AS, myocardial infarction (MI) and stroke.
TMAO
TMAO was first identified as a contributor to CVD in a large clinical cohort of 1,876 subjects by Stanley L. Hazen team using an untargeted metabolomics platform (Wang et al., 2011). In a subsequent expansion study of 4,007 subjects undergoing elective coronary angiography indicated an association between elevated TMAO levels in plasma and increased risk for major adverse cardiovascular events (MACE) over a 3-year period in humans (Tang et al., 2013). Then, increasing clinical reports support an involvement of plasma TMAO levels in the etiology of various CVDs. For example, elevated plasma TMAO levels in patients predict a high atherosclerotic burden (Senthong et al., 2016a), long-term adverse event risk and incremental prognostic of peripheral artery disease (PAD) (Senthong et al., 2016b), higher long-term mortality risk of coronary artery disease (CAD) (Senthong et al., 2016c), and adverse clinical outcomes in heart failure (HF) (Tang et al., 2014, 2015b; Troseid et al., 2015). Higher TMAO levels provide clinical utility in risk stratification of acute coronary syndromes (ACS) (Li X. S. et al., 2017) showing a direct pro-thrombotic effect (Zhu et al., 2017), predict close association with poor prognosis of MI (Suzuki et al., 2017). A systematic review and meta-analysis reconfirmed elevated concentrations of TMAO and its precursor TMA were associated with increased risks of MACE (Heianza et al., 2017). Furthermore, gut microbiota played an obligatory role in the metabolism of TMA, eight species (Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Escherichia fergusonii, Proteus penneri, Providenciarett geri, and Edwardsiella tarda) in two different phyla (Firmicutes and Proteobacteria) and six genera correlated with choline consumption and TMA accumulation were identified (Romano et al., 2015). Undoubtedly, TMAO had become a new biomarker in diagnosis of CVD.
The conclusions of cumulative reports on the meta-organismal metabolic pathway for TMAO production and its possible mechanisms resulting in CVD are highlighted: ➀TMA production: phosphatidylcholine (PC), choline, and L-carnitine, generating the precursor TMA by gut microbiota cleavage, were abundant in dietary foods such as red meat, shellfish, egg yolk and high-fat dairy products (Wang et al., 2011). Until now, either choline or L-carnitine as substrate, TMA is produced by two identified distinct microbial enzyme systems. Catalytic unit (cutC) and a regulatory polypeptide (cutD) are required for TMA production from choline (Craciun and Balskus, 2012; Craciun et al., 2014). The catalytic protein (CntA) and the regulatory protein (CntB) are involved in TMA production form L-carnitine (Zhu et al., 2014). ➁TMA→TMAO: hepatic flavin monooxygenase 3 (FMO3) expression was up-regulated by bile acids (BAs) via nuclear receptor farnesoid X receptor (FXR) activation (Bennett et al., 2013). TMA was readily absorbed and traveled through the portal circulation to the liver and was oxidized into TMAO by FMO3 (Wang et al., 2011; Bennett et al., 2013). ➂TMAO induced or enhanced cell phenotypic changes: elevated plasma TMAO induced endothelial dysfunction via activating reactive oxygen species (ROS)/thioredoxin-interactive protein (TXNIP)/nod-likereceptor family pyrin domain containing 3 (NLRP3) inflammasome (Sun X. et al., 2016) and impairing endothelial nitric oxide synthase (eNOS)-derived NO bioavailability (Hu et al., 2015; Li T. et al., 2017). In addition, TMAO accelerated vascular inflammation through mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signaling (Seldin et al., 2016), reduced endothelial self-repair, and increased monocyte adhesion partly via the pathway of protein kinase C (PKC)/NF-κB/vascular cell adhesion molecule-1 (VCAM-1) activation (Ma et al., 2017). Moreover, TMAO contributed to macrophage cholesterol accumulation and foam cell formation (Wang et al., 2011). Furthermore, TMAO elevated platelet hyperreactivity, enhancing agonists-induced platelet activation through intracellular Ca2+ mobilization (Zhu et al., 2016). These changes in cell phenotype contribute to atherosclerotic CVD. ➃TMAO promoted CAD in animal studies: TMAO accelerated AS by reversing cholesterol transport and altering bile acids (BAs) composition (Koeth et al., 2013), enhanced thrombosis formation by activating platelet (Zhu et al., 2016), exacerbated pressure overload–induced heart failure by inducting adverse cardiac remodeling (Organ et al., 2016). Attention should be paid to the gender identity in the study of TMAO synthesis, since FMO3 expression is higher in females than males in both human and mouse (Bennett et al., 2013).
For further research, pharmacologic inhibition of TMAO producition will be a potential therapeutic strategy to reduce CVD events by targeting microbial community, microbial enzyme and/or FMO3 expression. The mechanisms of TMAO pathway (PC, choline, L-carnitine→TMA→FMO3→TMAO) linking the specific microbiota to cardiovascular functionis are very important and need to be further elucidated. In addition to the new finding of TMAO, other microbial metobolites such as SCFAs (Marques et al., 2017), BAs (Mayerhofer et al., 2017), and LPS (Pastori et al., 2017) which are beneficial for CVD will not be discussed in details here.
Gut microbiota in metabolic diseases
A considerable number of publications have reported correlations between gut microbiota and metabolic diseases (Moreno-Indias et al., 2014; Janssen and Kersten, 2015; Greenhill, 2016; Saad et al., 2016; Sonnenburg and Backhed, 2016; Woting and Blaut, 2016). Specific metabolic abnormalities such as pro-inflammatory states, insulin resistance, glucose intolerance, dyslipidemia, high blood pressure and NAFLD, which accompanies gut microbiota dysbiosis, often develop in obese people. Moreover, obesity and T2D are considered as a medical condition, which not only contributes to the risk of developing CVD and cancer, but also negatively affects longevity and quality of life. Here, we describe several crucial mechanisms (mainly about SCFAs, LPS and BAs) that contribute to understanding the correlation between gut microbiota and obestiy/T2D.
SCFAs
The intestinal microbial fermentation and degradation of dietary nondigestible fiber and polysaccharides to SCFAs (acetate, propionate and butyrate) are regarded as potential metabolic targets to prevent obesity/T2D in glucose metabolism and insulin resistance. Besides, several mechanisms correlate with SCFAs affect body weight via energy intake and energy harvesting, and link with insulin sensitivity through inflammatory resoponse, lipid storage and adipose tissue function (Canfora et al., 2015). SCFAs, serving as energy substrates, directly inhibit histone deacetylases (HDACs) and activate G-protein-coupled recepotors (GPCRs). Moreover, butyrate also has effect on epithelial barrier function by increasing mucus production and protein zonula occludens-1 (ZO)-1, occludin expression (Bordin et al., 2004; Peng et al., 2007). GPR41and GPR43 targets are of significance for SCFAs. Gut microbiota promotes adiposity and body weight via SCFAs receptor GPR41. The expression of peptide YY (or PYY), a key hormone involved in the elevation of intestinal transit rate and reduction in energy hearvest, is decreased in GPR41−/− mice (Samuel et al., 2008). On the contrary, SCFAs may prevent obesity via activation of GPR43. Normal diet GPR43−/− mice are obese, whereas HFD-fed GPR43+/+ mice remain lean. Insulin signaling in adipocytes and fat accumulation in white adipose tissue (WAT) are inhibited via acetate-medatied GPR43 (Kimura et al., 2013). These distinct differences remain to be analyzed in how the gut microbiota is modulated. Besides, GPCR43 activation by SCFAs promotes the release of glucagon-like peptide-1 (GPL-1) by intestinal enteroendocrine L cells, thereby leading to insulin release and stimulating glucose tolerance (Tolhurst et al., 2012). Furthermore, a recent paper reported that acetate contributes to GPR43-mediated intestinal IgA response to microbiota, leading to crucial role in intestinal homeostasis maintenance and intestines inflammation denfence (Wu et al., 2016). Apparently, SCFAs-mediated GPCRs signaling in mice shows extensive effects on obesity/T2D, but the role of GPR41/43 signaling in humans remains to be established.
LPS
Endotoxin LPS is a major component of the gram-negative bacterial (such as Escherichia coli) outer membrane. HFD-induced gut microbiota dysbiosis can alter gut permeability and then increase cirlulating LPS levels which promotes low-grade inflammation and insulin resistance and, ultimately, obesity and T2D in rodents and humans (Cani et al., 2007, 2008; Creely et al., 2007). In addition, Increased intestinal epithelial barrier permeability is due to increased endocannabinoid system tone (Muccioli et al., 2010) and tight junctions (ZO)-1, occludin and claudin-1 expression (Wang J. H. et al., 2014). LPS stimulates inflammatory response mainly by binding to CD14/Toll-like receptor 4 (TLR4) which is respnsible for the recruitment and activation of MyD88 adaptor and NF-κB transcription factor, inducing the pro-inflammatory factors interleukin-6 (IL-6), interleukin-1β (IL-1β) and monocyte chemoattractant protein-1 (MCP-1) secretion (Hennessy et al., 2010), and therefore it triggers matabolic diseases (Robbins et al., 2014; Kang et al., 2016). Metabolic characteristics of obesity and T2D in mice were not initiated by injecting LPS when CD14/TLR4 receptor was genetically deleted, showing the significant contribution of LPS/CD14/TLR4 signaling (Shi et al., 2006; Cani et al., 2007, 2008; Poggi et al., 2007). Unexpectedly, insulin is more sensitive in TLR4−/− (Shi et al., 2006) mice, but less in TLR6−/− (Vijay-Kumar et al., 2010) mice with the modulator of gut microbiota than wild-type controls.
BAs
BAs is produced in the liver from cholesterol and metabolized in the gut by the intestinal microbiota (Midtvedt, 1974). Inversely, BAs can modulate gut microbial composition via innate immune genes activation in the small intestine (Wahlstrom et al., 2016). Cholic acid (CA) and chenodeoxycholic acid (CDCA) are the primary BAs produced in humans, whereas CA and muricholic acids (MCAs) are generated in rodents. Besides, mice also produce ursodeoxycholic acid (UDCA) as primary BAs (Sayin et al., 2013), whereas as a secondary BA in human (Ishizaki et al., 2005). The primary BAs are converted into secondary BAs by gut microbial modifications. BAs play multiple roles in the control of obesity/T2D related glucose and lipid metabolism, and energy homeostasis by activating the nuclear FXR and the cytoplasmic G protein-coupled membrane receptor 5 (TGR5) which regulate a large number metabolic pathways in the host (Thomas et al., 2008, 2009; Wahlstrom et al., 2016). On one hand, FXR is activated mainly by the CA and CDCA (Makishima et al., 1999; Parks et al., 1999; Wang et al., 1999), while TGR5 is stimulated mostly by LCA and DCA which are secondary BAs metabolized from CA and CDCA (Maruyama et al., 2002; Chen X. et al., 2011). On another, Tα/βMCA (primary BAs in mice) and UDCA inhibit FXR activation (Li et al., 2013; Sayin et al., 2013; Mueller et al., 2015). Furthermore, GLP-1 synthesis is inhibited by FXR activition (Trabelsi et al., 2015), while it is activated and secreted by TGR5 activation in colonic L cells (Thomas et al., 2009). GLP-1 signaling may be exploited into a new therapy for T2D with the help of gut microbiota (Claus, 2017; Grasset et al., 2017). Hepatic cholesterol 7a-hydroxylase (CYP7A1) is regulated by intestinal FXR with the contribution of a fibroblast growth factor15 (FGF15) activity (Inagaki et al., 2005). What's more, recent study showed HFD-fed FXR−/− mice an obesity phenotype compared to the wild-type mice (Parséus et al., 2016). Thus, targeting BAs, FXR, and/or TGR5 signaling with microbiota, may shed a new light on preventing or treating metabolic diseases. At present, our knowledge on the mutual effects between BAs and gut microbiota is still far from complete.
In recent studies, the gut metabolite TMAO is also found to have an intimate relationship with metabolic diseases such as T2D (Dambrova et al., 2016; Tang et al., 2016; Schugar et al., 2017), NAFLD (Chen Y. M. et al., 2016), chronic kidney disease (CKD) (Tang et al., 2015a; Xu K. Y. et al., 2017) and bariatric surgery (Troseid et al., 2016), along with the metabolic functions including insulin resistance (Oellgaard et al., 2017) and BAs metabolism (Wilson et al., 2016). With the rapid development in the field of intestinal microbiota, the gut metabolites like TMAO, SCFAs, LPS, and BAs with their signaling interplay between microbiota, have evolved as promising avenues for prevention and treatment of CMDs. Herbal medicine and functionl food with the properity of muti-ingredient, muti-target and muti-pathway action may serve as a prebiotic-like remediation (Laparra and Sanz, 2010; Xu J. et al., 2017). How might they work in CMDs by modulating gut microbiota are discussed below.
Herbal medicine and gut microbiota
The effectiveness of antibiotics in modern medicine has diminished somewhat due to the development of multi-drug resistant bacteria after using for more than 70 years. New classes of antimicrobial drugs are unlikely to become widely available any time soon (Laxminarayan et al., 2016). If and when they do, bacteria, viruses and other microbes will again evolve antimicrobial resistance (AMR) through variety of ways including horizontal gene transfer of mobile genetic elements (Carroll et al., 2014; Jorgensen et al., 2016). Experimental evidence, particularly rodent studies, showed convincingly that prebiotics, non-digestible, fermentable carbohydrates and fibers are capable of enhancing the growth of specific beneficial gut bacteria, thus reducing body weight, reversing insulin resistance and exerting anti-inflammatory effects (Bindels et al., 2015; Sonnenburg and Backhed, 2016). However, these effects have yet to be confirmed by intervention studies in human. Recent investigations support the idea of the involvement of intestinal bacteria in host metabolism and preventative therapeutic potential of prebiotic interventions for CMDs. Herbal medicine may therefore serve as a potential prebiotic remedy to treat CMDs and complications.
Several herbal medicine formulae, herbals and nutraceuticals that contain fiber, polyphenol, polysaccharide and certain other substances have anti-obese, anti-diabetic and anti-atherosclerotic effects through the modulation of diverse gut microbiota. These herbals with their components have the potential to be a new source for CMD drugs discovery that target specifically the gut microbiota, as summarized in Tables 1–3. According to the early direct evidence in 187 T2D patients, a herbal formula Gegen Qinlian Decoction (GQD) including four herbs: Gegen (Radix Puerariae), Huangqin (Radix Scutellariae), Huanglian (RhizomaCoptidis) and Gancao (Honey-fried Licorice Root), showed the anti-T2D effect partly by enriching the amounts of specific beneficial bacteria Faecalibacterium spp. (Xu et al., 2015). Interestingly, both Rhizoma coptidis (as the major component of GQD) and berberine (as the main phytochemicals of Rhizoma coptidis) are confimed to have an anti-obese effect by inhibiting the ratio of Firmicutes/Bacteroidetes, and lowering the growth of Lactobacillus (a classical type of Firmicutes) in HFD-fed mice feces. In addition, Rhizoma coptidis and berberine can reduce HFD-induced body and visecral adipose weights, and blood glucose and lipid levels in mice (Xie et al., 2011). What's more, berberine increases putative SCFA-producing bacteria, including Blautia, Allobaculum, Bacteriodes, Blautia, Butyricoccus, and Phascolarctobacterium, possibly leading to anti-obese and anti-diabetic effects in the host (Zhang et al., 2012a, 2015). Rhizoma coptidis and berberine, also the main ingredients of GQD, may contribute to the significant resistance to metabolic disease by targeting intestinal microbiota, which need to be further confirmed in clinical trials. A recent study showed that berberine improved non-alcoholic steatohepatitis (NASH) by restoring Biffidobacteria and reducing Firmicutes/Bacteroidetes ratio (Cao et al., 2016). Another herbal formula Qushi Huayu Decoction (QHD), a mixture of five herbs (Artemisia capillaries Thunb, Gardenia jasminoides Ellis, Fallopia japonica, Curcuma longa L., and Hypericum japonicum Thunb.) and two active ingredients (geniposide and chlorogenic acid) reduces oxidative stress and inflammatory response in liver by inducing glutathione-generating enzymes, decreases lipid synthesis and elevates steatosis by inhibiting glucokinase expression, and ameliorates gut barrier function and alleviates liver inflammation by inducing Treg-producing baceria. In these studies, 12 phyla of gut bacteria were altered, including increased Fusobacteria, Lentisphaerae, Verrucomicrobia, Cyanobacteria, Deferribacteres, Proteobacteria, and Bacteroidetes, as well as decreased Firmicutes, Tenericutes and Actinobacteria (Yang et al., 2017).
Table 1.
Herbal formulae and gut microbiota.
| Formulae | Herbs & ingredients | Objects | Diseases | Physiological function related to gut microbiota | Gut microbiota | References |
|---|---|---|---|---|---|---|
| Gegen Qinlian Decoction (GQD) |
Herbs: Radix Puerariae (Ge Gen), Radix Scutellariae (Huang Qin), Rhizoma Coptidis (Huang Lian), Honey-fried Licorice Root (Gan Cao), Ingredients: Baicalin, Puerarin, Berberine |
187 T2D patients | T2D | ➀Enrich beneficial bacteria, ➁Reduce blood glucose and glycated hemoglobin. |
Increased: Faecalibacterium spp. |
Xu et al., 2015 |
| Sancai Lianmei Particle (SLP) |
Herbs: Panax ginseng (Ren Shen), Rhizoma Atractylodis Macrocephalae (Bai Zhu), Coptis chiinensis (Huang Lian) |
60 T2D patients | T2D | Regulate intestinal flora of T2D and have similar function with acarbose. | N/A (Not Applicable) | Fang et al., 2016 |
| Ginseng decoction |
Herbs: Panax ginseng (Ren Shen), Ingredients: Ginseng polysaccharides, Ginsenosides |
SD rat | Over-fatigue and acute cold stress model (OACS) | ➀Improve intestinal metabolism and absorption of certain ginsenosides, ➁Reinstate the holistic gut microbiota. |
Increased: Lactobacillus spp. Bacteroides spp. |
Zhou et al., 2016 |
| Daesiho-tang (Korea) |
Herbs: Bupleuri radix (Chai Hu), Pinelliae rhizome (Ban Xia), Zingiberis rhizome (Gan Jiang), Scutellariae radix (Huang Qin), Paeoniae radix (Shao Yao), Zizyphu sfructus (Da Zao), Ponciri fructus, Rheiundulati rhizome |
C57BL/6 mice | Obesity | ➀Ameliorate body weight gain and body fat, ➁Regulate adiponectin and leptin genes expression, ➂Exert an anti-diabetic effect by attenuating fasting glucose level and serum insulin level, ➃Reduce TC, TG and increase HDL, GPT and GOT levels and reduce fat droplets accumulation. |
Increased: Bacteroidetes/Firmicutes ratio, Bacteroidetes, Lactobacillus, Akkermansia, Bifidobacterium. Decreased: Firmicutes. |
Hussain et al., 2016 |
| Yupingfeng polysaccharides |
Herbs: Astragali radix (Huang Qi) Atractylodes macrocephala rhizome (Bai Zhu), Radix saposhnikoviae (Fang Feng), Ingredients: Yupingfeng polysaccharides |
Weaning rex rabbits | Immune-related diseases | ➀Promote growth and immune activities, improve intestinal microbiota homeostasis and maitain intestinal barrier functionality and integrity, ➁Enhance IL-2, IL-4, IL-10, TNF-α, TLR2, TLR4 mRNA levels; improve IL-1, IL-2, IL-4, IL-6, IL-10, IL-12, TNF-α, IFN-γ protein levels, and TLR2, TLR4 mRNA expressions. |
Increased: Cellulolytic bacteria Decreased: Streptococcus spp. Enterococcus spp. |
Sun H. et al., 2016 |
| Qushi Huayu Decoction (QHD) |
Herbs: Artemisia capillaries Thunb (Yin Chenhao), Gardenia jasminoides Ellis (Zhi Zi), Fallopia japonica (Hu Zhang), Curcuma longa L. (Jiang Huang), Hypericum japonicum Thunb. (Tian Jihuang), Ingredients: Geniposide, chlorogenic acid. |
SD rats | NAFLD | ➀Decrease serum LPS, hepatic lipid synthesis, and regulatory T cell inducing microbiota, ➁Improve gut barrier function and hepatic anti-oxidative mechanism. |
Increased: Fusobacteria, Lentisphaerae, Verrucomicrobia, Cyanobacteria, Deferribacteres, Proteobacteria, Bacteroidetes, Decreased: Firmicutes, Tenericutes, Actinobacteria. |
Feng et al., 2017 |
Table 3.
Herbal phytochemicals and gut microbiota.
| Phytochemicals | Catergory | Objects | Diseases | Physiological function related to gut microbiota | Gut microbiota | References |
|---|---|---|---|---|---|---|
| Resveratrol and epigallocatechin-3-gallate | Polyphenol | 37 obese men and women | Obestiy | Increase fat oxidation |
Decreased: Bacteroidetes, Faecalibacterium Prausnitzii. |
Most et al., 2017 |
| Resveratrol | Polyphenol | (1) C57BL/6J ApoE−/− mice | (1) AS | (1) AS-related ➀Reduce TMA production → decrease TMAO synthesis in liver → inhibit AS, ➁Increase BSH activity → promote generation of unconjugated BAs. ➂Decrease BA content → inhibit FXR-FGF15 axis → increase CYP7A1 expression → induce neosynthesis of hepatic BA → promote cholesterol homeostasis → attenuate AS. |
(1) Increased: Lactobacillus, Bifidobacterium. |
Qiao et al., 2014; Chen M. L. et al., 2016 |
| (2) Kunming mice | (2) Obesity | (2) Obesity-related ➀Decrease body and visceral adipose weights, and reduce lipid and blood glucose levels, ➁Increase Fiaf gene expression, and decreases LPL, SCD1, PPAR-γ, ACC1, Fas mRNA expression correlation with fatty acids synthesis, lipogenesis and adipogenesis. |
(2) Increased: Bacteroidetes/Firmicutes ratio, Lactobacillus, Bifidobacterium. Decreased: Enterococcus faecalis. |
|||
| Berberine | Alkaloid | (1) Wistar rats | (1) Obesity, insulin resistance |
(1) ➀Inhibit obesity and insulin resistance development, ➁Increase LPS-binding protein MCP-1, leptin levels, and decrease adiponectin level, ➂Elevate SCFA levels in the intestine. |
(1) Increased: Blautia, Allobaculum. |
Xie et al., 2011; Zhang et al., 2012a, 2015; Cao et al., 2016; Wang Y. et al., 2017 |
| (2) Wistar rats | (2) Obesity | (2) Enrich bacteria produced by SCFA and reduce microbial diversity | (2) Decreased: Allobaculum, Bacteroides, Blautia, Butyricoccus, Phascolarctobacterium |
|||
| (3) C57BL/6J mice | (3) Obesity | (3) Decrease dietary polysaccharides degradation, lower the intake of potential calorie, and activate the expressions of Fiaf protein and related genes of mitochondrial energy metabolism. | (3) Increased: Bacteroidetes/Firmicutes ratio, Decreased: Lactobacillus, Bacteroidetes/Firmicutes ratio, |
|||
| (4) BALB/C Mice | (4) NAFLD | (4) Reduce body weight, and lipids, glucose, insulin level in serums. Improve transaminase activity and NAFLD activity score through down-regulated CD14, IL-1, IL-6, TNF-α. | (4) Increased: Bacteroidetes/Firmicutes ratio, Bifidobacteria. |
|||
| (5) SD rat | (5) Energy metabolism | (5) ➀Promote butyrate production, ➁Reduce blood lipid and glucose level, ➂Regulate energy metabolism by suppressing bacterial ATP production and nicotinamide adenine dinucleotide phosphate (NADH) levels. |
(5) Increased: Enterobacter, Escherichia–Shigella. |
|||
| Quercetin | Polyphenol | Wistar rats | Obesity | Prevent body weight gain and reduce serum insulin levels and insulin resistance. |
Decreased: Firmicutes/Bacteroidetes ratio, Erysipelotrichaceae, Bacillus, Eubacteriumcylindroides |
Etxeberria et al., 2015a |
| Curcumin | Polyphenol | (1) 129/SvEv mice, germ-free Il10−/− mice | (1) Colitis and colon cancer | (1) Increase survival, decrease colon weight/length ratio, eliminate tumor burden. | (1) Increased: Lactobacillus, Lactobacillales, Bifidobacteriales, Erisipelotrichales, Coriobacteriales, Decreased: Clostridiales, Firmicutes. |
Ghosh et al., 2014; Mcfadden et al., 2015 |
| (2) LDLR−/− mice | (2) AS | (2) ➀Decrease LPS levels, ➁Increase intestinal barrier function by restoring intestinal alkaline phosphatase activity and tight junction proteins ZO-1 and Claudin-1 expression, ➂Reduce glucose intolerance and AS. |
(2) N/A | |||
| Ophiopogon japonicas polysaccharide MDG-1 | polysaccharide | C57BL /6J mice | Obesity | Improve gut microbiota diversity and promote proliferation. |
Increased: Taiwan lactobacillus, Lactobacillus murinus |
Shi et al., 2015 |
| Pterostilbene | Polyphenol | Zucker (fa/fa) rats | Obesity | Improved metabolic function (insulin sensitivity) and Anti-obesity. |
Increased: Akkermansia, Odoribacter, Verrucomicrobia, Decreased: Firmicutes. |
Etxeberria et al., 2016 |
| Rhein | Polyphenol | C57BL/6J | Obesity | ➀Reduce body weight and improve glucose tolerance, ➁Inhibit macrophage accumulation, anti-neuroinflammation and improve BDNF expression. |
Increased: Bifidobacterium spp. Lactobacillus spp. Decreased: Prevotella spp. Desulfovibrios spp. |
Wang et al., 2016 |
| Taurine | Amino acid | BALB/C mice | Neuroendocrine functions | Increase SCFA content in feces, decrease LPS content in serum. |
Decreased: Proteobacteria (especially Helicobacter) |
Yu et al., 2016 |
Table 2.
Herbs and gut microbiota.
| Herbs | Ingredients | Objects | Diseases | Physiological function related to gut microbiota | Gut microbiota | References |
|---|---|---|---|---|---|---|
| Rehmannia glutinosa Libosch (Shu Dihuang) | N/A | Twenty 40–65 years old female middle-aged subjects with obesity | Obesity | Decrease waist circumference. |
Increased: Actinobacteria, Bifidobacterium, Decreased: Firmicutes, Blautia. |
Han et al., 2015 |
| Ganoderma lucidum (Ling Zhi) | Polysaccharides | C57BL/6NCrlBltw mice | Obesity | ➀Reverse HFD-induced gut dysbiosis, ➁Anti-obesity. |
Decreased: Firmicutes/Bacteroidetes ratio, Proteobacteria. |
Chang C. J. et al., 2015 |
| Flos Lonicera (JinYinghua) | Flavonoids, Organic acids, Saponins, Iridoid glycosides |
SD rats | Obesity and metabolic endotoxemia | ➀Decrease body weights, ➁Lower endotoxin, aspartate transaminase, HDL, triglyceride levels, ➂Reduce lipid accumulation and alleviate urinary lactulose/mannitol ratio. |
Increased: Akkermansia spp., Bacteroidetes/Firmicutes ratio. |
Wang et al., 2014 |
| Rhizoma Coptidis (Huang Lian) | Berberine | C57BL/6J mice | Obesity | Lower degradation of dietary polysaccharides, decrease potential calorie intake, increase Fiaf protein and its related gene expressions of mitochondrial energy metabolism. |
Increased: Bacteroidetes/Firmicutes ratio, Decreased: Lactobacillus. |
Xie et al., 2011 |
| Lingonberry (Vaccinium vitis-idaea L.) | 20% lingonberries | C57BL/6J mice | Obesity | ➀Reduce endotoxemia and inflammation, ➁Anti-obesity. |
Increased: Akkermansia/Faecalibacterium ratio. |
Heyman-Linden et al., 2016 |
| Herba Epimdii (Yin Yanghuo) | Icariin, Epimedin A, B, C | SD rats | Osteoporosis | Enhance epimedium flavonoids absorption and antiosteoporosis activity. | N/A | Zhou et al., 2015 |
| Garcinia cambogia (Teng Huangguo) | Extract | C57BL/6J mice | Obesity | Alleviate weight gain and adiposity |
Decreased: Clostridium aminophilum. |
Heo et al., 2016 |
| Cassia obtusifolia L. (Jue Mingzi) | Anthraquinone | SD rats | NAFLD | ➀Evaluate Lipid metabolism and gut microbiota diversity, ➁Up-regulate FXR, CYP7A1, LDL-R mRNA and PPAR-α protein levels, down-regulate HMGCR, PPAR-γ and SREBP-1c expression. |
Increased: Lactobacillus, Bacteroides, Parabacteroides, Decreased: Oscillospira. |
Mei et al., 2015 |
|
Radix ginseng rubra (Hong Shen) and Semen Coicis (Yi Yiren) |
N/A | Wistar rat | Ulcerative colitis (UC) | Improve gut microbiota structure and relieve the ulcerative Colitis symptom. |
Increased: Bifidobacterium, Lactobacillus. |
Guo et al., 2015 |
| Polygonatum kingianum (Dian Huangjing) | Polysaccharides, Saponins | SD rats | T2D | ➀Reduce SCFAs production, ➁Decrease LPS level, ➂Partial recover insulin secretion and fasting blood glucose levels |
Increased: Family Ruminococcaceae, Genus Ruminococcus. |
Yan et al., 2017 |
| Adlay | Polyphenol extract | Wistar rats | High cholesterol-related disease | Ameliorate and LDL cholesterol restore HDL cholesterol. |
Decreased: Erysipelotrichales, Clostridia. |
Wang Q. et al., 2015 |
Resveratrol (RSV), a natural polyphenolic compound extrated from herbal medicine Rhixoma Polygoni Cuspidati or functional food peanut, grape, and Fructus Mori, exerts antioxidant, anti-inflammatory (Walker et al., 2014), anti-tumor, cardioprotective, aging-delay, and anti-obesity effects (Baur and Sinclair, 2006; Zhang et al., 2012b). On one hand, RSV decreases TMAO levels and increases hepatic BA neosynthesis via increasing the genera Lactobacillus and Bifidobacterium, thus attenuating TMAO-induced AS in ApoE−/− mice. RSV-induced BA neosynthesis was partially mediated through the enterohepatic FXR-fibroblast growth factor 15 (FGF15) axis (Chen M. L. et al., 2016). On another, RSV increases the ratio of Bacteroidetes/Firmicutes and the growth of Lactobacillus and Bifidobacterium. It also reduces the growth of Enterococcus faecalis through fasting-induced adipose factor (Fiaf, a key gene expresses in the intestine and negatievely regulated by interstinal flora) signaling, decelerating the development of obesity (Qiao et al., 2014). RSV is probably an unique and firstly reported natural product that mediates protection against both CVD and metabolic diseases via gut microbiota to date. In addition, quercetin, a key member of the polyphenol family, is discovered in numerous medicinal botanicals, including Ginkgo biloba, Hypericum perforatum, and Sambucus canadensi and also found in a variety of functional foods including apple, grape, berry, onion and tea (Li Y. et al., 2016). Intake of quercetin reduced body weight gain and attenuated serum insulin levels by reducing Firmicutes/Bacteroidetes ratio and inhibiting the growth of bacterial species Erysipelotrichaceae, Bacillus and Eubacterium cylindroides, which correlated with HFD-induced obesity (Etxeberria et al., 2015a). Moreover, it was shown in a recent study that curcumin, the major polyphenolic ingredient of an edible herb Curcuma longa L. improved intestinal barrier function by modulation of intracellular signaling, and organization of tight junctions, providing a mechanism that curcumin modulates chronic inflammatory diseases despite poor bioavailability (Wang J. et al., 2017). The details of some other herbal medicines, including formulae, herbals and phytochemicals reportedly to achieve their therapeutic effects for CMDs through gut microbiota modulation are summarized in Tables 1–3.
Functional food and microbiota
Functional food has the advantages of wide availability, ease of preparation and fewer adverse effects. They could be well suited for CMDs remedies due to their potential effects such as anti-inflammatory, antioxidants, antiestrogenics, immunomodulatory, whereas purified active compounds are preferable as pharmaceutical drugs for the treatment of severe chronic symptoms (Martel et al., 2016; Meyer and Bennett, 2016). Epidemiological studies have identified associations between frequent consumption of fruits, vegetables, whole grains and teas, which are rich in fiber, polyphenol, and polysaccharide could reduce the risk of CMDs (Woodside et al., 2013; Klinder et al., 2016). These phytochemicals and their metabolic products may inhibit pathogenic bacteria while stimulating the growth of beneficial bacteria for CMDs (Laparra and Sanz, 2010).
Apples are among the most frequently consumed fruits to prevent obestiy by modulating gut microbiota with their multiple components, including fiber, pectin (Jiang et al., 2016), procyanidins (Masumoto et al., 2016) and polysaccharides (Wang S. et al., 2017). Administration with apple procyanidins (a subclass of polyphenols) for 20 weeks was able to reduce obesity, decrease lipid metabolism related genes expression, lower LPS levels and gut permeabiliby through decreasing the Firmicutes/Bacteroidetes ratio and increasing Akkermansia proportion (Masumoto et al., 2016). In addition, treatment with apple polysaccharide inhibited chronic inflammation, gut permeability, and SCFAs production, leading to lower abundance of Firmicutes and Fusobacteium, and higher Bacteroidetes and Lactobacillus in HFD-fed rats (Wang S. et al., 2017). Furthermore, the reciprocity between apple ingredients and the gut microbioa may benefit cardiovascular health (Koutsos et al., 2015). Unexpectedly, diet apple fiber and flavone were positively associated with Blautia, Lactobacillus, Bifidobacterium, and Faecalibacterium, showing great significance for the patients who suffer from systemic lupus erythematosus (SLE) (Cuervo et al., 2015; Table 4). As another example, ingestion of laminarin, a kind of polysaccharides extracted from Laminaria japonica, by HFD-fed mice significantly increased genus Bacteroides and decreased Firmicutes, with elevated energy metabolism (Nguyen et al., 2016; Table 5). Besides, 3,3-dimethyl-1-butanol (DMB), the structural analog of choline detected in some functional food such as balsamic vinegars, red wines, and olive oils (Kitai and Tang, 2017), is an inhibitor of TMA formation through inhibition of microbial TMA lyases. Therefore, it inhibited choline diet-promoted macrophage foam cell formation and atherosclerotic lesion development without altering the circulating cholesterol levels (Wang Z. et al., 2015).
Table 4.
Functional food and gut microbiota.
| Functional Food | Ingredients | Objects | Diseases | Physiological function related to gut microbiota | Gut microbiota | References |
|---|---|---|---|---|---|---|
| Vegetable/fruit juice | Polyphenols, Oligosaccharides, Fber, Nitrate |
Twenty adults | Obesity | ➀Alter the intestinal microbiota associated with weight loss, ➁Increase in vasodilator NO, ➂Decrease in lipid oxidation. |
Increased: Bacteroidetes, Cyanobacteria, Decreased: Firmicutes, Proteobacteria. |
Henning et al., 2017 |
| Barley | β-Glucan | 30 volunteers | CVD | N/A |
Increased: Bacteroidetes, Prevotella, Decreased: Firmicutes, Dorea. |
Wang Y. et al., 2016 |
| Apple | (1) procyanidin | (1) C57BL/6J mice | (1) Obesity | (1) ➀Attenuate inflammatory effects and weight gain including gut permeability and lipopolysaccharide, ➁Decrease endogenous metabolites levels related with insulin resistance. |
(1) Increased: Akkermansia, Decreased: Firmicutes/Bacteroidetes ratio. |
Cuervo et al., 2015; Jiang et al., 2016; Masumoto et al., 2016; Wang S. et al., 2017 |
| (2) pectin | (2) SD rat | (2) Obesity | (2) ➀Attenuate weight gain and serum total cholesterol Level, ➁Improve intestinal alkalinephosphatase, claudin 1 expression, decrease TLR4 expression in ileal tissue, decrease inflammation (TNF-α) and metabolic endotoxemia. |
(2) Increased: Bacteroidetes, Decreased: Firmicutes. |
||
| (3) Polyphenols | (3) 20 Systemic lupus erythematosus patients | (3) Systemic lupus erythematosus | (3) N/A | (3) Increased: Bifidobacterium. |
||
| (4) Polysaccharide | (4) SD rat | (4) Microbial dysbiosis and chronic inflammation | (4) ➀Increase total SCFAs level, ➁Alleviate gut permeability and chronic inflammation (decrease LBP, up-regulation of occludin, down-regulation TNF, MCP-1, CXCL-1, IL-1β). |
(4) Increased: Bacteroidetes, Lactobacillus, Decreased: Firmicutes, Fusobacteium. |
||
| Oranges | Polyphenols | 20 Systemic lupuserythematosus patients | Systemic lupus erythematosus | N/A |
Increased: Lactobacillus, |
Cuervo et al., 2015 |
| Grape | (1) Pomace, Polyphenols | (1) Lamb | (1) N/A | (1) Decrease oxidative stress-induced damage to lipids and proteins such as TBARS and CARB. | (1) Decreased: Enterobacteriacae, Escherichia coli. |
Baldwin et al., 2016; Kafantaris et al., 2016 |
| (2) N/A | (2) C57BL/6J mice | (2) Obesity | (2) ➀Decrease triglyceride and liver weight levels and reduce GPAT1 expression, ➁Reduce hepatic mRNA PPAR-γ2, SCD1, FABP4 and GPAT1 levels |
(2) Increased: Akkermansia muciniphila, Allobaculum, Decreased: Desulfobacter spp. |
||
| Grape seed | Proanthocyanidin | C57BL/6 mice | Obesity | ➀Decrease plasma inflammatory factors TNF-α, IL-6 and MCP-1 levels, ➁Ameliorate macrophage infiltration, ➂Reduced epidydimal fat mass and improve insulin sensitivity. |
Increased: Clostridium XIVa, Roseburia, Prevotella. |
Liu et al., 2017 |
| Agave salmiana | Saponin | C57BL/6 mice | Obesity and hepatic steatosis | ➀Reduce fat mass and weight gain, Lower insulin, glucose, and LDL levels, ➁Lower hepatic lipid levels and HOMA index, increase fatty acid oxidation related genes expression, ➂Increase fatty acid oxidation, AMPK phosphorylation, white adipose tissue browning, mitochondrial activity and energy expenditure. |
Increased: Akkermansia muciniphila. |
Leal-Diaz et al., 2016 |
| Cranberry | Cranberry extract | C57BL/6J mice | Obesity | ➀Decrease liver weight and triglyceride accumulation involved in inflammation and blunted hepatic oxidative stress, ➁Improve insulin tolerance, decrease glucose-induced hyperinsulinaemia, ➂Lower intestinal triglyceride content and alleviate intestinal oxidative stress and inflammation. |
Increased: Akkermansia. |
Anhe et al., 2015 |
| Bamboo shoot | Fiber | C57BL/6J mice | Obesity | Lose weight. |
Increased: Bacteroidetes, Decreased: Verrucomicrobia. |
Li et al., 2016 |
| Nopal | Fiber, Polyphenols, Vitamin C |
Wistar rat | Obesity | ➀Modify gut microbiota and increase intestinal occludin-1, ➁Decrease in LPS, glucose insulinotropic peptide, glucose intolerance, lipogenesis, and metabolic inflexibility, ➂Reduce hepatic steatosis and oxidative stress in adipose tissue and brain, ➃Improve cognitive function. |
Increased: Bacteroidetes/Firmicutes ratio, Anaeroplasma, Prevotella, Ruminucoccus, Bacteroides fragilis, Decreased: Faecalibacterium, Clostridium, Butyricicoccus. |
Sanchez-Tapia et al., 2017 |
| Wheat | (1) Enzyme-treated wheat bran, | (1) C57BL/6J mice | (1) Obesity | (1) ➀Decrease body weight and liver TGs, increase index of liver reactive oxygen species, ➁Decrease liver antioxidants (glutathione and α-tocopherol) and liver carbohydrate metabolites (glucose); lower hepatic arachidonic acid; and increase liver and plasma β-hydroxybutyrate. |
(1) Increased: Bacteroidetes, Decreased: Firmicutes. |
Neyrinck et al., 2011; Kieffer et al., 2016 |
| (2) Arabinoxylan | (2) C57BL/6J mice | (2) Obesity | (2) ➀Regulate host metabolic parameters: reduce body weight gain, fat mass development, inflammation (serum IL-6, MCP-1), cholesterolemia and insulin resistance, and increase gut junction proteins. ➁Regulate host adipose tissue: reduce lipogenesis (Fatty acid synthase), fatty acid oxidation (carnitinepalmitoyl transferase-1), fatty acid uptake (lipoprotein lipase) and GPR-43 expression, and increase adipocyte area and rumenic acid. |
(2) Increased: Bacteroides/Prevotella ratio, Bifidobacteria, Roseburia spp. |
||
| Oat | N/A | SD rat | Obesity | ➀Decrease body weight, epididymal fat accumulation, and serum inflammatory factor (TNF-α) levels and significantly regulate serum lipid levels, ➁Increase the total SCFA concentration in colonic digesta. |
Increased: Bacteroidetes, Bacteroidetes/Firmicutes Ratio, Decreased: Firmicutes. |
Dong et al., 2016 |
| Tea Polyphenols | Polyphenols | C57BL/6 ApoE–/– mice | AS | ➀Decrease the total cholesterol and low-density lipoproteincholesterol, ➁Decrease the plaque area/lumen area ratios. |
Increased: Bifidobacteria. |
Liao et al., 2016 |
| Green tea and isomalto-oligosaccharides | N/A | HFD-induced male Swiss albino mice | Obesity | Prevent leaky gut phenotype and LPS, pro-inflammatory cytokines (e.g. resistin, adiponectin, TNF-α, IL-1β, IL-6) increase |
Increased: Lactobacillus, Bifidobacteria, Akkermansia, Roseburia spp., Prevotella/Bacteroides, Decreased: Firmicutes/Bacteriodetes ratio. |
Singh et al., 2017 |
| Yellow pea | Fiber | SD rat | Obesity | Lower final percent body fat. |
Decreased: Firmicutes. |
Eslinger et al., 2014 |
| Green tea, oolong tea, black tea | 8 phenolic acids, 12 flavanols, 9 flavonols, 2 alkaloids, 1 amino acids | C57BL/6J mice | Obesity | Trend to lose weight. |
Increased: Alistipes, Rikenella, Lachnospiraceae, Akkermansia, Bacteroides, Allobaculum, Parabacteroides. |
Liu et al., 2016 |
| Fuzhuan tea | N/A | Wistar rats | NAFLD | Reduce plasma leptin and prevent high saturated fat diet-induced inflammation. |
Increased: Lactobacillus spp. |
Foster et al., 2016 |
Table 5.
Functional food phytochemicals and gut microbiota.
| Phytochemicals | Category | Objects | Diseases | Physiological function related to gut microbiota | Gut microbiota | References |
|---|---|---|---|---|---|---|
| Laminarin | Polysaccharide | BALB/c mice | Obesity | Reduce energy metabolism |
Increased: Bacteroidetes (especially the genus Bacteroides), Decreased: Firmicutes. |
Ko et al., 2014; Nguyen et al., 2016 |
| Fucoidan | Polysaccharide | C57BL/6J mice | Intestinal dysbiosis | Reduce inflammatory response and antigen load, and decrease LPS-binding protein levels. |
Increased: Lactobacillus, Ruminococcaceae, Decreased: Peptococcus. |
Shang et al., 2016 |
| Melatonine | Alkaloid | C57BL/6J mice | Obesity | Change gut microbiota composition |
Increased: Akkermansia, Decreased: Firmicutes/Bacteroidetes ratio. |
Xu P. et al., 2017 |
| Fructans | Polysaccharide | C57BL/6J mice | Obesity | Improve intestinal physiology and shift gut microbiota. |
Increased: Actinobacteria, Verrucomicrobia (Akkermansia). |
Liu J. P. et al., 2016 |
| Capsaicin (Chili peppers) | Alkaloid | C57BL/6J mice | Obesity | ➀Prevent HFD-induced gut barrier dysfunction by inhibiting cannabinoid receptor type 1 (CB1). ➁Protect against HFD-induced obesity is transferrable. |
Increased: Ruminococcaceae, Lachnospiraceae. |
Kang et al., 2017 |
| 3,3-dimethyl-1-butanol (DMB) | Choline analogue | C57BL/6J mice | AS | Reduce microbial trimethylamine formation and inhibit choline diet-enhanced AS. | N/A | Wang Q. et al., 2015 |
Moreover, vegetables (e.g., bamboo shoot), whole grains (e.g., wheat, barley and oat), and teas (e.g., green tea, oolong tea, black tea and fuzhuan tea), exert positive effects on CMDs through modulating gut microbiota (Table 4). Numerous instances are detailed in Tables 4, 5. Although, intervention studies conducted both in animals and humans have demonstrated beneficial effects of functonal foods on anti-inflammation, vascular function, and energy metabolism, the apparent association with altered gut microbiota is still lacking.
Overlaping effects between herbal medicine and functional food on gut microbiota
Medicine and food deriving from the same source has been realized since ancient times. In TCM, food is conceptualized according to both nutritional and functional aspects, and can be used to treat illnesses. The “medicine-food homology” concept has given a new meaning since the discovery of human-microbiota existing as a whole symbiotic ecosystem. Interestingly, a series of overlapping characteristics through modulating gut microbiota for CMDs between herbal medicine and functional food are uncovered based on the description above (Tables 1–5): ➀shared components, ➁similar functions, ➂common mechanisms, and ➃same intestinal microbiota.
First and foremost, there is no absolute boundary between medicine and food. Some medicines are food whereas certain foods can be employed as medicine. Lonicera japonica Thunb, Cassia obtusifolia L., Semen Coicis, adlay, Zingiber officinale Roscoe (major ingredient: curcumin), and mulberry (main ingredient: RSV), are edible medicines, there are only dosage differences between edible and medicinal use. These herbs not only belong to medicine with valid efficacies for CMDs remedy, but also are delicious food with rich nutrients. Besides, some medicines have been developed into nutraceuticals, which contain important natural bioactive compounds that confer health-promoting and medical benefits to humans, such as Ganoderma lucidum, Herba Epimdii, Ophiopogon japonicas, Rehmannia glutinosa Libosch, Rheum rhabarbarum (major ingredient: rhein), and lingonberry, see Tables 1–3. Furthermore, many foods could serve as nutraceutical candidates, and some of those, such as pomegranate peel, bamboo shoot, grape (major ingredient: RSV), and laminarin have the potential to branch into medicines (Tables 4, 5).
Secondly, the components of fibers (e.g., bamboo shoot, nopal, and yellow pea), polyphenols (e.g., GQD, Flos Lonicera, adlay, apple, grape, orange, nopal and tea) and/or polysaccharides (e.g., Ginseng decoction, Yupingfeng, Ganoderma lucidum, Polygonatum kingianum, Ophiopogon japonicas, apple and barley) are shared in most herbals and foods, exerting prebiotics-like effects for CMDs, which can be seen in Tables 1–5. Moreover, polyphenol phytochemicals such as RSV and quercetin are present in both herbals and foods. These components are able to escape absorption in the upper gastrointestinal tract and reach the large intestine without breaking down. Thus, these components can also be converted by local microbiota to biologically active and bioavailable metabolites with systemic effects.
Thirdly, just as what we have introduced above, gut microbiota-derived metabolites such as SCFAs, LPS, Bas, and TMAO are the most likely microbial metabolites linking CMDs remedy to intestinal microbiota. Numerous herbals and foods are likely to prevent and treat CMDs through these mediators. Improved gut permeability and gut integrity in conjunction with the increased expression of ZO-1 and/or occludin-1 and/or claudin-1, resulted in reduction of circulating LPS levels and a series of inflammatory response, which are affected by herbals (Yupingfeng polysaccharides enhanced immunity, Sun H. et al., 2016). QHD was used in the treatment of NAFLD (Feng et al., 2017); Flos Lonicera ameliorated obesity (Wang J. H. et al., 2014); curcumin attenuated AS (Ghosh et al., 2014) and foods [apple derived polyphenols and polysaccharide prevented obesity, (Masumoto et al., 2016; Wang S. et al., 2017), nopal and capsaicin were used in combacting obesity (Kang et al., 2017; Sanchez-Tapia et al., 2017)]. SCFAs production was shown to restore aberrant levels of gut hormones such as GLP-1, PYY, and the activation of GPR43. SCFAs production are also promoted by berberine in energy metabolism, insulin resistance and obesity (Xie et al., 2011; Zhang et al., 2015; Xu J. H. et al., 2017); elevated by apple polysaccharide in chronic inflammation, and enriched by oat in obesity treatment (Wang S. et al., 2017). In addition, Polygonatum kingianum and taurine intervene with both SCFAs and LPS levels in different CMDs (Yu et al., 2016; Yan et al., 2017). TMAO levels were inhibited via the reduction of TMA formation by RSV and then attenuate AS (Wang Z. et al., 2015; Chen M. L. et al., 2016). At the same time, RSV increased BAs deconjugation and fecal excretion by enhancing the activity of hydrolase activity, which displayed correlation with the lowered BA content in ilealby suppressing FXR-FGF15 axis and promoting CYP7A1 expression (Chen M. L. et al., 2016). All of these interventions are along with the changed microbiota composition. An increasing number of metabolic pathway and potential mechanisms are studied on the mediators of SCFAs, LPS, BAs and TMAO. These studies provide a better understanding of how herbals and foods prevent or treat CMDs by gut microbiota. The cross-talk between these mediators and specific alteration of intestinal bacteria in host physiology, as well as the precise contributing elements in herbals and foods for CMDs remedy shoud be subjects for future studies.
Finally, previous work has established that genera Clostridium, Lactobacillus and Ruminococcus, as well as the butyrate producers Eubacterium, Fecalibacterium and Roseburia are the important members of Firmicutes. Bacteroidetes including the genus Bacteroides, Prevotella and Xylanibacter are known to be efficient degraders of dietary fiber (Simpson and Campbell, 2015). Genus Bifidobacterium is a major member of Actinobacteria. Proteobacteria contains Escherichia and Desulfovibrio, whereas Verrucomicrobia includes only the mucus-degrading genus Akkermansia so far (Schroeder and Backhed, 2016). Tables 1–5 show that the ratio of Firmicutes/Bacteroidetes is modulated in most herbal- and food-intervention studies for CVD as well as various metabolic diseases. For example, decreased ratio of Firmicutes/Bacteroidetes was observed in obesity after intervened by herbals [Daesiho-tang (Hussain et al., 2016), Ganoderma lucidum (Chang C. J. et al., 2015), Flos Lonicera (Wang J. H. et al., 2014), Rhizoma coptidis (Xie et al., 2011), Resveratrol (Qiao et al., 2014), Berberine (Xie et al., 2011; Zhang et al., 2012a), Quercetin (Etxeberria et al., 2015b)] and foods [apple (Jiang et al., 2016; Masumoto et al., 2016), nopal (Sanchez-Tapia et al., 2017), wheat (Kieffer et al., 2016), Laminarin (Nguyen et al., 2016)], as well as in QHD (Feng et al., 2017) and berberine (Cao et al., 2016) for NAFLD (Feng et al., 2017), and barley for CVD (Wang Y. et al., 2016). All of these studies confirmed that increase in gut bacteria phylum Bacteroidetes, and inhibition of Firmicutes, and alteration of Firmicutes/Bacteroidetes ratio helped to treat CMDs including obesity (Ley et al., 2006; Sweeney and Morton, 2013), insulin resistance (Greenhill, 2015), NAFLD (Liu J. P. et al., 2016) and CVD (Marques et al., 2017). In addition, an increase in the Akkermansia population was found to be in favorable treatment for T2D (Shin et al., 2014), obesity (Everard et al., 2013), AS (Li J. et al., 2016) and some other metabolic syndromes (Roopchand et al., 2015). A recent study showed that fat mass development, insulin resistance and dyslipidemia were reduced by purified membrane protein from Akkermansia (Plovier et al., 2017). Interestingly, the abundance of Akkermansia was dramatically increased not only by Daesiho-tang for T2D (Hussain et al., 2016) and agave salmiana for hepatic steatosis (Leal-Diaz et al., 2016), but also by Flos Lonicera (Wang J. H. et al., 2014), pterostilbene (Etxeberria et al., 2016), apple (Masumoto et al., 2016), grape (Baldwin et al., 2016), agave salmiana (Leal-Diaz et al., 2016), cranberry (Anhe et al., 2015), green tea (Liu Z. et al., 2016; Singh et al., 2017), melatonine (Xu P. et al., 2017) and capsaicin (Kang et al., 2017) for obesity. Moreover, various diseases such as obesity, diabetes and ballergies have been associated with lower numbers of Bifidobacterium at various stages of life (Arboleya et al., 2016). The therapeutical effects of RS for AS (Chen M. L. et al., 2016), berberine for NAFLD (Cao et al., 2016), and Rehmannia glutinosa Libosch (Han et al., 2015), Daesiho-tang (Hussain et al., 2016), rhein (Wang et al., 2016), tea polyphenols (Singh et al., 2017), green tea (Singh et al., 2017) for obesity were associated with the elevated abundance of Bifidobacterium. What's more, increased abundance of genus Lactobacillus, Bacteroides, and Prevotella which contribute to metabolic diseases and/or CVD, were also closely associated with digestion of herbals and foods, as shown in Tables 1–5. In summary, increasing the abundance of phylum Bacteroidetes, and genus Akkermansia, Bifidobacterium, Lactobacillus, Bacteroides, and Prevotella, while reducing phylum Firmicutes and Firmicutes/Bacteroidetes ratio may serve as the common characteristics for gut bacteria modulation of herbal medicine and functional food for CMDs. For future studies, the related gut microbiota species interplay with plants and mammalian hosts need to be further investigated.
Potential effects of herbal medicine and functional food on gut-organs axes
Commensal gut bacteria impact the host health especially CMDs processes in multiple organs. Several new concepts are proposed in recent reviews focusing on the relationship between gut and organs, such as gut-heart axis (Buglioni and Burnett, 2013), gut-brain axis (De Clercq et al., 2017; Dinan and Cryan, 2017), gut-liver axis (Wiest et al., 2017), gut-kidney axis (Katagiri et al., 2013; Budden et al., 2017) and gut-liver-lung axis (Young et al., 2016). The gut is no longer viewed as just a digestive organ, it is also considered as a metabolic and immunomodulatory organ. The major components of fiber, polyphenols and polysaccharides are present in large quantities in both herbal medicine and functianl food, which we have analyzed above. Besides, their muti-ingredient, muti-target and muti-pathway mode are well known and capable of meeting the complex system of the gut-organ interactions. Targetinig the gut-organs axes may also be responsible for CMD treatment. The potential effects are implicated by some latest reports. For instance, a recently published study found that chronic prebiotic treatment indeed exhibited both antidepressant and anxiolytic effects, reduced stress-induced corticosterone release, and modified specific gene expression in the hippocampus and hypothalamus. These effects were exerted via increased cecal acetate and propionate and reduced isobutyrate concentrations. These findings provided clear evidence supporting therapeutic targeting of the gut microbiota for gut-brain axis disorders (Burokas et al., 2017). Another recent finding (Marques et al., 2017) illustrated how HFD and supplementation with acetate influenced gut-heart-kidney axis in a mouse hypertension and heart failure model. It was found that both fiber and acetate decreased gut dysbiosis, measured by the ratio of Firmicutes to Bacteroidetes, and increased the prevalence of Bacteroides acidifaciens. Both HFD and acetate supplementation significantly reduced blood pressure, cardiac fibrosis, and left ventricular hypertrophy. Transcriptome analyses showed that the protective effects of high fiber and acetate were accompanied by the downregulation of cardiac and renal early growth response protein 1 (EGR1), a master cardiovascular regulator involved in cardiac hypertrophy, cardiorenal fibrosis, and inflammation. The upregulation of a network of genes involved in circadian rhythm in both tissues and downregulation of the renin-angiotensin system in the kidney and MAPK signaling in the heart presents an interesting example of gut-multi organs interactions that are simultaneously affected by diet via microbiota.
Althrough there are no reports on herbal medicine or functional food directly targeting gut-organs axes, more study should be carried out in the area to fully exploit the beneficial aspects of gut microbiota. Major components such as fiber and polysaccharide could be fermented and converted into SCFAs which has been shown to be beneficial on the treatment of CMDs. It is foreseeable that the influence of functional food and herbal medicine on the interactive and dynamic relationships between gut microbiota and essential organs will be elucidated in the future.
Conclusions and perspective
The concept of “medicine-food homology” has evolved from its ancient origin and is given a new prospective with newly revealed role of gut microbiota of the host. Human diseases, particularly CMDs, not only could be treated by herbal derived medicine, but also could be prevented by medicinal food via co-inhabiting and influcing gut microbiota.
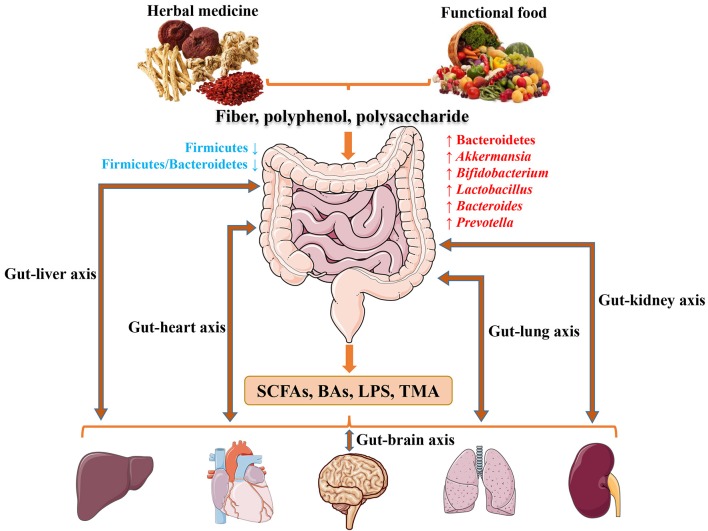
With the rapid advancement of sequencing technology and intense efforts by researchers, a significant understanding of host gut microbiota has been achieved (Xiao et al., 2017). It is also becoming apparent that herbal medicine and functional food may strongly influence gut microbiota associated with CMDs in humans ranging from obesity to T2D and CVD. Nevertheless, studies on the interaction between herbal derived bioactive compounds and gut microbes are still needed. Future investigation in the field may include, but not limited to the following directions: (1) disease-related and disease-specific microbiota and pathological mechanisms; (2) future research on herbal medicine and functional food should exploit molecular mechanisms and the relationship between microbiota and host behavior; (3) until now, most medicine and functional food research has focused on obesity and T2D rather than CVD, which deserves more careful studies and funding; (4) certain polyphenols (puerarin, paeoniflorin, baicalein, icariin, mangiferin, gallic acid, luteolin, cryptotanshinone, kaempferol, etc.) are similar to RSV and showed poor absorption into the bloodstream after oral administration, but they may have an impact on gut microbiota as well. In conclusion (Figure 1), herbal medicine and functional food with major ingredients including fiber, polyphenols and polysaccharides are inclined to increasing abundance of phylum Bacteroidetes, and genus Akkermansia, Bifidobacterium, Lactobacillus, Bacteroides, and Prevotella, while reducing phylum Firmicutes and Firmicutes/Bacteroidetes ratio to prevent or treat CMDs through SCFAs, BAs, LPS and TMAO signaling. The condition of health or disease in human is critically dependent on the balance between medicine/food by modulating gut microbiota. Human intake of herbal medicine and functional food can alter gut microbiota, and microbiome in turn can influce human health through microbial metabolites. The convergence between herbal medicine and functional food through microbiota reinforces the idea that CMDs are not only treatable but also preventable by maintaining the balance between the two.
Figure 1.
The potentially shared biological processes and underlying mechanisms of herbal medicine and functional food for CMDs through modulating microbiota.
Author contributions
YZ conceived, designed, organizedand revised the manuscript; ML conceived, designed, wrote and revised the manuscript; YW, GF, XW, and SX revised the manuscript and discussed interpretation.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by grants from National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (Nos. 2013ZX09201020 and 2015ZX09J15102-004-004), the National Science Foundation of China (NSFC 81274128), and the International Cooperation Project of MOST, China (2013DFA31620). We thank John Orgah for his expert editorial assistance.
Glossary
Abbreviations
- CMDs
Cardiometabolic diseases
- CVD
cardiovascular disease
- T2D
type 2 diabetes
- NAFLD
non-alcoholic fatty liver disease
- AS
atherosclerosis
- CKD
chronic kidney disease
- MI
myocardial infarction
- SCFAs
short-chain fatty acids
- Bas
bile acids
- LPS
lipopolysaccharide
- TMAO
trimethylamine–N-oxide
- TCM
traditional Chinese medicine
- MetaHIT
Metagenomics of the Human Intestinal Tract
- GI
gastrointestinal
- HFD
high-fat-diet
- UC
ulcerative colitis
- AMR
antimicrobial resistance
- RSV
resveratrol
- GQD
GegenQinlian Decoction
- FMO3
flavin-containing monooxygenase 3
- GLP-1
glucagon-like peptide-1
- MCP1
monocyte chemoattractant protein 1
- ZO-1
zona occludens-1
- FXR
farnesoid X receptor
- FGF15
fibroblast growth factor 15
- Fiaf
fasting-induced adipose factor
- TLR4
toll-like receptor 4
- GPAT1
glycerol-3-phosphate acyltransferase 1.
References
- Anhe F. F., Roy D., Pilon G., Dudonne S., Matamoros S., Varin T. V., et al. (2015). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64, 872–883. 10.1136/gutjnl-2014-307142 [DOI] [PubMed] [Google Scholar]
- Arboleya S., Watkins C., Stanton C., Ross R. P. (2016). Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 7:1204. 10.3389/fmicb.2016.01204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron-Wisnewsky J., Clement K. (2016). The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat. Rev. Nephrol. 12, 169–181. 10.1038/nrneph.2015.191 [DOI] [PubMed] [Google Scholar]
- Baldwin J., Collins B., Wolf P. G., Martinez K., Shen W., Chuang C. C., et al. (2016). Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J. Nutr. Biochem. 27, 123–135. 10.1016/j.jnutbio.2015.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J. A., Sinclair D. A. (2006). Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506. 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- Bennett B. J., De Aguiar Vallim T. Q., Wang Z., Shih D. M., Meng Y., Gregory J., et al. (2013). Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17, 49–60. 10.1016/j.cmet.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels L. B., Delzenne N. M., Cani P. D., Walter J. (2015). Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 12, 303–310. 10.1038/nrgastro.2015.47 [DOI] [PubMed] [Google Scholar]
- Bordin M., D'atri F., Guillemot L., Citi S. (2004). Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol. Cancer Res. 2, 692–701. [PubMed] [Google Scholar]
- Budden K. F., Gellatly S. L., Wood D. L., Cooper M. A., Morrison M., Hugenholtz P., et al. (2017). Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 15, 55–63. 10.1038/nrmicro.2016.142 [DOI] [PubMed] [Google Scholar]
- Buglioni A., Burnett J. C., Jr. (2013). A gut-heart connection in cardiometabolic regulation. Nat. Med. 19, 534–536. 10.1038/nm.3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A., Arboleya S., Moloney R. D., Peterson V. L., Murphy K., Clarke G., et al. (2017). Targeting the Microbiota-Gut-Brain Axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 82, 472–487. 10.1016/j.biopsych.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Canfora E. E., Jocken J. W., Blaak E. E. (2015). Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591. 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- Cani P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., et al. (2007). Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772. 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- Cani P. D., Bibiloni R., Knauf C., Waget A., Neyrinck A. M., Delzenne N. M., et al. (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481. 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- Cao Y., Pan Q., Cai W., Shen F., Chen G. Y., Xu L. M., et al. (2016). Modulation of gut microbiota by berberine improves steatohepatitis in high-fat diet-fed BALB/C mice. Arch. Iran. Med. 19, 197–203. [PubMed] [Google Scholar]
- Carroll S. P., Jorgensen P. S., Kinnison M. T., Bergstrom C. T., Denison R. F., Gluckman P., et al. (2014). Applying evolutionary biology to address global challenges. Science 346:1245993. 10.1126/science.1245993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. J., Lin C. S., Lu C. C., Martel J., Ko Y. F., Ojcius D. M., et al. (2015). Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 6:7489. 10.1038/ncomms8489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.-L., Yi L., Zhang Y., Zhou X., Ran L., Yang J., et al. (2016). Resveratrol attenuates trimethylamine-N-Oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 7:e02210–15. 10.1128/mBio.02210-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Lou G., Meng Z., Huang W. (2011). TGR5: a novel target for weight maintenance and glucose metabolism. Exp. Diab. Res. 2011:853501. 10.1155/2011/853501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M., Liu Y., Zhou R. F., Chen X. L., Wang C., Tan X. Y., et al. (2016). Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 6:19076. 10.1038/srep19076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I., Yamanishi S., Cox L., Methe B. A., Zavadil J., Li K., et al. (2012). Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626. 10.1038/nature11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus S. P. (2017). Will gut microbiota help design the next generation of GLP-1-based therapies for Type 2 diabetes? Cell Metab. 26, 6–7. 10.1016/j.cmet.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Craciun S., Balskus E. P. (2012). Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. U.S.A. 109, 21307–21312. 10.1073/pnas.1215689109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciun S., Marks J. A., Balskus E. P. (2014). Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes. ACS Chem. Biol. 9, 1408–1413. 10.1021/cb500113p [DOI] [PubMed] [Google Scholar]
- Creely S. J., Mcternan P. G., Kusminski C. M., Fisher F. M., Da Silva N. F., Khanolkar M., et al. (2007). Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 292, E740–E747. 10.1152/ajpendo.00302.2006 [DOI] [PubMed] [Google Scholar]
- Cuervo A., Hevia A., Lopez P., Suarez A., Sanchez B., Margolles A., et al. (2015). Association of polyphenols from oranges and apples with specific intestinal microorganisms in systemic lupus erythematosus patients. Nutrients 7, 1301–1317. 10.3390/nu7021301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambrova M., Latkovskis G., Kuka J., Strele I., Konrade I., Grinberga S., et al. (2016). Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp. Clin. Endocrinol. Diab. 124, 251–256. 10.1055/s-0035-1569330 [DOI] [PubMed] [Google Scholar]
- De Clercq N., Frissen M. N., Groen A. K., Nieuwdorp M. (2017). Gut Microbiota and the Gut-Brain Axis: new insights in the pathophysiology of metabolic syndrome. Psychosom Med. 79, 874–879. 10.1097/PSY.0000000000000495 [DOI] [PubMed] [Google Scholar]
- Dinan T. G., Cryan J. F. (2017). Gut-brain axis in 2016: brain-gut-microbiota axis - mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 14, 69–70. 10.1038/nrgastro.2016.200 [DOI] [PubMed] [Google Scholar]
- Dong J. L., Zhu Y. Y., Ma Y. L., Xiang Q. S., Shen R. L., Liu Y. Q. (2016). Oat products modulate the gut microbiota and produce anti-obesity effects in obese rats. J. Funct. Foods 25, 408–420. 10.1016/j.jff.2016.06.025 [DOI] [Google Scholar]
- Eslinger A. J., Eller L. K., Reimer R. A. (2014). Yellow pea fiber improves glycemia and reduces Clostridium leptum in diet-induced obese rats. Nutr. Res. 34, 714–722. 10.1016/j.nutres.2014.07.016 [DOI] [PubMed] [Google Scholar]
- Etxeberria U., Arias N., Boque N., Macarulla M. T., Portillo M. P., Martinez J. A., et al. (2015a). Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 26, 651–660. 10.1016/j.jnutbio.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Etxeberria U., Arias N., Boque N., Romo-Hualde A., Macarulla M. T., Portillo M. P., et al. (2015b). Metabolic faecal fingerprinting of trans-resveratrol and quercetin following a high-fat sucrose dietary model using liquid chromatography coupled to high-resolution mass spectrometry. Food Funct. 6, 2758–2767. 10.1039/C5FO00473J [DOI] [PubMed] [Google Scholar]
- Etxeberria U., Hijona E., Aguirre L., Milagro F. I., Bujanda L., Rimando A. M., et al. (2016). Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 61:1500906. 10.1002/mnfr.201500906 [DOI] [PubMed] [Google Scholar]
- Everard A., Belzer C., Geurts L., Ouwerkerk J. P., Druart C., Bindels L. B., et al. (2013). Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A. 110, 9066–9071. 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W., Wei C., Dong Y., Tang X., Zu Y. (2016). The effect on gut microbiota structure of primarily diagnosed type 2 diabetes patients intervened by sancai lianmei particle and acarbose: a randomized controlled trial. J. Clin. Trails 6:270 10.4172/2167-0870.1000270 [DOI] [Google Scholar]
- Feng Q., Liu W., Baker S. S., Li H., Chen C., Liu Q., et al. (2017). Multi-targeting therapeutic mechanisms of the Chinese herbal medicine QHD in the treatment of non-alcoholic fatty liver disease. Oncotarget 8, 27820–27838. 10.18632/oncotarget.15482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M. T., Gentile C. L., Cox-York K., Wei Y., Wang D., Estrada A. L., et al. (2016). Fuzhuan tea consumption imparts hepatoprotective effects and alters intestinal microbiota in high saturated fat diet-fed rats. Mol. Nutr. Food Res. 60, 1213–1220. 10.1002/mnfr.201500654 [DOI] [PubMed] [Google Scholar]
- Ghosh S. S., Bie J. H., Wang J., Ghosh S. (2014). Oral Supplementation with Non-Absorbable Antibiotics or Curcumin Attenuates Western Diet-Induced Atherosclerosis and Glucose Intolerance in LDLR-/- Mice - Role of Intestinal Permeability and Macrophage Activation. PLoS ONE 9:0108577. 10.1371/journal.pone.0108577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasset E., Puel A., Charpentier J., Collet X., Christensen J. E., Terce F., et al. (2017). A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 26:278. 10.1016/j.cmet.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Greenhill C. (2015). Gut microbiota: firmicutes and bacteroidetes involved in insulin resistance by mediating levels of glucagon-like peptide 1. Nat. Rev. Endocrinol. 11:254. 10.1038/nrendo.2015.40 [DOI] [PubMed] [Google Scholar]
- Greenhill C. (2016). Metabolism: intestinal microbiota affects host physiology. Nat. Rev. Endocrinol. 13:64. 10.1038/nrendo.2016.207 [DOI] [PubMed] [Google Scholar]
- Guarner F., Malagelada J. R. (2003). Gut flora in health and disease. Lancet 361, 512–519. 10.1016/S0140-6736(03)12489-0 [DOI] [PubMed] [Google Scholar]
- Guo M., Ding S., Zhao C., Gu X., He X., Huang K., et al. (2015). Red Ginseng and Semen Coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J. Ethnopharmacol. 162, 7–13. 10.1016/j.jep.2014.12.029 [DOI] [PubMed] [Google Scholar]
- Han K., Bose S., Kim Y. M., Chin Y. W., Kim B. S., Wang J. H., et al. (2015). Rehmannia glutinosa reduced waist circumferences of Korean obese women possibly through modulation of gut microbiota. Food Funct. 6, 2684–2692. 10.1039/C5FO00232J [DOI] [PubMed] [Google Scholar]
- Hansen T. H., Gobel R. J., Hansen T., Pedersen O. (2015). The gut microbiome in cardio-metabolic health. Genome Med 7:33. 10.1186/s13073-015-0157-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heianza Y., Ma W., Manson J. E., Rexrode K. M., Qi L. (2017). Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. 6:e004947. 10.1161/JAHA.116.004947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy E. J., Parker A. E., O'neill L. A. (2010). Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 9, 293–307. 10.1038/nrd3203 [DOI] [PubMed] [Google Scholar]
- Henning S. M., Yang J., Shao P., Lee R. P., Huang J., Ly A., et al. (2017). Health benefit of vegetable/fruit juice-based diet: role of microbiome. Sci. Rep. 7:2167. 10.1038/s41598-017-02200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J., Seo M., Park H., Lee W. K., Guan L. L., Yoon J., et al. (2016). Gut microbiota modulated by probiotics and garcinia cambogia extract correlate with weight gain and adipocyte sizes in high fat-fed mice. Sci. Rep. 6:33566. 10.1038/srep33566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman-Linden L., Kotowska D., Sand E., Bjursell M., Plaza M., Turner C., et al. (2016). Lingonberries alter the gut microbiota and prevent low-grade inflammation in high-fat diet fed mice. Food Nutr. Res. 60:29993. 10.3402/fnr.v60.29993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhao Y., Yuan L., Yang X. (2015). Protective effects of tartary buckwheat flavonoids on high TMAO diet-induced vascular dysfunction and liver injury in mice. Food Funct 6, 3359–3372. 10.1039/C5FO00581G [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project, C. (2012). A framework for human microbiome research. Nature 486, 215–221. 10.1038/nature11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Yadav M. K., Bose S., Wang J. H., Lim D., Song Y. K., et al. (2016). Daesiho-Tang is an effective herbal formulation in attenuation of obesity in mice through alteration of gene expression and modulation of intestinal microbiota. PLoS ONE 11:e0165483. 10.1371/journal.pone.0165483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T., Choi M., Moschetta A., Peng L., Cummins C. L., Mcdonald J. G., et al. (2005). Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225. 10.1016/j.cmet.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Ishizaki K., Imada T., Tsurufuji M. (2005). Hepatoprotective bile acid 'ursodeoxycholic acid (UDCA)' Property and difference as bile acids. Hepatol. Res. 33, 174–177. 10.1016/j.hepres.2005.09.029 [DOI] [PubMed] [Google Scholar]
- Janssen A. W., Kersten S. (2015). The role of the gut microbiota in metabolic health. FASEB J. 29, 3111–3123. 10.1096/fj.14-269514 [DOI] [PubMed] [Google Scholar]
- Jiang T., Gao X., Wu C., Tian F., Lei Q., Bi J., et al. (2016). Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients 8:126. 10.3390/nu8030126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. S., Wernli D., Carroll S. P., Dunn R. R., Harbarth S., Levin S. A., et al. (2016). Use antimicrobials wisely. Nature 537, 159–161. 10.1038/537159a [DOI] [PubMed] [Google Scholar]
- Kafantaris I., Kotsampasi B., Christodoulou V., Kokka E., Kouka P., Terzopoulou Z., et al. (2016). Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. (Berl). 101, e108–e121. 10.1111/jpn.12569 [DOI] [PubMed] [Google Scholar]
- Kang C., Wang B., Kaliannan K., Wang X., Lang H., Hui S., et al. (2017). Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. MBio 8:e00470–17. 10.1128/mBio.00470-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. E., Kim J. M., Joung K. H., Lee J. H., You B. R., Choi M. J., et al. (2016). The Roles of Adipokines, Proinflammatory Cytokines, and Adipose Tissue Macrophages in Obesity-Associated Insulin Resistance in Modest Obesity and Early Metabolic Dysfunction. PLoS ONE 11:e0154003. 10.1371/journal.pone.0154003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri D., Hamasaki Y., Doi K., Okamoto K., Negishi K., Nangaku M., et al. (2013). Protection of glucagon-like peptide-1 in cisplatin-induced renal injury elucidates gut-kidney connection. J. Am. Soc. Nephrol. 24, 2034–2043. 10.1681/ASN.2013020134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer D. A., Piccolo B. D., Marco M. L., Kim E. B., Goodson M. L., Keenan M. J., et al. (2016). Obese mice fed a diet supplemented with enzyme-treated wheat bran display marked shifts in the liver metabolome concurrent with altered gut bacteria. J. Nutr. 146, 2445–2460. 10.3945/jn.116.238923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I., Ozawa K., Inoue D., Imamura T., Kimura K., Maeda T., et al. (2013). The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 4:1829. 10.1038/ncomms2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai T., Tang W. H. W. (2017). The role and impact of gut microbiota in cardiovascular disease. Rev. Esp. Cardiol. (Engl. Ed). 70, 799–800. 10.1016/j.recesp.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Klinder A., Shen Q., Heppel S., Lovegrove J. A., Rowland I., Tuohy K. M. (2016). Impact of increasing fruit and vegetables and flavonoid intake on the human gut microbiota. Food Funct. 7, 1788–1796. 10.1039/C5FO01096A [DOI] [PubMed] [Google Scholar]
- Ko S. J., Kim J., Han G., Kim S. K., Kim H. G., Yeo I., et al. (2014). Laminaria japonica combined with probiotics improves intestinal microbiota: a randomized clinical trial. J. Med. Food 17, 76–82. 10.1089/jmf.2013.3054 [DOI] [PubMed] [Google Scholar]
- Koeth R. A., Wang Z., Levison B. S., Buffa J. A., Org E., Sheehy B. T., et al. (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585. 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopen A. M., Groen A. K., Nieuwdorp M. (2016). Human microbiome as therapeutic intervention target to reduce cardiovascular disease risk. Curr. Opin. Lipidol. 27, 615–622. 10.1097/MOL.0000000000000357 [DOI] [PubMed] [Google Scholar]
- Koutsos A., Tuohy K. M., Lovegrove J. A. (2015). Apples and cardiovascular health–is the gut microbiota a core consideration? Nutrients 7, 3959–3998. 10.3390/nu7063959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laparra J. M., Sanz Y. (2010). Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 61, 219–225. 10.1016/j.phrs.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Laxminarayan R., Matsoso P., Pant S., Brower C., Rottingen J. A., Klugman K., et al. (2016). Access to effective antimicrobials: a worldwide challenge. Lancet 387, 168–175. 10.1016/S0140-6736(15)00474-2 [DOI] [PubMed] [Google Scholar]
- Leal-Diaz A. M., Noriega L. G., Torre-Villalvazo I., Torres N., Aleman-Escondrillas G., Lopez-Romero P., et al. (2016). Aguamiel concentrate from Agave salmiana and its extracted saponins attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice. Sci. Rep. 6:34242. 10.1038/srep34242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I. (2006). Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- Li F., Jiang C., Krausz K. W., Li Y., Albert I., Hao H., et al. (2013). Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 4:2384. 10.1038/ncomms3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lin S., Vanhoutte P. M., Woo C. W., Xu A. (2016). Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe−/− Mice. Circulation 133, 2434–2446. 10.1161/CIRCULATIONAHA.115.019645 [DOI] [PubMed] [Google Scholar]
- Li T., Chen Y., Gua C., Li X. (2017). Elevated circulating trimethylamine N-Oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front. Physiol. 8:350. 10.3389/fphys.2017.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. S., Obeid S., Klingenberg R., Gencer B., Mach F., Raber L., et al. (2017). Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 38, 814–824. 10.1093/eurheartj/ehw582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Guo J., Ji K., Zhang P. (2016). Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci. Rep. 6:32953. 10.1038/srep32953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yao J., Han C., Yang J., Chaudhry M. T., Wang S., et al. (2016). Quercetin, inflammation and immunity. Nutrients 8:167. 10.3390/nu8030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z. L., Zeng B. H., Wang W., Li G. H., Wu F., Wang L., et al. (2016). Impact of the Consumption of Tea Polyphenols on Early Atherosclerotic Lesion Formation and Intestinal Bifidobacteria in High-Fat-Fed ApoE−/− Mice. Front. Nutr. 3:42 10.3389/fnut.2016.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. P., Zou W. L., Chen S. J., Wei H. Y., Yin Y. N., Zou Y. Y., et al. (2016). Effects of different diets on intestinal microbiota and nonalcoholic fatty liver disease development. World J. Gastroenterol. 22, 7353–7364. 10.3748/wjg.v22.i32.7353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. W., Cephas K. D., Holscher H. D., Kerr K. R., Mangian H. F., Tappenden K. A., et al. (2016). Nondigestible fructans alter gastrointestinal barrier function, gene expression, histomorphology, and the microbiota profiles of diet-induced obese C57BL/6J Mice. J. Nutr. 146, 949–956. 10.3945/jn.115.227504 [DOI] [PubMed] [Google Scholar]
- Liu W., Zhao S., Wang J., Shi J., Sun Y., Wang W., et al. (2017). Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 61:1601082. 10.1002/mnfr.201601082 [DOI] [PubMed] [Google Scholar]
- Liu Z., Chen Z., Guo H., He D., Zhao H., Wang Z., et al. (2016). The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 7, 4869–4879. 10.1039/C6FO01439A [DOI] [PubMed] [Google Scholar]
- Ma G., Pan B., Chen Y., Guo C., Zhao M., Zheng L., et al. (2017). Trimethylamine N-oxide in atherogenesis: impairing endothelial Self-repair capacity and enhancing monocyte adhesion. Biosci Rep. 37:BSR20160244. 10.1042/BSR20160244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., et al. (1999). Identification of a nuclear receptor for bile acids. Science 284, 1362–1365. 10.1126/science.284.5418.1362 [DOI] [PubMed] [Google Scholar]
- Marques F. Z., Nelson E., Chu P. Y., Horlock D., Fiedler A., Ziemann M., et al. (2017). High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135, 964–977. 10.1161/CIRCULATIONAHA.116.024545 [DOI] [PubMed] [Google Scholar]
- Martel J., Ojcius D. M., Chang C. J., Lin C. S., Lu C. C., Ko Y. F., et al. (2016). Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 13, 149–160. 10.1038/nrendo.2016.142 [DOI] [PubMed] [Google Scholar]
- Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., et al. (2002). Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298, 714–719. 10.1016/S0006-291X(02)02550-0 [DOI] [PubMed] [Google Scholar]
- Masumoto S., Terao A., Yamamoto Y., Mukai T., Miura T., Shoji T. (2016). Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 6:31208. 10.1038/srep31208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer C. C. K., Ueland T., Broch K., Vincent R. P., Cross G. F., Dahl C. P., et al. (2017). Increased Secondary/Primary Bile Acid-Ratio in Chronic Heart Failure. J Card Fail. 23, 666–671. 10.1016/j.cardfail.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Mcfadden R. M., Larmonier C. B., Shehab K. W., Midura-Kiela M., Ramalingam R., Harrison C. A., et al. (2015). The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel Dis. 21, 2483–2494. 10.1097/MIB.0000000000000522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L., Tang Y., Li M., Yang P., Liu Z., Yuan J., et al. (2015). Co-Administration of Cholesterol-Lowering Probiotics and Anthraquinone from Cassia obtusifolia L. Ameliorate Non-Alcoholic Fatty Liver. PLoS ONE 10:e0138078. 10.1371/journal.pone.0138078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. A., Bennett B. J. (2016). Diet and gut microbial function in metabolic and cardiovascular disease risk. Curr. Diab. Rep. 16:93. 10.1007/s11892-016-0791-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R., Penalvo J. L., Cudhea F., Imamura F., Rehm C. D., Mozaffarian D. (2017). Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 317, 912–924. 10.1001/jama.2017.0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midtvedt T. (1974). Microbial bile acid transformation. Am. J. Clin. Nutr. 27, 1341–1347. [DOI] [PubMed] [Google Scholar]
- Moreno-Indias I., Cardona F., Tinahones F. J., Queipo-Ortuno M. I. (2014). Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front. Microbiol. 5:190. 10.3389/fmicb.2014.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J., Penders J., Lucchesi M., Goossens G. H., Blaak E. E. (2017). Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur. J. Clin. Nutr. 71, 1040–1045. 10.1038/ejcn.2017.89 [DOI] [PubMed] [Google Scholar]
- Muccioli G. G., Naslain D., Backhed F., Reigstad C. S., Lambert D. M., Delzenne N. M., et al. (2010). The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 6:392. 10.1038/msb.2010.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M., Thorell A., Claudel T., Jha P., Koefeler H., Lackner C., et al. (2015). Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol. 62, 1398–1404. 10.1016/j.jhep.2014.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrinck A. M., Possemiers S., Druart C., Van De Wiele T., De Backer F., Cani P. D., et al. (2011). Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 6:e20944. 10.1371/journal.pone.0020944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen S. G., Kim J., Guevarra R. B., Lee J.-H., Kim E., Kim S.-I., et al. (2016). Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 7, 4193–4201. 10.1039/C6FO00929H [DOI] [PubMed] [Google Scholar]
- Oellgaard J., Winther S. A., Hansen T. S., Rossing P., Von Scholten B. J. (2017). Trimethylamine N-oxide (TMAO) as a new potential therapeutic target for insulin resistance and cancer. Curr Pharm Des. 23, 3699–3712. 10.2174/1381612823666170622095324 [DOI] [PubMed] [Google Scholar]
- Organ C. L., Otsuka H., Bhushan S., Wang Z., Bradley J., Trivedi R., et al. (2016). Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ. Heart Fail 9:e002314. 10.1161/CIRCHEARTFAILURE.115.002314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., et al. (1999). Bile acids: natural ligands for an orphan nuclear receptor. Science 284, 1365–1368. 10.1126/science.284.5418.1365 [DOI] [PubMed] [Google Scholar]
- Parséus A., Sommer N., Sommer F., Caesar R., Molinaro A., Stahlman M., et al. (2016). Microbiota-induced obesity requires farnesoid X receptor. Gut 66, 429–437. 10.1136/gutjnl-2015-310283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori D., Carnevale R., Nocella C., Novo M., Santulli M., Cammisotto V., et al. (2017). Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: effect of adherence to mediterranean diet. J. Am. Heart Assoc. 6:e005784. 10.1161/JAHA.117.005784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., He Z., Chen W., Holzman I. R., Lin J. (2007). Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 61, 37–41. 10.1203/01.pdr.0000250014.92242.f3 [DOI] [PubMed] [Google Scholar]
- Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., et al. (2017). A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113. 10.1038/nm.4236 [DOI] [PubMed] [Google Scholar]
- Poggi M., Bastelica D., Gual P., Iglesias M. A., Gremeaux T., Knauf C., et al. (2007). C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50, 1267–1276. 10.1007/s00125-007-0654-8 [DOI] [PubMed] [Google Scholar]
- Qiao Y., Sun J., Xia S., Tang X., Shi Y., Le G. (2014). Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct 5, 1241–1249. 10.1039/c3fo60630a [DOI] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K. S., Manichanh C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins G. R., Wen H., Ting J. P. (2014). Inflammasomes and metabolic disorders: old genes in modern diseases. Mol. Cell 54, 297–308. 10.1016/j.molcel.2014.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano K. A., Vivas E. I., Amador-Noguez D., Rey F. E. (2015). Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 6:e02481. 10.1128/mBio.02481-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopchand D. E., Carmody R. N., Kuhn P., Moskal K., Rojas-Silva P., Turnbaugh P. J., et al. (2015). Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 64, 2847–2858. 10.2337/db14-1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M. J., Santos A., Prada P. O. (2016). Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda) 31, 283–293. 10.1152/physiol.00041.2015 [DOI] [PubMed] [Google Scholar]
- Samuel B. S., Shaito A., Motoike T., Rey F. E., Backhed F., Manchester J. K., et al. (2008). Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. U.S.A. 105, 16767–16772. 10.1073/pnas.0808567105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Tapia M., Aguilar-Lopez M., Perez-Cruz C., Pichardo-Ontiveros E., Wang M., Donovan S. M., et al. (2017). Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci. Rep. 7:4716. 10.1038/s41598-017-05096-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. (1977). Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31, 107–133. 10.1146/annurev.mi.31.100177.000543 [DOI] [PubMed] [Google Scholar]
- Sayin S. I., Wahlstrom A., Felin J., Jantti S., Marschall H. U., Bamberg K., et al. (2013). Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235. 10.1016/j.cmet.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Schroeder B. O., Backhed F. (2016). Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 22, 1079–1089. 10.1038/nm.4185 [DOI] [PubMed] [Google Scholar]
- Schugar R. C., Shih D. M., Warrier M., Helsley R. N., Burrows A., Ferguson D., et al. (2017). The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 19, 2451–2461. 10.1016/j.celrep.2017.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin M. M., Meng Y., Qi H., Zhu W., Wang Z., Hazen S. L., et al. (2016). Trimethylamine N-Oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J. Am. Heart Assoc. 5:e002767. 10.1161/JAHA.115.002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthong V., Li X. S., Hudec T., Coughlin J., Wu Y., Levison B., et al. (2016a). Plasma Trimethylamine N-Oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J. Am. Coll. Cardiol. 67, 2620–2628. 10.1016/j.jacc.2016.03.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthong V., Wang Z., Fan Y., Wu Y., Hazen S. L., Tang W. H. (2016b). Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J. Am. Heart Assoc. 5:e004237. 10.1161/JAHA.116.004237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthong V., Wang Z., Li X. S., Fan Y., Wu Y., Tang W. H., et al. (2016c). Intestinal microbiota-generated metabolite trimethylamine-n-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J. Am. Heart Assoc. 5:e002816. 10.1161/JAHA.115.002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Q., Shan X., Cai C., Hao J., Li G., Yu G. (2016). Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct 7, 3224–3232. 10.1039/C6FO00309E [DOI] [PubMed] [Google Scholar]
- Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. (2006). TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025. 10.1172/JCI28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.-L., Wang Y., Feng Y. (2015). [Effect of MDG-1, a polysaccharide from Ophiopogon japonicas, on diversity of lactobacillus in diet-induced obese mice]. Zhongguo Zhong Yao Za Zhi 40, 716–721. 10.4268/cjcmm20150426 [DOI] [PubMed] [Google Scholar]
- Shin N. R., Lee J. C., Lee H. Y., Kim M. S., Whon T. W., Lee M. S., et al. (2014). An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735. 10.1136/gutjnl-2012-303839 [DOI] [PubMed] [Google Scholar]
- Simon G. L., Gorbach S. L. (1984). Intestinal flora in health and disease. Gastroenterology 86, 174–193. [PubMed] [Google Scholar]
- Simpson H. L., Campbell B. J. (2015). Review article: dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 42, 158–179. 10.1111/apt.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. P., Singh J., Boparai R. K., Zhu J., Mantri S., Khare P., et al. (2017). Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol. Res. 123, 103–113. 10.1016/j.phrs.2017.06.015 [DOI] [PubMed] [Google Scholar]
- Sonnenburg J. L., Backhed F. (2016). Diet-microbiota interactions as moderators of human metabolism. Nature 535, 56–64. 10.1038/nature18846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Ni X., Song X., Wen B., Zhou Y., Zou F., et al. (2016). Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl. Microbiol. Biotechnol. 100, 8105–8120. 10.1007/s00253-016-7619-0 [DOI] [PubMed] [Google Scholar]
- Sun X., Jiao X., Ma Y., Liu Y., Zhang L., He Y., et al. (2016). Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 481, 63–70. 10.1016/j.bbrc.2016.11.017 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Heaney L. M., Jones D. J., Ng L. L. (2017). Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin. Chem. 63, 420–428. 10.1373/clinchem.2016.264853 [DOI] [PubMed] [Google Scholar]
- Sweeney T. E., Morton J. M. (2013). The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg. 148, 563–569. 10.1001/jamasurg.2013.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H., Wang Z., Fan Y., Levison B., Hazen J. E., Donahue L. M., et al. (2014). Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 64, 1908–1914. 10.1016/j.jacc.2014.02.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H., Wang Z., Kennedy D. J., Wu Y., Buffa J. A., Agatisa-Boyle B., et al. (2015a). Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 116, 448–455. 10.1161/CIRCRESAHA.116.305360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H., Wang Z., Levison B. S., Koeth R. A., Britt E. B., Fu X., et al. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584. 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H., Wang Z., Li X. S., Fan Y., Li D. S., Wu Y., et al. (2016). Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin. Chem. 63, 297–306. 10.1373/clinchem.2016.263640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H., Wang Z., Shrestha K., Borowski A. G., Wu Y., Troughton R. W., et al. (2015b). Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail 21, 91–96. 10.1016/j.cardfail.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., et al. (2009). TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177. 10.1016/j.cmet.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. (2008). Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693. 10.1038/nrd2619 [DOI] [PubMed] [Google Scholar]
- Tolhurst G., Heffron H., Lam Y. S., Parker H. E., Habib A. M., Diakogiannaki E., et al. (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371. 10.2337/db11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi M. S., Daoudi M., Prawitt J., Ducastel S., Touche V., Sayin S. I., et al. (2015). Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 6:7629. 10.1038/ncomms8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troseid M., Hov J. R., Nestvold T. K., Thoresen H., Berge R. K., Svardal A., et al. (2016). Major increase in microbiota-dependent proatherogenic metabolite tMAO one year after bariatric surgery. Metab. Syndr. Relat. Disord 14, 197–201. 10.1089/met.2015.0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troseid M., Ueland T., Hov J. R., Svardal A., Gregersen I., Dahl C. P., et al. (2015). Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 277, 717–726. 10.1111/joim.12328 [DOI] [PubMed] [Google Scholar]
- Turnbaugh P. J., Ley R. E., Hamady M., Fraser-Liggett C. M., Knight R., Gordon J. I. (2007). The human microbiome project. Nature 449, 804–810. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M., Aitken J. D., Carvalho F. A., Cullender T. C., Mwangi S., Srinivasan S., et al. (2010). Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231. 10.1126/science.1179721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom A., Sayin S. I., Marschall H. U., Backhed F. (2016). Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50. 10.1016/j.cmet.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Walker J., Schueller K., Schaefer L. M., Pignitter M., Esefelder L., Somoza V. (2014). Resveratrol and its metabolites inhibit pro-inflammatory effects of lipopolysaccharides in U-937 macrophages in plasma-representative concentrations. Food Funct. 5, 74–84. 10.1039/C3FO60236B [DOI] [PubMed] [Google Scholar]
- Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. (1999). Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3, 543–553. 10.1016/S1097-2765(00)80348-2 [DOI] [PubMed] [Google Scholar]
- Wang J. H., Bose S., Kim G. C., Hong S. U., Kim J. H., Kim J. E., et al. (2014). Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PLoS ONE 9:e86117. 10.1371/journal.pone.0086117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ghosh S., Ghosh S. (2017). Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Ph 312, C438–C445. 10.1152/ajpcell.00235.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Du Z., Zhang H., Zhao L., Sun J., Zheng X., et al. (2015). Modulation of gut microbiota by polyphenols from adlay (Coix lacryma-jobi L. var. ma-yuen Stapf.) in rats fed a high-cholesterol diet. Int. J. Food Sci. Nutr. 66, 783–789. 10.3109/09637486.2015.1088941 [DOI] [PubMed] [Google Scholar]
- Wang S., Huang X. F., Zhang P., Wang H., Zhang Q., Yu S., et al. (2016). Chronic rhein treatment improves recognition memory in high-fat diet-induced obese male mice. J. Nutr. Biochem. 36, 42–50. 10.1016/j.jnutbio.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Wang S., Li Q., Zang Y., Zhao Y., Liu N., Wang Y., et al. (2017). Apple Polysaccharide inhibits microbial dysbiosis and chronic inflammation and modulates gut permeability in HFD-fed rats. Int. J. Biol. Macromol. 99, 282–292. 10.1016/j.ijbiomac.2017.02.074 [DOI] [PubMed] [Google Scholar]
- Wang Y., Ames N. P., Tun H. M., Tosh S. M., Jones P. J., Khafipour E. (2016). High Molecular Weight Barley beta-Glucan Alters Gut Microbiota Toward Reduced Cardiovascular Disease Risk. Front. Microbiol. 7:129. 10.3389/fmicb.2016.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Shou J. W., Li X. Y., Zhao Z. X., Fu J., He C. Y., et al. (2017). Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism 70, 72–84. 10.1016/j.metabol.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Wang Y., Tong J., Chang B., Wang B., Zhang D., Wang B. (2014). Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol. Med. Rep. 9, 2352–2356. 10.3892/mmr.2014.2126 [DOI] [PubMed] [Google Scholar]
- Wang Z. N., Klipfell E., Bennett B. J., Koeth R., Levison B. S., Dugar B., et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–82. 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Roberts A. B., Buffa J. A., Levison B. S., Zhu W., Org E., et al. (2015). Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 163, 1585–1595. 10.1016/j.cell.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest R., Albillos A., Trauner M., Bajaj J., Jalan R. (2017). Targeting the gut-liver axis in liver disease. J. Hepatol. 67, 1084–1103. 10.1016/j.jhep.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Wilson A., Mclean C., Kim R. B. (2016). Trimethylamine-N-oxide: a link between the gut microbiome, bile acid metabolism, and atherosclerosis. Curr. Opin. Lipidol. 27, 148–154. 10.1097/MOL.0000000000000274 [DOI] [PubMed] [Google Scholar]
- Wink M. (2015). Modes of action of herbal medicines and plant secondary metabolites. Medicines 2:251. 10.3390/medicines2030251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside J. V., Young I. S., Mckinley M. C. (2013). Fruit and vegetable intake and risk of cardiovascular disease. Proc. Nutr. Soc. 72, 399–406. 10.1017/S0029665113003029 [DOI] [PubMed] [Google Scholar]
- Woting A., Blaut M. (2016). The intestinal microbiota in metabolic disease. Nutrients 8:202. 10.3390/nu8040202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Sun M., Chen F., Cao A. T., Liu H., Zhao Y., et al. (2016). Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 10, 946–956. 10.1038/mi.2016.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M., Yang J., Feng Y., Zhu Y., Chai X., Wang Y. (2017). Metaproteomic strategies and applications for gut microbial research. Appl. Microbiol. Biotechnol. 101, 3077–3088. 10.1007/s00253-017-8215-7 [DOI] [PubMed] [Google Scholar]
- Xie W., Gu D., Li J., Cui K., Zhang Y. (2011). Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS ONE 6:e24520. 10.1371/journal.pone.0024520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. H., Liu X. Z., Pan W., Zou D. J. (2017). Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol. Med. Rep. 15, 2765–2787. 10.3892/mmr.2017.6321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Chen H. B., Li S. L. (2017). Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med. Res. Rev. 37, 1140–1185. 10.1002/med.21431 [DOI] [PubMed] [Google Scholar]
- Xu J., Lian F., Zhao L., Zhao Y., Chen X., Zhang X., et al. (2015). Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 9, 552–562. 10.1038/ismej.2014.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K. Y., Xia G. H., Lu J. Q., Chen M. X., Zhen X., Wang S., et al. (2017). Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 7:1445. 10.1038/s41598-017-01387-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Wang J., Hong F., Wang S., Jin X., Xue T., et al. (2017). Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal. Res. 62:e12399. 10.1111/jpi.12399 [DOI] [PubMed] [Google Scholar]
- Yan H., Lu J., Wang Y., Gu W., Yang X., Yu J. (2017). Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine 26, 45–54. 10.1016/j.phymed.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Yang Y., Chen G., Yang Q., Ye J., Cai X., Tsering P., et al. (2017). Gut microbiota drives the attenuation of dextran sulphate sodium-induced colitis by Huangqin decoction. Oncotarget. 8, 48863–48874. 10.18632/oncotarget.16458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. P., Hopkins R. J., Marsland B. (2016). The Gut-Liver-Lung Axis. Modulation of the Innate Immune Response and Its Possible Role in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 54, 161–169. 10.1165/rcmb.2015-0250PS [DOI] [PubMed] [Google Scholar]
- Yu H., Guo Z., Shen S., Shan W. (2016). Effects of taurine on gut microbiota and metabolism in mice. Amino Acids 48, 1601–1617. 10.1007/s00726-016-2219-y [DOI] [PubMed] [Google Scholar]
- Zhang X. H., Huang B., Choi S. K., Seo J. S. (2012b). Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr. Res. Pract. 6, 286–293. 10.4162/nrp.2012.6.4.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao Y., Xu J., Xue Z., Zhang M., Pang X., et al. (2015). Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 5:14405. 10.1038/srep14405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao Y., Zhang M., Pang X., Xu J., Kang C., et al. (2012a). Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 7:e42529. 10.1371/journal.pone.0042529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Ma Y. H., Zhou Z., Chen Y., Wang Y., Gao X. (2015). Intestinal absorption and metabolism of epimedium flavonoids in osteoporosis rats. Drug Metab. Dispos. 43, 1590–1600. 10.1124/dmd.115.064386 [DOI] [PubMed] [Google Scholar]
- Zhou S. S., Xu J., Zhu H., Wu J., Xu J. D., Yan R., et al. (2016). Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci. Rep. 6:22474. 10.1038/srep22474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Gregory J. C., Org E., Buffa J. A., Gupta N., Wang Z., et al. (2016). Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 165, 111–124. 10.1016/j.cell.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Wang Z., Tang W. H. W., Hazen S. L. (2017). Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation 135, 1671–1673. 10.1161/CIRCULATIONAHA.116.025338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Jameson E., Crosatti M., Schafer H., Rajakumar K., Bugg T. D., et al. (2014). Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. U.S.A. 111, 4268–4273. 10.1073/pnas.1316569111 [DOI] [PMC free article] [PubMed] [Google Scholar]