Abstract
Esculentoside A (EsA), a saponin isolated from Phytolacca esculenta, can attenuate acute liver and lung injury. However, whether EsA has a protective effect against sepsis-induced acute kidney injury (AKI) has not been reported. In this study, EsA (2.5, 5, or 10 mg/kg) was given to rats with sepsis induced by cecal ligation and puncture (CLP). We found that EsA improved the survival of septic rats in a dose-dependent manner. In addition, EsA lowered the kidney tubular damage score and decreased blood urea nitrogen and creatinine. Moreover, EsA inhibited excessive generation of pro-inflammatory tumor necrosis factor-α, IL-1β, and IL-6 in the serum and downregulated cyclooxygenase-2 and inducible nitric oxide synthase in the renal tissues of septic rats. EsA also suppressed the production of malonaldehyde and the activity of myeloperoxidase in the septic kidney and enhanced the activity of superoxide dismutase and glutathione. The anti-inflammatory and antioxidative effects of a high dose of EsA were comparable to those of dexamethasone. Mechanically, EsA inhibited CLP-induced increases in high-mobility group box 1, Toll-like receptor-4, and myeloid differentiation primary response 88 and nuclear accumulation of nuclear factor kappa B p65 in renal tissues. In vitro, lipopolysaccharide-induced alteration of AKI-related factors in HK-2 cells, which had been evaluated in vivo, was inhibited after EsA administration. Taken together, our study suggests that EsA effectively protects rats against septic AKI caused by CLP.
Keywords: acute kidney injury, esculentoside A, HMGB1/TLR/NF-kB pathway, sepsis
Introduction
Sepsis is usually caused by a pathogenic microorganism, leading to septic shock and multiple organ dysfunction [11]. Acute kidney injury (AKI) is a major complication that causes death in patients with sepsis [10]. Although a great improvement has been made in the treatment of septic AKI, the overall survival of patients remains low [26]. Thus, it is urgent to develop novel strategies for the treatment of septic AKI.
Esculentoside A (EsA) is a saponin isolated from the roots of the Chinese herb Phytolaca esculenta [39]. Previous reports have demonstrated that EsA has the effect of regulating immune response and anti-inflammatory and antioxidative effects in various experimental models [19, 20, 32, 34, 36]. EsA treatment inhibits the secretion of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL-1β, IL-2, IL-6, and prostaglandin E2 (PGE2) [35]. EsA attenuates carbon tetrachloride (CCl4) and galactosamine (GalN)/lipopolysaccharide (LPS)-induced acute liver injury in mice [38]. Furthermore, EsA can also relieve LPS-induced acute lung injury in mice via suppression of nuclear factor kappa B (NF-κB)/mitogen-activated protein kinase (MAPK) signal pathways [40]. However, whether EsA has a protective effect on sepsis-induced AKI has not been reported. In the present study, the effect of EsA was evaluated in rats with sepsis induced by cecal ligation and puncture (CLP) and in LPS-stimulated HK-2 cells, and the possible mechanisms were investigated as well.
Materials and Methods
Animals
Male Sprague–Dawley rats (8 weeks old, weighing 200–220 g) were purchased from Liaoning Changsheng Biological Technology co., ltd. (China, certificate No. SCXK (Liao) 2015–0001). The animals were housed in standard cages for rodents containing no more than five animals, with a 12-h artificial light/dark cycle and a constant temperature of 22°C. This study was approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Harbin Medical University and performed according to its Guidelines for the Care and Use of Laboratory Animals.
The rats were randomized into 6 groups (n=16): (i) sham group, (ii) CLP-induced AKI group, (iii) CLP-induced AKI + dexamethasone (DXM; 2 mg/kg) group, (iv) CLP-induced AKI + EsA (2.5 mg/kg) group, (v) CLP-induced AKI + EsA (5 mg/kg) group, and (vi) CLP-induced AKI + EsA (10 mg/kg) group.
CLP-induced sepsis and drug treatment
CLP surgery was carried out as previously described [27]. In brief, laparotomy was performed to isolate the cecum of rats anesthetized with 10% chloral hydrate. Approximately 1/3 of the cecal tip was ligated with a 4–0 silk suture. The cecum was punctured twice gently and squeezed to expel approximately a 1 mm column of fecal material. In sham-operated rats, the cecum was isolated without ligation or puncture. Immediately after the surgery, the rats in the DXM group received an intraperitoneal injection of DXM, the rats in the EsA groups received an intraperitoneal injection of their respective doses of EsA, and the rats in the sham and AKI groups received equal volumes of saline. Twenty-four hours after EsA, DXM, or saline administration, 6 rats in each group were randomly selected and scarified. The renal tissues and blood were collected. The other 10 rats in each group were used for a survival study. The rats were observed twice a day for 4 days, and survival was recorded.
Periodic acid-Schiff (PAS) stain
Pathological changes of the kidney were assessed with PAS staining as previously described [22]. The degree of renal damage was estimated at 200× magnification with five randomly selected fields by the following criteria: 0, normal; 1, damage involving less than 25% of tubules; 2, damage involving 25% to 50% of tubules; 3, damage involving 50% to 75% of tubules; 4, damage involving 75% to 100% of tubules.
Serum creatinine (CR) and blood urea nitrogen (BUN) examinations
The levels of CR and BUN in the serum were examined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Cell culture
HK-2 human kidney tubular epithelial cells were purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA). The cells were maintained in an incubator with a constant humidified atmosphere and 5% CO2 at 37°C.
Four groups were designed as follows: control group, 1 µg/ml LPS group, 1 µg/ml LPS + normal saline (NS) group, and 1 µg/ml LPS + 20 µmol EsA group. The cells were treated with LPS and EsA for 24 h simultaneously for malondialdehyde (MDA), TNF-α, IL1-β, and IL-6 detection. The cells were treated with LPS for 24 h and subsequently treated with EsA for another 24 h for other analyses.
Oxidative stress and myeloperoxidase (MPO) activity assay
The contents of MDA and glutathione (GSH) and the activities of MPO and superoxide dismutase (SOD) were determined using commercial biochemical kits (Nanjing Jiancheng, Nanjing, China) according to the instructions of the manufacturer. MPO activity was assayed using a Myeloperoxidase Assay Kit (Nanjing Jiancheng) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay (ELISA)
The levels of TNF-α and IL-6 were detected using a commercial ELISA kit purchased from USCN (Wuhan, China). The level of IL-1β was detected using a commercial ELISA kit purchased from Boster Biological Technology (Wuhan, China). In all cases, ELISA was performed according to the manufacturer’s protocols.
Western blotting assay
A western blotting assay was applied to detect protein expression in kidney tissue and HK-2 cells. In short, total protein and nucleoprotein were extracted with RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) and a nuclear and cytoplasmic protein extraction kit (Beyotime Institute of Biotechnology) according to the protocols of the manufacturer. Protein concentrations were calculated using an enhanced BCA protein assay kit (Beyotime Institute of Biotechnology). The lysates were separated on SDS-PAGE gels and transferred onto PVDF membranes. Nonfat milk (5%) was used to block membranes. The blots were incubated with anti-inducible nitric oxide synthase (iNOS) antibody (1:400; BA0362, Boster Biological Technology), anti-cyclooxygenase (COX)-2 antibody (1:400; BA0738, Boster Biological Technology), anti-high-mobility group box 1 (HMGB1) antibody (1:400; BA4277, Boster Biological Technology), anti-p65 antibody (1:400; BA0610, Boster Biological Technology), anti-inhibitors of NF-κBα (IκBα) antibody (1:500; bs-1287R, Bioss Antibodies Inc., Woburn, MA, USA), anti-p-p65 antibody (1:500; bs-0982R, Bioss Antibodies Inc.), anti-Toll-like receptor (TLR) 4 antibody (1:200; sc-293072, Santa Cruz Biotechnology, Dallas, TX, USA), anti-myeloid differentiation primary response 88 (MyD88) antibody (1:200; sc-8196, Santa Cruz Biotechnology), and anti-β-actin antibody (1:1,000; sc-47778, Santa Cruz Biotechnology) at 4°C overnight. The membranes were then washed and incubated with secondary antibodies (1:5,000, Beyotime Institute of Biotechnology, Haimen, China) for 45 min at room temperature. The blots were detected using enhanced chemiluminescence (ECL). The expression levels of target proteins were normalized to β-actin.
Total RNA extraction and real-time RT-PCR
An RNA pure High-purity Total RNA Rapid Extraction Kit (BioTeke, Beijing, China) was used to isolate total RNA from kidney tissue according to the manufacturer’s instructions. Complementary DNA was reverse transcribed using the purified RNA and M-MLV reverse transcriptase (BioTeke). PCR was performed using 1 µl template cDNA, 10 µl SYBR GREEN master mix (2X), and 0.5 µl forward and reverse primers (10 µM per primer concentration) in a 20 µl system. The optimal reaction condition was set as follows: 10 min at 95°C and then 40 cycles of 10 s at 95°C, 20 s at 60°C, and 30 s at 72°C. Quantitative analysis was performed using an Exicycler™ 96 quantitative fluorescence instrument (Bioneer, Daejeon, Korea). Relative gene expression was normalized to β-actin and calculated using the 2−ΔΔCt method.
Immunohistochemical analysis
Immunohistochemical analysis was performed to assess the expressions of COX-2, HMGB1, and iNOS in kidney tissue. Briefly, tissue samples embedded with paraffin were cut into sections 5 µm thick and fixed on the slide. Histological sections were deparaffinized with xylene and rehydrated with serial gradient ethanol. The sections were boiled in 10 mM sodium citrate for 10 min using a microwave oven to perform the antigen retrieval. After incubation with 3% H2O2 for 15 min at room temperature to eliminate endogenous peroxidase activity, the sections were blocked with goat serum and incubated in primary antibodies against COX-2 (1:200; BA0738, Boster Biological Technology), HMGB1 (1:50; D260488, Sangon Biotech, Shanghai, China), and iNOS (1:100; bs-2072R, Bioss Antibodies Inc.) overnight at 4°C in darkness. Subsequently, the sections were incubated with horseradish peroxidase-labeled secondary antibodies, and staining was visualized by reaction with diaminobenzidine tetrahydrochloride (DAB). After counterstaining with hematoxylin, the sections were observed under a light microscope (DP73, Olympus, Tokyo, Japan).
Immunofluorescent assay
For immunofluorescence staining, deparaffinized tissue sections were blocked with goat serum and incubated with primary antibodies against p65 (1:100; bs-20355R, Bioss Antibodies Inc.) overnight at 4°C in darkness. The sections were then incubated in Cy3 fluorescently labeled secondary antibody in darkness for 1 h at room temperature. DAPI was used to stain the cell nuclei. The staining was observed using a fluorescence microscope (BX53, Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA). Student’s t-test and one-way analysis of variance (ANOVA) were used to compare the means of normally distributed continuous variables. The data are presented as the mean ± SD, and each experiment was repeated three times. Survival data were analyzed using Kaplan–Meier curves and log-rank tests. A P value less than 0.05 was regarded as statistically significant.
Results
EsA improved the survival rate of rats with CLP-induced sepsis
In the present study, we first determined the effect of EsA on the survival rate of rats with CLP-induced sepsis. As shown in the Fig. 1A, the number of surviving rats was significantly reduced after CLP compared with the number of surviving sham rats. Administration of DXM and 5 mg/kg and 10 mg/kg EsA significantly increased the number of surviving rats. There was no significant difference in the number of surviving rats between the DXM and 10 mg/kg EsA groups.
Fig. 1.
Administration of EsA improved the survival of rats with CLP-induced sepsis. Survival was analyzed using Kaplan–Meier analysis and the log-rank test.
EsA alleviated kidney damage caused by CLP in rats
Next, we explored the effect of EsA on renal function of rats. The results of PAS staining showed that renal tubule structures were markedly damaged in rats with CLP-induced sepsis. However, treatment with DXM (2 mg/kg) or EsA (5 mg/kg and 10 mg/kg) significantly attenuated injury of renal tubules (Fig. 2A). The levels of BUN and CR, two indicators of renal function, were remarkably increased in septic rats, while their increases were markedly inhibited after DXM (2 mg/kg) or EsA (5 mg/kg and 10 mg/kg) treatment (Fig. 2B).
Fig. 2.
Treatment with EsA attenuated AKI in rats with CLP-induced sepsis. (A) Representative photomicrographs of PAS staining of the kidney in different groups. Scale bars: 100 µm. (B) The levels of serum BUN and CR. i, Sham group; ii, CLP-induced AKI group; iii, CLP-induced AKI + DXM (2 mg/kg) group; iv, CLP-induced AKI + EsA (2.5 mg/kg) group; v, CLP-induced AKI + EsA (5 mg/kg) group; vi, CLP-induced AKI + EsA (10 mg/kg) group. Data are shown as the mean ± SD. *P<0.05; **P<0.01; ***P<0.001.
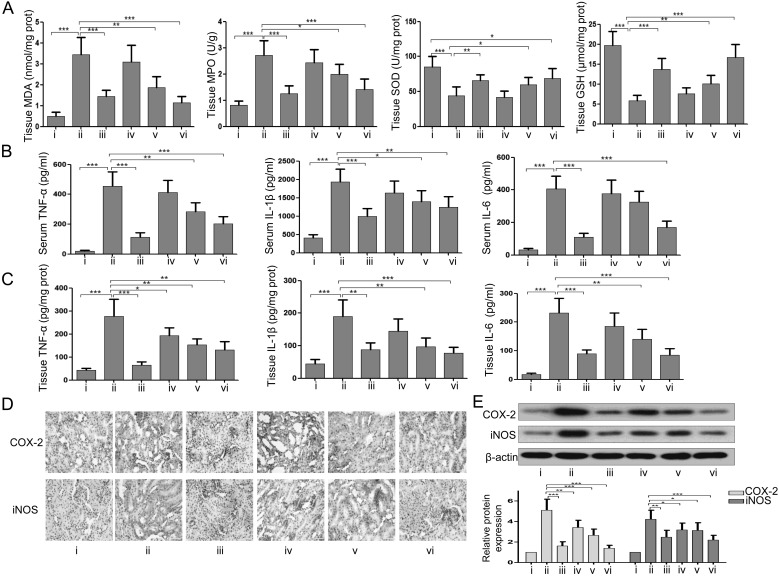
EsA suppressed inflammation and oxidative stress in CLP-induced septic rats
Next, we evaluated the effects of EsA on inflammation and oxidative stress in the kidney of septic rats. As shown in the Fig. 3A, the results indicated that the decreases in the activities of SOD and GSH and increases in MDA and MPO in tissue induced by CLP were partly restored by DXM (2 mg/kg) or EsA (5 mg/kg and 10 mg/kg) treatment. The elevated levels of TNF-α, IL1-β, and IL-6 in tissue and serum induced by CLP were also reduced after DXM (2 mg/kg) or EsA (5 mg/kg and 10 mg/kg) treatment (Figs. 3B and C). The results of immunohistochemistry and western blotting revealed that the overexpression of COX-2 and iNOS in tissue caused by CLP in rats was significantly reduced after DXM (2 mg/kg) or EsA (5 mg/kg and 10 mg/kg) treatment (Figs. 3D and E).
Fig. 3.
Administration of EsA suppressed inflammation and oxidative stress in the kidney of rats with CLP-induced sepsis. (A) The levels of MDA and GSH and activities of MPO and SOD. (B) The contents of serum TNF-α, IL-1β, and IL-6. (C) The levels of kidney tissue TNF-α, IL-1β, and IL-6. (D) Immunohistochemical analysis of COX-2 and iNOS in the kidney. Scale bars: 50 µm. (E) The expression levels of COX-2 and iNOS in the kidney were assessed using western blotting. i, Sham group; ii, CLP-induced AKI group; iii, CLP-induced AKI + DXM (2 mg/kg) group; iv, CLP-induced AKI + EsA (2.5 mg/kg) group; v, CLP-induced AKI + EsA (5 mg/kg) group; vi, CLP-induced AKI + EsA (10 mg/kg) group. Data are shown as the mean ± SD. *P<0.05; **P<0.01; ***P<0.001.
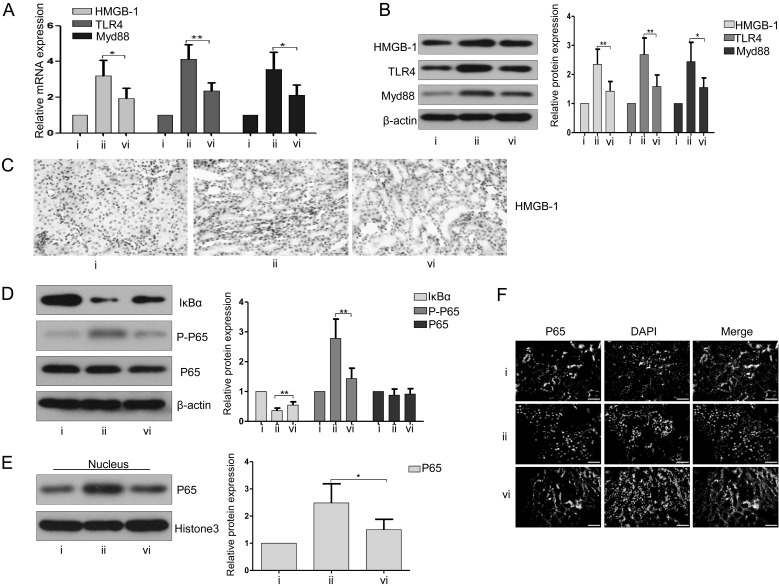
EsA inhibited the HMGB1/TLR/NF-kB pathway-related proteins in CLP-induced septic rats
As shown in Figs. 4A and B, the elevated levels of expression of mRNA and protein of HMGB1, TLR4, and Myd88 induced by CLP were significantly inhibited after 10 mg/kg EsA treatment. Immunohistochemical analysis also demonstrated that the overexpression of HMGB1 caused by CLP was markedly suppressed after administration of 10 mg/kg EsA (Fig. 4C). The results of western blotting showed that the decrease in IκBα expression and increase in p-p65 expression induced by CLP were remarkably inversed after 10 mg/kg EsA administration (Fig. 4D). Neither CLP nor EsA affected the expression of p65 in the whole protein of renal tissues (Fig. 4D). However, the amount of nuclear p65 was increased in the kidney of septic rats, while treatment with 10 mg/kg EsA negated the increase of nuclear p65 (Fig. 4E). This finding was confirmed by immunofluorescence staining (Fig. 4F).
Fig. 4.
Treatment with EsA inhibited the HMGB1/TLR/NF-kB pathway in rats with CLP-induced sepsis. (A) The mRNA expression levels of HMGB1, TLR4, and Myd88 in renal tissue were evaluated by RT-PCR. (B) Western blotting was performed to assess the expressions of HMGB1, TLR4, and Myd88 in the kidney. (C) Immunohistochemical staining of HMGB1 in the kidney. Scale bars: 50 µm. (D) The expressions of p65 and p-p65 were detected in the kidney by western blotting. (E) The expression of nuclear p65 in renal tissue was evaluated with immunoblotting. (F) Immunofluorescence was performed to assess the location and expression of p65 in renal tissue. Scale bars: 50 µm. i, Sham group; ii, CLP-induced AKI group; vi, CLP-induced AKI + EsA (10 mg/kg) group. Data are shown as the mean ± SD. *P<0.05; **P<0.01; ***P<0.001.
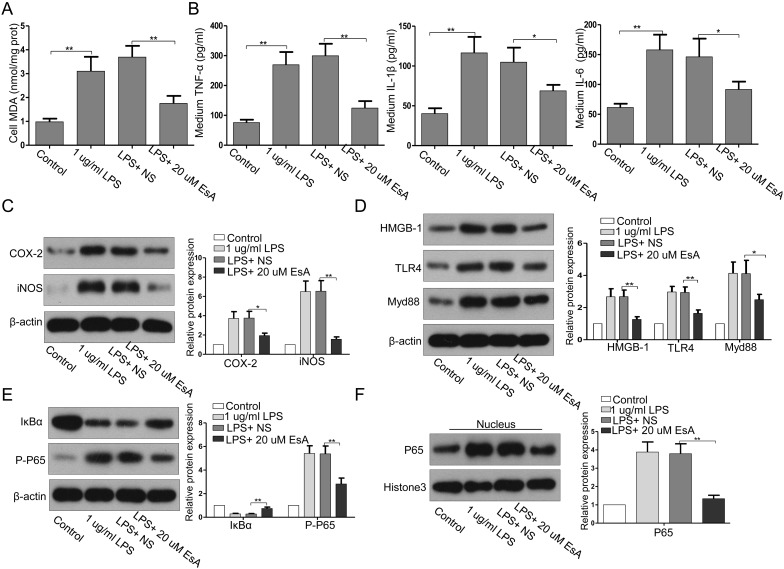
EsA reduced LPS-mediated oxidative stress and inflammatory responses in HK-2 cells
Our findings indicate that EsA can attenuate CLP-induced sepsis in rats and that the effect of EsA is exerted through the inhibition of inflammatory responses. As shown in Figs. 5A and B, LPS enhanced the release of MDA, TNF-α, IL-1β, and IL-6 in HK-2 cells. EsA decreased the LPS-induced MDA and pro-inflammatory cytokine production in HK-2 cells. In addition, EsA administration suppressed the LPS-induced upregulation of COX-2 and iNOS (Fig. 5C), which indicated that EsA can protect kidney tubular epithelial cells from LPS-induced oxidative stress and inflammatory responses.
Fig. 5.
Treatment with EsA inhibited LPS-stimulated alteration of stress-, inflammation-, and HMGB1/TLR/NF-kB pathway-related factors in HK-2 cells. (A, B) The levels of MDA, TNF-α, IL1-β, and IL-6. (C–F) The expression levels of COX-2, iNOS, HMGB1, TLR4, Myd88, IκBα, p-p65, and nuclear p65 were evaluated by western blotting. NS, normal saline; LPS, lipopolysaccharide. Data are shown as the mean ± SD. *P<0.05; **P<0.01; ***P<0.001.
EsA inhibited LPS-induced upregulation of HGB1/TLR4 and activation of NF-κB in HK2 cells
Consistent with the findings in the in vivo study, LPS induced increased expressions of HMGB1, TLR4, and Myd88 (Fig. 5D), decreased expression of IκBα, and enhanced phosphorylation and nuclear translocation of p65 in HK-2 cells (Figs. 5D and F). EsA treatment significantly reversed these changes.
Discussion
AKI is one of the most severe complications of sepsis, which is associated with high mortality [9, 29]. In the present study, CLP-induced rats, a well-established sepsis model [13] and LPS-induced HK-2 cells were used to explore the effects of EsA on septic AKI. DXM is usually used for treatment of inflammatory diseases and exhibits protective effects against sepsis in animal models [4, 5, 16]. Here, the effects of EsA were compared with those of DXM. The results showed that EsA attenuated renal tubular injury and improved renal function in septic rats, and the effects were similar to those of DXM. Mechanically, EsA inhibited oxidative stress, decreased the production of proinflammatory cytokines, and inhibited TLR4/NF-κB signal activation in both CLP-induced rats and LPS-injured HK-2 cells. Our findings indicate that EsA can attenuate CLP-induced sepsis in rats and that its anti-inflammatory effect and TLR4/NF-κB signal inhibition may contribute to this effect.
Inflammation plays a critical role in the pathogenesis of sepsis-induced AKI [18, 21]. Previous studies demonstrated that EsA attenuated LPS-induced acute hepatic and pulmonary inflammation and inhibited proinflammatory cytokine release in macrophages, indicating that EsA can suppress inflammation induced by bacterial LPS [14, 38, 40]. In agreement with these studies, we found that treatment with EsA significantly attenuated CLP-induced renal inflammation in septic rats, as evidenced by the decreased TNF-α, IL-1β, and IL-6. TLR4, a member of the TLR family, plays a key role in identifying bacterial LPS and mediating inflammatory responses and innate immunity [1]. Inhibition of the TLR4 pathway has been demonstrated to be associated with inflammation attenuation and mortality reduction in sepsis [12, 33, 37]. However, studies on TLR4 knockout mice have found that deficiency in TLR4 could inhibit secretion of proinflammatory cytokines but could not affect the survival of CLP mice [2, 6]. Based on these findings, the suppression of TLR4 induced by EsA may, at least partly, contribute to the anti-inflammatory effects of EsA. Whether or not the decreased mortality caused by EsA is associated with the inhibition of TLR4 needs to be studied in the future.
TLR4 signaling activates downstream effectors including NF-κB through the adaptor protein MyD88, resulting in the generation of pro-inflammatory cytokines [23]. The role of MyD88 in CLP-induced sepsis survival is controversial. In the study of Castoldi et al., MyD88 knockout mice showed improved survival after being subjected to CLP [2]. However, Echtenacher et al. found that MyD88 knockout worsened survival in CLP mice [25]. In our opinion, the environmental and operative technique differences between the two labs may have contributed to this discrepancy because we noted that there is a dramatic difference in the survival of wild type mice between the two studies. In our study, the survival rate was similar to that in the study of Castoldi et al., and we found that EsA treatment inhibited MyD88 expression. Thus, the improved survival in our study may be associated with the downregulation of Myd88, but the detailed mechanisms still need to be investigated.
HMGB1 is a nuclear protein that regulates multiple processes such as transcription, replication, and DNA repair [30]. It can also be passively or actively released into an extracellular medium upon cell stress or activation [8, 28]. Functions of HMGB1 in inflammatory responses are being debated: one argument is that it induces the release of proinflammatory cytokines; another is that highly purified HMGB1 has no cytokine-inducing activity. In contrast, it binds many pathogen-associated molecules such as LPS and bacterial lipopeptides and strengthens their effects. In any case, inhibition of HMGB1 release exerts a protective effect againstinflammatory responses [31]. HMGB1 secretion is mediated by TLR4 [15], and excessive HMGB-1 in turn induces cytokines production through binding with TLR4 and activates proinflammatory pathways like NF-κB [3]. In our study, HMGB1 expression was upregulated after LPS stimulation and CLP in vitro and in vivo, respectively. EsA administration suppressed the increase of HMGB1, which suggests that inhibition of HMGB1 may contribute to the anti-inflammatory effects of EsA in the kidney of septic rats.
NF-κB is a transcriptional protein that plays important roles in inflammatory responses [24]. Stress factors activate IκB kinase and in turn cause the phosphorylation of IκB proteins, the latter are degraded and release NF-κB. The activated NF-κB transfers to the nucleus and induces the transcription of target genes including proinflammatory cytokines [7, 24]. The released cytokines can also activate NF-κB, which forms a vicious circle [17]. In the present study, we found that CLP and LPS increased the expression levels of HMGB1, TLR4, and Myd88, but not p65, and EsA reversed these changes. Therefore, one possible explanation for these changes is that the increased levels of these proteins after stimulation enhance chances to initiate signaling through TLRs, leading to increased p65 phosphorylation, without any relation of EsA with TLR4 signal cascades. Our findings indicate that treatment with EsA could effectively relieve AKI and that its protective effects may be associated with regulation of the TLR4/MyD88/HMGB1 signaling pathway.
In summary, EsA treatment reduces mortality and protects the kidney against inflammation and oxidative stress in rats with CLP-induced sepsis. Our findings provide a potential strategy for the treatment of septic AKI.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Beutler B.2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430: 257–263. doi: 10.1038/nature02761 [DOI] [PubMed] [Google Scholar]
- 2.Castoldi A., Braga T.T., Correa-Costa M., Aguiar C.F., Bassi E.J., Correa-Silva R., Elias R.M., Salvador F., Moraes-Vieira P.M., Cenedeze M.A., Reis M.A., Hiyane M.I., Pacheco-Silva Á., Gonçalves G.M., Saraiva Câmara N.O.2012. TLR2, TLR4 and the MYD88 signaling pathway are crucial for neutrophil migration in acute kidney injury induced by sepsis. PLoS One 7: e37584. doi: 10.1371/journal.pone.0037584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q., Guan X., Zuo X., Wang J., Yin W.2016. The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm. Sin. B 6: 183–188. doi: 10.1016/j.apsb.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Du Y., Li Y., Wang X., Gao P., Yang G., Fang Y., Meng Y., Zhao X.2015. Panaxadiol saponin and dexamethasone improve renal function in lipopolysaccharide-induced mouse model of acute kidney injury. PLoS One 10: e0134653. doi: 10.1371/journal.pone.0134653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y., Meng Y., Lv X., Guo L., Wang X., Su Z., Li L., Li N., Zhao S., Zhao L., Zhao X.2014. Dexamethasone attenuates LPS-induced changes in expression of urea transporter and aquaporin proteins, ameliorating brain endotoxemia in mice. Int. J. Clin. Exp. Pathol. 7: 8443–8452. [PMC free article] [PubMed] [Google Scholar]
- 6.Echtenacher B., Freudenberg M.A., Jack R.S., Männel D.N.2001. Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect. Immun. 69: 7271–7276. doi: 10.1128/IAI.69.12.7172-7276.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuentes E., Rojas A., Palomo I.2016. NF-κB signaling pathway as target for antiplatelet activity. Blood Rev. 30: 309–315. doi: 10.1016/j.blre.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Gardella S., Andrei C., Ferrera D., Lotti L.V., Torrisi M.R., Bianchi M.E., Rubartelli A.2002. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 3: 995–1001. doi: 10.1093/embo-reports/kvf198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gómez H., Kellum J.A.2016. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 22: 546–553. doi: 10.1097/MCC.0000000000000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graziani G., Buskermolen M., Oldani S., Brambilla G.2006. [Sepsis, acute renal failure and multiple organ dysfunction syndrome]. G. Ital. Nefrol. 23:(Suppl 36): S13–S21(in Italian). [PubMed] [Google Scholar]
- 11.Gustot T.2011. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory response. Curr. Opin. Crit. Care 17: 153–159. doi: 10.1097/MCC.0b013e328344b446 [DOI] [PubMed] [Google Scholar]
- 12.Hong Z., Jiang Z., Liangxi W., Guofu D., Ping L., Yongling L., Wendong P., Minghai W.2004. Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int. Immunopharmacol. 4: 223–234. doi: 10.1016/j.intimp.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 13.Hubbard W.J., Choudhry M., Schwacha M.G., Kerby J.D., Rue L.W., 3rd, Bland K.I., Chaudry I.H.2005. Cecal ligation and puncture. Shock 24:(Suppl 1): 52–57. doi: 10.1097/01.shk.0000191414.94461.7e [DOI] [PubMed] [Google Scholar]
- 14.Ju D.W., Zheng Q.Y., Wang H.B., Guan X.J., Fang J., Yi Y.H.1994. [Inhibitory effects of esculentoside A on mouse macrophages and antibody production]. Yao Xue Xue Bao 29: 252–255(in Chinese). [PubMed] [Google Scholar]
- 15.Kim J.H., Kim S.J., Lee I.S., Lee M.S., Uematsu S., Akira S., Oh K.I.2009. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J. Immunol. 182: 2458–2466. doi: 10.4049/jimmunol.0801364 [DOI] [PubMed] [Google Scholar]
- 16.Kozan A., Kilic N., Alacam H., Guzel A., Guvenc T., Acikgoz M.2016. The Effects of Dexamethasone and L-NAME on acute lung injury in rats with lung contusion. Inflammation 39: 1747–1756. doi: 10.1007/s10753-016-0409-0 [DOI] [PubMed] [Google Scholar]
- 17.Lewis K., Caldwell J., Phan V., Prescott D., Nazli A., Wang A., Soderhölm J.D., Perdue M.H., Sherman P.M., McKay D.M.2008. Decreased epithelial barrier function evoked by exposure to metabolic stress and nonpathogenic E. coli is enhanced by TNF-alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G669–G678. doi: 10.1152/ajpgi.00382.2007 [DOI] [PubMed] [Google Scholar]
- 18.Li G., Fu J., Zhao Y., Ji K., Luan T., Zang B.2015. Alpha-lipoic acid exerts anti-inflammatory effects on lipopolysaccharide-stimulated rat mesangial cells via inhibition of nuclear factor kappa B (NF-κB) signaling pathway. Inflammation 38: 510–519. doi: 10.1007/s10753-014-9957-3 [DOI] [PubMed] [Google Scholar]
- 19.Li K., Cui N.N., Meng X.L., Ma J.N., Zhang S.S.2015. [Effect of Esculentoside A on Expression of AQP2 and AQP4 Protein of Kidney in Water-Loaded Rats]. Zhong Yao Cai 38: 1685–1689(in Chinese). [PubMed] [Google Scholar]
- 20.Li Y., Cao Y., Xu J., Qiu L., Xu W., Li J., Song Y., Lu B., Hu Z., Zhang J.2017. Esculentoside A suppresses lipopolysaccharide-induced pro-inflammatory molecule production partially by casein kinase 2. J. Ethnopharmacol. 198: 15–23. doi: 10.1016/j.jep.2016.12.041 [DOI] [PubMed] [Google Scholar]
- 21.Lingaraju M.C., Pathak N.N., Begum J., Balaganur V., Ramachandra H.D., Bhat R.A., Ram M., Singh V., Kandasamy K., Kumar D., Kumar D., Tandan S.K.2015. Betulinic acid attenuates renal oxidative stress and inflammation in experimental model of murine polymicrobial sepsis. Eur. J. Pharm. Sci. 70: 12–21. doi: 10.1016/j.ejps.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 22.Miyaji T., Hu X., Yuen P.S., Muramatsu Y., Iyer S., Hewitt S.M., Star R.A.2003. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 64: 1620–1631. doi: 10.1046/j.1523-1755.2003.00268.x [DOI] [PubMed] [Google Scholar]
- 23.Ouyang Y., Guo J., Lin C., Lin J., Cao Y., Zhang Y., Wu Y., Chen S., Wang J., Chen L., Friedman S.L.2016. Transcriptomic analysis of the effects of Toll-like receptor 4 and its ligands on the gene expression network of hepatic stellate cells. Fibrogenesis Tissue Repair 9: 2. doi: 10.1186/s13069-016-0039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S.D., Cheon S.Y., Park T.Y., Shin B.Y., Oh H., Ghosh S., Koo B.N., Lee S.K.2015. Intranuclear interactomic inhibition of NF-κB suppresses LPS-induced severe sepsis. Biochem. Biophys. Res. Commun. 464: 711–717. doi: 10.1016/j.bbrc.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 25.Peck-Palmer O.M., Unsinger J., Chang K.C., Davis C.G., McDunn J.E., Hotchkiss R.S.2008. Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. J. Leukoc. Biol. 83: 1009–1018. doi: 10.1189/jlb.0807528 [DOI] [PubMed] [Google Scholar]
- 26.Rajapakse S., Rodrigo C., Rajapakse A., Kirthinanda D., Wijeratne S.2009. Renal replacement therapy in sepsis-induced acute renal failure. Saudi J. Kidney Dis. Transpl. 20: 553–559. [PubMed] [Google Scholar]
- 27.Rittirsch D., Huber-Lang M.S., Flierl M.A., Ward P.A.2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4: 31–36. doi: 10.1038/nprot.2008.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaffidi P., Misteli T., Bianchi M.E.2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195. doi: 10.1038/nature00858 [DOI] [PubMed] [Google Scholar]
- 29.Shankar-Hari M., Rubenfeld G.D.2016. Understanding Long-Term Outcomes Following Sepsis: Implications and Challenges. Curr. Infect. Dis. Rep. 18: 37. doi: 10.1007/s11908-016-0544-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stros M.2010. HMGB proteins: interactions with DNA and chromatin. Biochim. Biophys. Acta 1799: 101–113. doi: 10.1016/j.bbagrm.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 31.Tsan M.F.2011. Heat shock proteins and high mobility group box 1 protein lack cytokine function. J. Leukoc. Biol. 89: 847–853. doi: 10.1189/jlb.0810471 [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Zhang S., Cheng H., Lv H., Cheng G., Ci X.2016. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radic. Biol. Med. 101: 401–412. doi: 10.1016/j.freeradbiomed.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 33.Wu Y., Liu Y., Huang H., Zhu Y., Zhang Y., Lu F., Zhou C., Huang L., Li X., Zhou C.2013. Dexmedetomidine inhibits inflammatory reaction in lung tissues of septic rats by suppressing TLR4/NF-κB pathway. Mediators Inflamm. 2013: 562154. doi: 10.1155/2013/562154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao Z., Su Y., Yang S., Yin L., Wang W., Yi Y., Fenton B.M., Zhang L., Okunieff P.2006. Protective effect of esculentoside A on radiation-induced dermatitis and fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 65: 882–889. doi: 10.1016/j.ijrobp.2006.01.031 [DOI] [PubMed] [Google Scholar]
- 35.Xiao Z.Y., Zheng Q.Y., Jiang Y.Y., Zhou B., Yin M., Wang H.B., Zhang J.P.2004. Effects of esculentoside A on production of interleukin-1, 2, and prostaglandin E2. Acta Pharmacol. Sin. 25: 817–821. [PubMed] [Google Scholar]
- 36.Xiao Z.Y., Zheng Q.Y., Zhang J.P., Jiang Y.Y., Yi Y.H.2002. Effect of esculentoside A on autoimmunity in mice and its possible mechanisms. Acta Pharmacol. Sin. 23: 638–644. [PubMed] [Google Scholar]
- 37.Yu M., Shao D., Yang R., Feng X., Zhu S., Xu J.2007. Effects of ketamine on pulmonary inflammatory responses and survival in rats exposed to polymicrobial sepsis. J. Pharm. Pharm. Sci. 10: 434–442. doi: 10.18433/J3RP46 [DOI] [PubMed] [Google Scholar]
- 38.Zhang F., Wang X., Qiu X., Wang J., Fang H., Wang Z., Sun Y., Xia Z.2014. The protective effect of Esculentoside A on experimental acute liver injury in mice. PLoS One 9: e113107. doi: 10.1371/journal.pone.0113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang K.F., Zhang L., Wu X.F., Zhang X.G., Yu H., Yi Y.H., Zhao S.Q.2004. [Therapeutical effects of esculentoside A on rats with MsPGN induced by anti-Thy1.1 antibody]. Sichuan Da Xue Xue Bao Yi Xue Ban 35: 662–664 (In Chinese). [PubMed] [Google Scholar]
- 40.Zhong W.T., Jiang L.X., Wei J.Y., Qiao A.N., Wei M.M., Soromou L.W., Xie X.X., Zhou X., Ci X.X., Wang D.C.2013. Protective effect of esculentoside A on lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 185: 364–372. doi: 10.1016/j.jss.2013.05.018 [DOI] [PubMed] [Google Scholar]