Abstract
In an earlier report, we demonstrated an antipsychotic-like activity of a methanolic extract of Morinda citrifolia Linn fruit in mouse models and postulated the contribution of its bioactive principles, scopoletin and rutin. Moreover, the antidopaminergic activities of scopoletin and rutin were reported in isolated vas deferens preparations. In the present study, scopoletin and rutin were assessed for antipsychotic-like activity using apomorphine-induced climbing behavior and methamphetamine-induced stereotypy in mice. The results of this study revealed that scopoletin and rutin (0.05, 0.1, 0.5, and 1 mg/kg, p.o.) had a “U-shaped” dose-dependent effect on climbing and stereotyped behaviors induced by apomorphine and methamphetamine, respectively, in mice. A significant reduction in climbing and stereotyped behaviors caused by scopoletin and rutin was observed only at a dose 0.1 mg/kg. This study suggests that scopoletin and rutin can alleviate positive symptoms of schizophrenia only at a specific dose. Further studies evaluating the effects of scopoletin and rutin on animal models for negative symptoms of schizophrenia are required for a novel drug discovery in the treatment of neuropsychiatric diseases.
Keywords: climbing behavior, psychosis, rutin, scopoletin, stereotypy
Introduction
According to National Institute of Mental Health (NIMH), schizophrenia is defined as a chronic mental disorder that impedes a person’s thinking, feelings, and behavior. The symptoms of schizophrenia are categorized as positive symptoms (hallucinations, delusions, thought disturbances, and agitated body movements), negative symptoms (defect in the expression of emotions, anhedonia, social withdrawal, and reduced speaking), and cognitive dysfunction. Conventional antipsychotics such as haloperidol, droperidol, fluphenazine, and thiothixene have minimal or no effect in alleviating negative symptoms and have severe adverse effects, particularly extrapyramidal symptoms (EPS). Second generation antipsychotics such as clozapine, olanzapine, quetiapine, risperidone, and aripiprazole can alleviate both positive and negative symptoms. However, these drugs cause more weight gain and QT prolongation than older agents [2].
Use of phytotherapy to treat various ailments including neuropsychiatric diseases is an emerging trend in medicine. Phytotherapy research is the basis for discovery and development of novels drugs in modern medicine. In the past, many drugs, such as morphine, avermectin, artemisinin, lovastatin, and paclitaxel, were developed from medicinal plants for the treatment of pain, parasites, malaria, and hyperlipidemias, respectively [8]. Recently, Yadav et al. extensively reviewed and listed 48 medicinal plants that possess antipsychotic activity [17]. However, so far, none of these medicinal plants have been studied further for novel drug development for effective treatment of schizophrenia.
In earlier studies, our research group established an antipsychotic-like activity of a methanolic extract of unripe Morinda citrifolia fruit (MMC) in mouse models of apomorphine-induced cage climbing behavior and methamphetamine-induced stereotyped behavior in mice [12]. Moreover, our research group also demonstrated the antidopaminergic effect of MMC and its bioactive principles, such as scopoletin and rutin. We proposed that these bioactive principles could be responsible for the antipsychotic-like activity of Morinda citrifolia fruit [13]. Therefore the present study was designed to evaluate the antipsychotic-like activity of scopoletin and rutin using mouse models of apomorphine-induced climbing behavior and methamphetamine-induced stereotypy.
Materials and Methods
Animal
Male ICR mice weighing from 25–30 g were used for this study. The animals were randomly transferred from the shipping container they were sent in into appropriate cages (n=4 in each cage) with free access to food and water and maintained in a controlled vivarium environment with a temperature of 22 ± 1°C, relative humidity of 45–60%, and a 12 h light: 12 h dark normal cycle (lights on at 7 AM) in a quarantine room. The quarantined animals were then randomly selected, marked to permit individual identification, and kept in their corresponding cages for at least two weeks prior to the start of the experiment to enable acclimatization to the laboratory conditions. Mice with abnormal conditions (excessive aggression/excessive licking/self-injury/any physical abnormalities) were excluded from the study. Selected mice were further randomized by body weight. The selected mice were assigned into different treatment groups (control and treatment) by randomized weight distribution so that the mean body weight of each group would not be statistically different from those of the other groups. All experimental protocols were approved by the University Animal Care and Use Committee, University of Malaya, Kuala Lumpur (ACUC Ethics No.2013–12 03/PHAR/R/VP). All experimental protocols and animal care adhered to the guidelines of the National Research Council of the National Academies of Sciences, Engineering, and Medicine, USA [5]. The behavioral experiments were performed during the light cycle between 10.00 AM and 6.00 PM. All efforts were made to minimize suffering of the mice.
Drugs and administration
Apomorphine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline containing sodium metabisulphite (0.125% w/v) and methamphetamine hydrochloride (Department of Chemistry, Ministry of Health, Malaysia) was dissolved in normal saline and administered intraperitoneally (i.p.) at a constant volume of 1 ml/100 g body weight of the animal. Scopoletin (7-hydroxy-6-methoxycoumarin) obtained from Friendemann Schmidt Chemicals and rutin hydrate (quercetin-3-rutinoside hydrate) procured from Sigma-Aldrich were prepared as suspensions in a 1% w/v aqueous solution of sodium carboxymethyl cellulose (CMC) and administered orally (p.o.).
Assessment of apomorphine-induced climbing behavior in mice
The naïve animals were segregated into six groups (n=7–10). Group I served as a saline control group and received a saline injection. Group II served as vehicle control and received 1% w/v CMC solution (1 ml/100 g, p.o.) 1 h prior to apomorphine injection (5 mg/kg, i.p.). Group III, IV, V, and VI animals received an oral gavage of scopoletin at doses of 0.05, 0.1, 0.5, and 1 mg/kg, respectively, 1 h before apomorphine injection. The naïve mice were first allowed to acclimatize to the cylindrical metal observation cages (18 × 19 cm, which had vertical (1 cm apart) and horizontal (4.5 cm apart) metal bars (2 mm) and an upper lid with a lower bottom), for 15 min prior to the experiment. Immediately after injection of apomorphine, the mice were individually placed at the base of their respective observation cages and observed. The climbing behavior induced by apomorphine was assessed every 5 min for 30 min and scored according to the method previously described by Pandy et al. using the following rating scale: 0 =four paws on the floor, 2 =two paws on the wall of the cage, and 4 =four paws on the wall of the cage (climbing). The score corresponding to the posture of the animal adopted for the longest period of time was recorded. The climbing index was recorded at every 5 min for 30 min, and the cumulative climbing index was calculated. The maximum possible cumulative climbing index was 24. Additionally, the time spent by the animal on the wall of the cage (keeping four paws on the wall) was recorded as the climbing time in seconds. The same procedure was repeated for rutin with a different set of animals at doses of 0.05, 0.1, 0.5, and 1 mg/kg.
Assessment of methamphetamine-induced stereotypy in mice
The procedure and apparatus used in this study were the same as described in our previous report by Pandy et al. with slight modifications. The naïve mice were distributed randomly into six groups (n=8). Group I received a normal saline injection and served as a saline control group. Group II served as a vehicle control and received 1% w/v of CMC 1 h prior to intraperitoneal injection of methamphetamine (5 mg/kg). Group III, IV, V, and VI animals were administered scopoletin at doses of 0.05, 0.1, 0.5, and 1 mg/kg (p.o.), respectively, 1 h before methamphetamine injection. The animals were first allowed to explore their respective observation cages freely for 15 min prior to the experiment. Immediately after injection of methamphetamine, the mice were individually placed back into their respective observation cages at the base and observed for stereotyped behavior. The intensity of stereotypy of individual animals was recorded at 15-min intervals for a period of 60 min by an observer who was unaware of the drug treatment. The stereotyped behavior was scored as described previously [12]: 0, no change compared with control; 1, discontinuous sniffing and constant exploratory activity; 2, continuous sniffing and periodic exploratory activity; 3, continuous sniffing and discontinuous biting, gnawing, or licking; and 4, continuous biting, gnawing, or licking and no exploratory activity. The same procedure was repeated for rutin with a different set of animals at doses of 0.05, 0.1, 0.5, and 1 mg/kg (p.o.).
Statistical analysis
Comparisons among different groups for climbing time and cumulative climbing index were made using one-way analysis of variance (ANOVA) followed by Dunnett’s test. As for stereotypes score, comparisons were made using the Kruskal-Wallis test followed by Dunn’s multiple comparison test. The data were analyzed using the GraphPad Prism, version 5.0, statistical software. A level of P<0.05 was considered statistically significant.
Results
Effect of scopoletin and rutin on apomorphine-induced climbing behavior in mice
ANOVA results revealed a significant effect of scopoletin on apomorphine-induced climbing time [F (5, 53)=4.486; P<0.001] and cumulative climbing index [F (5, 53)=7.353; P<0.0001]. Post hoc analysis revealed that scopoletin significantly (P<0.05) lowered the climbing time and cumulative climbing index in mice, but only at a dose of 0.1 mg/kg, as shown in Figs. 1a and 1b.
Fig. 1.
Effect of scopoletin (SCOP 0.05, 0.1, 0.5, and 1 mg/kg, p.o.) on cage climbing behavior induced by apomorphine in mice. (a) Total time spent on the wall of the cage. (b) The cumulative scores were measured for 30 min at 5-min interval after administration of apomorphine. All data are presented as means ± SEM of the scores obtained from eight to ten animals. ##: P<0.01, ###: P<0.001 when compared with the saline control group. *: P<0.05 when compared with the vehicle group.
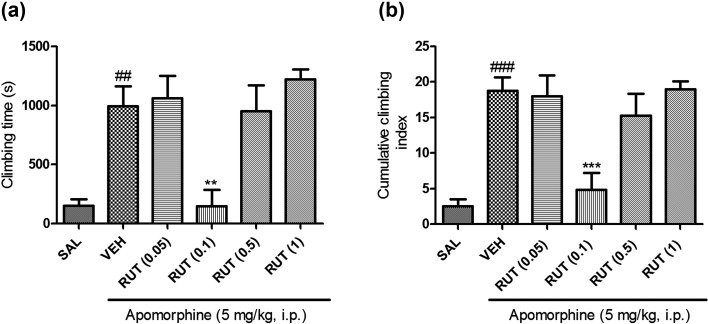
Similarly, rutin exhibited a significant effect on the apomorphine-induced climbing time [F (5, 41)=9.506; P<0.0001] and cumulative climbing index [F (5, 41)=10.98; P<0.0001]. Post hoc analysis revealed rutin (0.1 mg/kg, p.o.) significantly (P<0.05) lowered the climbing time and cumulative climbing index in mice (Figs. 2a and 2b).
Fig. 2.
Effect of rutin (RUT 0.05, 0.1, 0.5, and 1 mg/kg, p.o.) on cage climbing behavior induced by apomorphine in mice. (a) Total time spent on the wall of the cage. (b) The cumulative scores were measured for 30 min at 5-min intervals after administration of apomorphine. All data are presented as means ± SEM of the scores obtained from seven to eight animals. ##: P<0.01, ###: P<0.001 when compared with the saline group. **: P<0.01, ***: P<0.001 when compared with the vehicle group.
Effect of scopoletin and rutin on methamphetamine-induced stereotyped behavior in mice
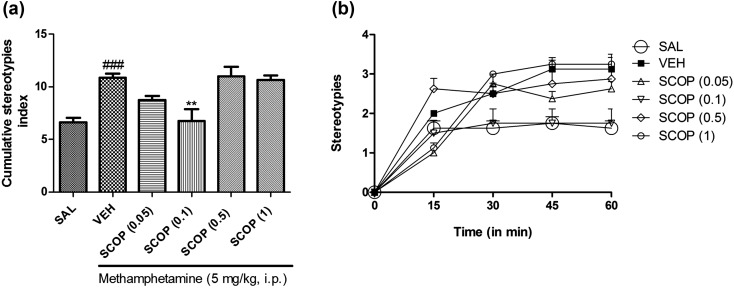
Kruskal-Wallis analysis revealed a significant (P<0.0001) effect of scopoletin treatment on a mouse model of methamphetamine-induced stereotypy. The post hoc Dunn’s test results emphasized that scopoletin at 0.1 mg/kg significantly (P<0.01) reduced the stereotyped behavior in mice, as shown in Figs. 3a and 3b.
Fig. 3.
Effect of scopoletin (SCOP 0.05, 0.1, 0.5, and 1 mg/kg, p.o.) on stereotypies induced by methamphetamine in mice. (a) The cumulative stereotypy scores were measured during 30 to 60 min after administration of methamphetamine. (b) Stereotype scores at different time points for 60 min. All data are presented as means ± SEM of eight animals. ###: P<0.001 when compared with the saline group. **: P<0.01 when compared with the vehicle group.
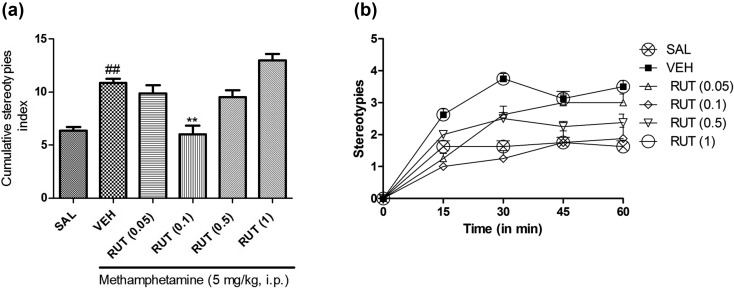
In another study, rutin also showed a significant (P<0.0001) effect on methamphetamine-induced stereotyped behavior in mice. Dunn’s test results revealed that rutin (0.1 mg/kg, p.o.) significantly (P<0.01) lowered the stereotypy in mice (Figs. 4a and 4b).
Fig. 4.
Effect of rutin (RUT 0.05, 0.1, 0.5, and 1 mg/kg, p.o.) on stereotypies induced by methamphetamine in mice. (a) The cumulative stereotypy scores were measured during 30 to 60 min after administration of methamphetamine. (b) Stereotype scores at different time points for 60 min. All data are presented as means ± SEM of eight animals. ##: P<0.01 when compared with the saline group. **: P<0.01 when compared with the vehicle group.
Discussion
Scopoletin is a naturally occurring coumarin found in Morinda citrifolia Linn. in addition to many edible plants such as Allium ampeloprasum, Avena sativa, Apium graveolens, Capsicum frutescens, Capsicum annuum, Citrus limon, Cichorium intybus, Citrus paradise, and Daucus carota [1]. Neuropharmacological studies revealed the importance of scopoletin in antidepressant [3], anxiolytic [11], memory enhancing [9], and anticonvulsant [10] activities in scopoletin-containing plants. Scopoletin has also been recommended as a marker constituent for the quality control and pharmacokinetic study of noni (M. citrifolia) products [6].
Apomorphine (D1 and D2 agonist)-induced cage climbing and methamphetamine-induced stereotypy mouse models are widely used to screen for potential antipsychotic-like activity in test compounds in terms of their effects on positive symptoms of schizophrenia [12, 16]. The dopamine hypothesis of schizophrenia revealed that hyperactivity of dopaminergic signal transduction (D2 receptors) in the mesolimbic pathway is responsible for positive symptoms of schizophrenia [16]. Methamphetamine induces positive symptoms of psychosis by reversing the action of the dopamine transporter, thereby enhancing dopaminergic neurotransmission in the mesolimbic pathway [15]. In the present study, acute oral administration of scopoletin at 0.1 mg/kg showed anticlimbing and anti-stereotypy effects on apomorphine-induced cage climbing and methamphetamine-induced stereotypy behaviors, respectively, in mice. This demonstrated antipsychotic-like activity of scopoletin, which has the potential to alleviate positive symptoms of schizophrenia. Moreover, in a previous ex vivo study, we showed that scopoletin (100 and 200 µg/ml) significantly reduced the dopamine-induced contractile response in isolated vas deferens [13] preparations, which suggested the antidopaminergic effect of scopoletin. Conversely, scopoletin at higher doses (0.5 and 1 mg/kg, p.o.) did not exhibit any antipsychotic-like activity in the present study. In a recent report, scopoletin at a high dose (80 mg/kg, i.p.) significantly increased dopamine and its metabolite (DOPAC) by inhibiting both MAO-A and MAO-B enzymes [1]. This could be a probable reason for the ineffectiveness of scopoletin at higher doses (0.5 and 1 mg/kg) on the animal models of psychosis in the present study.
Rutin (3,3′,4’,5,7-pentahydroxyflavone-3-rhamnoglucoside) is a flavonoid found in many plants and fruits including noni plant and fruit. Flavonoids have been reported to have many beneficial effects on the central nervous system, especially improvement of cognitive function mainly mediated by suppressing astrocytes and microglia activation and facilitating synaptic plasticity [4]. They have also been reported to have antidepressant activity mediated by their interaction with monoaminergic systems [4]. In the present study, rutin (0.1 mg/kg, p.o.) significantly reduced the intensity of climbing and stereotyped behaviors induced by apomorphine and methamphetamine, respectively, in mice, which indicates the antipsychotic-like activity of rutin. In our earlier ex vivo study using isolated rat vas deferens, rutin at 156 and 312 µg/ml significantly inhibited the dopamine-induced contractile response. This suggests that the antipsychotic-like effect of rutin could be mediated by its interaction with dopamine D2 receptors. However, similar to scopoletin, rutin at higher doses (0.5 and 1 mg/kg, p.o.) failed to demonstrate any antipsychotic-like effect in mice. This could be due to a facilitatory effect of rutin on the dopaminergic system at higher doses. The “U-shaped” pattern of response of apomorphine-induced climbing and methamphetamine-induced stereotypy behaviors to rutin (0.05–1 mg/kg, p.o.) in the present study is not atypical. A similar “U-shaped” dose-response pattern was observed for the antidepressant effect of H. citrina flower ethanolic (0%, 25%, 50%, 75%, and 100%) extracts containing 96.2, 73.2, 65.7, 74.2, and 69.2% w/w rutin [4]. Interestingly, different doses (100–1,600 mg/kg) of H. citrina flower ethanol (75%) extracts showed similar “U-shaped” dose-response profiles for monoamine transmitter levels including dopamine [4].
Moreover, we recently demonstrated the biphasic effect of the ethyl acetate fraction of a methanolic extract of unripe noni (Morinda citrifolia Linn.) fruit (EA-MMC) on the dopaminergic system in mice in which EA-MMC (5–100 mg/kg, p.o.) resulted in a dose-dependent U-shaped trend in the APO-induced cage climbing and METH-induced stereotyped behavior [14]. It was postulated that EA-MMC showed antidopaminergic activity at lower doses by blocking the postsynaptic dopaminergic D2 receptors and a dopaminergic facilitatory effect at higher doses by blocking the presynaptic autodopaminergic D2 receptors (i.e., inhibiting the negative feedback mechanism). This study also suggested the possible involvement of the bioactive principles of noni, scopoletin and rutin, in its biphasic effect on the dopaminergic system [14]. Recently, in silico molecular docking analysis of phytoconstituents (alizarin, ascorbic acid, beta-carotene, beta-sitosterol, morindin, morindone, rutin, and scopoletin) from Morinda citrifolia fruit extract and the standard typical and atypical antipsychotic drugs along with their target amino acid residues of the D2 dopamine receptor based on MolDock score and hydrogen bond interaction has been reported [7]. The phytoconstituents with the lowest MolDock scores and hydrogen bond interactions were considered to have maximum affinity for the target protein. The results of this in silico study revealed that rutin showed the lowest MolDock score (−120.52) when compared with the standard antipsychotics pimozide and aripiprazole, the scores of which were −116.13 and −115.04, respectively. Moreover, rutin showed the maximum hydrogen bond interaction with a value of −7.56, which was comparable to the standard antipsychotic drugs paliperidone and haloperidol, which showed interaction values of −4.52 and −3.23, respectively [7]. Therefore, the present results concerning the in vivo antipsychotic-like activity of scopoletin and rutin in mouse models are consistent with the report from this recent in silico study. Overall, this study highlighted the antipsychotic-like effects of rutin and scopoletin at 0.1 mg/kg body weight.
In conclusion, the bioactive principles of Morinda citrifolia Linn, scopoletin and rutin, exhibit antipsychotic-like activity at 0.1 mg/kg with the potential to lower positive symptoms of psychosis in mouse models, and it is suggested that these bioactive principles have antidopaminergic properties at a specific dose. However, further studies on negative symptoms of psychosis in animal models are warranted to develop scopoletin and rutin as novel drugs to treat neuropsychiatric diseases.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgments
This study was supported by University of Malaya research grants [PG232-2015B, PG035- 2014B and PG023-2014B] and an HIR MOHE grant [UM.C/625/1/HIR/MOHE/MED/05 (H-20001-E000088)]. We are grateful to the administration of the University of Malaya for providing financial assistance and necessary infrastructure to carry out this research.
Reference
- 1.Basu M., Mayana K., Xavier S., Balachandran S., Mishra N.2016. Effect of scopoletin on monoamine oxidases and brain amines. Neurochem. Int. 93: 113–117. doi: 10.1016/j.neuint.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 2.Campbell M., Young P.I., Bateman D.N., Smith J.M., Thomas S.H.L.1999. The use of atypical antipsychotics in the management of schizophrenia. Br. J. Clin. Pharmacol. 47: 13–22. doi: 10.1046/j.1365-2125.1999.00849.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capra J.C., Cunha M.P., Machado D.G., Zomkowski A.D., Mendes B.G., Santos A.R., Pizzolatti M.G., Rodrigues A.L.2010. Antidepressant-like effect of scopoletin, a coumarin isolated from Polygala sabulosa (Polygalaceae) in mice: evidence for the involvement of monoaminergic systems. Eur. J. Pharmacol. 643: 232–238. doi: 10.1016/j.ejphar.2010.06.043 [DOI] [PubMed] [Google Scholar]
- 4.Du B., Tang X., Liu F., Zhang C., Zhao G., Ren F., Leng X.2014. Antidepressant-like effects of the hydroalcoholic extracts of Hemerocallis citrina and its potential active components. BMC Complement. Altern. Med. 14: 326. doi: 10.1186/1472-6882-14-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garber J.C., Barbee R.W., Bielitzki J.T., Clayton L.A., Donovan J.C., Kohn D.F., Lipman N.S., Locke P., Melcher J., Quimby F.W., Turner P.V., Wood G.A., Würbel H.2011. Guide for the Care and Use of Laboratory Animals, 8th ed., The National Academies Press, Washington, D.C. [Google Scholar]
- 6.Issell B.F., Franke A., Fielding R.M.2008. Pharmacokinetic study of Noni fruit extract. J. Diet. Suppl. 5: 373–382. doi: 10.1080/19390210802519671 [DOI] [PubMed] [Google Scholar]
- 7.Jeyabalan S., Subramanian K., Cheekala U.M.R., Krishnan C.2017. GC-MS analysis and in-silico antipsychotic activity of Morinda citrifolia (Indian Noni). J. Appl. Pharm. Sci. 7: 89–95. [Google Scholar]
- 8.Katiyar C., Gupta A., Kanjilal S., Katiyar S.2012. Drug discovery from plant sources: An integrated approach. Ayu 33: 10–19. doi: 10.4103/0974-8520.100295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik J., Karan M., Vasisht K.2016. Attenuating effect of bioactive coumarins from Convolvulus pluricaulis on scopolamine-induced amnesia in mice. Nat. Prod. Res. 30: 578–582. doi: 10.1080/14786419.2015.1025398 [DOI] [PubMed] [Google Scholar]
- 10.Mishra N., Oraon A., Dev A., Jayaprakash V., Basu A., Pattnaik A.K., Tripapthi S.N., Akhtar M., Ahmad S., Swaroop S., Basu M.2010. Anticonvulsant activity of Benkara malabarica (Linn.) root extract: In vitro and in vivo investigation. J. Ethnopharmacol. 128: 533–536. doi: 10.1016/j.jep.2010.01.042 [DOI] [PubMed] [Google Scholar]
- 11.Monsef-Esfahani H.R., Amini M., Goodarzi N., Saiedmohammadi F., Hajiaghaee R., Faramarzi M.A., Tofighi Z., Ghahremani M.H.2013. Coumarin compounds of Biebersteinia multifida roots show potential anxiolytic effects in mice. Daru 21: 51. doi: 10.1186/2008-2231-21-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandy V., Narasingam M., Mohamed Z.2012. Antipsychotic-like activity of noni (Morinda citrifolia Linn.) in mice. BMC Complement. Altern. Med. 12: 186. doi: 10.1186/1472-6882-12-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandy V., Narasingam M., Kunasegaran T., Murugan D.D., Mohamed Z.2014. Effect of noni (Morinda citrifolia Linn.) fruit and its bioactive principles scopoletin and rutin on rat vas deferens contractility: an ex vivo study. Sci. World J. 2014: 909586. doi: 10.1155/2014/909586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandy V., Narasingam M., Vijeepallam K., Mohan S., Mani V., Mohamed Z.2017. The ethyl acetate fraction of a methanolic extract of unripe noni (Morinda citrifolia Linn.) fruit exhibits a biphasic effect on the dopaminergic system in mice. Exp. Anim. 66: 283–291. doi: 10.1538/expanim.16-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman R.B., Baumann M.H., Dersch C.M., Romero D.V., Rice K.C., Carroll F.I., Partilla J.S.2001. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39: 32–41. doi: [DOI] [PubMed] [Google Scholar]
- 16.Vijeepallam K., Pandy V., Kunasegaran T., Murugan D.D., Naidu M.2016. Mitragyna speciosa leaf extract exhibits antipsychotic-like effect with the potential to alleviate positive and negative symptoms of psychosis in mice. Front. Pharmacol. 7: 464. doi: 10.3389/fphar.2016.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav M., Parle M., Kadian M., Sharma K.2015. A review on psychosis and anti-psychotic plants. Asian J. Pharm. Clin. Res. 8: 24–28. [Google Scholar]