Abstract
The in vivo imaging of mice makes it possible to analyze disease progress non-invasively through reporter gene expression. As the removal of hair improves the accuracy of in vivo imaging, gene-modified mice with a reporter gene are often crossed with Hos:HR-1 mutant mice homozygous for the spontaneous Hrhr mutation that exhibit a hair loss phenotype. However, it is time consuming to produce mice carrying both the reporter gene and mutant Hrhr gene by mating. In addition, there is a risk that genetic background of the gene-modified mice would be altered by mating. To resolve these issues, we established a simple method to generate hairless mice maintaining the original genetic background by CRISPR technology. First, we constructed the pX330 vector, which targets exon 3 of Hr. This DNA vector (5 ng/µl) was microinjected into the pronuclei of C57BL/6J mice. Induced Hr gene mutations were found in many founders (76.1%) and these mutations were heritable. Next, we performed in vivo imaging using these gene-modified hairless mice. As expected, luminescent objects in their body were detected by in vivo imaging. This study clearly showed that hairless mice could be simply generated by the CRISPR/Cas9 system, and this method may be useful for in vivo imaging studies with various gene-modified mice.
Keywords: CRISPR/Cas9, hairless, in vivo imaging, mouse
Introduction
In vivo molecular imaging is an essential tool for detecting target biomolecules directly and non-invasively, and for visualizing molecular processes. Indocyanine green (ICG) is a common fluorescent molecule used in human medical diagnostics [15]. ICG is a cyanine dye that is removed exclusively by the liver and excreted into the bile [5]. ICG can be excited at 800 nm and emits fluorescence at 840 nm when bound with proteins [2]. Transient overexpression of near-infrared fluorescent protein (iRFP) causes bright fluorescence in cells, tissues, and the entire animal body without the addition of exogenous biliverdin [7]. The iRFP can be excited at 690 nm and emits fluorescence at 713 nm. An optimal fluorescent protein for in vivo imaging should have both excitation and emission maxima within a near-infrared window from ~650 nm to 900 nm, for which tissue has the lowest absorbance and less light scattering than at shorter wavelengths [12]. Therefore, iRFP is suitable for deep tissue imaging.
In vivo imaging with the iRFP makes it possible to detect fluorescent proteins in the living bodies of mice [14]. The hair blocks, absorbs, and scatters light. Especially, Black hair absorbs more light than other color hairs [22, 30]. As even white hair absorbs light, it is very difficult to detect fluorescence signals. Therefore, it is necessary to remove the hair from mice to detect the iRFP signal with in vivo imaging system. Nude mice lack hair, although they are immunodeficient [8, 23]. The FoxN1 gene, located on chromosome 11, is responsible for these phenotypes [4, 21, 29]. Hr gene (RefSeq Accession: NM_021877) mutants such as Hos:HR-1 mice also show hair loss without abnormalities in the immune system. These hairless mice are useful for not only skin, immunology, and cancer studies but also in vivo imaging [3, 9]. Gene-modified mice are often backcrossed to Hr mutant mice for in vivo imaging [20]. However, it is time consuming to produce mice carrying both a reporter gene for in vivo imaging and mutated Hr gene by mating. Moreover, if reporter gene is located on chromosome 14 on which Hr gene is located, it is difficult to introduce both mutations by mating. In addition, there is the risk that the genetic background of the reporter mice would be changed by mating.
The CRISPR/Cas9 system is very convenient for inducing specific mutations in various animals. This system is composed of a single guide RNA (sgRNA) and Cas9 protein, which cause site-specific DNA double-strand breaks (DSB), leading to indel mutations by non-homologous end joining (NHEJ) [11, 25]. To shorten the time for producing hairless gene-modified mice and to eliminate the risk of changes in genetic background, we generated hairless mice for in vivo imaging using the CRISPR/Cas9 system.
In this study, we tried to produce new hairless mice using the CRISPR/Cas9 system for detecting iRFP signal by in vivo imaging.
Materials and Methods
Animals
C57BL/6J and Jcl:CD1 (ICR) mice were purchased from Charles River Laboratories Japan (Kanagawa, Japan) and CLEA Japan (Tokyo, Japan), respectively. The iRFP transgenic mice were generated as described previously [30]. Hrhr/hr mice (RBRC01223) were provided by RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan. Mice were kept in plastic cages under pathogen-free conditions in a room maintained at 23.5 ± 2.5°C and 52.5 ± 12.5% relative humidity under a 14-h light:10-h dark cycle. Mice had free access to commercial chow (MF diet; Oriental Yeast, Tokyo, Japan) and filtered water. All mouse experiments were performed with the approval of the University of Tsukuba Animal Experiment Committee.
Vector construction
The pX330 plasmid, carrying both gRNA and Cas9 expression units, was a gift from Dr. Feng Zhang (Addgene plasmid 42230) [6]. The oligos, Hr51 CRISPR F (5′-caccAGCCCCTGTGAACGGCATTG-3′) and Hr51 CRISPR R (5′-aaacCAATGCCGTTCACAGGGGCT-3′), were annealed and inserted into the entry site of pX330 as described previously [18]. The resulting plasmid was designated as pX330-Hr51. The oligos, p2color-Hr51 F (5′-aattAGCCCCTGTGAACGGCATTGTGG-3′) and p2color-Hr51 R (5′-ggccCCACAATGCCGTTCACAGGGGCT-3′), were annealed and inserted into the entry site of p2color vector. This plasmid was designated as p2color-Hr51. Transfection into HEK293T cells and fluorescence observations were performed as described previously [18].
Microinjection
Female C57BL/6J mice were injected with pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) with a 48-h interval, and mated with male C57BL/6J mice. The zygotes were collected from the oviducts. Then, the pX330-Hr51 DNA vector (circular, 5 ng/µl) was injected into the pronuclei according to standard protocols [10]. The injected embryos (pronuclei stage) were then transferred into pseudopregnant ICR mice.
Genomic PCR and sequence analysis
Screening of founder mice (three weeks old) was performed by PCR using genomic DNA obtained from the tail. The PCR amplicons were passed through MCE-202 MultiNA (Shimadzu, Kyoto, Japan), which is a microchip electrophoresis system for DNA analysis. The off-target effect was examined by PCR and direct sequencing [18]. PCR was performed with AmpliTaq Gold® 360 Master Mix (Applied Biosystems, Foster City, CA) and the primers listed in Supplementary Table 1. The PCR products were purified with a Fast Gene Gel/PCR Extraction Kit, and sequences were analyzed using an Applied Biosystems 3130 Genetic Analyzer (Life Technologies, Palo Alto, CA) with a BigDye® Terminator v3.1 Cycle Sequencing Kit (Life Technologies).
Imaging of an ICG tube in hairless mice
The ICG tube was detected with an in vivo imaging system (IVIS Spectrum; PerkinElmer, Wellesley, MA) and a CT scanner (ALOKA La Theta LCT-100; Hitachi, Tokyo, Japan). All mice were anesthetized with isoflurane. The ICG tube was inserted into the abdominal cavity. Analysis of fluorescence signals was performed with Living Image Software 4.0 (Caliper LifeSciences, Hopkinton, MA). Three-dimensional (3D) reconstruction was performed with OsiriX Lite 7.5 (Pixmeo, Bernex, Switzerland).
Imaging of iRFP embryos
The iRFP fluorescence signal was detected with an in vivo imaging system (IVIS Spectrum). The iRFP-expressing embryos at 10.5 days post-coitum (dpc) in the uterus were assessed in female hairless mice crossed with iRFP-expressing male mice. All mice were anesthetized with isoflurane or sacrificed before imaging. The fluorescence signals were analyzed with Living Image Software 4.3.1 (Caliper LifeSciences).
RNA extraction and cDNA synthesis
Total RNA from skin was extracted using Isogen (Nippon Gene Co., Ltd., Tokyo, Japan) in accordance with the manufacturer’s instructions. cDNA was synthesized from 5 µg of RNA using Superscript II reverse transcriptase (Thermo Fisher Scientific, San Jose, CA) and oligo (dT) primer (Thermo Fisher Scientific). PCR was performed with AmpliTaq Gold® 360 Master Mix (Applied Biosystems) and the primers listed in Supplementary Table 1. The PCR products were purified with a Fast Gene Gel/PCR Extraction Kit, and sequences were analyzed using an Applied Biosystems 3130 Genetic Analyzer (Life Technologies) with a BigDye® Terminator v3.1 Cycle Sequencing Kit (Life Technologies).
Blood sampling
The blood was sampled at the same time of day (10:00 − 12:00) to control for the chronobiological variability of study parameters. Whole blood samples were taken with a 1-ml syringe and a 26-gauge needle from the caudal vena cava after laparotomy under anesthesia. For hematology analysis, the blood samples (0.1 ml) were placed in 1.5-ml tubes containing Na2-EDTA (Dojindo Molecular Technologies, Kumamoto, Japan) and analyzed immediately. The remainder of each sample was collected in a 1.5-ml tube for blood chemistry, and the blood was allowed to clot for 30 min and centrifuged (3,000 × g, 5 min) for serum separation. Serum samples were analyzed immediately [13].
Hematology
Hematology analyses were performed using Celltac α (MEK-6458; Nihon Kohden, Tokyo, Japan). The hematological parameters investigated were white blood cell count (WBC), red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count (PLT). The measurement method was same as previous report [30].
Blood chemistry
Blood chemistry parameters were measured using a Fuji Dri-chem 7000 (Fuji-Film, Tokyo, Japan). The blood chemistry parameters investigated were aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, blood glucose, blood urea nitrogen, creatinine, total protein, albumin, total bilirubin, calcium, inorganic phosphate, total cholesterol, triglyceride, sodium, potassium, and chloride. The measurement method was same as previous report [30].
Results
Generation of hairless mice by CRISPR/Cas9
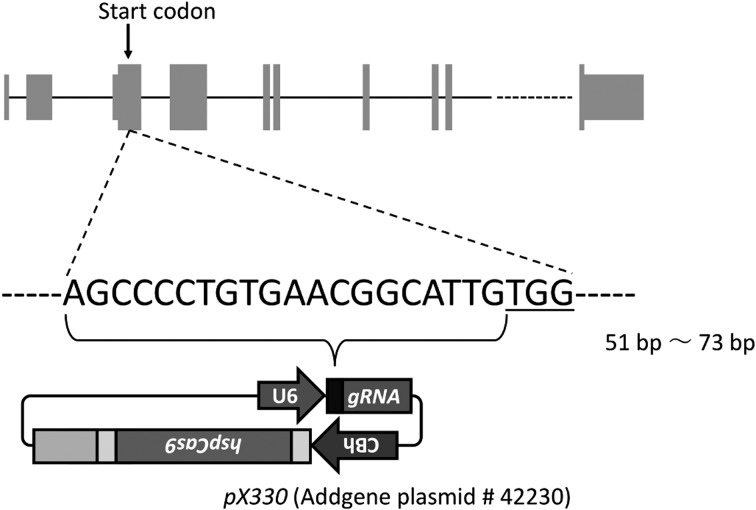
To generate hairless mice by the CRISPR/Cas9 system, we first targeted a sequence in the Hr gene. According to UCSC Genome Browser, the Hr gene located on chromosome 14 has 20 exons. We targeted exon 3, which contains the start codon (Fig. 1). To decrease off-target candidates, we used the CRISPRdirect web server to design CRISPR/Cas guide RNA [19]. The CRISPR target was located 51–73 bp downstream of the start codon of Hr. We inserted a 20-bp gRNA target into pX330, and the resultant plasmid was designated as pX330-Hr51. Next, the cleavage activity of pX330-Hr51 was examined by the traffic reporter system [11]. The results indicated that pX330-Hr51 could efficiently cleave the target site (Supplementary Fig. 1B).
Fig. 1.
Schematic for pX330-Hr51. The DNA sequence indicate the CRISPR guide RNA target site. The underlined letters indicate the protospacer adjacent motif (PAM) sequence. At the top of this figure, thin gray boxes indicate untranslated regions. Thick gray boxes indicate coding sequence regions.
We then microinjected 5 ng/µl of the pX330-Hr51 DNA vector (circular) into the pronuclei of 437 one-cell stage embryos obtained from C57BL/6J mice. No morphological abnormalities were observed in 395 of 437 one-cell embryos immediately after microinjection. These one-cell embryos with a normal appearance were transferred into the oviducts of pseudopregnant recipient ICR mice, and 88 neonates, including six without hair, were obtained. The hairless phenotype caused by Hr mutation was inherited in a recessive manner. Therefore, we confirmed Hr gene mutation even in founders with hair. The heteroduplex mobility assay (HMA) with MultiNA revealed indel mutations in 67 of 88 mice, including six with no hair (Table 1).
Table 1. Generation of CRISPR/Cas9-mediated Hr mutant mice.
| Injected DNA | Oocytes injected |
Oocytes transferred |
Number of newborns | Number of newborns without insertion of pX330 |
|
|---|---|---|---|---|---|
| pX330-Hr51 (5 ng/μl) | 437 | 395 | Mutated and hairless | 6 (6.8 %)a | 5 |
| Mutated | 61 (69.3 %)b | 58 | |||
| Wild-type | 21 (23.9 %)c | 21 | |||
| Sum | 88 | 84 (95.5 %)d | |||
aMutated and hairless/ Sum of newborns. bMutated newborns/ Sum of newborns. cWild-type newborns/ Sum of newborns. dNewborns without insertion of pX330/ Sum of newborns.
To determine whether the pX330-Hr51 DNA vector was integrated into the chromosomes, we performed PCR with founder mouse genomic DNA and a primer pair for Cas9 detection. PCR products were detected in four founder mice (Table 1). Therefore, we succeeded in generating 63 (including five hairless) mutant mice without insertion of pX330. In the mice carried pX330-Hr chromosomal integration, ubiquitously expression of the gRNA for Hr and Cas9 may result in accumulating the off-target mutations in each somatic and germ cells. Therefore, we refrained from using them. In addition, off-targets of one male hairless founder were checked and no mutations were found (Supplementary Table 4).
Inheritance and gene expression
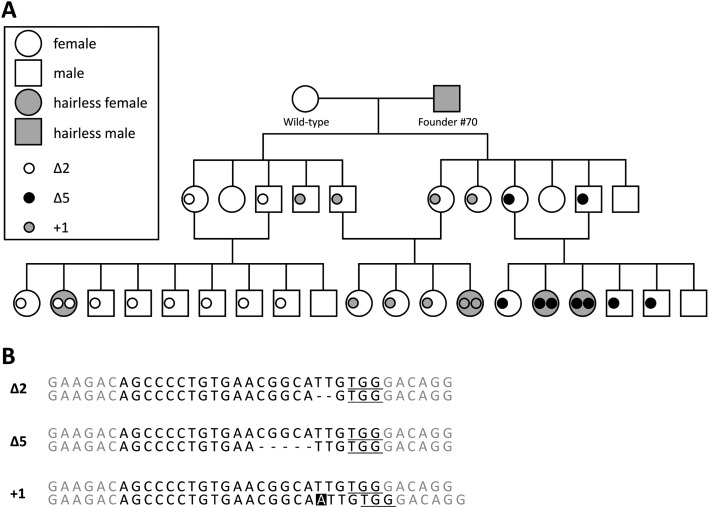
To confirm the hereditary and mutant sequence detail, male hairless founder #70 was crossed with a female wild-type mouse (Fig. 2A). We obtained 11 F1 mice with hair. Unexpectedly, three types of mutations were detected in these animals: Δ2 (TT deletion), Δ5 (CGGCA deletion), and +1 (A insertion) (Fig. 2B). The mice carrying the same mutation sequence were intercrossed and all F2 homozygous mutants were hairless. As no different phenotypes were found among them, we used the Δ5 hairless mouse line and their mutant allele was named Hrem1Utr.
Fig. 2.
Pedigree of founder #70 line. (A) Circles and squares represent female and male mice, respectively. The gray circles and squares represent hairless phenotypes. The small white, black, and gray circles indicate 2-bp deletion, 5-bp deletion, and 1-bp insertion alleles, respectively. (B) There were three types of mutation in the founder #70 line. The hyphens indicate deletion. The white letter indicates insertion.
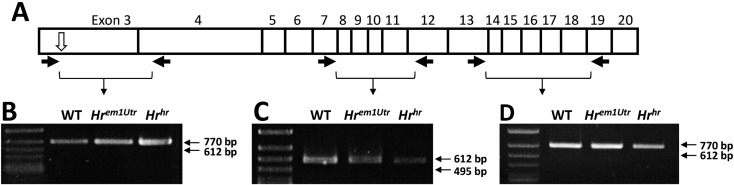
We also investigated the Hr RNA expression and its sequence from Hrem1Utr/em1Utr and Hrhr/hr mice. The Hrhr/hr mouse strain showed the hairless phenotype caused by spontaneous insertion mutation in intron 7 of the Hr gene, and has already been widely used in in vivo studies. The total RNAs of homozygous mutant skin were collected and RT-PCR was performed with three primer pairs (exon 3 to exon 4, exon 7 to exon 12, and exon 13 to exon 19). PCR products were detected under all conditions (Figs. 3B–3D). We then confirmed the sequences of these RT-PCR products and detected the CRISPR-induced 5-bp deletion mutation in Hrem1Utr/em1Utr mouse cDNA (Fig. 2B). The results indicated that the CRISPR-induced heritable genetic mutation resulted in mutant RNA expression and hairless phenotype.
Fig. 3.
Reverse transcription PCR of wild-type, Hrem1Utr/em1Utr, and Hrhr/hr mice. (A) Schematic for reverse transcription PCR. The white arrow indicates the deletion region. The black arrows indicate primers. (B) The PCR products of 755 bp from exon 3 to exon 4, (C) 588 bp from exon 7 to exon 12, and (D) 760 bp from exon 13 to exon 19 are shown to demonstrate the presence of cDNA. WT, wild-type mice; Hrem1Utr, Hrem1Utr/em1Utr mice; Hrhr, Hrhr/hr mice.
Hairless phenotype
We then confirmed that temporal changes in the hairless mouse phenotype in Hrem1Utr/em1Utr mice (Fig. 4 and Supplementary Fig. 2A) and Hrhr/hr mice (Supplementary Figs. 2B and 2C). First, Hrem1Utr/em1Utr mice lost their ventral hair at 4 weeks of age. Next, the hair loss progressive from the ventral side to the dorsal side in 2–4 weeks (Fig. 4 and Supplementary Fig. 2A). In contrast, the hair loss in Hrhr/hr mice started at 2 weeks of age [26]. It progressed rapidly from the rostral to the caudal side and Hrhr/hr mice were completely hairless by 4 weeks of age (Supplementary Figs. 2B and 2C) [28]. The Hrem1Utr/em1Utr mice grew slowly compared with wild-type controls (Supplementary Fig. 3). However, there was no difference in weight after 7 weeks of age. In hematology and blood chemistry analyses, we found minor differences related to red blood cells (Supplementary Table 2 and 3). This phenotype was consistent with Hrhr/hr mice [24, 27].
Fig. 4.
Temporal changes in Hrem1Utr/em1Utr mice. Female Hrem1Utr/em1Utr mice lost ventral hair at 4 weeks of age. After 6 weeks of age, they gradually lost dorsal hair.
In vivo imaging of Hrem1Utr/em1Utr mice with ICG tube implantation
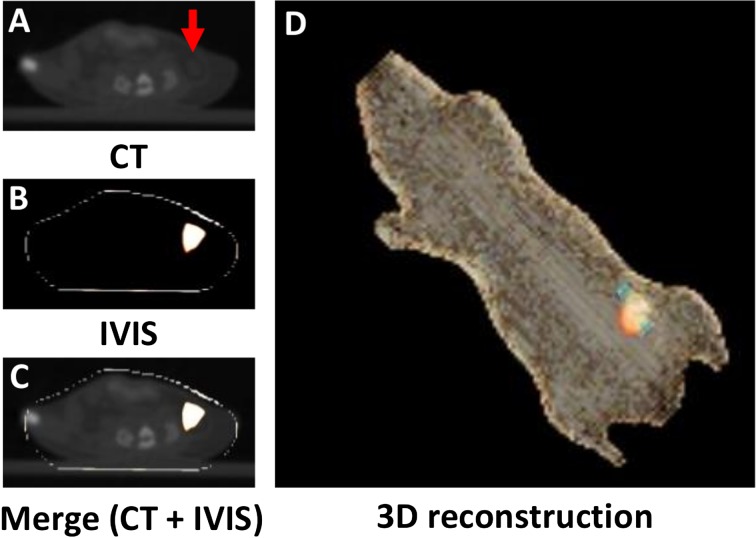
To confirm whether Hrem1Utr/em1Utr mice are available for in vivo imaging, ICG-filled artificial tubes were implanted into the abdominal cavity of Hrem1Utr/em1Utr mice. The tubes and fluorescence signals were detected by CT and IVIS, respectively. The position of the tube was consistent with that of ICG fluorescence (Figs. 5A–D). These results indicated that the ICG fluorescence could be easily detected in the abdominal cavity of Hrem1Utr/em1Utr mice.
Fig. 5.
In vivo imaging of Hrem1Utr/em1Utr mice with ICG tube implantation. (A) CT plane. The arrow indicates ICG tube. (B) IVIS plane. (C) Merge (CT + IVIS). (D) 3D reconstruction of merge by OsiriX Imaging Software. Orange light indicates fluorescence signal. Blue light indicates ICG tube. The ICG tube was easily identified using an IVIS Spectrum in vivo imaging system equipped with 745 nm excitation and 820 nm emission filters.
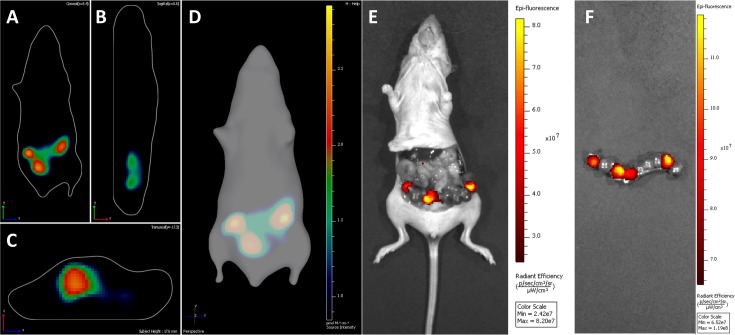
To confirm whether Hrem1Utr/em1Utr mice are available for detecting vital iRFP, female Hrem1Utr/em1Utr mice were crossed with iRFP-expressing male mice. The iRFP-expressing embryos in the uterus were detected at 10.5 dpc. The embryos with iRFP signals were detected in the coronal plane (Fig. 6A), the sagittal plane (Fig. 6B), and transverse plane (Fig. 6C). On 3D reconstruction of diffuse luminescence imaging tomography, three embryos expressing iRFP were detected (Fig. 6D). After celiotomy, the positions of iRFP-expressing embryos were almost consistent with those of iRFP fluorescence in 3D reconstruction (Fig. 6E). However, four rather than three embryos expressing iRFP were present in the uterus of the Hrem1Utr/em1Utr mouse (Fig. 6F). These results indicated that the iRFP-expressing embryos could be detected in the uterus of Hrem1Utr/em1Utr mice.
Fig. 6.
In vivo imaging of Hrem1Utr/em1Utr mice crossed with iRFP transgenic mice. (A) Coronal plane. (B) Sagittal plane. (C) Transverse plane. (D) 3D reconstruction of diffuse luminescence imaging tomography. (E), (F) Uterus after celiotomy. iRFP expression in embryos (10.5 dpc) was identified using an IVIS Spectrum in vivo imaging system equipped with 675 nm excitation and 720 nm emission filters.
Discussion
Using the CRISPR/Cas9 system, we generated a novel hairless mouse strain designated as Hrem1Utr/em1Utr mice. The complete hair loss in this mouse strain permitted in vivo imaging with ICG and iRFP. Although minor abnormalities were found in red blood cells of this mouse strain in comparison with Hrhr mice [24, 27], there were no major abnormal phenotypes that prevented the normal biological activity and long-term in vivo imaging studies. These results indicated that the hairless mice generated by CRISPR/Cas9 were useful for in vivo imaging.
It is necessary to remove hair from mice before in vivo imaging, because hair blocks, absorbs, and scatters light. There are several ways to remove the hair without the need for genetic modification. Shaving with a razor can be performed easily to remove the hair, but is associated with a risk of scab formation due to bleeding, which also block, absorb, and scatter light. Depilatory cream is also used, but this takes time. Especially, 3D in vivo imaging requires removal of hair from the whole body. In contrast, Hr mutant hairless mice make it possible to begin in vivo imaging immediately without removal of hair. This benefit becomes significant when many mice must be used or in vivo imaging must be repeated many times.
In general, female Hrhr/hr mice often fail to nurse their litters due to abnormal lactation. Although outbred female Hrhr/hr mice, such as Hos:HR-1 hairless mice, can nurse their litters, Hrhr/hr mice with the C57BL/6JJcl genetic background often fail to nurse their litters. In this study, female Hrem1Utr/em1Utr mice also failed to nurse their litters. Therefore, our observations support the suggestion that this low nursing activity is dependent on the genetic background [28].
Although the mechanism by which Hr mutations cause hair loss is not completely understood at the molecular level [16], several Hr mutant mouse stains have been reported. Null Hr mice (Hr−/−), generated by gene targeting, showed both hair loss and severe wrinkling of the skin [31]. In this strain, the genomic region containing exons 7–11 of Hr was deleted and there was no expression of Hr mRNA or Hr protein. The Hrrh-8j spontaneous mutant strain also showed both hair loss and wrinkling of the skin. This mutant carried a nonsense mutation of GA-to-TT substitution at positions 1,910 and 1,911 in exon 5 of Hr [1]. As Hrrh-8j mutant mRNA may be degraded by nonsense-mediated mRNA decay (NMD), small amounts of mutant mRNA were detected in their skin [31]. In contrast, Hrhr/hr mice did not show wrinkling of the skin but did show hair loss. The Hrhr allele has a spontaneous retroviral insertion (approximately 13 kb) into intron 7 [28]. Both wild-type Hr mRNA and two additional long mRNAs were expressed from this allele [31]. These additional long mRNAs may arise from aberrant splicing and act in a dominant negative manner to cause the observed loss of hair. These previous reports suggested that Hr null mutation yields both hair loss and severe wrinkling of the skin. Conversely, partial inhibition of Hr leads to hair loss but not to wrinkling of the skin. Interestingly, Hrhr/hr mice showed complete hair loss and very mild wrinkling of the skin. In addition, mutant Hrem1Utr mRNA was not degraded (Figs. 3B–3D). The CRISPR target sequence was designed according to exon 3 of Hr and a 5-bp deletion was induced within its target site. This deletion may induce a frameshift mutation with a stop codon in exon 3 (Supplementary Fig. 4A). In theory, this mutant mRNA should be degraded by NMD. However, there was no difference in the expression level of this mRNA between wild-type and Hrem1Utr/em1Utr mice (Fig. 3). These data suggest that an in-frame ATG, other than the original start codon, functions as an illegitimate start codon and an N-terminal truncated Hr protein results in the phenotype with hair loss and very mild wrinkling of the skin (Supplementary Figs. 4A and 4B). Thus, such as truncated protein which was induced by indel mutation was reported from another group [17]. On the other hands, there is also a possibility that this phenotypic difference is depended on genetic background because those of Hrhr/hr and Hrem1Utr/em1Utr mice are C57BL/6JJcl and C57BL/6J, respectively.
Although three iRFP-expressing embryos were identified in 3D reconstruction of diffuse luminescence imaging tomography (Fig. 6D), four iRFP-expressing embryos were identified in the uterus after celiotomy (Fig. 6F). In fact, our results indicated that it is difficult to distinguish adjacent iRFP-expressing embryos (10.5 dpc). In late pregnancy, it may be possible to distinguish adjacent iRFP-expressing embryos. Further studies are required to establish the method for analyzing the embryos in utero from implantation to perinatal stage.
In conclusion, we generated a novel hairless mouse strain, Hrem1Utr/em1Utr, using CRISPR/Cas9. These hairless mice enabled the in vivo imaging with ICG tubes and iRFP transgenic mice. The CRISPR/Cas9 system could be useful for generating genetically modified hairless mice for in vivo imaging studies.
Conflict of Interest
The authors declare there are no conflicts of interest.
Supplementary Material
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP26221004, JP15H04281, JP16K14589, and JP16H01630. We thank the members of the Sugiyama Laboratory for helpful discussions and encouragement.
References
- 1.Ahmad W., Panteleyev A.A., Sundberg J.P., Christiano A.M.1998. Molecular basis for the rhino (hrrh-8J) phenotype: a nonsense mutation in the mouse hairless gene. Genomics 53: 383–386. doi: 10.1006/geno.1998.5495 [DOI] [PubMed] [Google Scholar]
- 2.Arichi N., Mitsui Y., Ogawa K., Nagami T., Nakamura S., Hiraoka T., Yasumoto H., Shiina H.2014. Intraoperative fluorescence vascular imaging using indocyanine green for assessment of transplanted kidney perfusion. Transplant. Proc. 46: 342–345. doi: 10.1016/j.transproceed.2013.11.129 [DOI] [PubMed] [Google Scholar]
- 3.Benavides F., Oberyszyn T.M., VanBuskirk A.M., Reeve V.E., Kusewitt D.F.2009. The hairless mouse in skin research. J. Dermatol. Sci. 53: 10–18. doi: 10.1016/j.jdermsci.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd L.G.1993. Regional localization of the nu mutation on mouse chromosome 11. Immunogenetics 37: 157–159. doi: 10.1007/BF00216842 [DOI] [PubMed] [Google Scholar]
- 5.Chang C.C., Huang H.C., Liu K.L., Wu Y.M., Lee J.J., Jiang S.F., Su M.Y.2016. Clinical feasibility of Gd-EOB-DTPA-enhanced MR imaging for assessing liver function: validation with ICG tests and parenchymal cell volume. Clin. Imaging 40: 797–800. doi: 10.1016/j.clinimag.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F.2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. doi: 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filonov G.S., Piatkevich K.D., Ting L.M., Zhang J., Kim K., Verkhusha V.V.2011. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat. Biotechnol. 29: 757–761. doi: 10.1038/nbt.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanagan S.P.1966. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 8: 295–309. doi: 10.1017/S0016672300010168 [DOI] [PubMed] [Google Scholar]
- 9.Fujii M., Endo-Okuno F., Iwai A., Doi K., Tomozawa J., Kohno S., Inagaki N., Nabe T., Ohya S.2016. Hypomorphic mutation in the hairless gene accelerates pruritic atopic skin caused by feeding a special diet to mice. Exp. Dermatol. 25: 565–567. doi: 10.1111/exd.13015 [DOI] [PubMed] [Google Scholar]
- 10.Gordon J.W., Ruddle F.H.1981. Integration and stable germ line transmission of genes injected into mouse pronuclei. Science 214: 1244–1246. doi: 10.1126/science.6272397 [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa Y., Hoshino Y., Ibrahim A.E., Kato K., Daitoku Y., Tanimoto Y., Ikeda Y., Oishi H., Takahashi S., Yoshiki A., Yagami K., Iseki H., Mizuno S., Sugiyama F.2016. Generation of CRISPR/Cas9-mediated bicistronic knock-in ins1-cre driver mice. Exp. Anim. 65: 319–327. doi: 10.1538/expanim.16-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jöbsis F.F.1977. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198: 1264–1267. doi: 10.1126/science.929199 [DOI] [PubMed] [Google Scholar]
- 13.Khokhlova O.N., Tukhovskaya E.A., Kravchenko I.N., Sadovnikova E.S., Pakhomova I.A., Kalabina E.A., Lobanov A.V., Shaykhutdinova E.R., Ismailova A.M., Murashev A.N.2017. Using Tiletamine-Zolazepam-Xylazine Anesthesia Compared to CO2-inhalation for Terminal Clinical Chemistry, Hematology, and Coagulation Analysis in Mice. J. Pharmacol. Toxicol. Methods 84: 11–19. doi: 10.1016/j.vascn.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 14.Lai C.W., Chen H.L., Yen C.C., Wang J.L., Yang S.H., Chen C.M.2016. Using Dual Fluorescence Reporting Genes to Establish an In Vivo Imaging Model of Orthotopic Lung Adenocarcinoma in Mice. Mol. Imaging Biol. 18: 849–859. doi: 10.1007/s11307-016-0967-4 [DOI] [PubMed] [Google Scholar]
- 15.Landsman M.L., Kwant G., Mook G.A., Zijlstra W.G.1976. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 40: 575–583. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Sundberg J.P., Das S., Carpenter D., Cain K.T., Michaud E.J., Voy B.H.2010. Molecular basis for hair loss in mice carrying a novel nonsense mutation (Hrrh-R ) in the hairless gene (Hr). Vet. Pathol. 47: 167–176. doi: 10.1177/0300985809352970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makino S., Fukumura R., Gondo Y.2016. Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9. Sci. Rep. 6: 39608. doi: 10.1038/srep39608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuno S., Dinh T.T., Kato K., Mizuno-Iijima S., Tanimoto Y., Daitoku Y., Hoshino Y., Ikawa M., Takahashi S., Sugiyama F., Yagami K.2014. Simple generation of albino C57BL/6J mice with G291T mutation in the tyrosinase gene by the CRISPR/Cas9 system. Mamm. Genome 25: 327–334. doi: 10.1007/s00335-014-9524-0 [DOI] [PubMed] [Google Scholar]
- 19.Naito Y., Hino K., Bono H., Ui-Tei K.2015. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31: 1120–1123. doi: 10.1093/bioinformatics/btu743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakanishi T., Kokubun K., Oda H., Aoki M., Soma A., Taniguchi M., Kazuki Y., Oshimura M., Sato K.2012. Bioluminescence imaging of bone formation using hairless osteocalcin-luciferase transgenic mice. Bone 51: 369–375. doi: 10.1016/j.bone.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Nehls M., Pfeifer D., Schorpp M., Hedrich H., Boehm T.1994. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 372: 103–107. doi: 10.1038/372103a0 [DOI] [PubMed] [Google Scholar]
- 22.Ozeki H., Ito S., Wakamatsu K., Thody A.J.1996. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Res. 9: 265–270. doi: 10.1111/j.1600-0749.1996.tb00116.x [DOI] [PubMed] [Google Scholar]
- 23.Pantelouris E.M.1968. Absence of thymus in a mouse mutant. Nature 217: 370–371. doi: 10.1038/217370a0 [DOI] [PubMed] [Google Scholar]
- 24.Reske-Kunz A.B., Scheid M.P., Boyse E.A.1979. Disproportion in T-cell subpopulations in immunodeficient mutant hr/hr mice. J. Exp. Med. 149: 228–233. doi: 10.1084/jem.149.1.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh P., Schimenti J.C., Bolcun-Filas E.2015. A mouse geneticist’s practical guide to CRISPR applications. Genetics 199: 1–15. doi: 10.1534/genetics.114.169771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundberg J.P., King L.E., Jr2001. Morphology of hair in normal and mutant laboratory mice. Eur. J. Dermatol. 11: 357–361. [PubMed] [Google Scholar]
- 27.Suzu S., Tanaka-Douzono M., Nomaguchi K., Yamada M., Hayasawa H., Kimura F., Motoyoshi K.2000. p56(dok-2) as a cytokine-inducible inhibitor of cell proliferation and signal transduction. EMBO J. 19: 5114–5122. doi: 10.1093/emboj/19.19.5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki O., Koura M., Noguchi Y., Uchio-Yamada K., Matsuda J.2013. Zygosity determination in hairless mice by PCR based on Hr(hr) gene analysis. Exp. Anim. 62: 267–273. doi: 10.1538/expanim.62.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi Y., Shimizu A., Sakai T., Endo Y., Osawa N., Shisa H., Honjo T.1992. Mapping of the nu gene using congenic nude strains and in situ hybridization. J. Exp. Med. 175: 873–876. doi: 10.1084/jem.175.3.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran M.T., Tanaka J., Hamada M., Sugiyama Y., Sakaguchi S., Nakamura M., Takahashi S., Miwa Y.2014. In vivo image analysis using iRFP transgenic mice. Exp. Anim. 63: 311–319. doi: 10.1538/expanim.63.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarach J.M., Beaudoin G.M., 3rd, Coulombe P.A., Thompson C.C.2004. The co-repressor hairless has a role in epithelial cell differentiation in the skin. Development 131: 4189–4200. doi: 10.1242/dev.01303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.