Abstract
Type 1 diabetes mellitus is a chronic state of insulin deficiency which results from destruction of beta cells by the immune system. The long term microvascular and macrovascular complications can be devastating. Since the discovery of insulin almost 100 years ago new medical therapies have improved the long-term survival for people with type 1 diabetes. Each year we come closer to discovering a cure but much work still needs to be done to eliminate this disease.
Keywords: Type 1 diabetes, insulin, complications
Introduction
Prior to the discovery of insulin, a diagnosis of diabetes was fatal within a few weeks to months due to insulin deficiency. With the discovery of insulin people with type 1 diabetes were able to live productive lives for many decades. However in 2017, the life expectancy of people with type 1 diabetes is still approximately 12 years less on average than the rest of the general population (1). The Diabetes Control and Complications Trial showed us that intensive control of type 1 diabetes leads to a decrease in microvascular complications such as retinopathy, nephropathy, and neuropathy (2). The Epidemiology of Diabetes Interventions and Complications study showed that intensive blood glucose control reduces the risk of cardiovascular disease (3). The age adjusted relative risk for cardiovascular disease in people with type 1 diabetes is still 10 times that of the general population (4). The increased mortality and the burden of long-term diabetes care indicate that there is still much we need to learn about type 1 diabetes prevention, treatment and finding a true cure for this disease.
Etiology
T cell mediated autoimmune destruction of pancreatic beta cells is thought to be the final pathway in the development of type 1 diabetes (5). Multiple beta cell autoantibodies are frequently present in patients with type 1 diabetes. Antigens for these antibodies include insulin, glutamic acid decarboxylase (GAD-65), islet antigen 2, and zinc-transporter 2 (6). The role of these antibodies in causing beta cell destruction is not completely understood. How this autoimmune process is triggered is not known, although both genetic and environmental factors are thought to be necessary. These holes in our knowledge are critical impairments in our ability to prevent type 1 diabetes. Figure 1 shows a diagram of the proposed mechanisms behind the development of type 1 diabetes.
Figure 1.
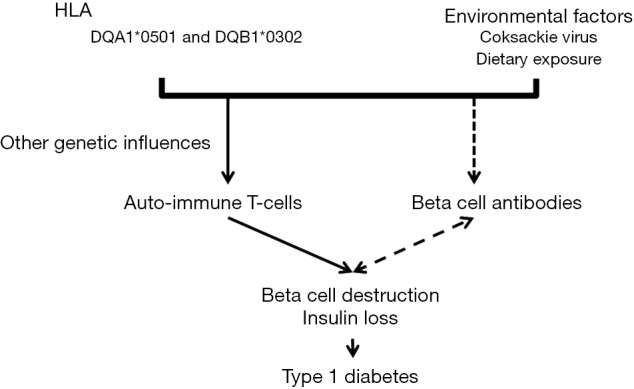
Proposed etiology of type 1 diabetes. Environmental factors in genetically susceptible individual trigger auto-immune T-cells that lead to beta cell destruction and insulin loss. HLA confers most of the genetic risk although genetics risk factors have been identified. It is unknown how humoral immunity and beta cell antibodies relate to beta-cell destruction. They may play an active role or may simply be a marker of ongoing destruction.
The major genetic determinants of type 1 diabetes are alleles at the HLA-DRB1 and DQB1 loci. DQA1*0501 and DQB1*0302 confer very high risk for type 1 diabetes (7). Kingery et al. have suggested a role for complement component 4 (C4) copy number variation in the development of the disease. This is interesting since the C4 gene is closely associated to the HLA locus (8). Polymorphisms in multiple other genes including the insulin gene have also been found to play a role, although their relative contribution is small (9).
The exact environmental factors involved in type 1 diabetes initiation are far less known. One plausible hypothesis is that viral infections trigger beta cell autoimmunity in genetically susceptible individuals. Several viruses have been associated with type 1 diabetes, including enteroviruses such as coxsackievirus B (10). Enterovirus infections are more frequent in siblings who develop type 1 diabetes compared with siblings without diabetes (11). Enterovirus antibodies are elevated in pregnant mothers whose children develop diabetes, particularly in cases diagnosed before 3 years of age (11). Coxsackievirus B is one of the most common enteroviral strains found in people with pre-diabetes and diabetes, and enteroviral RNA has been found in samples taken from children at the onset of type 1 diabetes (12,13). Although there have been viruses found in pancreatic beta cells, viruses may not necessarily be the trigger for type 1 diabetes and could possibly be protective since some countries with different sanitary standards and lower socioeconomic status tend to have more infections but a lower prevalence of type 1 diabetes (10). The role of the complement system and enteroviruses in the development of type 1 diabetes was recently explored by Abdel-Latif et al. who compared type 1 diabetes children who were enterovirus positive children with those children who had type 1 diabetes but were enterovirus negative and looked at autoantibodies, cytokines, complement activation products, and anti-coxsackievirus immunoglobulin IgG. The higher serum levels of complement components C3d and sC5b-9 indicated increased complement activity in diabetes enterovirus positive children versus diabetic enterovirus negative children. The enterovirus negative children with diabetes did not show any significant differences in complement levels compared to healthy controls (14).
Prevention
In spite of not knowing the true cause of the disease, several attempts have been made to try to prevent or delay the development of type 1 diabetes. Many of these attempts have been based on work done in the NOD mouse which is the most common animal model of type 1 diabetes, although there is concern regarding how closely it models human type 1 diabetes (15). The NOD mouse was discovered as a useful animal model for investigating type 1 diabetes in the early 1980s (16). Table 1 lists some of the type 1 diabetes prevention trials (DPT-1) and their outcomes.
Table 1. List of type 1 diabetes (T1D) prevention trials.
| Study | Objective | Conclusions/results |
|---|---|---|
| TRIGR (trial to Reduce IDDM in the genetically at risk) (17) | To determine if avoidance of cow’s milk protein will protect high risk newborns from initiation of B cell autoimmunity and prevent T1D | Expected 2017 |
| FINDIA (18) | To test if weaning to a bovine insulin-free cow’s milk formula reduces T1D associated antibodies in genetically at risk children | Compared to regular cow’s milk formula weaning to insulin-free cow’s milk formula reduced autoantibodies by age 3 years |
| BABYDIET (19) | Delay of introduction of gluten to prevent islet autoimmunity in infants with 1st degree relative with T1D and high risk HLA genotype | No difference in islet autoantibodies or development of diabetes |
| ENDIT (20) | Nicotinamide vs. placebo in ICA-positive relative of patients with T1D to prevent T1D | No difference in the development of T1D in those treated with nicotinamide compared to placebo |
| DPT-1 (21) | Oral insulin and parenteral insulin to alter the immune response and reduce islet destruction in 1st and 2nd degree relatives of patients with T1D | Oral/parenteral insulin did not delay or prevent T1D |
| DiAPREV-IT trial (22) | Prevention trial with Diamyd a GAD65 “vaccine” in children with positive islet cell antibodies | Does not prevent development of T1D |
| TrialNet oral insulin (23) | Oral insulin prevention trial in first and second degree relatives with insulin antibodies | No effect in most subjects |
The DPT-1 involved two studies looking at parenteral insulin and oral insulin therapy for the prevention of type 1 diabetes in relatives with high or intermediate risk of developing type 1 diabetes. Neither treatment was able to prevent or delay the development of type 1 diabetes although there was a hint that oral insulin might be effective in preventing diabetes in subjects with insulin antibodies (24). A subsequent study by the Trialnet group found that oral insulin did not delay or prevent the onset of diabetes in most insulin autoantibody positive first degree relatives but did delay onset in the small subgroup with decreased insulin secretion at study onset (23). TrialNet is identifying subjects for participation in DPT-1 trials and assembling a large cohort of at-risk persons that will yield new natural history information about pre-type 1 diabetes (25).
The European Nicotinamide Diabetes Intervention Trial (ENDIT) was a double-blind placebo controlled trial where nicotinamide was used in 552 relatives with confirmed islet cell antibody (ICA) levels and non-diabetic oral glucose tolerance tests. These participants were given either high doses of nicotinamide or placebo. Nicotinamide was not found to prevent the onset of type 1 diabetes in these participants (20).
The Trial to Reduce Incidence of Diabetes in Genetically at Risk Study, randomized genetically at risk infants to hydrolyzed formula versus cow’s milk formula and found no difference in pancreatic antibody development up to 10 years later (26). A variety of other trials using a wide variety of agents such as omega 3 fatty acids, GAD, Vitamin D, and other are in progress to evaluate their role in prevention of diabetes (27).
In patients with new onset type 1 diabetes, a variety of immunosuppressive agents have also been tried including steroids and immuran, cyclosporine, rituximab, and teplizumab. These studies have demonstrated short-term preservation of insulin secretion (27,28). It is not clear that these therapies by themselves have a favorable risk: benefit ratio. A single trial using autologous, nonmyeloablative, hematopoietic stem cell transplantation showed some benefit but included significant risks (29).
New therapies
Until we come up with a means to prevent the development of type 1 diabetes we need to develop ways to improve glycemic control and ease the burden of diabetes. Research is being pursued in three main areas: new insulins and insulin delivery systems; islet cell replacement; and adjunctive therapies to insulin.
Insulin was discovered in 1921 by Frederick Banting and Charles Best. Dr. Arnold Kadish invented the first insulin pump in the early 1960s. Multiple improvements have been made to insulin pumps since that time. A meta-analysis has shown that insulin pump use does moderately improve glycemic control although not all studies agree (30,31). Another new technology in use is continuous glucose monitoring. It can be used either with an insulin pump or injection therapy. Use of continuous glucose monitoring has been shown to improve glycemic control in subjects over 24 years (32). The main reason for failure to improve diabetes control in younger subjects was failure to wear the sensor at least 6 days per week.
Combining these two technologies into an “artificial pancreas” or closed-loop insulin delivery system has been a goal for many years. Several steps have been taken along this route with the low glucose and low glucose predictive suspend pumps that stop the insulin infusion when the sensor detects a low glucose level or a rapidly falling glucose level that would lead to hypoglycemia (33). More importantly, a hybrid closed-loop insulin pump was just released in 2017 which adjusts basal rates every 5 minutes with the assistance of a continuous glucose monitor. The patient still must enter pre-meal boluses. In pre-approval studies the system has been shown to reduce hemoglobin A1C and glucose variability (34). Multiple other systems are being developed including a bihormonal system using insulin and glucagon (35).
For patients using insulin injection therapy, the variety of different insulin analogues have made it easier for insulin levels in patients with type 1 diabetes to more closely mimic those in healthy subjects (36). The use of these long acting and rapid acting insulin analogues has been associated with reduced hypoglycemia. Recent advances have led to newer insulin preparations becoming available such as extended duration basal insulin such as degludec and an ultrafast insulin aspart which are now available in Europe and Canada. The ultrafast insulin aspart will be exceptionally beneficial for the closed loop systems where the delay in insulin action is a confounder. “Smart insulins” are being developed which have a glucose responsive element that increases insulin action with increasing glucose levels (37).
Islet cell transplantation has been shown to normalize blood sugar levels but issues with intrahepatic islet cell transplantation such as immediate blood-mediated inflammatory reactions, deleterious effects of chronic immunosuppressive drugs, lack of sustained insulin independence in some islet cell recipients, and lack of sufficient number of islet cell donors are all barriers to this as a viable treatment for the majority of people with type 1 diabetes (38). Islet transplantation can eliminate severe hypoglycemia in patients with type 1 diabetes (39). Recently as part of an ongoing study (Allogeneic Islet Cells Transplanted onto the Omentum; Clinicaltrials.gov number, NCT02213003), a 43-year-old woman with type 1 diabetes for 25 years underwent islet cell transplantation onto the omentum. Her insulin injections were able to be discontinued 17 days after the transplantation. At 12 months she had a fasting glucose level of 120 mg/dL and a 90-min glucose of 266 mg/dL after a mixed meal tolerance test. This study is of still ongoing as of May of 2017.
Glucose responsive beta cells have been generated from human pluripotent stem cells (hPSC) and when inserted into mice secrete human insulin in a glucose-regulated manner and decrease hyperglycemia in diabetic mice (40). Manzar et al. used induced pluripotent stem cells derived from patients with type 1 diabetes to generate glucose-responsive, insulin producing cells. The cells were initially resistant to differentiation but demethylation treatment was able to increase the yield of insulin producing cells. When these cells were transplanted into diabetic mice they became normoglycemic within 28 days. Stem cells produced from self that do not require immunosuppression provide a safer more realistic possibility for future treatment of type 1 diabetes and eliminate the need for cadaveric pancreatic tissue donors (41).
Adjunctive therapies in addition to insulin are also being studied. Of particular interest is metformin, to reduce insulin resistance in overweight and obese individuals with type 1 diabetes. In overweight adolescents the addition of metformin had little benefit with reduced insulin doses and weight but no improvement in glycemic control. Subjects receiving metformin not surprisingly had more gastrointestinal side effects (42).
Sodium glucose cotransporter-2 inhibitors (SGLT2) are a class of medications used to treat type 2 diabetes. They lower blood sugar by causing the kidneys to remove glucose from the body through the urine. SGLT2 inhibitors have shown some utility in type 1 diabetes but have a higher incidence of diabetic ketoacidosis (DKA). The U.S. Food and Drug Administration (FDA) issued a drug safety communication of increased risk of euglycemic DKA with the use of all the approved SGLT2 inhibitors (43). Peters et al. looked at the incidence of DKA with the addition of canagliflozin as an add on to insulin in adults with type 1 diabetes. All episodes of DKA occurred in the presence of circumstances that are known to potentially precipitate DKA such as infection and pump failure. The blood glucose levels of the patients in DKA ranged from 170 to 800 mg/dL. Empagliflozin treatment for 8 weeks improved glycemic control and reduced hypoglycemic events, weight and insulin doses in patients with type 1 diabetes (44). Dual SGLT1 and SGLT2 inhibition with sotagliflozin was shown to improve glycemic control and the CGM profile with bolus insulin dose reduction, weight loss, and no increase in hypoglycemia in patients with type 1 diabetes (45).
Conclusions
Our understanding of the mechanisms that cause type 1 diabetes is still incomplete in 2017, almost 100 years after the discovery of insulin. The improvements in insulin and insulin delivery systems have allowed for better control of blood glucose levels but better therapies are still needed to prevent the devastating microvascular and macrovascular complications that can occur with long standing type 1 diabetes mellitus.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Huo L, Harding JL, Peeters A, et al. Life expectancy of type 1 diabetic patients during 1997-2010: a national Australian registry-based cohort study. Diabetologia 2016;59:1177-85. 10.1007/s00125-015-3857-4 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53. 10.1056/NEJMoa052187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014;37:2843-63. 10.2337/dc14-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roep BO. The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia 2003;46:305-21. 10.1007/s00125-003-1089-5 [DOI] [PubMed] [Google Scholar]
- 6.Wenzlau JM, Hutton JC. Novel diabetes autoantibodies and prediction of type 1 diabetes. Curr Diab Rep 2013;13:608-15. 10.1007/s11892-013-0405-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erlich H, Valdes AM, Noble J, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008;57:1084-92. 10.2337/db07-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingery SE, Wu YL, Zhou B, et al. Gene CNVs and protein levels of complement C4A and C4B as novel biomarkers for partial disease remissions in new-onset type 1 diabetes patients. Pediatr Diabetes 2012;13:408-18. 10.1111/j.1399-5448.2011.00836.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet 2016;387:2331-9. 10.1016/S0140-6736(16)30582-7 [DOI] [PubMed] [Google Scholar]
- 10.Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes 2008;57:2863-71. 10.2337/db07-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyöty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes 1995;44:652-7. 10.2337/diab.44.6.652 [DOI] [PubMed] [Google Scholar]
- 12.Clements GB, Galbraith DN, Taylor KW. Coxsackie B virus infection and onset of childhood diabetes. Lancet 1995;346:221-3. 10.1016/S0140-6736(95)91270-3 [DOI] [PubMed] [Google Scholar]
- 13.Andréoletti L, Hober D, Hober-Vandenberghe C, et al. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J Med Virol 1997;52:121-7. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Latif M, Abdel-Moneim AA, El-Hefnawy MH, et al. Comparative and correlative assessments of cytokine, complement and antibody patterns in paediatric type 1 diabetes. Clin Exp Immunol 2017;190:110-21. 10.1111/cei.13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.In't Veld P. Insulitis in human type 1 diabetes: a comparison between patients and animal models. Semin Immunopathol 2014;36:569-79. 10.1007/s00281-014-0438-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makino S, Kunimoto K, Muraoka Y, et al. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 1980;29:1-13. 10.1538/expanim1978.29.1_1 [DOI] [PubMed] [Google Scholar]
- 17.Wherrett DK. Trials in the prevention of type 1 diabetes: current and future. Can J Diabetes 2014;38:279-84. 10.1016/j.jcjd.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaarala O, Ilonen J, Ruohtula T, et al. Removal of Bovine Insulin From Cow's Milk Formula and Early Initiation of Beta-Cell Autoimmunity in the FINDIA Pilot Study. Arch Pediatr Adolesc Med 2012;166:608-14. 10.1001/archpediatrics.2011.1559 [DOI] [PubMed] [Google Scholar]
- 19.Hummel S, Pflüger M, Hummel M, et al. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care 2011;34:1301-5. 10.2337/dc10-2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gale EA, Bingley PJ, Emmett CL, et al. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004;363:925-31. 10.1016/S0140-6736(04)15786-3 [DOI] [PubMed] [Google Scholar]
- 21.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care 2005;28:1068-76. 10.2337/diacare.28.5.1068 [DOI] [PubMed] [Google Scholar]
- 22.Larrson H. DIAPREV-IT Trial with GAD-Vaccine-Results and Conclusions. Oral Presentation 77th American Diabetes Association Scientific Session, San Diego CA, 2017. [Google Scholar]
- 23.Schatz D. Type 1 Diabetes TrialNet Oral Insulin Trial-Results and Conclusions. Oral Presentation 77th American Diabetes Association Scientific Session, San Diego CA, 2017. [Google Scholar]
- 24.Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685-91. 10.1056/NEJMoa012350 [DOI] [PubMed] [Google Scholar]
- 25.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97-104. 10.1111/j.1399-5448.2008.00464.x [DOI] [PubMed] [Google Scholar]
- 26.Knip M, Åkerblom HK, Becker D, et al. Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA 2014;311:2279-87. 10.1001/jama.2014.5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skyler JS. Primary and secondary prevention of Type 1 diabetes. Diabet Med 2013;30:161-9. 10.1111/dme.12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverstein J, Maclaren N, Riley W, et al. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med 1988;319:599-604. 10.1056/NEJM198809083191002 [DOI] [PubMed] [Google Scholar]
- 29.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297:1568-76. 10.1001/jama.297.14.1568 [DOI] [PubMed] [Google Scholar]
- 30.Hirsch IB, Bode BW, Garg S, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005;28:533-8. 10.2337/diacare.28.3.533 [DOI] [PubMed] [Google Scholar]
- 31.Heller S, White D, Lee E, et al. A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the REPOSE Trial. Health Technol Assess 2017;21:1-278. 10.3310/hta21200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group , Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464-76. 10.1056/NEJMoa0805017 [DOI] [PubMed] [Google Scholar]
- 33.Choudhary P, Rickels MR, Senior PA, et al. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care 2015;38:1016-29. 10.2337/dc15-0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther 2017;19:155-63. 10.1089/dia.2016.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs PG, El Youssef J, Castle J, et al. Automated control of an adaptive bihormonal, dual-sensor artificial pancreas and evaluation during inpatient studies. IEEE Trans Biomed Eng 2014;61:2569-81. 10.1109/TBME.2014.2323248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramanian S, Baidal D, Skyler JS, et al. The Management of Type 1 Diabetes. 2016. In: De Groot LJ, Chrousos G, Dungan K, et al. editors. South Dartmouth (MA): MDText.com, Inc., 2000. [Google Scholar]
- 37.Wu W, Zhou S. Responsive materials for self-regulated insulin delivery. Macromol Biosci 2013;13:1464-77. 10.1002/mabi.201300120 [DOI] [PubMed] [Google Scholar]
- 38.Agarwal A, Brayman KL. Update on islet cell transplantation for type 1 diabetes. Semin Intervent Radiol 2012;29:90-8. 10.1055/s-0032-1312569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016;39:1230-40. 10.2337/dc15-1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell 2014;159:428-39. 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manzar GS, Kim EM, Zavazava N. Demethylation of induced pluripotent stem cells from type 1 diabetic patients enhances differentiation into functional pancreatic β cells. J Biol Chem 2017;292:14066-79. 10.1074/jbc.M117.784280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libman IM, Miller KM, DiMeglio LA, et al. Effect of Metformin Added to Insulin on Glycemic Control Among Overweight/Obese Adolescents With Type 1 Diabetes: A Randomized Clinical Trial. JAMA 2015;314:2241-50. 10.1001/jama.2015.16174 [DOI] [PubMed] [Google Scholar]
- 43.Rosenstock J, Ferrannini E. Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern With SGLT2 Inhibitors. Diabetes Care 2015;38:1638-42. 10.2337/dc15-1380 [DOI] [PubMed] [Google Scholar]
- 44.Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014;37:1480-3. 10.2337/dc13-2338 [DOI] [PubMed] [Google Scholar]
- 45.Sands AT, Zambrowicz BP, Rosenstock J, et al. Sotagliflozin, a Dual SGLT1 and SGLT2 Inhibitor, as Adjunct Therapy to Insulin in Type 1 Diabetes. Diabetes Care 2015;38:1181-8. 10.2337/dc14-2806 [DOI] [PMC free article] [PubMed] [Google Scholar]


