Abstract
Background
To determine the optimal scanning protocols of dual energy computed tomography angiography (DECTA) in terms of radiation dose and image quality assessment at different keV levels, and compare it with conventional computed tomography angiography (CTA) in patients treated with peripheral arterial stents.
Methods
Twenty-nine patients with previous stent placement in peripheral arteries were evaluated with DECTA. Images were reconstructed with virtual monochromatic spectral imaging (VMS) at 65, 68, 70 and 72 keV and adaptive statistical iterative reconstruction (ASIR) at 50% compared with CTA. Image quality comprising image noise, signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were assessed, and radiation dose was compared. Effects of different type of peripheral arterial stents on image quality were also evaluated. Fifty-six uniquely identified stents that were located in common iliac arteries (CIA), external iliac arteries (EIA) and superficial femoral arteries (SFA) were evaluated.
Results
Within subjects, the results showed that DECTA images (VMS) had less noise than the CTA images for CIA, EIA and SFA stents, with the lowest noise at 72 keV. Also, the VMS images had greater SNR than the CTA images for the EIA stents (P<0.05); and the VMS images had greater CNR than the CTA images for CIA, EIA, and SFA stents (P<0.001). Also, on CT attenuation, VMS continued to outperform CTA, but to a lesser extent. Between subjects, average VMS noise varied significantly with the type of the stent used (P=0.025) for CIA stents. Radiation dose was highly significant between DECTA and conventional CTA scans (6.98 vs. 7.40 mSv, P=0.047).
Conclusions
We conclude that an optimal scanning protocol consisting of 72 keV and 50% ASIR leads to better image quality for DECTA in peripheral arterial stenting when compared to conventional CTA.
Keywords: Dual-energy CT, image quality, peripheral arterial stents, radiation dose
Introduction
Arterial stenosis and occlusions of the lower extremities are frequently treated with either balloon angioplasty or stents. In-stent restenosis after peripheral artery angioplasty is considered one of the major problems of this procedure. Patency rates for iliac artery stenosis in the follow-up range from 78% in the first year to 61% after five years (1). Digital subtraction angiography (DSA) is the standard follow-up procedure for peripheral arterial disease (PAD), but there are some drawbacks to this technique, including invasiveness and limited assessment of the vascular structures. Less invasive imaging techniques, such as multi-detector computed tomography (MDCT), are increasingly used in clinical practice to serve as an alternative to DSA (2).
MDCT has advantages of shorter procedural time, and few motion artifacts, and generation of 3D visualizations (3). Despite these benefits, it has its own limitation, such as the potential risk of contrast medium-induced nephrotoxicity, the presence of blooming artifacts caused by stent struts, and high radiation dose. Further, MDCT has difficulty in differentiating different materials related to peripheral arterial stents. As assessment of in-stent restenosis is closely related to stent materials, this may lead to an overestimation of the lesion severity. Blooming and beam hardening artifacts which are commonly seen in the conventional CT angiography (CTA) hamper the accurate assessment of in-stent restenosis (4), but these artifacts can be eliminated in dual-energy CT (DECT) applications. Huang et al. reported an improvement of correction with DECT (4). In a recent study by Mangold et al., reduction of blooming artifacts in imaging peripheral arterial stents with improved image quality was achieved when 70 or 80 keV was used (5). Stent lumen visibility for small stents was reported in a phantom study at high keV when the third generation of dual-source CT and DECT was used (6). For large stent evaluation such as those in peripheral arteries, DECT may improve stent visualization and decrease blooming artifacts better than that for small stents.
DECT is a recently developed technique that offers a diversity of applications for improving image visualization and material differentiation, based on CT attenuation obtained from two tube voltages (2,7,8). The bone removal algorithm of DECT plays a major role in confirming the diagnosis and evaluation of the disease progress (8-10). Image quality, in general, is improved with DECT due to the utilization of low energies. Creation of different kilo-electron volt (keV) as monochromatic images may allow optimization of image quality. To the best of our knowledge, only a few studies have been published on the role of DECT angiography (DECTA) in peripheral arterial stents (5,6,11,12), and evidence of optimal scanning protocols of DECTA in peripheral arterial stents as compared to conventional CTA is lacking. Thus, the aim of this study was to determine the optimal scanning protocols of DECTA in terms of radiation dose and image quality assessment at different keV levels in comparison to conventional CTA in patients treated with peripheral arterial stents.
Methods
Patients population and stent characteristics
Twenty-nine patients [27 males and 2 females, mean age, 57.88±9.7 years, ranging from 38 to 77 years and with a mean body mass index (BMI) of 27.76±7.14 kg/m2] were prospectively enrolled in this study between September 2014 and December 2015. All patients had been diagnosed with PAD and were treated with peripheral arterial stents; 93.1% of the patients were also diagnosed with diabetes mellitus (DM). For comparison with conventional CTA, we retrospectively reviewed 24 patients (23 males and 1 female, mean age 58.04±10.53 years, between 38 and 77 years of age and with a mean BMI of 27.38±6.86 kg/m2) from the same population as the prospective study, but who had undergone conventional CTA for lower extremities prior to our data collection. Exclusion criteria for subjects included renal dysfunction or renal failure and contraindication to intravenous administration of the iodine contrast medium. The study was approved by the Curtin University Human Research Ethics (HR 167/2013) and King Fahad Specialist Hospital (KFSH-D) Committees (IRB-RAD029-FB). Informed consent was obtained from all participants.
Stent manufacturer and stent details were available for 86% (48/56) of the stents. The mean stent diameter was 6.9±1.3 mm (range, 4.0–9.0 mm), and the mean length was 54.0±29.6 mm (range, 9.0–150.0 mm). Five different stent types were used in this group of patients: ev3 Protege Everflex self-expandable-nitinol (ev3 Endovascular Inc, Plymouth, MN, USA) n=10; Abbott Omnilink (balloon mounted) cobalt chromium n=4; Cordis Genesis Balloon mounted nitinol (Cordis, Miami, FL, USA) n=19; Cordis SMART self-expandable stainless steel (Cordis, Miami, FL, UAS) n=8, and Boston Scientific Wall Stent self-expandable cobalt-based alloy (Boston Scientific Corp., Natick, MA) n=7. Thirty-five (62.5%) stents were located in the common iliac artery (CIA), 6 (10.7%) in the external iliac artery (EIA), and 15 (26.8%) in the superficial femoral artery (SFA).
DECT scanning protocol
All CT procedures were performed in the DE mode with fast kilovoltage-switching 64-slice CT scanner (Discovery CT HD 750; Gemstone Spectral Imaging, GE Healthcare, Milwaukee, Wisconsin, USA) at KFSH-D. Details of the DECTA protocol have been previously described in our recent study (13). Four sets of virtual monochromatic spectral (VMS) images were reconstructed at 65, 68, 70 and 72 keV with adaptive statistical iterative reconstruction (ASIR) at 50%. DECT images were initially reconstructed with the filtered back projection (FBP) and were subsequently performed by multiple iterations with IR algorithms to determine the effect of image noise on image quality.
Intravenous non-ionic iodinated contrast agent (1.5 mL/kg, 350 mgI/mL Xenetix, Guerbet, Sulzbach, Germany) was administered at a flow rate of 4–5 mL/s followed by 40 mL of a saline chaser at the same flow of contrast medium, using a power injector (Envision CT Injector, Medrad) through a minimum of 20 G catheter in the cubital vein. A region of interest (ROI) was placed within the aorta close to the celiac trunk level with a threshold of 150 HU as the triggering threshold to initiate the scan.
Conventional CTA scanning protocol
CT examinations were performed on two types of scanners; Discovery CT HD 750 (GE Healthcare, Milwaukee, Wisconsin, USA), and 64-slice CT scanner (Brilliance 64, Philips Medical Systems) at KFSH-D, with the following protocols: section thickness: 1.0 mm, pitch values: 0.516 and 0.890, reconstruction interval: 50% overlap of the section thickness. Tube voltage was 120 kVp, with auto-mAs and tube current modulation for all scans. Images of conventional CTA were not reconstructed with any iterative reconstructions.
Image reconstruction and image quality assessment
The images were transferred to a 3D workstation (Gemstone Spectral Imaging Viewer, GE) for analysis. Post-processing reconstructions in multiple formats were obtained for all patients, including axial CT images, multiplanar reformation (MPR) and curved planar reformation (CPR). Images were demonstrated with a window level and window width of 200/1,200 HU to improve visualization of the stent.
If the stent lumen appeared darker than the contrast-enhanced vessel lumen proximal to the stent, then the stent was considered to be occluded. Homogeneous enhancement inside the stent lumen or the absence of in-stent restenosis if it was similar to the reference vessel was considered to be normal with stent patency.
According to the image quality in terms of stent lumen visibility, each stent was classified as patent or non-patent. The stent was considered patent when the lumen was noticeable, and the contrast density of the lumen could be assessed without the effect of partial volume artifacts, metal artifacts caused by the stents and calcification in the arterial wall. Each peripheral artery was sub-divided into three zones based on the existing stent, including CIA, EIA and SFA locations to allow appropriate analysis.
Qualitative assessment
Qualitative evaluations were performed independently by two experienced radiologists with 21 and 15 years in cardiovascular CT imaging, respectively, on a workstation with dedicated software (Gemstone Spectral Imaging Viewer, GE). Each reader evaluated the different VMS image groups of each patient randomly. Because different VMS values could be easily detected by visual inspection of the images, it was considered unnecessary to blind the readers.
The image quality of the different VMS series was evaluated in three parts; the assessments were subjective and based on a 3-point scale as follows: 1—non-diagnostic; 2—moderate but sufficient for diagnosis; 3—excellent. Image noise within the stent was also evaluated on a 3-point scale: 1—poor; 2—adequate; 3—good. Stent patency or lumen visualization was evaluated on a 4-point scale: 1—stenosis or occlusion; 2—50% or less lumen is opacified; 3—50–75% lumen is visualized or opacified; 4—≥75% excellent opacification or visualization. A score of 2 or above in all three assessments was considered clinically diagnostic.
Quantitative assessment
CT attenuation values were performed on a workstation by a reader with 15 years of experience in CT. A circular ROI was placed over the enhanced area of the stent on axial images. Measurement of image noise (the standard deviation of the CT number in Hounsfield units) was attained for each peripheral arterial stent by placing a defined ROI on all VMS series. The area of the ROI on all VMS series and conventional CTA based on the stent size was marked as large as possible in the vessel lumen. When calcification was noticed the ROI was placed in a different region to avoid the artifact. Additional CT attenuation values were measured at the level of the artery proximal to the stent, and other CT attenuation values for the background image noise were taken at the muscle closest to the stent.
Mean CT attenuation and image noise were computed for individual stents by averaging the values for each stent location resulting from both sides of the arteries. The signal-to-noise ratio (SNR) was calculated as a mean CT value of ROI divided by the mean image noise in terms of standard deviation (SD), while the contrast-to-noise ratio (CNR) was calculated as a mean CT value of stent minus the CT value of background muscle divided by the mean image noise (SD) of a stent, which is:
| CNR = (Mean CT valuestent–Mean CT valuemuscle)/SDstent. |
Radiation dose estimation
The volume CT dose index (CTDvol) and dose length product (DLP) were recorded from the CT console for each subject. The multiplication product of DLP and a conversion factor for lower extremities DECTA and CTA examinations [k=0.0056 mSv/(mGy×cm)] yielded the effective radiation dose (14).
Statistical analysis
A commercial software SPSS (SPSS 24.0, IBM Corporation, Armonk, NY, USA) was used for data analysis in this study. A P value of <0.05 was considered to indicate a statistically significant difference. Quantitative variables were considered as mean ± standard deviation. Categorical variables were presented as frequencies and percentages.
Statistical analyses were undertaken at two levels as follows:
Patients were regarded as ‘blocks’ in a randomized block analysis of variance, with five imaging processes (four DECT, one conventional CT) as ‘treatments’. Duncan’s Multiple Range Test was employed to aid interpretation in those analyses exhibiting statistically significant treatment effects.
The effects of observed patient attributes (e.g., sex, BMI, stent type) on expressions of image quality were assessed via analysis of variance/covariance (the General Linear Model procedure in SPSS). Image quality for a given measure was taken as the arithmetic average of the corresponding four values within each patient from the DECT processes. Conventional CT values were excluded. Because of severe skewness in the observed frequencies of categorical attributes (e.g., 27 males, 2 females; 25 hypertensives, 4 non-hypertensives) a forward stepping search procedure was adopted, starting with each variable (whether factor or covariate) fitted alone. The variable yielding the most significant single contribution to the model was then fitted. Each of the remaining variables in the candidate pool was then also fitted, one at a time, to that model, then removed, and the variable yielding the most significant single contribution from among those was then fitted, along with the first variable so identified. This process continued, provided each term fitted made a significant improvement to the current model. As and when any two factors were fitted, then a term representing the interaction effects between them became eligible and was added to the pool of candidate terms. The same protocol was employed as and when any factor and any covariate were fitted.
A repeated measures analysis of variance, combining patient attributes (between-subjects level) and VMS profiles (within-subjects level) into a single analysis was considered, but frequency imbalances and variance/covariance heterogeneity issues rendered this inappropriate.
Inter-observer variability was assessed with Cohen’s kappa statistic to measure the degree of ‘more-than-chance’ agreement between the two readers for various parameters, and interpreted as follows: (k=0.01–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; 0.81–1.0, excellent agreement).
Results
All procedures were completed in all subjects without the incidence of any complications, and all examinations were of diagnostic image quality with good to excellent for stents visibility. A total of 56 stents (one to three stents per patient) were evaluated. Patient characteristics are shown in Table 1.
Table 1. Patient characteristics of the study groups.
| Variables | Dual-energy CTA (n=29) | Conventional CTA (n=24) |
|---|---|---|
| Patient characteristics | ||
| Male:female | 27:2 | 23:1 |
| Age (years) | 57.88±9.71 | 58.04±10.53 |
| Height (cm) | 166.45±8.06 | 166.46±7.68 |
| Weight (kg) | 75.33±16.59 | 74.23±16.38 |
| BMI (kg/m2) | 27.76±7.14 | 27.38±6.86 |
| Radiation dose | ||
| DLP (mGy·cm) | 1,246.93±44.12 | 1,322.31±431.82 |
| Effective dose (mSv)* | 6.98±0.24 | 7.40±2.41 |
*, conversion factor for peripheral DECTA and conventional CTA =0.0056 mSv/mGy·cm. CTA, computed tomography angiography; BMI, body mass index; DLP, dose length product.
For quantitative analysis between subjects based on personal attributes associated with variations in DECT image quality, we found the average CNR varied significantly with BMI (P=0.027): a unit increase in BMI was associated with a decrease of 0.843 units of CNR, while average SNR appeared not to vary significantly with any of the available factors or covariates. The average CT value varied significantly with BMI (P=0.043), a unit increase in BMI was associated with a decrease of 5.747 HU. Average image noise varied significantly with the type of stent (P=0.025). CT attenuation of all VMS was found to vary significantly with DM (P=0.037) and with BMI (P=0.010). On average, diabetic patients exhibited 190.056 HU more than non-diabetic patients, once controlled for BMI. Controlling for DM, each additional unit of BMI was associated with a reduction of 7.158 HU. The model accounted for 41% of total variation. Image noise was found to vary significantly with DM (P=0.018). On average, patients with diabetes exhibited 18.66 noise units fewer than non-diabetic patients. The model accounted for 29% of the total variation. The small number of observations for DECT and conventional CTA at CIA [19, 14], EIA [3, 2] and SFA [7, 6] combined with considerable clustering of cases in the binary categories of factors (gender, BMI, and DM) rendered almost all factor interaction effects untestable as shown in Table 2.
Table 2. Quantitative assessment of image quality based on stents location.
| Stent location | DECTA protocols | CT attenuation | Image noise | SNR | CNR |
|---|---|---|---|---|---|
| 1 CIA | |||||
| DECTA [19] | 65 | 462.20±101.62 | 42.10±17.72 | 12.88±8.53 | 20.44±16.80 |
| 68 | 420.50±90.43 | 38.45±16.17 | 12.93±9.11 | 19.37 ±13.40 | |
| 70 | 397.50±87.52 | 38.51±15.47 | 11.65±7.62 | 17.65±10.67 | |
| 72 | 374.50±82.84 | 38.59±15.54 | 10.93±7.35 | 15.72±8.81 | |
| Conventional CTA [14] | 428.70±92.17 | 48.46±23.70 | 11.11±5.60 | 9.62±4.92 | |
| 2 EIA | |||||
| DECTA [3] | 65 | 562.70±36.50 | 32.78±13.20 | 18.40±8.49 | 20.20±2.14 |
| 68 | 508.80±33.10 | 31.52±13.27 | 17.42±8.28 | 19.61±3.33 | |
| 70 | 476.40±31.20 | 31.34±13.59 | 16.47±8.01 | 18.38±4.03 | |
| 72 | 446.20±29.42 | 31.53±14.04 | 15.33±7.49 | 16.82±4.55 | |
| Conventional CTA [2] | 637.00±447.72 | 61.52±39.67 | 10.12±0.75 | 8.93±1.43 | |
| 3 SFA | |||||
| DECTA [7] | 65 | 320.80±199.60 | 48.19±23.42 | 8.83±9.65 | 18.87±19.06 |
| 68 | 288.90±181.78 | 45.25±21.04 | 8.46±9.44 | 17.63±17.81 | |
| 70 | 269.70±171.10 | 43.95±19.55 | 8.04±9.05 | 16.14±16.23 | |
| 72 | 251.70±161.14 | 42.97±18.26 | 8.90±8.88 | 14.57±14.88 | |
| Conventional CTA [6] | 427.10±147.84 | 63.69 ±17.84 | 7.52±3.78 | 6.42±3.58 |
CT number in Hounsfield units; SNR, signal-to-noise ratio in the stented area; CNR, contrast-to-noise ratio. Image process: comparison between 4 keVs and conventional CTA. CIA, common iliac artery; EIA, external iliac artery; SFA, superficial femoral artery.
Image quality assessment
Image quality was assessed by taking into account the overall mean CT value and mean image noise for all stent zones. The image quality of 22 (39.3%) stents was good, moderate in 27 (48.2%) stents; the stent lumen was occluded in the remaining 7 (12%) stents. The reason for the interpretability of the images of these seven stents was due to the stent occlusion.
Regarding quantitative image quality, DECTA images had less noise than CTA images, for CIA stents with high significance (P<0.001) and EIA stents with significance (P=0.044). There were no significant differences for image noise of SFA stents between DECTA and CTA, but DECTA had greater CNR than the CTA images for both CIA and EIA stents, and very highly significant differences from the VMS images (P<0.001), and (P=0.005) respectively. Post hoc analyses using Duncan’s Multiple Range Test indicated that the mean CNR for the four VMS and conventional CTA was significantly different from the other four CNR means; although the test did not detect significant differences between the CNR means of all VMS sets, it was very close (P=0.053). For SNR there was no evidence of a difference between the VMS and conventional CTA for CIA and SFA stents, but SNR was found highly significant {F[2,7]=46.81, P=0.006} for EIA stents. The main effect of CT attenuation on CIA stents was found to be a very highly significant difference between VMS on both CIA and SFA stents {F[18,67]=16.22, P<0.001}, and {F[4,23]=4.80, P=0.006}, respectively. Details of ANOVA results for quantitative image quality are shown in Table 3, Figure 1 and Figure 2, with an image example in Figure 3.
Table 3. Results of ANOVA.
| Source | Degrees of freedom | CT value | Image noise | SNR | CNR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P value | F | P value | F | P value | F | P value | |||||
| CIA [19] | [18,67] | 9.963 | 0.001 | 19.22 | 0.001 | 12.25 | 0.001 | 11.928 | 0.001 | |||
| Image process [5] | [4,67] | 6.966 | 0.001 | 6.066 | 0.001 | 1.274 | 0.289 | 7.445 | 0.001 | |||
| EIA [3] | [2,7] | 0.984 | 0.420 | 8.467 | 0.014 | 46.81 | 0.001 | 7.089 | 0.021 | |||
| Image process [5] | [4,7] | 0.486 | 0.747 | 4.341 | 0.044 | 9.707 | 0.006 | 9.900 | 0.005 | |||
| SFA [7] | [6,23] | 11.93 | 0.001 | 9.383 | 0.001 | 37.52 | 0.001 | 66.00 | 0.001 | |||
| Image process [5] | [4,23] | 4.804 | 0.006 | 1.804 | 0.162 | 0.795 | 0.541 | 2.556 | 0.066 | |||
CT value, CT number in Hounsfield units; SNR, signal-to-noise ratio in the stented area; CNR, contrast-to-noise ratio; F, value of test statistic of F-test for corresponding effect; P, corresponding P value; image process, comparison between 4 keVs and conventional CT. CIA, common iliac artery; EIA, external iliac artery; SFA, superficial femoral artery.
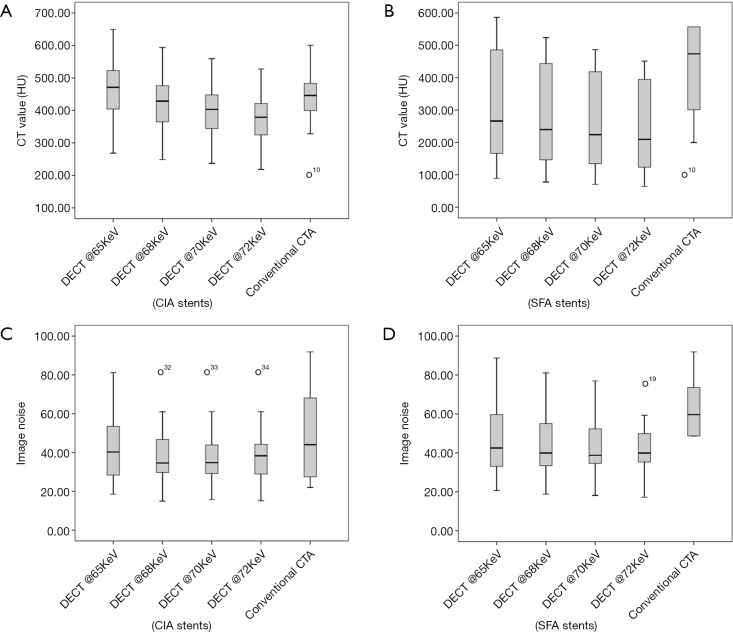
Figure 1.
Box-and-whisker plots of image quality. (A-D) Comparison between the 4 VMS and conventional CTA of CT attenuation value (HU) and image noise in measured in CIA and SFA stents. Box-and-whisker plots (O, outliers) show difference of image quality in different stent locations. VMS, virtual monochromatic spectral; CTA, CT angiography; CIA, common iliac artery; SFA, superficial femoral artery.
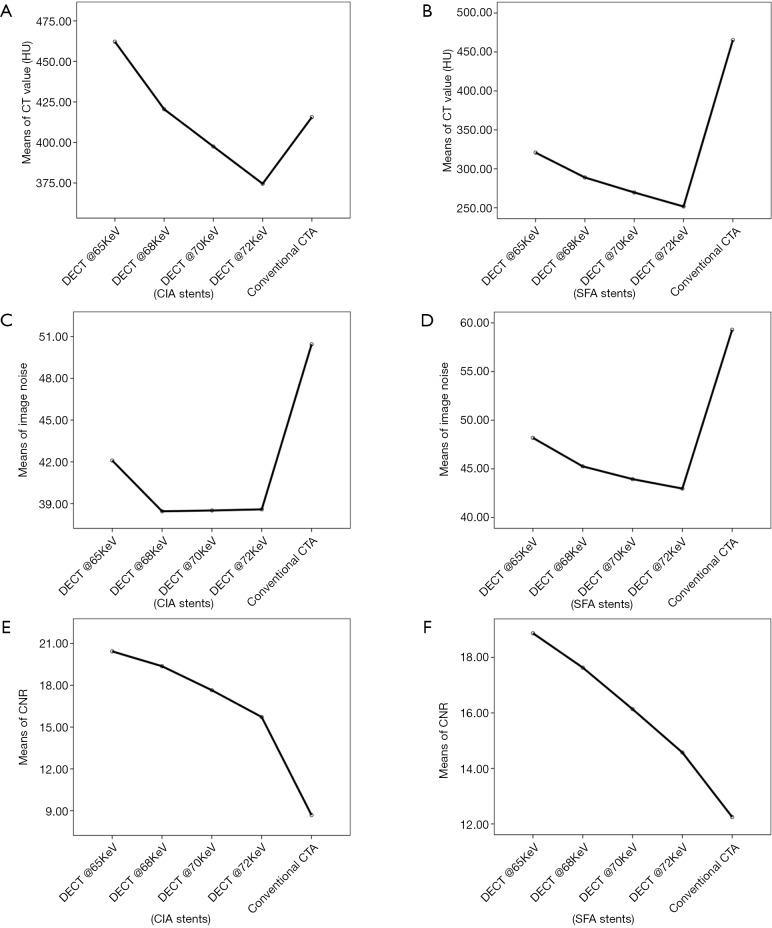
Figure 2.
A line graph of comparison between the 4 VMS and conventional CTA for stents at CIA and SFA. (A,B) CT attenuation (HU) shows the highest CT value at the lower keV; (C,D) image noise demonstrates that the lowest image noise was found at 72 keV protocol and the highest image noise at the conventional CTA, followed by 65 keV protocol; and (E,F) contrast-to-noise ratio (CNR) of CIA shows that CNR decreased with increase in VMS, however, all of the VMS were found to be better than conventional CTA. VMS, virtual monochromatic spectral; CTA, CT angiography; CIA, common iliac artery.
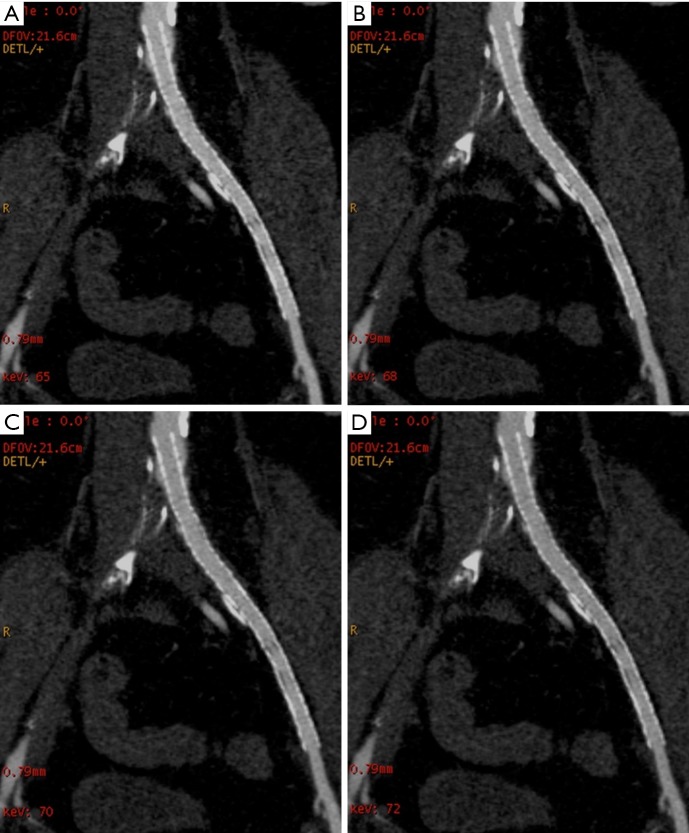
Figure 3.
Example of mage quality of multiplanar reformatted images at different VMS of CIA stents at (A) 65 keV, (B) 68 keV, (C) 70 keV, and (D) 72 keV. The protocol of 72 keV shows the optimal image quality. VMS, virtual monochromatic spectral; CIA, common iliac artery.
Reduction in CT values at all stent zones was associated with an increase in keV, with a reduction of 18% to 21% between 65 and 72 keV for all stent zones. Image noise was found to be highest at 65 keV and a very minimal change between 70 and 72 keV, as shown in Table 3.
With regard to image quality based on stent type, CNR was obviously higher for all VMS than conventional CTA for all stents, while image noise was found to be low for all VMS compared with conventional CTA. The Omnilink stents achieved the highest CNR, and the Wallstent stents recorded the lowest image noise. Cordis SMART stents received the lowest CT attenuation for all VMS while conventional CTA achieved high CT attenuation, as presented in Table 4.
Table 4. Quantitative assessment of image quality based on the stents type.
| Stent type | keV | CNR | SNR | CT attenuation | Image noise |
|---|---|---|---|---|---|
| Missing stent details [8] | 65 | 12.13±9.12 | 6.26±6.50 | 272.42±212.08 | 33.33±25.85 |
| 68 | 11.37 ±8.43 | 6.37±6.94 | 248.58 ±195.50 | 30.90±24.48 | |
| 70 | 10.44 ±7.59 | 6.15 ±6.78 | 232.70 ±183.85 | 29.90±23.71 | |
| 72 | 9.39±6.74 | 5.79 ±6.38 | 217.84 ±172.95 | 29.27 ±23.08 | |
| Conventional CTA [6] | 10.38±2.70 | 12.00±3.15 | 472.76±29.69 | 42.96±15.41 | |
| ev3 Protege Everflex (self-expandable) [10] | 65 | 14.06±7.01 | 8.68±8.22 | 395.17±168.70 | 44.92±23.87 |
| 68 | 14.14±6.63 | 8.22±4.65 | 359.52±154.19 | 43.33±23.46 | |
| 70 | 13.21±6.10 | 7.69±4.30 | 337.98 ±145.46 | 43.01±23.35 | |
| 72 | 12.13±5.53 | 7.09±3.88 | 317.87±137.36 | 43.29±43.64 | |
| Conventional CTA [5] | 7.54±5.34 | 9.31±6.05 | 383.13±68.87 | 52.13±18.19 | |
| Abbott Omnilink (balloon mounted) [4] | 65 | 21.12±4.67 | 18.72±7.38 | 527.08±80.65 | 33.32 ±11.17 |
| 68 | 21.04±4.09 | 16.87 ±7.13 | 481.79 ±65.63 | 32.34±10.94 | |
| 70 | 20.21±3.78 | 15.57±6.78 | 454.42±56.66 | 31.53 ±10.73 | |
| 72 | 18.95±3.71 | 14.25 ±6.24 | 428.84±48.29 | 30.75±10.21 | |
| Conventional CTA [3] | 7.17±1.10 | 8.56±1.50 | 377.60±84.49 | 47.42±20.62 | |
| Cordis genesis (balloon mounted) [19] | 65 | 20.25±17.91 | 14.13±9.53 | 463.74±150.95 | 33.61±14.66 |
| 68 | 19.87±14.39 | 13.70±7.92 | 421.43±136.53 | 32.32±13.17 | |
| 70 | 17.83±11.91 | 12.79 ±7.07 | 396.12±128.06 | 31.95±12.70 | |
| 72 | 15.78±10.21 | 12.90 ±9.64 | 373.43±120.84 | 31.70 ±13.28 | |
| Conventional CTA [17] | 10.73±4.12 | 12.23±4.62 | 481.51±133.57 | 46.52±23.68 | |
| Cordis SMART (self-expandable) [8] | 65 | 9.99±13.78 | 6.29±9.91 | 244.65±226.47 | 42.32 ±36.80 |
| 68 | 8.64 ±10.32 | 6.85±10.90 | 222.82 ±207.30 | 37.65 ±33.98 | |
| 70 | 8.21±9.99 | 6.55±10.32 | 208.93 ±194.51 | 36.50±33.04 | |
| 72 | 7.39±9.15 | 6.10±9.57 | 195.94 ±182.66 | 35.80 ±32.14 | |
| Conventional CTA [4] | 7.35±3.70 | 8.38±3.91 | 418.14±154.87 | 57.91±20.41 | |
| Boston Scientific Wallstent (self-expandable) [7] | 65 | 19.68 ±19.31 | 12.87 ±12.92 | 409.63±267.01 | 34.12 ±21.86 |
| 68 | 18.70 ±17.91 | 13.20 ±12.98 | 375.90 ±246.53 | 30.76±19.54 | |
| 70 | 17.15±16.19 | 12.92±12.96 | 355.52±234.33 | 30.58±19.73 | |
| 72 | 15.50±14.75 | 12.59±12.74 | 336.57±223.10 | 30.56 ±20.23 | |
| Conventional CTA [4] | 4.60±1.79 | 5.45±1.95 | 336.94±107.18 | 50.02±21.70 |
A total of 116 keV images of the stents were evaluated with 564 measurements in our study. The mean scores of protocol assessment for all VMS sets were 2.69±0.46, 2.69±0.46, 2.45±0.46, 2.46±0.49, respectively, and for stent patency assessment the scores of all VMS sets were 1.96±0.49, 2.22±0.56, 2.63±0.48 and 2.81±0.40, respectively. The scores of stent lumen assessment were 3.00±0.76, 3.17±0.77, 3.36±0.81 and 3.50±0.78, respectively. Both readers ranked the 72 keV results as excellent. Inter-reader agreement was good on protocol assessment (k=0.71), good on stent patency assessment (k=0.78), and excellent on stent lumen assessment (k=0.82). Overall, the qualitative assessments of image quality showed that all images were satisfactory for clinical use.
Radiation dose
By our protocols, DLP and effective radiation dose for lower extremities CTA acquisition in DECTA were 1,246.93±64.33 mGy·cm, and 6.98±0.24 mSv, respectively with fixed CTDIvol of 9.05 mGy. These values were lower than those in conventional CTA with the CTDIvol, DLP, and the effective radiation dose being 9.60±3.36 mGy, 1,322.31±431.82 mGy·cm and 7.40±2.41 mSv, respectively, with significant difference (P=0.047).
Discussion
In this study, 56 peripheral arterial stents were evaluated using 64-slice single source DECT, and 49 (88%) of these images were interpretable. The image quality of peripheral arterial stents using DECT relies on optimized keV settings. This implies that a perfect visualization of the stent lumen may not always be seen in a single keV level, and might vary depending on the stent characteristics.
For a technically adequate angiographic imaging, a CT attenuation of 250 HU was used for vascular assessment. The results of this study demonstrated that a DECTA protocol of 70–72 keV with 50% ASIR and a minimum average CT attenuation of 251.70 HU, results in diagnostic images that can distinguish between the stent material and contrast, with optimal CT attenuation and low image noise in comparison with the other VMS levels, and with high CNR compared to conventional CTA.
The outcome of the study was consistent with previous findings in a phantom study, which demonstrated the acquisition of optimal image quality of VMS and was achieved between 65 and 70 keV (11). Throughout the VMS, an increase in keV was associated with a decrease in the mean all-stent attenuations. Higher in-stent attenuation probably results from beam-hardening artifacts affected by the reconstruction algorithm (15). With DECT, blooming artifacts in stents depend on the keV level, as higher keV is associated with lower blooming artifacts; however, the higher keV level leads to a significant reduction of CT attenuation, which may affect the diagnosis of in-stent stenosis evaluation. In the present study, use of 70–72 keV in the differentiation between stent materials, contrast medium and neointimal hyperplasia in a run-off DECTA gave better results than the other 2 keV evaluated.
Only a few studies have used DECT and monochromatic imaging in the evaluation of peripheral arterial stents, and most of these evaluate imaging of coronary stents (16-18). Their results vary in the identification of the optimal keV, ranging from as low as 55 keV to >80 keV. However, a recent study found 70 and 80 keV achieved optimal image quality with the use of DECTA in 31 patients with 45 stents. It was stated that increasing keV to over 80 (90–150 keV) did not show any improvement in either image quality or diagnostic confidence (5). Studies that have evaluated lower extremities with DECT found that optimal image quality falls between 55 to 70 keV (13,19,20).
In this study, DECTA protocols of 72 keV acquired with single-source DE with rapid kV switching were considered optimal for evaluating peripheral arterial stents as it returned the lowest image noise as well as the good differentiation between the contrast medium within the stents and the neointima within the stents. Also, the subjective judgment of image quality by both readers indicates that the 72 keV was the protocol achieving the best image quality. For the objective evaluation in CIA, EIA and SFA stents lower image noise was found with 70 keV, but the type of stent was a significant factor: the Boston Scientific Wallstent, Cordis Genesis, and Abbott Omnilink had the lowest image noise and highest SNR and CNR in comparison with conventional CTA and other VMS values. The findings of this study support the use of higher keV in DECTA of peripheral arterial stents.
Radiation dose was significantly lower with DECT, with a mean DLP of 1,246.93 mGy·cm compared with 1,318.45 mGy·cm for conventional CTA. This is consistent with the literature that radiation dose is not significantly increased using DECT than when using single-energy CT (21,22). The conversion coefficient used for calculation of the most effective dose for CTA of lower extremities followed most recent approaches and seemed to result in smaller quantities than are used in literature. As stated earlier, this new conversion coefficient is provided for lower extremities allowing for estimation of effective dose for clinical CTA, and it was lower than reported in the literature as a high conversion coefficient factor was used.
Our study has several potential limitations. The main limitation is the small number of participated subjects; this was unavoidable as our primary aim was to exploit a homogenous group in terms of implanted stent type and size. Moreover, as subjects could only be recruited from a single center, a limited number of cases were available for the study. There is also extreme imbalance, in the personal attributes of the subject factors; for example, of the 19 subjects with first stents in the CIA zone only one was female, and 18 were males; and 2 non-diabetics and 17 diabetics in the DM factor. Further, a very limited number of stent types was evaluated in this study. A further concern is that we were not able to assess the treatment outcome, because we did not perform any DSA procedures, and no direct comparison could be drawn between DECTA and DSA.
In conclusion, this prospective clinical study of DECTA in imaging peripheral arterial stents was carried out by comparing VMS with conventional CTA. The outcomes demonstrate that the image quality of VMS achieved with DECTA is clinically acceptable. Results of this study indicate that the image quality of DECTA in peripheral arterial stents is achieved at 70 keV and 50% ASIR, and is higher compared with other VMS and conventional CTA. This protocol is considered optimal to achieve lower image noise with adequate clinically diagnostic images. Further studies based on a large population group are warranted.
Acknowledgements
We are grateful to Mr. Gil Stevenson for his assistance in the data analysis.
Ethical Statement: The study was approved by the Curtin University Human Research Ethics (HR 167/2013) and King Fahad Specialist Hospital (KFSH-D) Committees (IRB-RAD029-FB). Informed consent was obtained from all participants.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kalmar PI, Portugaller RH, Schedlbauer P, Bohlsen D, Deutschmann HA. Placement of hemoparin-coated stents in the iliac arteries: early experience and midterm results in 28 patients. Eur J Radiol 2014;83:1205-8. 10.1016/j.ejrad.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 2.Brockmann C, Jochum S, Sadick M, Huck Z, Ziegler P, Fink C, Schoenberg SO, Diehl SJ. Dual-energy CT angiography in peripheral arterial occlusive disease. Cardiovasc Intervent Radiol 2009;32:630-7. 10.1007/s00270-008-9491-5 [DOI] [PubMed] [Google Scholar]
- 3.Maintz D, Burg MC, Seifarth H, Bunck AC, Ozgun M, Fischbach R, Jurgens KU, Heindel W. Update on multidetector coronary CT angiography of coronary stents: in vitro evaluation of 29 different stent types with dual-source CT. Eur Radiol 2009;19:42-9. 10.1007/s00330-008-1132-5 [DOI] [PubMed] [Google Scholar]
- 4.Huang SY, Nelson RC, Miller MJ, Kim CY, Lawson JH, Husarik DB, Boll DT. Assessment of vascular contrast and depiction of stenoses in abdominopelvic and lower extremity vasculature: comparison of dual-energy MDCT with digital subtraction angiography. Acad Radiol 2012;19:1149-57. 10.1016/j.acra.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 5.Mangold S, De Cecco CN, Schoepf UJ, Yamada RT, Varga-Szemes A, Stubenrauch AC, Caruso D, Fuller SR, Vogl TJ, Nikolaou K, Todoran TM, Wichmann JL. A noise-optimized virtual monochromatic reconstruction algorithm improves stent visualization and diagnostic accuracy for detection of in-stent re-stenosis in lower extremity run-off CT angiography. Eur Radiol 2016;26:4380-9. 10.1007/s00330-016-4304-8 [DOI] [PubMed] [Google Scholar]
- 6.Mangold S, Cannaó PM, Schoepf UJ, Wichmann JL, Canstein C, Fuller SR, Muscogiuri G, Varga-Szemes A, Nikolaou K, De Cecco CN. Impact of an advanced image-based monoenergetic reconstruction algorithm on coronary stent visualization using third generation dual-source dual-energy CT: a phantom study. Eur Radiol 2016;26:1871-8. 10.1007/s00330-015-3997-4 [DOI] [PubMed] [Google Scholar]
- 7.Ho LM, Yoshizumi TT, Hurwitz LM, Nelson RC, Marin D, Toncheva G, Schindera ST. Dual energy versus single energy MDCT: measurement of radiation dose using adult abdominal imaging protocols. Acad Radiol 2009;16:1400-7. 10.1016/j.acra.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 8.Kau T, Eicher W, Reiterer C, Niedermayer M, Rabitsch E, Senft B, Hausegger KA. Dual-energy CT angiography in peripheral arterial occlusive disease-accuracy of maximum intensity projections in clinical routine and subgroup analysis. Eur Radiol 2011;21:1677-86. 10.1007/s00330-011-2099-1 [DOI] [PubMed] [Google Scholar]
- 9.Meyer BC, Werncke T, Hopfenmuller W, Raatschen HJ, Wolf KJ, Albrecht T. Dual energy CT of peripheral arteries: effect of automatic bone and plaque removal on image quality and grading of stenoses. Eur J Radiol 2008;68:414-22. 10.1016/j.ejrad.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto S, McWilliams J, Arellano C, Marfori W, Cheng W, Mcnamara T, Quinones-Baldrich WJ, Ruehm SG. Dual-energy CT angiography of pelvic and lower extremity arteries: dual-energy bone subtraction versus manual bone subtraction. Clin Radiol 2009;64:1088-96. 10.1016/j.crad.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 11.Almutairi A, Sun Z, Al Safran Z, Poovathumkadavi A, Albader S, Ifdailat H. Optimal Scanning Protocols for Dual-Energy CT Angiography in Peripheral Arterial Stents: An in Vitro Phantom Study. Int J Mol Sci 2015;16:11531-49. 10.3390/ijms160511531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohler M, Burg MC, Bunck AC, Heindel W, Seifarth H, Maintz D. Dual-Source CT Angiography of Peripheral Arterial Stents: In Vitro Evaluation of 22 Different Stent Types. Radiol Res Pract 2011;2011:103873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almutairi A, Sun Z, Poovathumkadavi A, Assar T. Dual Energy CT Angiography of Peripheral Arterial Disease: Feasibility of Using Lower Contrast Medium Volume. PLoS One 2015;10e0139275. [DOI] [PMC free article] [PubMed]
- 14.Saltybaeva N, Jafari ME, Hupfer M, Kalender WA. Estimates of effective dose for CT scans of the lower extremities. Radiology 2014;273:153-9. 10.1148/radiol.14132903 [DOI] [PubMed] [Google Scholar]
- 15.Lenhart M, Volk M, Manke C, Nitz WR, Strotzer M, Feuerbach S, Link J. Stent appearance at contrast-enhanced MR Angiography: In vitro examination with 14 stents. Radiology 2000;217:173-8. 10.1148/radiology.217.1.r00se28173 [DOI] [PubMed] [Google Scholar]
- 16.Ebersberger U, Tricarico F, Schoepf UJ, Blanke P, Spears JR, Rowe GW, Halligan WT, Henzler T, Bamberg F, Leber AW, Hoffmann E, Apfaltrer P. CT evaluation of coronary artery stents with iterative image reconstruction: improvements in image quality and potential for radiation dose reduction. Eur Radiol 2013;23:125-32. 10.1007/s00330-012-2580-5 [DOI] [PubMed] [Google Scholar]
- 17.Fuchs TA, Stehli J, Fiechter M, Dougoud S, Gebhard C, Ghadri JR, Husmann L, Gaemperli O, Kaufmann PA. First experience with monochromatic coronary computed tomography angiography from a 64-slice CT scanner with Gemstone Spectral Imaging (GSI). J Cardiovasc Comput Tomogr 2013;7:25-31. 10.1016/j.jcct.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Stehli J, Fuchs TA, Singer A, Bull S, Clerc OF, Possner M, Gaemperli O, Buechel RR, Kaufmann PA. First experience with single-source, dual-energy CCTA for monochromatic stent imaging. Eur Heart J Cardiovasc Imaging 2015;16:507-12. 10.1093/ehjci/jeu282 [DOI] [PubMed] [Google Scholar]
- 19.Pinho DF, Kulkarni NM, Krishnaraj A, Kalva SP, Sahani DV. Initial experience with single-source dual-energy CT abdominal angiography and comparison with single-energy CT angiography: image quality, enhancement, diagnosis and radiation dose. Eur Radiol 2013;23:351-9. 10.1007/s00330-012-2624-x [DOI] [PubMed] [Google Scholar]
- 20.Sudarski S, Apfaltrer P, Nance JW, Jr, Schneider D, Meyer M, Schoenberg SO, Fink C, Henzler T. Optimization of keV-settings in abdominal and lower extremity dual-source dual-energy CT angiography determined with virtual monoenergetic imaging. Eur J Radiol 2013;82:e574-81. 10.1016/j.ejrad.2013.04.040 [DOI] [PubMed] [Google Scholar]
- 21.Machida H, Tanaka I, Fukui R, Shen Y, Ishikawa T, Tate E, Ueno E. Dual-energy spectral CT: various clinical vascular applications. Radiographics 2016;36:1215-32. 10.1148/rg.2016150185 [DOI] [PubMed] [Google Scholar]
- 22.Foley WD, Shuman WP, Siegel MJ, Sahani DV, Boll DT, Bolus DN, De Cecco CN, Kaza RK, Morgan DE, Schoepf J, Vrtiska TJ, Yeh B, Berland LL. White paper of the Society of computed body tomography and magnetic resonance on dual-energy CT, part 2: radiation dose and iodine sensitivity. J Comput Assist Tomogr 2016;40:846-50. 10.1097/RCT.0000000000000539 [DOI] [PubMed] [Google Scholar]