Abstract
Diabetes and cancer are prevalent diseases whose incidence is increasing globally. Diabetic women have a moderate risk increase in ovarian cancer, suggested to be due to an interaction between these two disorders. Furthermore, patients manifesting both diseases have associated worse prognosis, reduced survival and shorter relapse-free survival. According to current recommendations, incretin drugs such as Exenatide, a synthetic analog of Exendin-4, and Liraglutide are used as therapy for the type 2 diabetes (T2D). We studied the effects of GLP-1 and Exendin-4 on migration, apoptosis and metalloproteinase production in two human ovarian cancer cells (SKOV-3 and CAOV-3). Exendin-4 inhibited migration and promoted apoptosis through caspase 3/7 activation. Exendin-4 also modulated the expression of key metalloproteinases (MMP-2 and MMP-9) and their inhibitors (TIMP-1 and TIMP-2). Vascular endothelial cells, which contribute to the formation and progression of metastasis, were also analyzed. TNF-α-stimulated endothelial cells from iliac artery after Exendin-4 treatment showed reduced production of adhesion molecules (ICAM-1 and VCAM-1). Additionally, incretin treatment inhibited activation of apoptosis in TNF-α-stimulated endothelial cells. In the same experiment, MMPs (MMP-1 and MMP-9), which are relevant for tumor development, were also reduced. Our study demonstrated that incretin drugs may reduce cancer cell proliferation and dissemination potential, hence limiting the risk of metastasis in epithelial ovarian cancer.
Keywords: Exenatide, ovarian cancer, endothelial cells, apoptosis, MMPs
Background
Cancer and diabetes are growing health problems worldwide. Epidemiological data show an increase in the prevalence of those diseases. Their coexistence is more common than estimated on the basis of their frequency in the population suggesting a cause–effect interaction between cancer and type 2 diabetes (T2D) (1, 2). Furthermore, the coexistence of both diseases is associated with worse prognosis and higher mortality (3). In the clinical setting, several groups of drugs are used in the treatment of diabetes including insulin, sulfonylurea and metformin. Insulin and sulfonylurea treatment in patients under anti-cancer therapy have been associated with worse prognosis, lower overall survival rate and shorter time-to-relapse as compared to patients on metformin or patients without diabetes. To date, metformin has proven to give beneficial effects in patients with cancer, leading to longer survival rates, better prognosis and in vitro inducing apoptosis of some tumor cell lines (4).
Alternatively, incretin mimetic drugs are a relatively new group of drugs used in the treatment of diabetes that are currently recommended by American Diabetes Association in dual therapy with metformin for the treatment of T2D (5, 6). The mechanism of action of incretin mimetic drugs is through the binding to glucagon-like peptide-1 receptor (GLP-1R) in pancreatic beta cells stimulating insulin secretion. The two most important natural incretin hormones are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). However, GIP and GLP-1 are not used as drugs, due to their rapid hydrolysis by dipeptidyl peptidase-IV (DPP-IV). Consequently, pharmacological approaches are focused on the use of GLP-1 analogs, such as Liraglutide and Exenatide (a synthetic version of Exendin-4), with extended half-life and resistance to DDP-IV enzymatic degradation. Interestingly, GLP-1R is present on various types of cells, among which include cancer cells (7, 8).
The high mortality rate in ovarian cancer patients is mostly due to a late diagnosis, at which time the cancer has metastasized throughout the peritoneal cavity and omentum (9). Epithelial ovarian cancer can disseminate via transcoelmic, hematogenous or lymphatic route (10). Cancer metastasis is facilitated by the remodeling of the extracellular matrix (ECM) at the tumor site (11) and during invasion of tissues (12). Breakdown of the ECM components is carried out by matrix metalloproteinases (MMPs), a family of proteolytic enzymes. MMP activity is tightly controlled mainly by tissue inhibitors of metalloproteinase (TIMPs). Ovarian cancer cells alter MMP/TIMP ratio creating a microenvironment promoting cancer cell migration and metastasis (11, 13). Interestingly, pro-inflammatory environment modulates ovarian cancer cells (9) and endothelial cells (14, 15) to stimulate the synthesis or activation of various MMPs to aid in tumor growth, invasion and eventual metastasis. Furthermore, increase in MMP-2 and MMP-9 production has been associated with increased angiogenic response by VEGF expression potentially affecting metastatic potential of cancer cells (16).
The role of GLP-1 analogs on cancer cell growth and invasion both in vitro and in vivo is yet to be elucidated. Incretins have shown to inhibit growth and enhance apoptosis of cancer cells through inhibition of the PI3K/Akt pathway for some cell lines of breast (17), colon cancer (18) and ovarian cancer (19). Thus, understating of the role of GLP-1 analogs has important clinical implication in the design of novel anti-cancer therapies emphasizing the potential benefits of combining both incretins with chemotherapy-cytostatic drugs. In the present study, we investigated whether incretin agonist, Exendin-4, influenced ovarian cancer and vascular endothelium and this had a carry-on effect on tissue remodeling.
Materials and methods
Cell lines
Human ovarian cancer cell lines, SKOV-3 and CAOV-3 (ATCC), were cultured in DMEM (Sigma) or McCoy (Sigma) medium, respectively, with 1% antibiotics (Sigma) and 10% FBS (Sigma) at 37°C in 5% CO2 humidified incubator. Human Iliac artery endothelial cells and human aortic endothelial cells (Lonza, Basel, Switzerland) were cultured in EBM-2 culture medium as previously described (20). Three ovarian tumor cell lines were isolated de novo from anonymous patients undergoing surgical removal of the ovaries due to ovarian cancer with patients’ consent. Biopsies were obtained after approval of the ethic committee of the Medical University of Silesia (KNW/0022/KB1/122/14). Prior to incubation, the ovarian cancer biopsies were washed in HEPES buffer to remove blood. Then, the sections were cut into pieces with a diameter of approx. 1 mm and placed in a culture Petri dish. Tumor cells were isolated using the ‘explant method’ (21, 22) and cultured under the same conditions as SKOV-3 cells.
Transwell migration assays
Ovarian cancer cell migration was conducted using an 8-μm transwell system (Greiner Bio-One, Kremsmünster, Austria) in a 24-well plate format. For migration assay, 2 × 105 cells were placed in the upper chamber of the transwell in 200 μL medium (0.5% FBS), Exendin-4 (50 nM, Sigma), GLP-1 (100 nM, Sigma), GLP-1 antagonist 9–36 (50 nM) (Tocris, Bristol, UK). Cells were pre-incubated with Exendin-4 for 24 h when appropriate. Cells were induced to actively migrate through membrane into the lower compartment containing 600 μL of DMEM and monocyte chemoattractant protein (MCP-1) at 10 nM (23). Cells were cultured for 24 h. Migrated cells in the underside of the membrane were detached with trypsin and fluorescently labeled with Calcein-AM (8 μM) for 45 min. A number of migrating cells were assessed by measuring fluorescence (excitation wavelength of 485 nm and an emission wavelength of 520 nm) with a microplate reader on Infinite M200 (TECAN, Männedorf, Switzerland).
Viability, cytotoxicity and apoptosis assays
Ovarian cancer cells were seeded into a 96-well plate at a total density of 2 × 104 cells per well in appropriate cell culture medium. Cells were pre-incubated with Exendin-4 for 24 h when appropriate and then incubated with Camptothecin (100 nM) or Exendin-4 (50 nM) for 24 h. Viability, cytotoxicity and caspase activation were determined using ApoTox-Glo Triplex assay (Promega) according to the manufacturer’s protocol.
Immunoblotting
Cells were lysed in PathScan lysis buffer (Cell Signaling Technology) containing phenylmethylsulfonyl fluoride (Sigma) and protease inhibitor (Roche). The protein samples were separated by SDS-PAGE. Proteins were transferred into low autofluorescence Immobilon-FL PVDF membrane (Millipore) and incubated with either antibody, GLP-1R (ab39072, Abcam) at 1:1000 or GAPDH (Sigma) at 1:5000. Goat anti-rabbit secondary antibody conjugated with fluorescent dye IRDye800 (LI-COR Biosciences, Lincoln, NE, USA) was used. The proteins of interest were visualized by using LICOR Odyssey Infrared Imaging System.
Fluorescent bead-based Luminex cytokine assay
Analysis of MMPs and TIMPs protein concentration was performed in cell medium using multiplex, bead-based (Luminex) assays on a Bio-Plex200 suspension array system according to each manufacturer’s instructions. Data were acquired on a validated and calibrated Bio-Plex 200 system (Bio-Rad Laboratories) and analyzed with Bio-Plex Manager 6.0 software (Bio-Rad Laboratories) with a detection target of 50 beads per region, low RP1 target for CAL2 calibration and recommended doublet discriminator gates of 5000–25,000 for Bio-Plex. The median fluorescence intensity was measured. Bio-Plex Pro Human MMP 9-Plex Panel (Bio-Rad Laboratories) was used for detection of MMPs. Bio-Plex Pro Human Cytokine 27-plex assay (Bio-Rad Laboratories) was used for detection of TNF-α and VEGF. Analysis of phosphoIκB (Ser32) and IκB was performed in cell medium using multiplex, bead-based (Luminex xMAP, Merck) as described earlier and according to manufacturer’s instructions.
Determination of adhesion molecule levels
ICAM-1 and VCAM-1 protein concentrations in IAEC cells were quantified by Enzyme Linked ImmunoSorbent Assay (ELISA) by using DuoSet ELISA kits (R&D Systems), according to the manufacturer’s instructions. ET-1 was assayed using RnD’s QuantiGlo assay (R&D Systems).
Statistical analysis
Data are presented as mean values ± s.e.m. Data were analyzed with GraphPad-Prism 5.0 (Graphpad Software), and differences were considered statistically significant at P < 0.05.
Results
Exendin-4 reduces migration of ovarian cancer cells through the GLP-1R
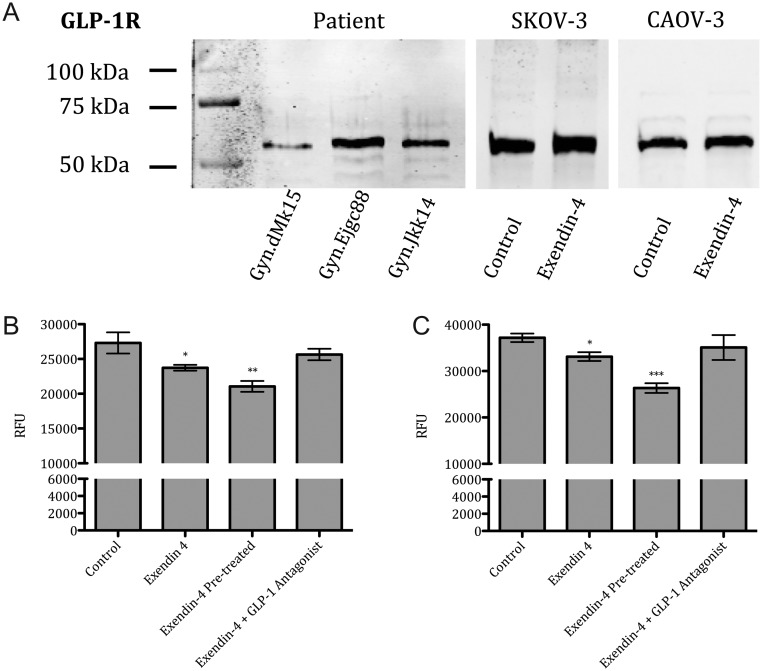
The main effects of GLP-1 analogs are achieved through binding to the GLP-1R. In SKOV-3 and CAOV-3 cell lines, GLP-1R receptor presence was shown in Western blot analysis. GLP-1R was also observed in ovaries of patients with adenocarcinoma (Fig. 1A).
Figure 1.
GLP-1R Western blot and transwell-based migration assay. Representative Western blot image of protein extracts from SKOV-3, CAOV-3 and patient biopsies (A). Ovarian cancer cell lines were stimulated for 24 h with Exendin-4 (50 nM), Exendin-4 (50 nM) pre-treatment for 24 h and GLP-1 Antagonist 9–36 (50 nM) in both SKOV-3 (B) and CAOV-3 (C). Relative fluorescence units (RFU). Mean values ± s.e.m. are shown. n = 3 per group. *P < 0.05, **P < 0.01, ***P < 0.001 Control vs different conditions. One-way ANOVA followed with Dunnett’s post hoc.
In transwell-based migration assay, Exendin-4 attenuated the MCP-1 induced migration in both SKOV-3 and CAOV-3 cell lines (Fig. 1B and C). Exendin-4 (50 nM) was added into the upper well and invasiveness was reduced by 13% in SKOV-3 cells and by 11% in CAOV-3 cells. Pre-treatment of Exendin-4 for 24 h further attenuated the migration potential by 22% in SKOV-3 cells and by 29% in CAOV-3 cells. In addition, GLP-1R antagonist (50 nM) reversed the inhibitory effects induced by Exendin-4 in both cell lines.
Incretin effects on apoptotic and viability
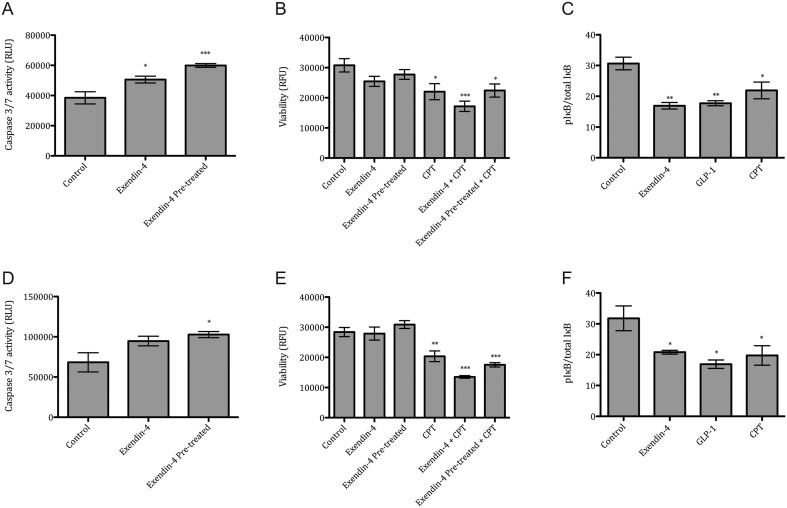
To assess the influence of incretin drugs on apoptosis and cell viability, SKOV-3 and CAOV-3 cells were stimulated with 50 nM of Exendin-4 for 24 h with or without the pre-treatment with Exendin-4 for 24 h. In both analyzed cancer cell lines, Exendin-4 induced the activation of caspase 3/7 by 31% in SKOV-3 cells and by 38% in CAOV-3 cells. Pre-treatment with Exendin-4 further increased activation of caspases by 56% in SKOV-3 cells and by 50% in CAOV-3 cells (Fig. 2A and D). Both cell lines displayed a similar pattern of viability.
Figure 2.
Exendin-4 effects on apoptosis, cell viability and signaling pathway. Ovarian cancer cell lines were stimulated for 24 h with Exendin-4 (50 nM), Exendin-4 (50 nM) pre-treatment for 24 h and Camptothecin (CPT, 100 nM) in both SKOV-3 (A, B, C) and CAOV-3 (D, E, F). Caspase 3/7 activation in SKOV-3 (A) and CAOV-3 (D), cell viability in SKOV-3 (B) and CAOV-3 (E) and phosphoIκB in SKOV-3 (C) and CAOV-3 (F) was analyzed. Relative fluorescence units (RFU). Mean values ± s.e.m. are shown. n = 3 per group. *P < 0.05, **P < 0.01, ***P < 0.001 Control vs different conditions. One-way ANOVA followed with Dunnett’s post hoc.
In both cancer cell lines, stimulation with Exendin-4 with or without 24 h pre-treatment caused no significant changes in viability. Camptothecin (CPT) treatment caused a reduction in viability in SKOV-3 cells by 27% and by 28% in CAOV-3. Interestingly, Exendin-4 and CPT together further reduced viability of SKOV-3 cells by 44% and by 52% in CAOV-3. Additionally, cells pre-treated with Exendin-4 and incubated with Exendin-4 and CPT together caused a reduction in viability of SKOV-3 cells by 27% and by 38% in CAOV-3 (Fig. 2B and E).
In parallel, activation of GLP-1R signaling pathway was determined by multiplex analysis. Exendin-4 treatment decreased the phospho-IκB protein levels by 43% in SKOV-3 and by 36% in CAOV-3. Similarly, GLP-1 (100 nM) decreased the phospho-IκB protein levels by 40% in SKOV-3 and by 46% in CAOV-3 (Fig. 2C and F). The group treated with Camptothecin, a potent chemotherapeutic drug, also displayed a reduced pIκB/IκB ratio leading to an increased inhibition of NF-κB (24).
Distinct metalloproteinase production in Exendin-4-stimulated ovarian cancer cells
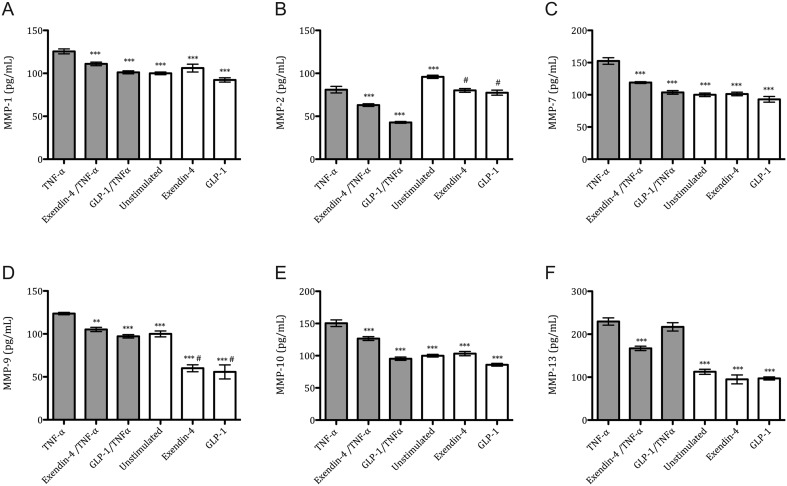
Ovarian cancer cells, SKOV-3 and CAOV-3, were incubated with TNF-α and incretin mimetic drugs and MMP levels were evaluated. MMP-1, MMP-2, MMP-7, MMP-9, MMP-10 and MMP-13 protein concentrations of cell culture mediums were assessed in both cancer cell lines upon stimulation with TNF-α, Exendin-4 and GLP-1 separately or in combination. Only detectable levels of MMPs were observed in the SKOV-3 cell line, whereas MMP levels were under detection limits for CAOV-3. After TNF-α incubation, treatment with either Exendin-4 or GLP-1 moderately decreased the protein level of all MMPs when compared with the TNF-α (Fig. 3). Upon TNF-α incubation, either Exendin-4 or GLP-1 caused an MMP-1 reduction by 14% and 19%, respectively (Fig. 3A). MMP-2 reduction was 23% and 47%, respectively (Fig. 3B). MMP-7 reduction was 22% and 32%, respectively (Fig. 3C). MMP-9 reduction was 15% and 21%, respectively (Fig. 3D). MMP-10 reduction was 16% and 37%, respectively (Fig. 3E). MMP-13 reduction by 27% was observed only when Exendin-4 was given (Fig. 3F). Interestingly, both Exendin-4 and GLP-1 caused a reduction in MMP-2 and MMP-9 protein levels in non-TNF-α groups when compared with the unstimulated group (Fig. 4B and D); this reduction is not observed for the other MMPs.
Figure 3.
TNF-α-mediated metalloproteinase profile after Exendin-4 treatment in SKOV-3 cancer cells. Protein levels of MMP-1 (A), MMP-2 (B), MMP-7 (C), MMP-9 (D), MMP-10 (E) and MMP-13 (F) were analyzed by multiplex analysis after 24 h incubation with TNF-α (10 ng/mL), Exendin-4 (50 nM) and GLP-1 agonist (100 nM). Mean values ± s.e.m. are shown. n = 3–6 per group. Gray bars, TNF-α-stimulated. White bars, non-TNF-α-stimulated. *P < 0.05, **P < 0.01, ***P < 0.001 TNF-α vs different conditions. #P < 0.05 Unstimulated vs Exendin-4 or GLP-1 group. One-way ANOVA followed with Dunnett’s post hoc.
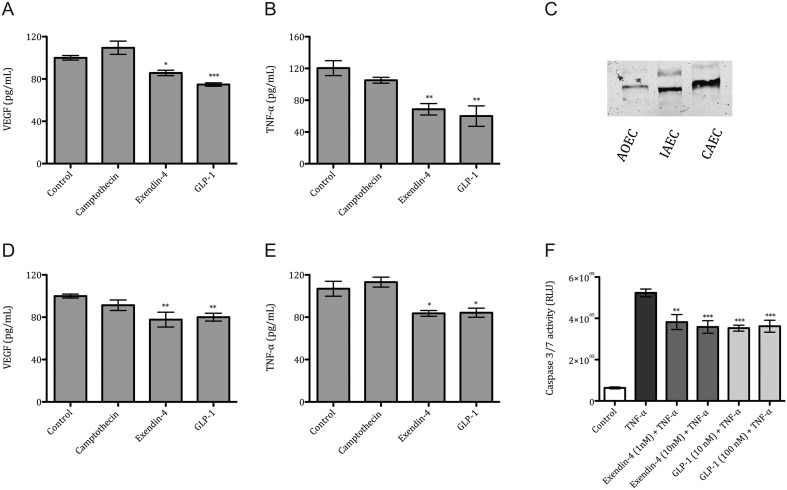
Figure 4.
VEGF, TNF-α concentration in ovarian cancer cells and Caspase 3/7 activation in endothelium. VEGF protein levels in SKOV-3 (A) and CAOV-3 (D) and TNF-α protein levels in SKOV-3 (B) and CAOV-3 (E) after 24 h incubation with Camptothecin (100 nM), Exendin-4 (50 nM) and GLP-1 (100 nM). Representative Western blot image of GLP-1R expression in human Aortic Endothelial Cells (AoEC), Iliac Artery Endothelial Cells (IAEC) and Coronary Artery Endothelial Cells (CAEC) (C). Caspase 3/7 activation in TNF-α (10 ng/mL) stimulated IAEC after incubation with Exendin-4 (1 nM and 10 nM), and GLP-1 (10 nM and 100 nM) (F). Mean values ± s.e.m. are shown. n = 3–6 per group. *P < 0.05, **P < 0.01, ***P < 0.001 TNF-α vs different conditions. One-way ANOVA followed with Dunnett’s post hoc.
Exendin-4 modulates angiogenic factors and inhibits TNF-α endothelial cell apoptosis
We assessed the protein levels of VEGF in both cancer cell lines after stimulation with CPT and incretin agonists. VEGF protein levels were reduced in both cell lines when compared to the control group (Fig. 4). Exendin-4 caused a reduction by 14% and GLP-1 by 25% in SKOV-3 cells (Fig. 4A) and a reduction by 22% and GLP-1 by 19% in CAOV-3 cells (Fig. 4D). We also assessed the TNF-α in both cancer cell media after stimulation with CPT and incretin drugs. TNF-α protein levels were reduced in both cell lines when compared to the control group. Exendin-4 caused a reduction by 43% and GLP-1 by 50% in SKOV-3 cells (Fig. 4B), whereas in CAOV-3 cells Exendin-4 caused a reduction by 22% and GLP-1 by 21% (Fig. 4E). No changes were observed in inflammatory cytokines such as IL-6 and IL-8 (Supplementary data 1, see section on supplementary data given at the end of this article).
Interestingly, endothelial cells from aorta, coronary artery and iliac artery showed differences in the expression levels of the GLP-1R (Fig. 4C). GLP-1R agonist treatment reduced the TNF-α-mediated apoptosis in iliac artery endothelium. Exendin-4 treatment at 1 nM or 10 nM reduced caspase 3/7 activation by 27% and 31%, respectively. GLP-1 treatment at 10 nM or 100 nM reduced caspase 3/7 by 32% and 30%, respectively (Fig. 4F).
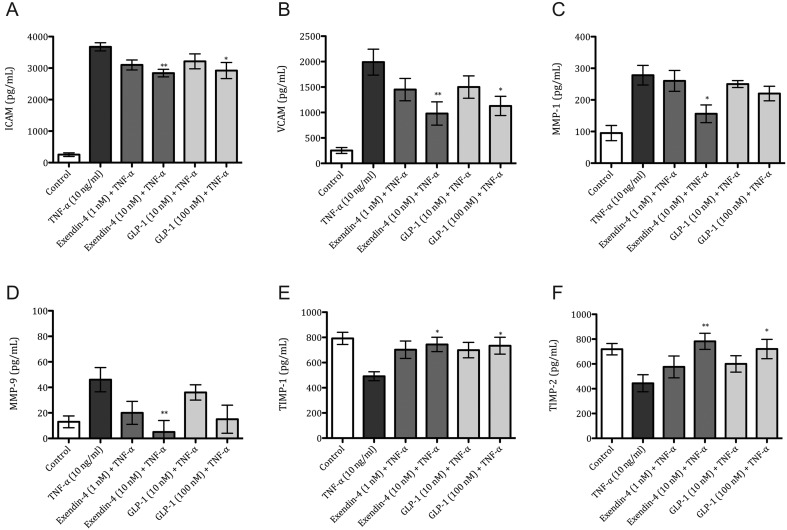
Modulation of adhesion molecules, metalloproteinases and inhibitors of metalloproteinase production in endothelial cells after GLP-1 agonist treatment
The protein levels of selected adhesion molecules were quantified in TNF-α-stimulated endothelial cells (Fig. 5). Upon treatment with GLP-1R agonists (10 nM and 100 nM), only the higher concentration of either analog significantly reduced the levels of intracellular adhesion molecule 1 (ICAM-1) by 22% and 20%, respectively (Fig. 2A). Vascular adhesion molecule was also reduced by 50% and 43%, respectively (Fig. 2B). The levels of other adhesion molecules, P-selectin and Endothelin-1 (ET-1), remained unchanged (Supplementary data 2).
Figure 5.
Exendin-4 modulates endothelial-cell response after TNF-α stimulation. Iliac Artery Endothelial Cells (IAEC) were stimulated for 24 h with TNF-α (10 ng/mL), Exendin-4 (1 nM and 10 nM) and GLP-1 (10 nM and 100 nM). Protein concentration of VCAM-1 (A), ICAM-1 (B), MMP-1 (C), MMP-9 (D), TIMP1 (E) and TIMP-2 (F). Mean values ± s.e.m. are shown. n = 6 per group. *P < 0.05, **P < 0.01 TNF-α vs different conditions. One-way ANOVA followed with Dunnett’s post hoc.
Endothelial cells in the presence of TNF-α treated with Exendin-4 at 10 nM only significantly reduced the levels of MMP-1 by 43% (Fig. 5C) and MMP-9 by 89% (Fig. 5D). Conversely, the levels of TIMP-1 and TIMP-2 (Fig. 5E and F), but not TIMP-3 (Supplementary data 2), were markedly increased in the higher concentration of both incretins. In the presence of TNF-α, Exendin-4 and GLP-1 caused an increase in TIMP-1 by 51% and 50%, respectively (Fig. 5E) and an increase in TIMP-2 by 65% and 52%, respectively (Fig. 5F).
Discussion
Evidence and epidemiological observational studies show an increase in the prevalence of both diabetes and cancer; thus, clinicians will have to face treatment of both conditions in the patient at the same time. In clinical environment, GLP-1 analogs and DDP-4 inhibitors are currently being used to treat T2D. Early data obtained from health register analysis and observational studies have suggested that incretin drugs increase incidence of cancer, especially pancreatic cancer, in patients on incretin therapies (25). However, randomized clinical trials showed that incretin-based drugs were not associated with an increased risk of pancreatic cancer (26). Overall, the scarce in vitro data limit our understanding of the role of incretins and cancer. Although the mechanisms of action of GLP-1 analogs are yet to be elucidated, GLP-1 analogs have been shown to influence cancer cells by altering proliferation, apoptosis, ECM remodeling and the response to chemotherapy (17, 18, 19).
We studied several aspects of ovarian cancer progression such as apoptosis and migration, as well as metalloproteinase production associated during these processes. Consistent with the work of He and collaborators (19), we show that Exendin-4 suppresses ovarian cancer migration and induces apoptosis by the activation of GLP-1R in human ovarian cancer cells. Ovarian cancer patients are currently treated with platinum-based drugs and taxol (50); however, the results of this study indicate potential additive or synergic effect of incretin-chemotherapy treatment. Besides its anti-cancer effects, we observed anti-inflammatory effects, which are partially mediated by modulation of the NF-κB signaling pathway.
During early ovarian carcinogenesis, inflammatory mediators such TNF-α and TLR4 (27, 28) activate NF-κB signaling pathway by activation of the IKK complex, which then phosphorylates IκB. Phosphorylated IκB is then ubiquitinated and degraded by the proteasome system, leading to the release of NF-κB. The NF-κB molecule then translocates to the nucleus and initiates the transcription genes associated with metastasis, proliferation angiogenesis and suppression of apoptosis (29). We observed that treatment with Exendin-4 inhibits phosphorylation of IκB, hence the activation of the NF-κB signaling pathway.
Reasons for the high mortality rate associated with ovarian cancer include a late diagnosis, at which time cancer has metastasized throughout the peritoneal cavity. Ovarian tumor cells and the surrounding stromal cells stimulate the synthesis or activation of a family of proteolytic enzymes known as the MMPs to aid in tumor growth, invasion and eventual metastasis (12, 30). The MMP activity is controlled by their inhibitors, TIMPs. Thus, any disturbances in MMP/TIMP balance determine the type of tumor microenvironment which may facilitate metastasis (12). Studies have shown that MMP-9 overexpression is associated with an increased metastatic potential of ovarian tumors, which leads to poor prognosis and decreased survival (31). In another study, elevated levels of MMP-2 in serum were used as an indicator of the severity of invasion in breast cancer (32). Although the expression pattern of each individual MMP varies depending upon the type of tumor, tumor stage, patient diagnosis and even potentially the patient population (33), studies have shown that a reduction in MMP production reduces the metastatic potential of cancer cells (34). In this study, we have shown that incretin drugs can reduce the expression of several MMPs under TNF-α-mediated inflammatory environment in SKOV-3 cancer cell line. Furthermore, we were not able to assess detectable protein levels of MMPs in CAOV-3.
We studied the potential crosstalk between cancer cells and endothelial cells by analyzing the production of MMPs, cytokines such as VEGF, TNF-α, IL-6 by both cells types in vitro. These inflammatory mediators are recognized as an important component of the tumor–stroma interaction which may predispose to angiogenesis, invasiveness and increases in metastasis potential (35, 36, 37). We found that VEGF protein levels, a key molecule involved in neovascularization (38, 39, 40), were reduced in cancer cells after incretin drug treatment. Ovarian cancer inflammatory mediators such as TNF-α and IL-6, which are elevated in ovarian cancers (41), were also reduced upon incretin drug treatment.
Interestingly, recent investigations revealed a new mechanism by which cancer cells interact with endothelial cells during metastasis progression. In contrast to leukocyte transendothelial diapedesis, the research indicated that cancer cells can induce programmed cell death of the endothelial cells, hence promoting extravasation and metastasis (42). Through a series of in vitro experiments, the authors demonstrated that cancer cells can trigger the death of endothelial cells in a process mediated by TNF-α and MMPs (43, 44). In our study, we found that incretin drugs can reduce the TNF-α protein levels in both cancer cell lines, whereas the levels of other inflammatory cytokines were not affected. Furthermore, incretin drugs can influence vascular biology due to the presence of GLP-1R in endothelial cells. Consequently, in order to reproduce the effects that tumor cell may exert on endothelium at the moment of extravasation and metastasis, endothelial cells were stimulated with TNF-α and treated with GLP-1R agonists. Contrarily to the effects in ovarian cancer cells, we have observed that incretin drugs prevent apoptosis of endothelial cells.
Ovarian cancer progression is characterized by the production of inflammatory mediators such as TNF-α and IL-1 (45). Studies have shown that these inflammatory molecules mediate the expression of endothelial adhesion molecules VCAM-1 and ICAM-1 (46, 47). Changes in the expression of cell adhesion molecules have been implicated in all steps of tumor progression, including migration of cells from primary tumor site to circulation, intravasation into the blood stream, extravasation to secondary metastatic places and formation of new tumors (48). Interestingly, we have shown that incretin agonists can reduce the expressions of both VCAM-1 and ICAM-1 adhesion molecules in endothelial cells suggesting a potential role of incretin drugs in reducing the metastatic potential of cancer cells. Additionally, production of metalloproteinases by endothelial cells is also a critical event during angiogenesis that occurs under normal and tumorigenic conditions (49). We observed a decrease in MMP-1 and MMP-9 production accompanied by an increase in the corresponding TIMPs.
Due to the hidden potential of incretin drugs as anti-cancer drugs, this study aimed to provide further understanding of the role of incretin drugs on tumor progression. Our work indicates that the pleiotropic effects of incretin drugs are not limited only to glucose-lowering actions, but incretins also modulate the actions of both ovarian cancer cells and endothelial cells involving several aspects of tumor progression. Although the synergic effects of anti-tumor and incretin therapy on tumor progression yet remain to be studied, incretin alone has shown great potential in the ovarian cancer field.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by grants from the Medical University of Silesia KNW-1-076/K/6/0 and KNW-1-018/N/5/0.
References
- 1.Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, Svensson A-M, Ljung R, Wild SH, Kerssens JJ, Harding JL, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9000 cancers in type 1 diabetic individuals. Diabetologia 2016. 59 980–988. ( 10.1007/s00125-016-3884-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 2015. 38 264–270. ( 10.2337/dc14-1996) [DOI] [PubMed] [Google Scholar]
- 3.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiological Reviews 2015. 95 727–748. ( 10.1152/physrev.00030.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farooki A, Schneider SH. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: response to Bowker Diabetes Care 2006. 29 1989–1990. ( 10.2337/dbib6-0874) [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Recommendations. Approaches to glycemic treatment. Sec. 7. In Standards of Medical Care in Diabetes 2016. Diabetes Care 2016. 39 (Supplement 1) S52–S59. [DOI] [PubMed] [Google Scholar]
- 6.Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs in Context 2015. 4 1–19. ( 10.7573/dic.212283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacological Reviews 2008. 60 470–512. ( 10.1124/pr.108.000604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-like-peptide-1 receptor expression in normal and diseased human thyroid and pancreas. Modern Pathology 2015. 28 391–402. ( 10.1038/modpathol.2014.113) [DOI] [PubMed] [Google Scholar]
- 9.Lengyel E. Ovarian cancer development and metastasis. American Journal of Pathology 2010. 177 1053–1064. ( 10.2353/ajpath.2010.100105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weidle UH, Birzele F, Kollmorgen G, Rueger R. Mechanisms and targets involved in dissemination of ovarian cancer. Cancer Genomics and Proteomics 2016. 13 407–424. ( 10.21873/cgp.20004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Alem L, Curry TE. Ovarian cancer: involvement of the matrix metalloproteinases. Reproduction 2015. 150 R55–R64. ( 10.1530/REP-14-0546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung T-L, Leung CS, Yip K-P, Au Yeung CL, Wong STC, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: cell and molecular processes in cancer metastasis. American Journal of Physiology: Cell Physiology 2015. 309 C444–C456. ( 10.1152/ajpcell.00188.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worzfeld T, Pogge von Stand mann E, Huber M, Adhikary T, Wagner U, Reinartz S, Müller R. The unique molecular and cellular microenvironment of ovarian cancer. Frontiers in Oncology 2017. 7 24 ( 10.3389/fonc.2017.00024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010. 141 52–67. ( 10.1016/j.cell.2010.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alarcon CR, Tavazoie SF. Endothelial-cell killing promotes metastasis. Nature 2016. 536 154–155. ( 10.1038/nature19465) [DOI] [PubMed] [Google Scholar]
- 16.Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A, Asosingh K, Erzurum S, Anand-Apte B. Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. American Journal of Pathology 2010. 176 496–503. ( 10.2353/ajpath.2010.080642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidan-Yaylali G, Dodurga Y, Seçme M, Elmas L. Antidiabetic exendin-4 activates apoptotic pathway and inhibits growth of breast cancer cells. Tumor Biology 2016. 37 2647–2653. ( 10.1007/s13277-015-4104-9) [DOI] [PubMed] [Google Scholar]
- 18.Koehler JA, Kain T, Drucker DJ. Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology 2011. 152 3362–3372. ( 10.1210/en.2011-1201) [DOI] [PubMed] [Google Scholar]
- 19.He W, Yu S, Wang L, He M, Cao X, Li Y, Xiao H. Exendin-4 inhibits growth and augments apoptosis of ovarian cancer cells. Molecular and Cellular Endocrinology 2016. 436 240–249. ( 10.1016/j.mce.2016.07.032) [DOI] [PubMed] [Google Scholar]
- 20.Garczorz W, Francuz T, Siemianowicz K, Kosowska A, Kłych A, Aghdam MRF, Jagoda K. Effects of incretin agonists on endothelial nitric oxide synthase expression and nitric oxide synthesis in human coronary artery endothelial cells exposed to TNFα and glycated albumin. Pharmacological Reports 2015. 67 69–77. ( 10.1016/j.pharep.2014.08.007) [DOI] [PubMed] [Google Scholar]
- 21.Planz B, Tabatabaei S, Kirley SD, Aretz HT, Wang Q, Lin C-W, McDougal WS, Marberger M. Studies on the differentiation pathway and growth characteristics of epithelial culture cells of the human prostate. Prostate Cancer and Prostatic Diseases 2004. 7 73–83. ( 10.1038/sj.pcan.4500704) [DOI] [PubMed] [Google Scholar]
- 22.Sharma M, Shubert DE, Sharma M, Rodabaugh KJ, McGarrigle BP, Vezina C., Bofinger DP, Olson JR. Antioxidant inhibits tamoxifen–DNA adducts in endometrial explant culture. Biochemical and Biophysical Research Communications 2003. 307 157–164. ( 10.1016/S0006-291X(03)01134-3) [DOI] [PubMed] [Google Scholar]
- 23.Furukawa S, Soeda S, Kiko Y, Suzuki O, Hashimoto Y, Watanabe T, Nishiyama H, Tasaki K, Hojo H, Abe M, et al. MCP-1 promotes invasion and adhesion of human ovarian cancer cells. Anticancer Research 2013. 33 4785–4790. [PubMed] [Google Scholar]
- 24.Jayasooriya RGPT, Park SR, Choi YH, Hyun J-W, Chang W-Y, Kim G-Y. Camptothecin suppresses expression of matrix metalloproteinase-9 and vascular endothelial growth factor in DU145 cells through PI3K/Akt-mediated inhibition of NF-κB activity and Nrf2-dependent induction of HO-1 expression. Environmental Toxicology and Pharmacology 2015. 39 1189–1198. ( 10.1016/j.etap.2015.04.011) [DOI] [PubMed] [Google Scholar]
- 25.Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Research and Clinical Practice 2012. 98 271–284. ( 10.1016/j.diabres.2012.09.008) [DOI] [PubMed] [Google Scholar]
- 26.Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, Durand M, Juurlink DN, Targownik LE, Turin TC, et al. Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ 2016. 352 i581 ( 10.1136/bmj.i581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009. 30 1073–1081. ( 10.1093/carcin/bgp127) [DOI] [PubMed] [Google Scholar]
- 28.Mandai M, Yamaguchi K, Matsumura N, Baba T, Konishi I. Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. International Journal of Clinical Oncology 2009. 14 383–391. ( 10.1007/s10147-009-0935-y) [DOI] [PubMed] [Google Scholar]
- 29.Alvero AB. Recent insights into the role of NF-kappaB in ovarian carcinogenesis. Genome Medicine 2010. 2 56 ( 10.1186/gm177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinkamp MP, Winner KK, Davies S, Muller C, Zhang Y, Hoffman RM, Shirinifard A, Moses M, Jiang Y, Wilson BS. Ovarian tumor attachment, invasion, and vascularization reflect unique microenvironments in the peritoneum: insights from xenograft and mathematical models. Frontiers in Oncology 2013. 3 97 ( 10.3389/fonc.2013.00097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu X, Li D, Zhang W, Zhou J, Tang B, Li L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Archives of Gynecology and Obstetrics 2012. 286 1537–1543. ( 10.1007/s00404-012-2456-6) [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Yang S, Yeh K, Yeh C, Chiou H. Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clinica Chimica Acta 2006. 371 92–96. ( 10.1016/j.cca.2006.02.026) [DOI] [PubMed] [Google Scholar]
- 33.Henderson BE, Lee NH, Seewaldt V, Shen H. The influence of race and ethnicity on the biology of cancer. Nature Reviews Cancer 2012. 12 648–653. ( 10.1038/nrc3341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almholt K, Juncker-Jensen A, Laerum OD, Dano K, Johnsen M, Lund LR, Romer J. Metastasis is strongly reduced by the matrix metalloproteinase inhibitor Galardin in the MMTV-PymT transgenic breast cancer model. Molecular Cancer Therapeutics 2008. 7 2758–2767. ( 10.1158/1535-7163.MCT-08-0251) [DOI] [PubMed] [Google Scholar]
- 35.Szlosarek PW. Expression and regulation of tumor necrosis factor in normal and malignant ovarian epithelium. Molecular Cancer Therapeutics 2006. 5 382–390. ( 10.1158/1535-7163.MCT-05-0303) [DOI] [PubMed] [Google Scholar]
- 36.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002. 420 860–867. ( 10.1038/nature01322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005. 7 211–217. ( 10.1016/j.ccr.2005.02.013) [DOI] [PubMed] [Google Scholar]
- 38.Bamberger ES, Perrett CW. Angiogenesis in epithelian ovarian cancer. Molecular Pathology 2002. 55 348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markowska A, Sajdak S, Markowska J, Huczynski A. Angiogenesis and cancer stem cells: new perspectives on therapy of ovarian cancer. European Journal of Medicinal Chemistry 2017 [in press]. ( 10.1016/j.ejmech.2017.06.030) [DOI] [PubMed] [Google Scholar]
- 40.Gómez-Raposo C, Mendiola M, Barriuso J, Casado E, Hardisson D, Redondo A. Angiogenesis and ovarian cancer. Clinical and Translational Oncology 2009. 11 564–571. ( 10.1007/s12094-009-0406-y) [DOI] [PubMed] [Google Scholar]
- 41.Gupta M, Babic A, Beck AH, Terry K. TNF-α expression, risk factors, and inflammatory exposures in ovarian cancer: evidence for an inflammatory pathway of ovarian carcinogenesis? Human Pathology 2016. 54 82–91. ( 10.1016/j.humpath.2016.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, Pasparakis M, Offermanns S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 2016. 536 215–218. ( 10.1038/nature19076) [DOI] [PubMed] [Google Scholar]
- 43.Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death and Differentiation 2017. 24 1184–1195. ( 10.1038/cdd.2017.65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter ME, Hadji A, Murmann AE, Brockway S, Putzbach W, Pattanayak A, Ceppi P. The role of CD95 and CD95 ligand in cancer. Cell Death and Differentiation 2015. 22 549–559. ( 10.1038/cdd.2015.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacologica Sinica 2008. 29 1275–1288. ( 10.1111/j.1745-7254.2008.00889.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsoyi K, Jang HJ, Nizamutdinova IT, Park K, Kim YM, Kim HJ, Seo HG, Lee JH, Chang KC. PTEN differentially regulates expressions of ICAM-1 and VCAM-1 through PI3K/Akt/GSK-3β/GATA-6 signaling pathways in TNF-α-activated human endothelial cells. Atherosclerosis 2010. 213 115–121. ( 10.1016/j.atherosclerosis.2010.07.061) [DOI] [PubMed] [Google Scholar]
- 47.Mantovani A. Cancer: inflaming metastasis. Nature 2009. 457 36–37. ( 10.1038/457036b) [DOI] [PubMed] [Google Scholar]
- 48.Makrilia N, Kollias A, Manolopoulos L, Syrigos K. Cell adhesion molecules: role and clinical significance in cancer. Cancer Investigation 2009. 27 1023–1037. ( 10.3109/07357900902769749) [DOI] [PubMed] [Google Scholar]
- 49.Kobawala TP, Trivedi TI, Gajjar KK, Patel DH, Patel GH, Ghosh NR. Significance of TNF-α and the adhesion molecules: L-selectin and VCAM-1 in papillary thyroid carcinoma. Journal of Thyroid Research 2016. 2016 1–17. ( 10.1155/2016/8143695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Therapeutic Advances in Medical Oncology 2014. 6 229–239. ( 10.1177/1758834014544121) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a