Abstract
Purpose
Our aim was to improve access to genetic services in an underserved region by developing a collaborative telegenetic service delivery model with a pediatrician, medical geneticist, and genetics counselor (GC).
Methods
Protocols for the evaluation of common genetic indications were developed. Patients referred with indications suggestive of a syndromic etiology were scheduled to see the geneticist directly via telegenetics. Other patients were scheduled to see the pediatrician and GC in person before follow-up with the geneticist if indicated. Patients seen by the geneticist and/or pediatrician/GC were enumerated and the next available appointment was tracked. Patient satisfaction surveys were conducted.
Results
Of the 265 patients evaluated during the study period, 116 (44%) were evaluated by a pediatrician and GC in person first, after which 82 (71% of those evaluated) required further follow-up with the geneticist. The next available appointment with a pediatrician and GC never exceeded 6 weeks, while new appointments with a geneticist ranged from 3 to 9 months. All patients reported high satisfaction with this genetic service model.
Conclusion
The pediatrician/GC clinic provides a model of collaborative care that is a medical home neighbor and exemplifies the integration of genetics into primary care. The telegenetics clinic offers a viable solution to providing competent and convenient access to a geneticist for patients in chronically underserved regions.
Keywords: genetic counseling, genetic service model, medical home, pediatrics, telegenetics
Introduction
Pediatric genetic services in the United States are concentrated in metropolitan centers, leading to inequity of access to care.1 Using telemedicine technology to provide a clinical genetic service (telegenetics) offers a model to improve access for patients and families living in underserved regions.2 A systematic review of telegenetic services concluded that patient satisfaction with telegenetics is high and comparable to in-person visits. High satisfaction is influenced by the benefit of receiving a service that would otherwise require substantial cost, time and inconvenience of travel to a metropolitan center.2
Telegenetics has the potential for use in neonatal and pediatric patient care, but this modality is underutilized in pediatric genetic services.2, 3, 4 A 2010 survey by the National Society of Genetic Counselors determined that only 2.5% (2 of 80) of pediatric genetic counselors (GCs) used telegenetics.5 A 2013 survey of geneticists and GCs, conducted by the National Coordinating Center Telegenetics Work Group, reported that approximately one-third of their 233 respondents used telegenetics.6 The majority of the respondents used telemedicine for prenatal and cancer genetic counseling.6 In models of pediatric telegenetic clinics, the geneticist or GC conducts the evaluation using telemedicine technology with a facilitator at the patient site who could be a GC, nurse, pediatric specialist or telehealth presenter.6, 7, 8, 9
In April 2010, a GC employed by the Kansas University School of Medicine–Wichita (KUSM–W) established a telegenetics clinic at the Wesley Medical Center (Wichita, Kansas) in collaboration with a pediatric geneticist from the University of Arkansas for Medical Science (Little Rock, Arkansas). In this clinic, the GC is in the room with the patient, and the geneticist evaluates patients using telemedicine videoconferencing technology (televideo) on two half days per month. Within the first year of operation, the wait time for an appointment was over 6 months because of the limited availability of the geneticist.
This paper describes a strategy for optimizing the consultation time with the geneticist in a telegenetics clinic. In this model, a pediatrician and GC evaluate patients in person using protocols for genetic testing and evaluation. This promotes the efficient utilization of geneticists’ time, enabling them to focus on more complicated cases in the telegenetics clinic, and to provide supervision and support for the pediatrician/GC diagnostic team. The inclusion of a pediatrician in a telegenetics clinic is an example of collaborative primary-specialty care,which promotes the medical home neighbor concept.10 Here, we elaborate on the development, outcome and patient satisfaction of a telegenetics clinic and a pediatrician/GC clinic,which enhanced access to genetic services.
Materials and methods
Clinic development and implementation
The authors received a one-year grant in June 2011 from the Heartland Genetics and Newborn Screening Collaborative (supported in part by a grant from the Health Resources and Services Administration; U22MC03962) to develop a novel approach to the delivery of pediatric telegenetics services. A KUSM–W faculty general pediatrician was added to work with the GC in the genetics clinic in July 2011. In this clinic model, there are three providers and appointment types. The pediatrician and GC have a genetics clinic in which they evaluate patients in person together on two half days per month without the geneticist. The geneticist and GC have a telegenetics clinic on two alternate half days per month in which the geneticist evaluates patients by televideo. In this telegenetics clinic, the GC is in the room with the patient and facilitates the evaluation with the geneticist. The GC also makes independent genetic counseling appointments available on two half days per week. Each half-day clinic can accommodate three to five patients based on clinical indication.
The study period was from August 2011 to August 2013. The GC triaged pediatric cases based on the indication for referral and a review of records. Patients whose records suggested a nonsyndromic pattern of birth defects, developmental delay, autism, hearing loss, evaluation for Marfan syndrome, or neurofibromatosis were first scheduled to see the pediatrician and GC in person for primary genetic evaluation. The pediatrician and GC initiated primary genetic testing if indicated based on protocols developed in collaboration with the geneticist. Primary genetic testing in this service model is defined as genetic testing that is appropriate before further evaluation of syndromic etiology.
Based on the results of primary genetic testing, patients were further triaged to see the geneticist, GC, or another pediatric specialist. Patients with positive genetic test results requiring discussion of medical management were scheduled to see the geneticist within 3 months. Patients with negative genetic testing results who had features that warranted further evaluations were scheduled to see the geneticist by televideo within 3 months to a year based on clinical acuity. Patients with positive genetic test results that correlated with the clinical findings but did not require medical management by a geneticist were scheduled to see only the GC. If the results of primary evaluation by the pediatrician and GC determined that a different subspecialist was better suited to evaluate the patient, those patients were not scheduled to see the geneticist. Thus, in this workflow, the pediatrician’s primary role was to conduct primary genetic evaluation and not long-term follow-up.
Patients whose indications suggested a syndromic etiology or complex medical history were scheduled to see the geneticist by televideo first. These patients continued to follow-up only with the geneticist and GC if indicated. The pediatrician was not involved in the initial evaluation or follow-up for these patients.
The GC and geneticist scheduled biweekly meetings to discuss the cases seen by the pediatrician and GC, and to aid in reaching a consensus on diagnosis and management. Feedback from this meeting was relayed to the pediatrician. Feedback was also given to the pediatrician on cases who followed up with the geneticist after primary genetic testing. This consistent flow of information allowed for ongoing training of the pediatrician, and facilitated updates of the genetic testing protocols.
Genetics training and protocols
Genetics training for the pediatrician consisted of didactics, participating in genetic testing protocol development, observing patient evaluations by the geneticist, and the aforementioned case discussions. In July 2011, the pediatrician and GC went to theUniversity of Arkansas for Medical Scienceto train with the geneticist for four days. During this visit the team developed protocols for primary genetic testing. The pediatrician continued genetics training by observing patient evaluations conducted by the geneticist in the telegenetics clinic for two half days per month from August 2011 to July 2012. After July 2012, grant funding for the pediatrician’s time to observe the geneticist in the telegenetics clinic ended, but biweekly case discussion continued through August 2013. In this manner the pediatrician had ongoing exposure to genetics education for the duration of the study.
Clinic assessment
Patient satisfaction surveys were conducted for 12 months between August 2011 and July 2012. Separate surveys were used for patients attending an appointment with the geneticistor an appointment with the pediatrician and GC (see Supplementary File online). The surveys were adapted from validated patient satisfaction surveys from the Hawaii telegenetics program11 and were only available in English. They were approved for use by the institutional review board at the Wichita Medical Research and Education Foundation.
The surveys probed different aspects of the clinic visit. The survey about the appointment with the geneticist had questions about the telegenetics clinic equipment, the effect of technology on the evaluation, and barriers to care (Table 3). Parents were permitted multiple responses for questions about access to specialty services and what actions they would have taken to receive specialty care if the telegenetics clinic was not an option. The survey about the appointment with the pediatrician and GC asked if parents were notified during scheduling that they would be seeing a pediatrician and GC, and about the conduct of the providers during the appointment, confidence in the recommendations, overall satisfaction, barriers in access to care, and whether they would recommend this service to others (Table 2).
When parents checked in for the appointment with the pediatrician and GC or geneticist, the receptionist asked all English-speaking parents if they were interested in participating in a satisfaction survey. Parents who expressed interest in the survey were approached by the GC after the appointment to obtain informed consent. Parents were provided with the survey and requested to drop it into a sealed box in the clinic reception area.
All quantitative data were entered into a spreadsheet with no identifiers, before being tabulated and summarized. Using qualitative content analysis, two authors independently reviewed all the comments from the patient satisfaction surveys for themes. Authors coded comments as either positive (i.e., in favor of the clinic) or negative. Discrepancies between authors were resolved through consensus.
In addition to conducting patient satisfaction surveys for one year, the investigators enumerated the number of patients evaluated between August 2011 and August 2013 (25 months), and tracked the next available appointments for evaluation by a pediatrician and GC, geneticist, and GC. Patients were categorized into those who were evaluated only by the geneticist, and those who were evaluated by the pediatrician and GC first and then had follow-up with the geneticist, GC or another specialist provider.
Clinic funding and billing
The pediatrician and GC were full-time faculty employed by KUSM–W. The geneticist was under contract with KUSM–W for 0.1 full-time equivalent. The geneticist’s nonbillable time for supervision and education was provided as part of the existing contract for services with KUSM–W. The Heartland Genetics and Newborn Screening Collaborative grant funded the pediatrician’s nonbillable time and effort for training in genetics,as well as the GC’s effort for development and assessment of the program. The grant also permitted purchase of additional equipment (television and high-definition camera) for the telegenetics clinic.
The pediatrician billed for the visits conducted with the GC using typical evaluation and management codes. The geneticist also billed using evaluation and management codes, and added the telemedicine modifier code. The GC did not bill for patients seen by the pediatrician and GC. The GC billed insurance using current procedural terminology code 96040 only for patients seen independently for genetic counseling relating to test results. The investigators did not track insurance reimbursement or revenues separate from other clinic activities because the multiple factors that influence reimbursement and funding for genetics were beyond the scope of this project.
Results
Clinic service model
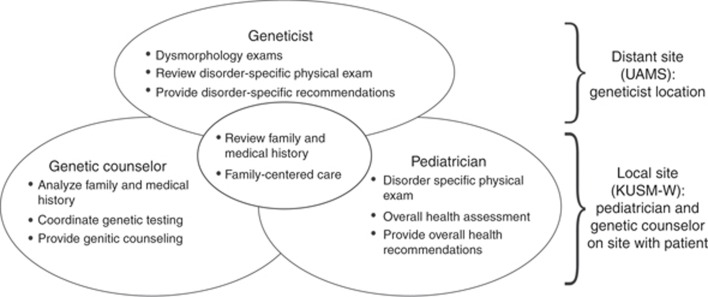
The pediatrician, GC and geneticist bring unique and overlapping skills to the telegenetics clinic model. The pediatrician focuses on clinical assessment and physical examination, including evaluation of neurological status, which is critical in making decisions about genetic testing strategy. The GC focuses on analyzing medical history, family history and discussion of the benefits and limitations of genetic testing with the family. The geneticist provides expert advice about genetic evaluation, management and supervision of the pediatrician and GC. This teamwork approach promotes the efficient utilization of each provider’s time and expertise, and facilitates comprehensive family-centered evaluation (Figure 1).
Figure 1.
Overlapping and complimentary roles of the pediatrician, genetic counselor and geneticist in the telegenetics clinic. Adapted with permission from Williamson L and LeBlanc DB, 2008.28
Primary genetic evaluation protocols
Primary genetic testing protocols were developed based on the established guidelines of professional organizations, such as the American Academy of Pediatrics and the American College of Medical Genetics and Genomics. For clinical conditions without existing guidelines, the literature was reviewed and the team developed its own algorithms that reflected the current standard of practice and the geneticist’s expertise. The algorithms were revised during the project based on updates in clinical practice and the availability of genetic testing (Table 1).
Table 1. Protocols for genetic testing.
| Indication | Primary genetic testing |
|---|---|
| Global developmental delay | Microarray and/or fragile X14, 17, 18 |
| Global developmental delay with hypotonia and features that could overlap with Prader–Willi syndrome | Microarray and methylation analysis14, 17, 18, 19, 20 |
| Global developmental delay with features that could overlap with Angelman syndrome | Microarray and methylation analysis,a14, 17, 18, 21, 22 |
| Multiple anomalies not specific to a well-delineated syndrome | Microarray14, 17 |
| Autism | Microarray and/or fragile X, PTEN23 |
| Nonsyndromic hearing loss | GJB2 sequencing and GJB6-D13S1830 deletion15 |
| Connective-tissue disorder with features that are suggestive of Marfan syndrome | FBN1 sequencingb16, 24, 25 |
| Neurofibromatosis type 1 | NF1 sequencingc26, 27 |
In select cases where features are characteristic of Angelman syndrome and if 15 methylation analysis is negative, use UBE3A sequencing or the Rett−Angelman syndrome panel.
If features are suggestive of overlapping connective-tissue disorders, use the Marfan−TAAD panel.
Use if patient is in early childhood and does not fulfill diagnostic criteria for neurofibromatosis.
Primary genetic evaluation and/or testing done by the pediatrician and GC on all cases was reviewed by the geneticist and determined to be the appropriate evaluation before any further evaluation with the geneticist.
Clinic data
Patients were categorized as those whose first visit was with the pediatrician and GC, and those whose first visit was with the geneticist/GC in the telegenetics clinic (Figure 2). From August 2011 to August 2012, the pediatrician observed 91 patients in the telegenetics clinic alongside the geneticist and GC as part of the genetics training. These patients have not been counted toward the total number of patients seen by the pediatrician, since the pediatrician was not the primary provider evaluating these patients.
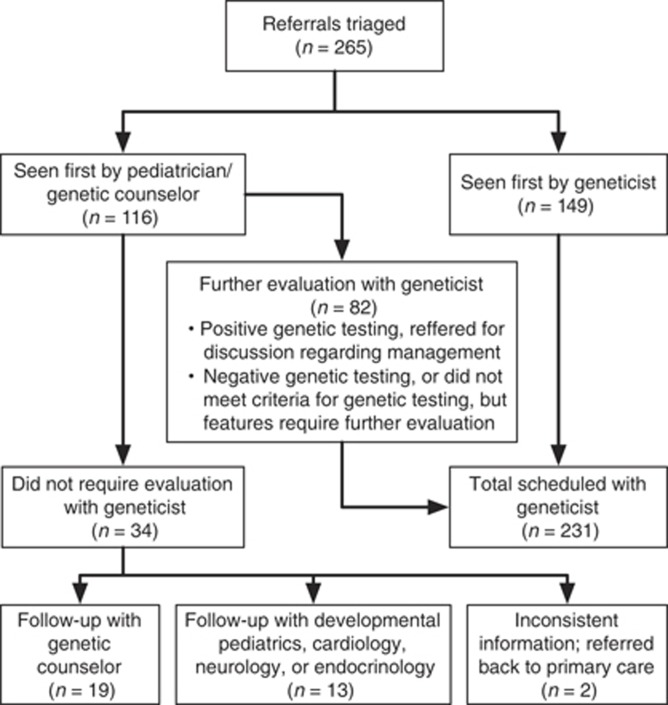
Figure 2.
Referral and follow up flow chart.
From August 2011 to August 2013, 265 patients were evaluated (Figure 2). One-hundred-and-forty-nine patients (56%) were scheduled to see the geneticist first. These patients continued to followup with the geneticist and GC as indicated. A total of 116 patients (44%) were scheduled to see the pediatrician and GC first. Of these, 82 patients (71%) were recommended to have a follow-up evaluation with the geneticist. For 19 of the 116 patients first seen by a pediatrician and GC (16%), the results of genetic testing correlated with clinical findings and these patients were scheduled to see the GC. The results of primary genetic evaluation in 15 patients (13%) concluded that there was either insufficient information to warrant a genetic evaluation at this time or that the patient was better evaluated by a different subspecialist. Overall, 34 (13%) of the 265 patients seen in the study period were determined not to require evaluation by the geneticist.
During the study period, the next available appointment with the pediatrician and GC was typically 6 weeks. The next available new appointment with the geneticist ranged from 3 to 9 months. The next available follow-up appointment with the geneticist ranged from 3 months to 1 year. Appointments with the geneticist were scheduled based on clinical acuity and/or the results of genetic testing. Assessing the statistical significance of wait times for appointments would not be informative given the variable nature of scheduling for the geneticist, and since this model was designed to accommodate patients for evaluation by the pediatrician and GC sooner than an appointment with a geneticist.
Patient satisfaction survey
From August 2011 to July 2012, 58 patients were seen by the pediatrician and GC. Fifty-two parents provided informed consent to participate in the survey about patient satisfaction and, of these, 30 (52%) responded. All parents reported agreeing or strongly agreeing with each of the different components of genetic evaluation provided by the pediatrician and GC (Table 2), with the exception of a question about having received adequate information about the nature of the visit before it took place. Five of the 30 parents who responded (17%) did not agree that they felt adequately informed before the visit. Despite this, all patients agreed or strongly agreed that they were confident in the quality of care, were satisfied with the visit and would recommend the genetics clinics to other families. In total, 20 parents (67%) answered open-ended questions about the visit. Two major themes emerged regarding the quality of the facilities and the genetic consultation itself. Of the 20 open-ended responses, 15 (75%) made positive comments about the evaluation and the clinic visits, such as: “I feel very confident in the pediatrician and counselor to handle my child’s case” and “Everybody I dealt with at the clinic was kind and professional.” Nine parents (45%) had negative comments, of which seven were about the facility, such as parking. The additional two comments pertained to not receiving adequate information before the appointment and concerns about the distance needed to travel to the appointment. There were no negative comments about the genetic evaluation.
Table 2. Results of the survey on appointments with the pediatrician and genetic counselor, n (%).
| Indicate how much you agree with each of the following statements | Strongly disagree | Disagree | Agree | Strongly agree |
|---|---|---|---|---|
| The information I received before the visit helped me understand what was going to happena | 2 (7) | 3 (11) | 10 (37) | 12 (44) |
| Before we started, each person introduced themselves and described their roleb | 0 (0) | 0 (0) | 4 (14) | 25 (86) |
| The pediatrician and genetic counselor listened and respected the information I provided | 0 (0) | 0 (0) | 3 (10) | 27 (90) |
| The pediatrician and genetic counselor responded to all of my questions | 0 (0) | 0 (0) | 6 (20) | 24 (80) |
| Privacy of information about my child and my family was protected | 0 (0) | 0 (0) | 6 (20) | 24 (80) |
| I feel confident in the quality of the care provided today | 0 (0) | 0 (0) | 5 (17) | 25 (83) |
| I feel confident in the recommendations from today’s visit | 0 (0) | 0 (0) | 7 (23) | 23 (77) |
| I would recommend the genetics clinic to other familiesb | 0 (0) | 0 (0) | 7 (24) | 22 (76) |
| Overall, I was satisfied with the visit todayb | 0 (0) | 0 (0) | 7 (24) | 22 (76) |
All data are number (%).
Three parents reported “Not applicable.”
One parent did not respond.
From August 2011 to July 2012, 91 patients were seen by the geneticist. A total of 86 parents consented to participate in the survey about telegenetics appointments with geneticist, and 71 (78%) responded. The cost and time associated with having to travel and the need to take additional time off from work were concerns for 41 parents (58%). In the absence of the telegenetics service, 43 parents (61%) would have had to travel a longer distance and, more worryingly, 15 parents (21%) would have opted not to seek the specialty service at all. All respondents agreed that telemedicine consultation had made it easier on the families, with 40 parents (56%) specifically stating that they saved time and money associated with travel. Forty-six parents (65%) preferred the telemedicine consultation to travelling to see a specialist. All but one respondent (99%) felt that the equipment worked well and was not a barrier to getting the most out of the visit. Qualitative analysis of the comments from 69 parents revealed three major themes: travel, experience with technology, and the genetic consultation. Forty-three parents (62%) stated that telegenetics made it easier to access care because they did not have to travel, and two parents (3%) stated that they would have liked to have had telegenetics closer to where they lived. The impact of travel on access to care is exemplified by the comment “If this would have not have been available in Wichita, we probably would not have pursued genetics if it involved travel.” Aside from two parents (3%) who commented on the technical delay with the voice from telemedicine equipment, and the small size of the room and screen, 12 parents (17%) had positive comments about the technology, such as: “This is the first telemedicine visit we have had. The experience was great.Everyone was caring and answered all our questions and concerns. I will not hesitate to do another telemedicine visit.” Comments about the genetic consultation and providers were expressed by 12 (17%) parents and were all positive. Negative comments when expressed by five parents (7%) were about the building facilities, such as parking and room temperature (Table 3).
Table 3. Results of the survey about telegenetics appointments with the geneticist, n (%).
| Indicate how much you agree with each of the following statements | Strongly disagree | Disagree | Agree | Strongly agree |
|---|---|---|---|---|
| The information I received before the visit helped me understand what was going to happena,b | 0 (0) | 0 (0) | 33 (51) | 32 (49) |
| The equipment worked well | 0 (0) | 1 (1) | 13 (18) | 57 (80) |
| Before we started, each person introduced themselves and described their role | 0 (0) | 0 (0) | 13 (18) | 58 (82) |
| The use of technology did not get in the way with being able to have a good conversation with the specialist | 0 (0) | 1 (1) | 14 (20) | 56 (79) |
| The specialist listened and respected the information I provided | 0 (0) | 0 (0) | 12 (17) | 59 (83) |
| The specialist responded to all of my questions | 0 (0) | 0 (0) | 12 (17) | 59 (83) |
| Privacy of information about my child and my family was protected | 0 (0) | 0 (0) | 15 (21) | 56 (79) |
| I feel confident in the quality of the care provided today | 0 (0) | 1 (1) | 14 (20) | 56 (79) |
| I feel confident in the recommendations from today’s telemedicine visit | 0 (0) | 0 (0) | 13 (18) | 58 (82) |
| I would recommend telemedicine to other families | 0 (0) | 0 (0) | 14 (20) | 57 (80) |
| Overall, I was satisfied with the visit today | 0 (0) | 0 (0) | 15 (21) | 56 (79) |
| Despite the obstacles to receiving care in person, I would still prefer to travel to see the specialistc | 17 (25) | 29 (43) | 12 (18) | 9 (13) |
One parent reported “Not applicable.”
Five parents did not respond.
Four parents did not respond.
Discussion
This paper describes the development of a telegenetics service model, whichwas successful in increasing access to genetic services and associated with a high degree of patient satisfaction. The primary purpose was to demonstrate a telegenetics service model that improved the timely access for primary genetic evaluation and/or testing of children referred for nonsyndromic developmental delay, autism, hearing loss and connective-tissue disorders. To the best of our knowledge, our service model differs from previously published telegenetics clinics in having a pediatrician and GC evaluate patients in person using consultation and genetic testing protocols before they attend a telegenetics appointment with the geneticist. The protocols, along with condition-specific training, enabled the pediatrician and GC to initiate appropriate primary genetic testing for patients who were referred for common pediatric genetic indications. Previously, these patients had to wait for up to 6 months to see the geneticist to initiate an evaluation. The revised system was designed such that the pediatrician and GC evaluated patients within 6 weeks of referral and prioritized syndromic cases for evaluation by the geneticist. This resulted in the geneticist appointments being more effectively utilized for patients with complex histories or for further management after appropriate primary genetic testing had been done by the pediatrician and GC. As of October 2016, over 800 evaluations have been conducted using this model.
This model identified that 6% of cases evaluated (15 of 265) were more appropriately treated by other subspecialties. It also determined follow-up intervals for patients. For an underserved area, this is valuable in more appropriately assigning patients to the geneticist’s schedule. Dysmorphic facial features are best assessed by a geneticist, but a pediatrician and GC with continued training and decision support from a geneticist can determine the appropriate interval for follow-up with the geneticist for a child with developmental delay and/or birth defects. In a region with decreased access to geneticists, appropriate triage of cases permits better utilization of geneticist expertise for cases with increased clinical acuity. Primary genetic testing done by a pediatrician and GC detected positive findings in 16% (19of 116) of patients who then had follow-up with the GC. The emergent need for a diagnosis and genetic counseling was addressed and all patients were referred to developmental pediatrics, neurology, endocrinology or other appropriate subspecialists for further evaluation and coordination of care.
The learning collaborative formed by this team has been suggested as a genetics education model and a method to increase primary care expertise in the screening and management of genetic conditions.12, 13 Pediatric residents on the genetics rotation learn about the application of telemedicine in a pediatric setting. They observe the pediatrician practicing guidelines and management recommended by the American Academy of Pediatrics, such as genetic evaluation of intellectual disability, neurofibromatosis or Marfan syndrome.14, 15, 16 They also rotate with the pediatrician in the general pediatric clinic and observe the implementation of genetics into a primary care practice. The pediatrician is a resource to colleagues about the appropriate genetic testing that can be initiated in primary care practices. This telegenetics service model is also an example of the medical home neighbor concept, in which the primary care practice is strengthened by including telemedicine and thus improving the primary care practice/specialist interaction.10 This, in turn, guides the efficient, appropriate and effective flow of necessary patient care information. It facilitates consultations by providing timely decision support, and guides determination of responsibility in comanagement situations.10 Thus, the educational and community benefits of this model of care have been substantial.
The satisfaction surveys regarding appointments with the pediatrician and GC and with the geneticist showed that almost all parents agreed or strongly agreed with almost all statements about the genetic evaluation, including overall satisfaction with the visit. A conservative interpretation is that patients were highly satisfied with access to a genetic service that did not necessitate extensive travel, and that they were confident in the recommendations for genetic testing and evaluation. Satisfaction with the pediatrician and GC clinic should be interpreted with the caveat that only 52% of parents who gave informed consent to participate responded to the survey. This study is limited by the inability to compare satisfaction with an in-person geneticist consultation or to compare the experience of parents who received initial evaluation with the pediatrician and GC and then followed up with the geneticist. This study was not designed to measure clinical efficacy or the diagnostic yield of the genetic testing protocols owing to the relatively small number of cases, the range of indications for referrals, and the complexity of insurance coverage. As this was a proof-of-concept study, the investigators did not collect longer-term variables, such as financial, behavioral and psychosocial factors that could affect both the access and the outcome of genetic services. Further studies are needed to better define the outcomes of pediatric genetic evaluations and to specify which outcomes are most appropriate in determining the satisfaction and efficacy of telegenetics evaluation compared with in-person genetic evaluations.
This genetics service model has been sustained past the period of grant funding and continues to this day because it demonstrated improved access and because both the KUSM–W and the Wesley Medical Center support the provision of genetics services in an underserved region. This model has the potential for replication in other communities based on provider availability and organizational support to develop telegenetics. In communities where there is a lack of access to both geneticists and GCs, it is conceivable to have both genetic providers connect to the pediatrician and patient in their community by televideo. Future studies would benefit from including tools to measure primary care provider competency during genetic evaluations and satisfaction with collaborative care. This would help to support the development of pediatric telegenetics service models. It would be informative to collect data about cost, reimbursement and health care savings, which could help to address the concern about financial viability, which is perceived to be a hindrance in the development of telegenetics.
Conclusions
The pediatrician and GC clinic provides a model of collaborative care that increases access to the geneticist, is a medical home neighbor, and exemplifies the integration of genetics into primary care. The telegenetics clinic offers a viable solution to providing competent, satisfactory and convenient access to a geneticist for patients living in regions that are chronically underserved. We believe our service model is unique because it incorporates a pediatrician/GC team to evaluate cases using standardized assessment protocols before the patient attends atelegenetics appointment. This enables the timely evaluation of common genetic conditions and allows more medically complex cases to be seen sooner by the geneticist. This telegenetics service delivery model achieves these aims with a high degree of patient satisfaction. We recommend that the establishment of a pediatrician and GC clinic be considered in other regions that utilize telegenetics for the evaluation of pediatric patients.
Acknowledgments
The authors gratefully acknowledge Matt Engel for his critical review, statistics and figures. This work was funded with a grant from the Heartland Genetics and Newborn Screening Collaborative (supported in part by a grant from the Health Resources and Services Administration; U22MC03962).
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/gim
The authors declare no conflict of interest.
Supplementary Material
References
- Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med 2005;7:439–443. [DOI] [PubMed] [Google Scholar]
- Hilgart JS, Hayward JA, Coles B, Iredale R. Telegenetics: a systematic review of telemedicine in genetics services. Genet Med 2012;14:765–776. [DOI] [PubMed] [Google Scholar]
- Burke BLJr, Hall RW, and the Section on Telehealth Care. Telemedicine: pediatric applications. Pediatrics 2015;136:e293–e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger TL, Gerdes J, Taub K, Swarr DT, Deardorff MA, Abend NS. Telemedicine for genetic and neurologic evaluation in the neonatal intensive care unit. J Perinatol 2014;34:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SA, Marvin ML, Riley BD, Vig HS, Rousseau JA, Gustafson SL. Identification of genetic counseling service delivery models in practice: a report from the NSGC Service Delivery Model Task Force. J.GenetCouns 2013;22:411–421. [DOI] [PubMed] [Google Scholar]
- Mann S, Keehn A, Andersson H. Who is using telegenetics in the United States: a national survey. 142nd APHA Annual Meeting and Exposition, New Orleans, LA, 17 November 2014.
- Flannery D. The Telemedicine Experience,2015. http://southeastgenetics.org/presentation.php/66/The_Telemedicine_Experience. Accessed 1 November 2016.
- Lea DH, Johnson JL, Ellingwood S, Allan W, Patel A, Smith R. Telegenetics in Maine: successful clinical and educational service delivery model developed from a 3-year pilot project. Genet Med 2005;7:21–27. [DOI] [PubMed] [Google Scholar]
- Stalker HJ, Wilson R, McCune H, Gonzalez J, Moffett M, Zori RT. Telegenetic medicine: improved access to services in an underserved area. J Telemed Telecare 2006;12:182–185. [DOI] [PubMed] [Google Scholar]
- Schaefer GB, Larson IA, Bolick J, Williamson-Dean L. What is the role of clinical genetics in the patient-centered medical home?: a commentary from the Medical Home Workgroup of the Heartland Regional Genetics and Newborn Screening Collaborative. Genet Med 2016;18:440–442. [DOI] [PubMed] [Google Scholar]
- Mann S, Hasegawa L, Spencer A. Maximizing access to genetic services using state and federal resources. AMCHP 2008 Annual Conference: Washington, DC, March 1st to 5th 2008. [Google Scholar]
- Haga SB, Burke W, Agans R. Primary-care physicians’access to genetic specialists: an impediment to the routine use of genomic medicine? Genet Med 2013;15:513–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper AR, Trotter TL, Lloyd-Puryear MA, Kyler P, Feero WG, Howell RR. A blueprint for maternal and child health primary care physician education in medical genetics and genomic medicine: recommendations of the United States secretary for health and human services advisory committee on heritable disorders in newborns and children. Genet Med 2010;12:77–80. [DOI] [PubMed] [Google Scholar]
- Manning M, Hudgins L, and the Professional Practice and Guidelines Committee. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med 2010;12:742–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford RL, Arnos KS, Fox M et al, American College of Medical Genetics and Genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genet Med 2014;16:347–355. [DOI] [PubMed] [Google Scholar]
- Pyeritz RE, and the American College of Medical Geteics and Genomics, Evaluation of the adolescent or adult with some features of Marfan syndrome. Genet Med 2012;14:171–177. [DOI] [PubMed] [Google Scholar]
- Miller DT, Adam MP, Aradhya S et al, Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet 2010;86:749–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeschler JB, Shevell M, and the Committee on Genetics. Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics 2014;134:e903–e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DJ, Miller JL, Schwartz S, Cassidy SB. Prader-Willi syndrome. GeneReviews. University of Washington: Seattle, WA, 1997–2013. [Google Scholar]
- McCandless SE, and the Committee on Genetics. Clinical report-health supervision for children with Prader-Willi syndrome. Pediatrics 2011;127:195–204. [DOI] [PubMed] [Google Scholar]
- Dagli A, Mueller J, Williams C. Angelman syndrome. GeneReviews. University of Washington: Seattle, WA, 1997–2013. [Google Scholar]
- Williams CA, Beaudet AL, Clayton-Smith J et al, Angelman syndrome 2005: updated consensus for diagnostic criteria. Am J Med Genet A 2006;140:413–418. [DOI] [PubMed] [Google Scholar]
- Schaefer GB, Mendelsohn NJ, and the Professional Practice and Guidelines Committee. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med 2013;15:399–407. [DOI] [PubMed] [Google Scholar]
- Dietz H. Marfan syndrome. GeneReviews. University of Washington: Seattle, WA, 1997–2013. [Google Scholar]
- Tinkle BT, Saal HM, and the Committee on Genetics. Health supervision for children with Marfan syndrome. Pediatrics 2013;132:e1059–e1072. [DOI] [PubMed] [Google Scholar]
- Friedman J. Neurofibromatosis 1. GeneReviews. University of Washington: Seattle, WA, 1997–2013. [Google Scholar]
- Hersh JH, and the American Academy of Pediatrics Committee on Genetics. Health supervision for children with neurofibromatosis. Pediatrics 2008;121:633–642. [DOI] [PubMed] [Google Scholar]
- Williamson L, LeBlanc DB. A genetic services practice model: advanced practice nurse and genetic counselor team. Newborn Infant Nurs Rev 2008;8:30–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.