Abstract
Objective To assess whether weight loss interventions for adults with obesity affect all cause, cardiovascular, and cancer mortality, cardiovascular disease, cancer, and body weight.
Design Systematic review and meta-analysis of randomised controlled trials (RCTs) using random effects, estimating risk ratios, and mean differences. Heterogeneity investigated using Cochran’s Q and I2 statistics. Quality of evidence assessed by GRADE criteria.
Data sources Medline, Embase, the Cochrane Central Register of Controlled Trials, and full texts in our trials’ registry for data not evident in databases. Authors were contacted for unpublished data.
Eligibility criteria for selecting studies RCTs of dietary interventions targeting weight loss, with or without exercise advice or programmes, for adults with obesity and follow-up ≥1 year.
Results 54 RCTs with 30 206 participants were identified. All but one trial evaluated low fat, weight reducing diets. For the primary outcome, high quality evidence showed that weight loss interventions decrease all cause mortality (34 trials, 685 events; risk ratio 0.82, 95% confidence interval 0.71 to 0.95), with six fewer deaths per 1000 participants (95% confidence interval two to 10). For other primary outcomes moderate quality evidence showed an effect on cardiovascular mortality (eight trials, 134 events; risk ratio 0.93, 95% confidence interval 0.67 to 1.31), and very low quality evidence showed an effect on cancer mortality (eight trials, 34 events; risk ratio 0.58, 95% confidence interval 0.30 to 1.11). Twenty four trials (15 176 participants) reported high quality evidence on participants developing new cardiovascular events (1043 events; risk ratio 0.93, 95% confidence interval 0.83 to 1.04). Nineteen trials (6330 participants) provided very low quality evidence on participants developing new cancers (103 events; risk ratio 0.92, 95% confidence interval 0.63 to 1.36).
Conclusions Weight reducing diets, usually low in fat and saturated fat, with or without exercise advice or programmes, may reduce premature all cause mortality in adults with obesity.
Systematic review registration PROSPERO CRD42016033217.
Introduction
Adults with obesity have an increased risk of premature mortality, cardiovascular disease, some cancers, type 2 diabetes, and many other diseases.1 2 These associations inform the need for programmes to prevent obesity, but, apart from prevention of type 2 diabetes,3 4 limited evidence from randomised controlled trials (RCTs) shows that weight loss interventions can prevent serious harm for people with obesity. Evidence from cohort studies has led to debate that deliberate weight loss for people who are overweight or obese, with body mass index (BMI) ≤35 kg/m2, might actually be harmful.5 Studies show that older people,6 and those with cardiovascular disease7 who are less markedly obese, might experience adverse consequences from deliberate weight loss. Recent analyses by the Global BMI Mortality Collaboration, however, tried to limit confounding and corrected for reverse causality, finding that the risk of premature mortality was lowest at BMIs of 20-25.8
Association studies cannot tell us if deliberate weight loss in adults with obesity can reduce their risk of premature mortality, cardiovascular disease, or cancer. Only one systematic review and meta-analysis of RCTs of intentional weight loss in adults with obesity has examined this question.9 That review included 15 trials, reporting a 15% relative reduction in premature mortality (risk ratio 0.85, 95% confidence interval 0.73 to 1.00), but did not evaluate causes of death or cardiovascular and cancer outcomes.9 We knew of many other weight loss RCTs with mortality data, as well as cancer and cardiovascular outcomes, from our database of long term RCTs of weight loss interventions for adult obesity, which was developed for health technology assessments10 11 and is continually updated. We systematically reviewed long term (≥1 year) RCTs of weight loss interventions for adults with obesity to examine the effects of any type of weight loss diet on all cause, cardiovascular, and cancer mortality, cardiovascular disease, cancer, and body weight.
Methods
We adhered to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines for systematic reviews of interventions.12 We used a prespecified protocol, registered with PROSPERO (CRD42016033217).13
Search strategy and selection criteria
We included RCTs with adults (mean or median age ≥18 years) and a minimum follow-up of one year. Participants had a mean BMI ≥30 at baseline. Included trials had to be focused clearly on weight loss with a weight reducing diet, with or without advice for increasing physical activity and/or provision of a physical activity programme to attend, compared with a control intervention. We didn’t include trials in pregnant or postpartum women.
We sought summary data for three primary outcomes: all cause mortality, cardiovascular mortality, and cancer mortality. Secondary outcomes were participants with a new cardiovascular event, participants with a new cancer, and weight change. In our main analysis we used cardiovascular mortality and events as defined by the investigators but did not include the development of hypertension. We undertook post hoc analyses of cardiovascular mortality and cardiovascular events as defined in the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.14
We identified RCTs by searching the full texts of trial reports in our database of all long term (≥1 year) RCTs of weight loss interventions for adults with obesity used in our previous systematic reviews and health technology assessments. Our database is derived from previous search strategies compiled from Medline, Embase, and the Cochrane Central Register of Controlled Trials, from 1966 to December 2015.10 11 We performed an updated search from August 2015 to December 2016. We didn’t apply any language exclusions. In 2016-17 we contacted the authors of 48 RCTs to clarify data or request unpublished outcome data, where trial reports implied that relevant data might be available; for example, when the trial reported hospital admissions or adverse events without giving further details.
Data analysis
AA and CM independently confirmed study eligibility. CM, FS, CR, and PS extracted data, which were then checked by a second author (AA, CM). Cancer outcome and cardiovascular outcome data (including coding outcomes defined by the ACC/AHA guideline14) were further adjudicated by MB, with differences resolved by Andrew Grey (associate professor in the Department of Medicine, University of Auckland). Two authors (AA, CM, FS, CR, PS) independently assessed quality using the Cochrane risk of bias tool.15 All differences were resolved by discussion.
We used random effects meta-analysis to analyse pooled outcome data. For binary outcomes, we estimated risk ratios and 95% confidence intervals, using all participants randomised for the denominators. We estimated weighted mean differences and 95% confidence intervals for continuous outcomes, giving preference to intention to treat data and data taking account of dropouts (preferentially baseline observation carried forward) if these were provided. We included outcome data from two cluster RCTs16 17 using the correction method described in the Cochrane Handbook18 and the intraclass correlation coefficients reported in the original trial publications. We assessed heterogeneity between studies using Cochran’s Q statistic and the I2 test. We originally planned meta-regression to investigate heterogeneity in disease outcomes, but I2 tests for disease outcomes were 0%, so it was not appropriate. We carried out a sensitivity analysis with a random effects bayesian logistic regression model (with non-informative priors) using WinBUGS 1.4.319 because some trials reported few events, which may cause sparse data bias. We performed all other analyses using Stata Release 1420 and used funnel plots to examine small study bias.
For all outcomes we performed prespecified subgroup analyses for sex, age (<60 v ≥60), BMI (<40 v ≥40, later changed to <35 v ≥35 as we found no trial with BMI ≥40), glycaemic control (normal v impaired glucose tolerance or impaired fasting glucose v type 2 diabetes), ethnicity (defined if ≥80% of participants belonged to an ethnic group, otherwise defined as mixed), physical activity interventions (none v advice only v exercise programme provided).
In post hoc additional analyses we added trials in any Asian population group if the mean BMI was ≥25, as diseases associated with obesity are known to occur at lower BMI in Asian populations than other ethnic groups.21 No single BMI cut-off has been agreed to define obesity in Asian populations. Although the World Health Organization recommends 27.5 as a BMI threshold for a high risk of comorbidities,21 it also suggests that Asian countries develop their own specific BMI cut-offs for obesity. India and Japan have set ≥25 as the threshold for obesity,22 23 and in China the risk of comorbidities has been found to increase for BMI over 28.24
For all outcomes we performed two prespecified sensitivity analyses for allocation concealment (low risk of bias vs other risk of bias) and follow-up (<80% vs ≥80%).
We used GRADE (grading of recommendations, assessment, development, and evaluations) to judge the quality of the evidence for mortality, cardiovascular, and cancer outcomes.25
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing this report. CM and AA had full access to all study data and had final responsibility for the decision to submit for publication.
Patient involvement
No patients or members of the public were involved in the development of research questions, the design of the study, or the development of outcome measures. No patients were asked to advise on interpretation or writing up of results. There are plans to disseminate the results of the research to the relevant patient community.
Results
Trial characteristics
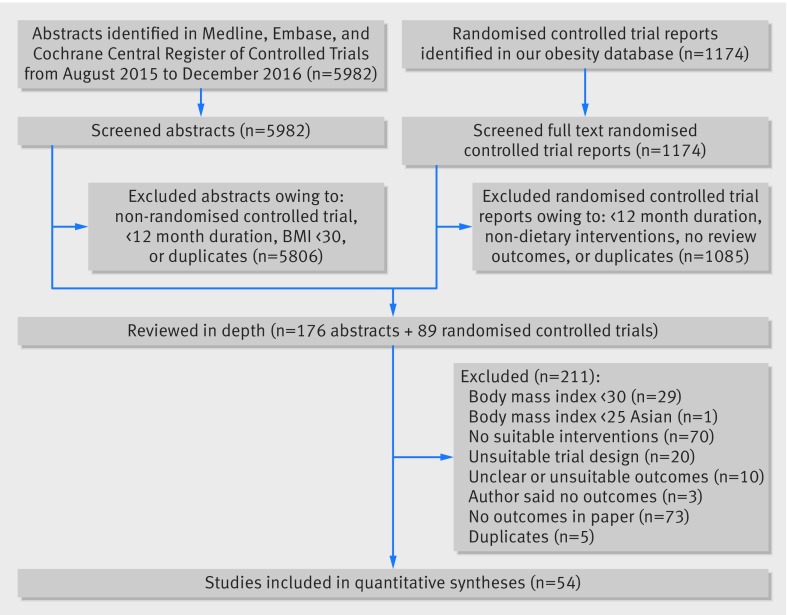
We screened 1174 full text trial reports and 5982 titles and abstracts (fig 1) and identified 54 RCTs for inclusion3 4 16 17 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 in the final review.
Fig 1 Study selection
Table 1 provides details of the included studies, involving 30 206 adults with obesity. Nine trials (16.7%) included women only,26 44 45 50 51 52 77 88 94 and two (3.7%) men only.58 72 Twelve trials (22.2%) recruited participants with no reported existing medical conditions or no reported increased risk of developing comorbidities related to obesity. Other trials recruited participants with increased risk of type 2 diabetes or hypertension or included participants that already had at least one of the following conditions: hypertension, type 2 diabetes, hyperlipidaemia, breast cancer, colorectal adenoma, psychiatric illnesses, cognitive impairment, osteoarthritis of the knee, coronary heart disease, or urinary incontinence.
Table 1.
Characteristics of randomised controlled trials
| Trial | n | Dropout (%) | Follow-up (months) | Comorbidities and drugs | Mean age (SD) | Female (%) | Smoke (%) | Ethnicity | Mean BMI, kg/m2 (SD) | Main weight loss interventions | Comparisons |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbenhardt, 2013, USA26 27 | 439 | 9.1 | 12 | NR | 58.0 (5.0) | 100 | 0 | White | 30.9 (4.0) | Group 1—Diet based on DPP and Look AHEAD (1200-2000 kcal/d, <30% calories from fat, <10% from saturated fat, for 10% weight loss) Group 2—Diet as above plus exercise programme |
Group 3—Control Group 4—Exercise programme |
| Abed, 2013, Australia28 | 150 | 46.0 | 15 | Atrial fibrillation | 60.1 (9.9) | 33 | 5 | White | 33.3 (3.8) | Group 1—Diet (800-1200 kcal/d for 8 weeks as 2 VLCD sachets and 1 low GI meal; weight maintenance low GI and low fat diet) and exercise advice |
Group 2—Control |
| Ackerman, 2015, USA29 | 509 | 15.5 | 12 | Impaired glucose tolerance or impaired fasting glucose | 51.0 (12.1) | 70.7 | NR | Mixed | 36.8 (8.5) | Group 1—Diet based on DPP (low calorie, <25% calories from fat, low saturated fat, for 5-7% weight loss) and exercise advice | Group 2—Control |
| Andrews, 2011, UK30 | 593 | 2.4 | 12 | Type 2 diabetes | 60.0 (10.1) | 35 | 8 | White | 31.7 (5.7) | Group 1—Diet based on Diabetes UK and Food Standards Agency (<35% calories from fat, <10% from saturated fat, low GI, for 5-10% weight loss) Group 2—Diet as above and exercise advice |
Group 3—Control |
| Appel, 2003, USA (PREMIER)31-33 | 810 | 11.4 | 18 | Above normal blood pressure | 50.3 (8.9) | 62 | 5 | Mixed | 33.1 (5.8) | Group 1—Diet (≤30% calories from fat, ≤10% from saturated fat, ≥6.8 kg weight loss) and exercise programme Group 2—Diet based on DASH (≤25% calories from fat, ≤7% from saturated fat, ≥6.8 kg weight loss) and exercise programme |
Group 3—Control |
| Ard, 2016, USA34 | 167 | 11.4 | 12 | Drugs for diabetes, lipids, or blood pressure | 70.3 (4.7) | 62.2 | NR | Mixed | 33.7 (2.95) | Group 1—Diet (28% calories from fat and 500 kcal/d deficit) and exercise programme Group 2—Diet (28% fat for weight maintenance) and exercise programme |
Group 3—Exercise programme |
| Aveyard, 2016, UK35 | 1882 | 24.6 | 12 | NR | 56.0 (16.1) | 57.2 | NR | White | 34.9 (4.8) | Group 1—Diet mainly Slimming World (low fat and low saturated fat) and exercise advice | Group 2—Control |
| Bennett, 2012, USA36 | 365 | 14.0 | 24 | Drugs for blood pressure | 54.6 (10.9) | 69 | 18 | Mixed | 37.0 (5.1) | Group 1—Diet (low fat and low saturated fat, weight reducing diet) and exercise advice | Group 2—Control |
| Anderson, 2014, UK (BeWEL)37 | 329 | 7.3 | 12 | Colorectal adenoma removal | 63.6 (6.8) | 26 | NR | White | 30.7 (4.2) | Group 1—Diet based on British Heart Foundation (600 kcal/d deficit, low fat, low saturated fat, for 7% weight loss) and exercise advice | Group 2—Control |
| Bhopal, 2014, UK16 | 171 | 2.3 | 36 | Impaired glucose tolerance or impaired fasting glucose | 52.5 (10.3) | 54 | 6 | Asian | 30.6 (4.8) | Group 1—Diet based on the Counterweight programme (500-600 kcal/d deficit, 30% calories from fat, for 5-10% weight loss) and exercise advice | Group 2—Control |
| Blonk, 1994, Netherlands38 | 60 | 11.7 | 24 | Type 2 diabetes | 58.8* | 57 | NR | White | 32.0* | Group 1—Diet (500 kcal/d deficit, 30% calories from fat, low saturated fat) and exercise programme | Group 2—Control |
| Burke, 2008, Australia39 | 241 | 41.9 | 40 | Drugs for blood pressure | 56.2 (7.4) | 56 | 3 | White | 30.1 (2.7) | Group 1—Diet based on DASH (<30% calories from fat, low sat fat, for 5-10% weight loss) and exercise advice | Group 2—Control |
| Daumit, 2013, USA40 | 291 | 4.1 | 18 | Psychiatric illness rehabilitation | 45.3 (11.3) | 50 | NR | Mixed | 36.3 (7.3) | Group 1—Diet based on DASH (low fat, low saturated fat, for 4.5 kg weight loss) and exercise programme | Group 2—Control |
| Davis, 1993, USA (TAIM)41 | 587 | 31.0 | 30 | Mild hypertension | 47.9 (NR) | 46 | 16 | Mixed | 30.2 (NR) | Group 1—Diet (low fat, for 4.5kg or 10% weight loss) and exercise advice | Group 2—Control |
| de Vos, 2014, Netherlands42-44 | 407 | 39.3 | 80 | Free of knee osteoarthritis or rheumatic diseases | 55.7 (3.2) | 100 | 17.9 | White | 32.4 (4.3) | Group 1—Diet (tailored low fat or low calorie reducing diet) and exercise programme | Group 2—Control |
| de Waard, 1993, Netherlands, Poland45 | 107 | 28.6 | 36 Netherlands, 12 Poland | Breast cancer | 50-69† | 100 | NR | White | 30.2 (NR) | Group 1—Diet (1000-1500 kcal/d balanced diet) | Group 2—Control |
| DPP, 2002, USA4 46 47 | 3234 | 9.9 | 34 | Impaired glucose tolerance | 50.6 (10.7) | 67 | 7 | Mixed | 34.0 (6.7) | Group 1—Diet (low calorie, <25% calories from fat, low saturated fat, for 7% weight loss) and exercise programme | Group 2—Control and metformin placebo |
| Finnish DPS, 2009, Finland3 48 49 | 523 | 16.4 | 138 | Impaired glucose tolerance | 55.2 (7.2) | 67 | 7 | White | 31.3 (4.5) | Group 1—Diet (<30% calories from fat, <10% from saturated fat, for ≥5% weight loss) and exercise programme | Group 2—Control |
| Fitzgibbon, 2010, USA50 | 213 | 10.8 | 18 | NR | 46.0 (8.4) | 100 | NR | Black | 39.2 (5.7) | Group 1—Diet (low calorie, low fat, for 7% weight loss) and exercise programme | Group 2—Control |
| Gabriel, 2011, USA51 | 508 | 10.2 | 48 | Postmenopausal, some on hormone replacement therapy | 57.0 (2.9) | 100 | 6 | White | 30.8 (3.8) | Group 1—Diet (<17% calories from fat, <4% from saturated fat, 1300-1500 kcal/d) and exercise advice | Group 2—Control |
| Goodwin, 2014, Canada, USA52 | 338 | 12.7 | 24 | Breast cancer, letrozole therapy | 61.0 (7.2) | 100 | 7 | White | 31.3 (5.2) | Group 1—Diet based on DPP (20% calories from fat, 500-1000 kcal/d deficit) and exercise advice | Group 2—Control |
| Greaves, 2015, UK53 | 108 | 11.1 | 12 | High cardiovascular risk | 65.1 (7.0) | 30.6 | NR | White | 32.7 (3.1) | Group 1—Diet (low fat, low sat fat diet, for ≥5% weight loss) and exercise advice | Group 2—Control |
| Green, 2015, USA54 | 200 | 18.0 | 24 | Antipsychotic drugs | 47.2 (10.6) | 72 | 33 | White | 38.3 (8.3) | Group 1—Diet based on DASH (≤30% calories from fat, ≤10% from saturated fat, for 4.5-6.8 kg weight loss) and exercise programme | Group 2—Control |
| Heller, 1988, UK55 | 87 | 13.8 | 12 | Diabetes | 56.5 (7.7) | 52 | NR | White | 31.6 (3.3) | Group 1—Diet (low sugar, high fibre for weight loss) | Group 2—Control |
| Heshka, 2003, USA56 | 423 | 27.0 | 24 | NR | 44.5 (10.0) | 85 | 9 | White | 33.7 (3.6) | Group 1—Diet (Weight Watchers for weight loss of 0.9 kg/w) and exercise advice | Group 2—Control |
| Horie, 2016, Brazil57 | 80 | 6.3 | 12 | Mild cognitive impairment | 68.1 (4.9) | 83.8 | 1.3 | Mixed | 35.5 (4.4) | Group 1—Diet (<30% calories from fat, 500 kcal/d deficit) and exercise advice | Group 2—Control |
| Hunt, 2014, UK (FFIT)58 | 748 | 17.7 | 12 | NR | 47.1 (8.0) | 0 | NR | White | 35.3 (4.9) | Group 1—Diet (low fat, 600 kcal/d deficit, healthy eating) and exercise programme | Group 2—Control |
| Hydrie, 2012, Pakistan59 | 317 | 13.6 | 18 | Impaired glucose tolerance | 43.6 (9.9) | 25 | NR | Asian | 27.0 (5.0) | Group 1—Diet (<30% calories from fat, for ≥5% weight loss) and exercise programme | Group 2—Control |
| Katula, 2013, USA60 | 301 | 13.3 | 24 | Impaired fasting glucose | 57.9 (9.5) | 58 | 5 | Mixed | 32.7 (4.0) | Group 1—Diet based on DPP (low calorie, 25-30% fat, 7% saturated fat, 1200-1800 kcal/d, for 5-7% weight loss) and exercise advice | Group 2—Control |
| Li Da Qing, 2014, China17 61-63 | 577 (33 clinics) | 8.1 | 72 | Impaired glucose tolerance | 45.0 (9.1) | 47 | 41 | Asian | 25.8 (3.8) | Group 1—Diet (BMI <25, 25-30 kcal/kg, 25-30% calories from fat; BMI ≥25 reduce calories to lose 0.5-1 kg/month, until BMI=23). Group 2—Diet as above and exercise advice |
Group 3—Control Group 4—Exercise advice |
| Logue, 2005, USA64 | 665 | 34.6 | 24 | NR | 52.3 (NR) | 69 | NR | Mixed | 34.2 (NR) | Group 1—Diet based on DGA (low calorie, low fat, low saturated fat, portion control) and exercise advice | Group 2—Control |
| Look AHEAD, 2013, USA65 66 | 5145 | 3.7 | 115 | Type 2 diabetes | 58.8 (6.9) | 60 | 4 | Mixed | 36.0 (5.9) | Group 1—Diet (1200-1800 kcal/d, <30% calories from fat, <10% from saturated fat, for 7% weight loss, meal replacements) and exercise programme | Group 2—Control |
| Ma, 2013, USA67 | 241 | 34.4 | 24 | Impaired fasting glucose or metabolic syndrome | 52.9 (10.6) | 47 | NR | White | 32.0 (5.4) | Group 1—Diet based on DPP (500-1000 kcal/d deficit, 25% calories from fat, for 7% weight loss) and exercise programme Group 2—Self directed diet and exercise as above |
Group 3—Control |
| Mengham, 1999, UK68 | 75 | 1.3 | 12 | Diabetes | 60.6 (12.5) | 45 | NR | White | 31.6 (4.6) | Group 1—Diet based on British Dietetic Association recommendations (low fat) | Group 2—Control |
| Messier, 2013, USA69 | 454 | 12.1 | 18 | Knee osteoarthritis | 66 (NR)6 | 72 | NR | White | 33.6 (3.7) | Group 1—Diet based on DGA (800-1000 kcal/d deficit, meal replacements ≤2/day, <30% calories from fat) Group 2—Diet as above and exercise programme |
Group 3—Exercise programme |
| Oldroyd, 2006, UK70 | 78 | 30.8 | 24 | Impaired glucose tolerance | 57.9 (NR) | 44 | NR | White | 30.2 (5.3) | Group 1—Diet (aim for BMI <25, ≤30% calories from fat, PUFA/SFA ratio ≥1.0) and exercise programme | Group 2—Control |
| O’Neil, 2016, USA71 | 563 | 14.0 | 12 | Type 2 diabetes | 55.1 (19.1) | 71.0 | NR | Mixed | 37.1 (5.7) | Group 1—Diet (Weight Watchers, hypoenergetic healthy eating) and exercise advice | Group 2—Control |
| Patrick, 2011, USA72 | 441 | 29.9 | 12 | NR | 43.9 (8.0) | 0 | NR | White | 34.3 (4.1) | Group 1—Diet based on DGA (saturated fat ≤20 g/day) and exercise advice | Group 2—Control |
| Penn, 2009, UK73 | 102 | 48.0 | 37 | Impaired glucose tolerance | 57.1 (NR) | 60 | NR | White | 33.8 (5.1) | Group 1—Diet (reduced saturated fat and <30% calories from fat until BMI <25) and exercise advice | Group 2—Control |
| Perri, 2014, USA74 | 612 | 19.6 | 24 | NR | 52.3 (11.5) | 78 | NR | White | 36.3 (4.0) | Groups 1-3—Diet based on DPP (1200-1800 kcal/d, low fat) and exercise advice. Three groups received different intensities of both diet and exercise interventions | Group 4—Control |
| Ramachandran, 2006, India75 | 531 | 5.5 | 30 | Impaired glucose tolerance | 45.9 (5.7) | 21 | 22 | Asian | 25.8 (3.5) | Group 1—Diet (low calorie, low fat) and exercise advice Group 2—Diet as above and exercise advice and metformin |
Group 3—Control Group 4—Metformin |
| Rejeski, 2011, USA (CLIP)76 | 288 | 13.5 | 18 | Recent cardiovascular disease, metabolic syndrome, or mobility limitation | 67.1 (4.8) | 67 | NR | White | 32.8 (3.8) | Group 1—Diet based on DGA (1200-1800 kcal/d, low fat, for 7-10% weight loss) and exercise programme | Group 2—Control Group 3—Exercise programme |
| Rock, 2015, USA77 78 | 698 | 15.9 | 24 | Breast cancer | 56 (NR)9 | 100 | 3.45 | White | 31.5 (4.7) | Group 1—Diet (500-1000 kcal/d deficit, low energy density, for 7% weight loss) and exercise advice | Group 2—Control |
| Ross, 2012, Canada (PROACTIVE)79 | 490 | 19.2 | 24 | NR | 51.8 (11.4) | 70 | NR | White | 32.3 (4.2) | Group 1—Diet (balanced Mediterranean type diet for weight loss, 5-10% reduction in waist circumference) and exercise advice | Group 2—Control |
| Saito, 2011, Japan80 | 641 | 9.2 | 36 | Impaired fasting glucose | 49.0* | 29 | 27 | Asian | 27.0 (2.6) | Group 1—Diet (low calorie, 20-25% calories from fat, for 5% weight loss) and exercise advice | Group 2—Control |
| Shea, 2010, USA (ADAPT)81 82 | 318 | 20.8 | 96 | Knee osteoarthritis | 68.7 (5.4) | 72 | NR | Mixed | 34.2 (5.5) | Group 1—Diet based on TONE based (500 kcal/d deficit, fat and portion control, for 5% weight loss) Group 2—Diet as above and exercise programme |
Group 3—Control Group 4—Exercise programme |
| Shea, 2011, USA (TONE) 83-85 | 585 | <20 | 152 | Drugs for blood pressure | 66.0 (4.3) | 53 | 5 | Mixed | 31.2 (2.3) | Group 1—Diet (low calorie, low fat, for ≥4.5 kg weight loss) and exercise programme Group 2—Diet (low calorie, low fat, for ≥4.5 kg weight loss), exercise programme, and sodium restriction |
Group 3—Control Group 4—Sodium restriction |
| TOHP II, 2007, USA86 87 | 2382 | 23.0 | 144 | High normal diastolic blood pressure | 43.6 (6.1) | 34 | 9 | Mixed | 30.9 (3.1) | Group 1—Diet (low calorie, low fat, for ≥4.5 kg weight loss) and exercise programme Group 2—Diet (low calorie, low fat, for ≥4.5 kg weight loss), exercise programme, for sodium restriction |
Group 3—Control Group 4—Sodium restriction |
| Toobert, 2000, USA88 | 28 | 10.7 | 24 | Coronary heart disease | 63.6 (10.4) | 100 | 7 | White | 32.0 (4.8) | Group 1—Diet based on Ornish (vegetarian diet, <10% calories from fat) and exercise programme | Group 2—Control |
| Uusitupa, 1993, Finland89-91 | 90 | 8.9 | 24 | Type 2 diabetes | 53.2 (6.6) | 43 | 17 | White | 33.5 (5.1) | Group 1—Diet (≤30% calories from fat, ≤10% from saturated fat) and exercise advice | Group 2—Control |
| Villareal, 2011, USA92 | 107 | 13.1 | 12 | Frailty | 69.7 (4.0) | 63 | NR | White | 37.2 (5.0) | Group 1—Diet (balanced diet, 500-750 kcal/d deficit, for 10% weight loss) Group 2—Diet as above and exercise programme |
Group 3—Control Group 4—Exercise programme |
| Wadden, 2011, USA93 | 390 | 13.8 | 24 | Metabolic syndrome | 51.5 (11.5) | 80 | NR | Mixed | 38.5 (4.7) | Group 1—Diet based on DPP (1200-1800 kcal/d, 20-35% calories from fat) and exercise advice (brief counselling) Group 2—Diet based on DPP (1200-1800 kcal/d, 20-35% calories from fat; meal replacements, orlistat or sibutramine if required) and exercise advice (enhanced counselling) |
Group 3—Control |
| Wing, 2010, USA (PRIDE) 94 95 | 338 | 14.5 | 18 | Urinary incontinence | 52.7 (10.3) | 100 | 5 | Mixed | 36.3 (5.3) | Group 1—Diet based on DPP and Look AHEAD (1200-1800 kcal/d, <30% calories from fat, for 7-9% weight loss, SlimFast vouchers) and exercise advice | Group 2—Control |
| Yardley, 2014, UK96 | 179 | 31.3 | 12 | NR | 51.2 (13.1) | 64 | NR | White | 35.7 (5.5) | Group 1—Diet ( self selected from: 600k cal/d deficit by reducing portions; or traffic light system; or low carbohydrate diet ≤50 g/d with traffic light system) and exercise advice (web based) Group 2—Diet as above and exercise advice (basic nurse support) Group 3—Diet as above and exercise advice (regular nurse support) |
Group 4—Control |
1 kcal=4.18 kJ
*Median
†Range
ADAPT=arthritis, diet, and activity promotion trial; CLIP=community level interventions for pre-eclampsia; DASH=dietary approaches to stop hypertension; DGA=dietary guidelines for Americans; DPP=diabetes prevention program; DPS=diabetes prevention study; FFIT=football fans in training; GI=glycaemic index; Look AHEAD=look action for health in diabetes; NR=not reported; PRIDE=program to reduce incontinence by diet and exercise; PUFA/SFA=polyunsaturated fatty acids/saturated fatty acids; SD=standard deviation; TAIM=trial of antihypertensive interventions and management; TONE=trial of nonpharmacologic intervention in the elderly; TOHP=trials of hypertension prevention; VLCD=very low calorie diet.
Five trials (9.3%) were undertaken in Asian populations,16 17 59 75 80 but only one with BMI ≥30,2 16 one trial (1.9%) was in a population of black people in the USA,50 31 (57.4%) in populations of white people, and 17 (31.5%) in mixed population groups. Thirty one (57.4%) trials took place in North America, 16 (29.6%) in Europe, two (3.7%) in Australia, and one (1.9%) in Brazil. The four trials in Asian populations outside the UK had mean BMIs between 25 and 30. 17 59 75 80 Thirty six (66.7%) trials had participants with a mean or median BMI <35, and 14 (25.9%) had BMIs ≥35 (table 1).
Most trials recruited predominantly middle aged adults. Fourteen (25.9%) had a mean or median age at baseline of 60 years or more, none had a mean or median age of under 40 years. Thirty one (57.4%) trials followed participants for two years or longer, and seven (13.0%) trials (9,937 participants) followed participants for five years or longer. In 39 trials (72.2%) the drop-out rate was <20% at trial completion.
Detailed descriptions of the weight loss diets were not always clearly provided in the trials. All but one of the trials described at least one of their interventions as being a low fat weight reduction diet (usually ≤30% of energy as fat, although this was not always specified) or had sufficient information to establish that a reduction in fat intake was prescribed. Most trials also described the prescription of a reduction in saturated fat. One trial described using a balanced Mediterranean diet.79 One trial included the option to undertake a diet with ≤50 g/day of carbohydrate.96 Two weight loss trials specifically described diets to reduce low glycaemic index as part of their intervention,26 30 whereas other trials generally described diets that would be compatible with lowering glycaemic indices by increasing intake of complex carbohydrates and dietary fibre. Four trials (7.4%) were based on the DASH (dietary approaches to stop hypertension) diet.31 39 40 54 Eight (14.8%) trials based their diets on those of the US Diabetes Prevention Program,4 26 52 60 67 74 93 94 and four trials (7.4%) described basing their content in part on different editions of the Dietary Guidelines for Americans.64 69 72 76
Only three trials (5.6%) did not report providing exercise advice or an exercise programme.45 55 68 Twenty two trials (40.7%) provided an exercise programme for participants to attend, and 29 trials (53.7%) described providing advice to increase exercise only, without an exercise programme.
Supplementary figure 1 provides our risk of bias assessments for individual studies. Only 15 trials (27.8%) reported methods of randomisation and allocation concealment judged to be at low risk of bias. Blinding of participants and study personnel was rarely possible, but we judged that lack of blinding of outcome assessment would rarely have been a source of bias except for weight outcomes. Only 10 (18.5%) trials were judged to be at low risk for attrition bias, and 12 (22.2%) at low risk for reporting bias. Seven (13.0%) trials were judged to be at high risk of bias as a result of premature trial termination,52 65 75 change in the primary outcome,16 influence of a drug placebo in the control group,4 or trial investigators reporting that they were sponsored by grants from a commercial weight loss programme71 or that they were co-owners of a company developing products related to the research.72
Meta-analyses
Details of our adjudication processes for cardiovascular and cancer outcomes are provided in supplementary tables 1-3. Supplementary table 1 compares all cause mortality, cardiovascular mortality, and cancer mortality across all trials, showing that we were not always able to obtain causes of death from authors.
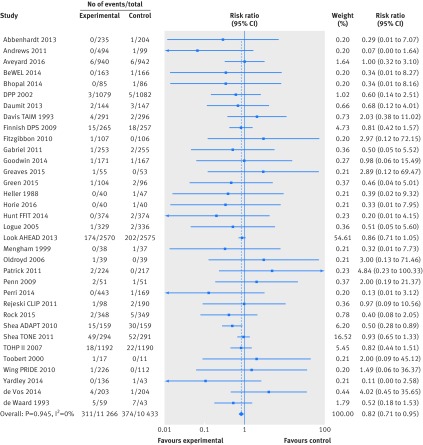
Based on the GRADE approach for judging quality of the evidence (supplementary table 4) we found high quality evidence from 34 trials (21 699 participants) providing data on all cause mortality (fig 2), which showed a decrease in premature mortality with weight loss interventions (n=34 trials, 685 events; risk ratio 0.82, 95% confidence interval 0.71 to 0.95; I2=0%). The Look AHEAD trial had 54.6% of the weighting in the meta-analysis.65 66 Without this trial weight loss interventions were still associated with decreased all cause mortality (n=33 trials, 309 events; risk ratio 0.78, 95% confidence interval 0.63 to 0.96; I2=0%). The funnel plot showed no evidence of small study bias (Egger’s test P=0.269, supplementary figure 2).
Fig 2 Random effects meta-analysis of the effects of weight loss interventions on all cause mortality. ADAPT=arthritis, diet, and activity promotion trial; CLIP=community level interventions for pre-eclampsia; DPP=diabetes prevention program; DPS=diabetes prevention study; FFIT=football fans in training; Look AHEAD=look action for health in diabetes; PRIDE=program to reduce incontinence by diet and exercise; TAIM=trial of antihypertensive interventions and management; TOHP=trials of hypertension prevention; TONE=trial of nonpharmacologic intervention in the elderly.
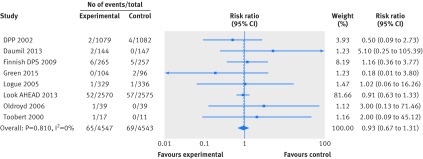
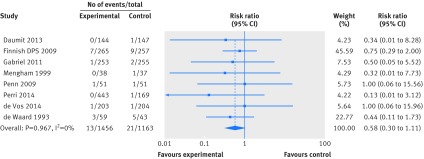
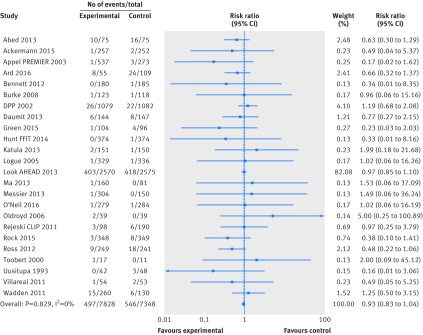
Fewer trials reported data for cardiovascular mortality and cancer mortality, resulting in considerable uncertainty in the estimates of effects of weight loss interventions on these outcomes. We found moderate quality evidence for an effect on cardiovascular mortality (n=8 trials, 134 events; risk ratio 0.93, 95% confidence interval 0.67 to 1.31; I2=0%) and very low quality evidence for an effect on cancer mortality (n=8 trials, 34 events; risk ratio 0.58, 95% confidence interval 0.30 to 1.11; I2=0%) (figs 3 and 4). Limiting cardiovascular mortality to ACC/AHA defined events did not influence this result, as the data were identical (n=8 trials, 134 events; risk ratio 0.93, 95% confidence interval 0.67 to 1.31; I2=0%).
Fig 3 Random effects meta-analysis of the effects of weight loss interventions on cardiovascular mortality. DPP=diabetes prevention program; DPS=diabetes prevention study.
Fig 4 Random effects meta-analysis of the effects of weight loss interventions on cancer mortality. DPS=diabetes prevention study.
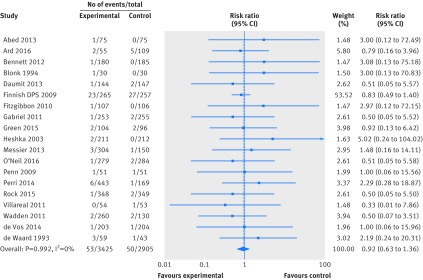
Twenty four trials (15 176 participants) reported high quality evidence on participants developing new cardiovascular events (n=24, 1043 events; risk ratio 0.93, 95% confidence interval 0.83 to 1.04; I2=0%). Using events classified according to ACC/AHA definitions, results were very similar (fig 5, supplementary figure 3). Nineteen trials (6330 participants) provided very low quality evidence on participants developing new cancers (n=19, 103 events; risk ratio 0.92, 95% confidence interval 0.63 to 1.36; I2=0%) (fig 6). Bayesian meta-analyses for all of the above outcomes provided similar results (supplementary table 5).
Fig 5 Random effects meta-analysis of the effects of weight loss interventions on participants with a cardiovascular event. CLIP=community level interventions for pre-eclampsia; DPP=diabetes prevention program; FFIT=football fans in training.
Fig 6 Random effects meta-analysis of the effects of weight loss interventions on participants developing cancer. DPS=diabetes prevention study.
Interventions had a beneficial effect on weight change after one year (n=44, mean difference −3.42 kg; 95% confidence interval −4.09 to −2.75 kg; I2=92%), after two years (n=20, mean difference −2.51 kg; 95% confidence interval −3.42 to −1.60 kg; I2=89%) and after three or more years (n=8, mean difference −2.56 kg; 95% confidence interval −3.50 to −1.62 kg; I2=87%) (supplementary figures 4 to 6). Heterogeneity for each of these meta-analyses was very high (I2=87% to 92%), reflecting the wide diversity of weight loss interventions and their effects on weight.
Sensitivity analyses
Sensitivity analyses for allocation concealment (low risk of bias versus other risk of bias) and completion of follow-up (<80% v ≥80% of participants completed) did not show any statistically significant heterogeneity for mortality, cardiovascular outcomes, or cancer outcomes (supplementary table 6).
Weight change at final follow-up was lower in trials with low risk of bias for allocation concealment (n=17, mean difference −2.33 kg; 95% confidence interval −2.87 to −1.79 kg) than for trials with high or unclear risk of bias for allocation concealment (n=31, mean difference −3.24 kg; 95% confidence interval −4.00 to −2.49 kg).
Weight change at final follow-up was lower in trials with completed follow-up of less than 80% (n=15, MD −2.09 kg; 95% CI: −2.80 to −1.37 kg) than for trials with follow-up of 80% or more (n=33, MD −3.13 kg; 95% CI: −3.71 to −2.55 kg).
Subgroup analyses
We undertook many subgroup analyses, including post hoc analyses with the addition of trials in Asian populations with BMI ≥25 (supplementary table 6, supplementary figures 7-9). Tests for subgroup differences for mortality, cardiovascular outcomes, and cancer outcomes provided weak evidence that participants without type 2 diabetes might be at lower risk of a new cardiovascular event than participants with type 2 diabetes or those with impaired glucose tolerance or impaired fasting glycaemia. Similarly, we found weak evidence that groups of white participants may be at lower risk of a new cardiovascular event than black, mixed, or Asian population groups when following weight loss interventions.
Subgroup analyses for weight change at final follow-up provided weak evidence that participants aged 60 or over lost more weight than younger participants and that participants in trials in Asian populations lost less weight than those in trials with other population groups. Similarly, we found weak evidence of better long term weight loss with trials that provided a physical activity programme, compared with trials that gave only physical activity advice or did not report providing physical activity advice.
Discussion
We found high quality evidence that weight reducing diets for adults with obesity, usually low in fat and low in saturated fat, were associated with a 18% relative reduction in premature mortality over a median trial duration of two years, corresponding to six fewer deaths per 1000 participants (95% confidence interval two to 10). This evidence provides a further reason for weight reducing diets to be offered alongside their already proven benefits, such as type 2 diabetes prevention. We were unable to show effects on cardiovascular and cancer mortality, or participants developing cardiovascular events or new cancers, although fewer trials reported events for these outcomes, resulting in much uncertainty around their effect estimates.
We identified 34 trials reporting mortality data compared with 15 in the previous systematic review by Kritchevsky and colleagues,9 which included weight loss interventions irrespective of baseline BMI, and we made very considerable efforts to clarify data and retrieve unpublished data from 48 trialists. We used a comprehensive search strategy with full text searching of trials in our obesity database. The trials we included were not necessarily designed to collect data on mortality, cardiovascular, and cancer outcomes, although larger trials generally were.65 66 81 82 83 84 85 86 87 We might have failed to identify all trials with outcome data, if trialists did not present these outcomes or presented them as unspecified adverse events. This may have biased results, although we could not see obvious funnel plot asymmetry for all cause mortality. Trials generally excluded participants with a recent diagnosis of cancer, but this was not always clear, so some participants may have had a recurrence of cancer, rather than a new event. Many of the trials had quite intensive control group interventions, and the unblinded nature of the interventions could have led to more medical treatment in control groups, tending to reduce differences between groups.65 Using GRADE to assess the quality of the evidence aids interpretation of the limitations of the evidence. We undertook sensitivity and subgroup analyses, including post hoc analyses, which should be regarded with caution. Individual patient data meta-analyses are required for further exploration of these subgroup findings.
In systematic reviews of controlled cohort studies, bariatric surgery has been associated with significant reductions in mortality, cardiovascular events, myocardial infarction, stroke, and risk of cancer.97 98 A systematic review and meta-analysis of population prospective cohort studies by Flegal and colleagues found that BMIs of 30 to <35 were not associated with higher mortality, compared with BMIs of 18.5 to <25.5 By contrast, the Global BMI Mortality Collaboration found that obesity (BMI 30 to <35) was associated with higher mortality; the investigators reduced reverse causality by examining data in non-smokers and excluding the first five years of follow-up.8 Their findings were consistent for men and women, up to 89 years, and in the four continents examined. Similar findings were seen for deaths due to coronary heart disease, stroke, cancer, and respiratory disease. Our findings for BMI from RCT evidence are consistent with data from the Global BMI Mortality Collaboration.8 Epidemiological studies can demonstrate the risks of higher BMIs and, therefore, the necessity for preventing obesity, but epidemiological associations between changes in body weight and changes in disease and mortality are often limited by the lack of information on the intentionality of that weight loss. Furthermore, treatment effects found in RCTs might differ from those expected in epidemiological studies, whereby epidemiological studies might overestimate benefits.99
Evidence from systematic reviews indicates that physical activity as an adjunct to weight reducing diets might be more effective than diets alone, in terms of weight loss and improvements in blood lipids and blood pressure.100 We were unable to show differences for mortality, cardiovascular disease, and cancer between weight reducing diets alone, diets plus advice on exercise, and diets plus an exercise programme for people to attend, for which we had limited statistical power. The majority of RCTs of weight loss interventions for obesity in adults have used low fat, weight reducing diets. But a recent systematic review by Tobias and colleagues101 found that low carbohydrate weight reducing diets were more effective for weight loss than low fat, weight reducing diets, but found no difference between low fat, weight reducing diets (defined as <30% fat) and higher fat, weight reducing diets on weight loss. Recent US guidelines102 have been criticised for the lack of evidence from RCTs to support guidance.103 Thus, we must consider whether the type of weight loss diet, particularly low fat, weight reducing diets, usually with <10% of energy as saturated fat, affects important health outcomes beyond cardiovascular risk factors or weight.100 That all except one of the interventions included here used a low fat, weight reducing diet provides important evidence on all cause mortality for weight reduction with this type of diet. We do not have the evidence to establish whether other forms of weight reducing diets have this effect, and we cannot dissociate the effects of weight loss from the use of low fat diets in our results.
We encourage investigators studying weight reducing diets to adhere to CONSORT guidance on reporting harms by always reporting clinically important outcomes and adverse events, irrespective of whether they think these events are related to the interventions.104 Collecting and reporting major disease outcomes in weight reducing trials for obesity is important, particularly cardiovascular disease and cancer. We did not have sufficient data to examine whether other types of diet or physical activity influence outcomes or whether certain groups in the population are more or less likely to benefit.
In conclusion, weight reducing diets, usually low in fat and low in saturated fat, with or without an exercise component, may reduce premature all cause mortality in adults who are obese. By implication, our data support public health measures to prevent weight gain and facilitate weight loss using these types of diet.
What is already known on this subject
Whether recommendations to follow weight reducing diets can reduce premature mortality, cardiovascular disease, and cancer for adults who are obese is unclear
What this study adds
Weight reducing diets, usually low in fat and saturated fat, with or without exercise advice or programmes, may reduce premature all cause mortality in adults who are obese
Our data provide supporting evidence for public health measures to prevent weight gain and facilitate weight loss using diets low in fat and saturated fat
Web extra.
Extra material supplied by authors
Appendix: Supplementary figures 1-11 and supplementary tables 1-6
We thank Andrew Grey for helping to resolve discrepancies in data extraction and interpretation for cardiovascular events and cancer events. We thank trialists from 16 studies for clarifying or providing additional information for this review (Andrews 2011, Aveyard 2016, Bennett 2012, de Vos 2014, Finnish Diabetes Prevention Study 2009, Goodwin 2014, Green 2015, Horie 2016, Hunt (FFIT) 2014, Katula 2013, Li (Da Qing) 2014, Logue 2005, Ma 2013, O’Neil 2016, Rejeski (CLIP) 2011, Uusitupa 1993) and others who provided information, but their trials were later found not to fulfil our inclusion criteria.
Contributors and sources: AA, CM, MJB, CF, and GM designed this study. CM, AA, and CF searched the literature. CM, AA, FS, CR, PS, and MJB extracted data. CM, AA, JH, MJB, and GM analysed data. CM and AA wrote the first draft of the manuscript. All authors contributed to revisions of the manuscript. AA is the guarantor.
Funding: The Health Services Research Unit is funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: The Health Services Research Unit is funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate. No author has financial relationships with any organisations that might have an interest in the submitted work in the previous three years.
Data sharing: All data are included in the paper or supplementary appendix. No additional data are available.
Transparency: AA and CM affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. World Cancer Research Fund 2007;Second Expert Report.AICR, 2007. [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88 10.1186/1471-2458-9-88 pmid:19320986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50. 10.1056/NEJM200105033441801 pmid:11333990. [DOI] [PubMed] [Google Scholar]
- 4. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403. 10.1056/NEJMoa012512 pmid:11832527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71-82. 10.1001/jama.2012.113905 pmid:23280227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson G, Hedberg P, Öhrvik J. Survival of the fattest: unexpected findings about hyperglycaemia and obesity in a population based study of 75-year-olds. BMJ Open 2011;1:e000012 10.1136/bmjopen-2010-000012 pmid:22021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol 2014;63:1345-54. 10.1016/j.jacc.2014.01.022 pmid:24530666. [DOI] [PubMed] [Google Scholar]
- 8.Di Angelantonio E, Bhupathiraju ShN, Wormser D, et al. Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776-86. 10.1016/S0140-6736(16)30175-1 pmid:27423262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One 2015;10:e0121993 10.1371/journal.pone.0121993 pmid:25794148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8:iii-iv, 1-182. 10.3310/hta8210 pmid:15147610. [DOI] [PubMed] [Google Scholar]
- 11.Robertson C, Archibald D, Avenell A, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18:v-vi, xxiii-xxix, 1-424. 10.3310/hta18350 pmid:24857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 10.1136/bmj.b2535 pmid:19622551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avenell A, Ma C, Stewart F, et al. Systematic review of the effects of weight loss trials on mortality, cardiovascular and cancer outcomes for adults with obesity (PROSPERO protocol). PROSPERO protocol 2016;CRD42016033217.
- 14.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol 2015;66:403-69. 10.1016/j.jacc.2014.12.018 pmid:25553722. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928. pmid:22008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhopal RS, Douglas A, Wallia S, et al. Effect of a lifestyle intervention on weight change in south Asian individuals in the UK at high risk of type 2 diabetes: a family-cluster randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:218-27. 10.1016/S2213-8587(13)70204-3 pmid:24622752. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474-80. 10.1016/S2213-8587(14)70057-9 pmid:24731674. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011].2011. [Google Scholar]
- 19.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37 10.1023/A:1008929526011. [DOI] [Google Scholar]
- 20.StataCorp. Stata Statistical Software. 2015;Release 14.
- 21. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. 10.1016/S0140-6736(03)15268-3 pmid:14726171. [DOI] [PubMed] [Google Scholar]
- 22.Misra A, Chowbey P, Makkar BM, et al. Consensus Group. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India 2009;57:163-70.pmid:19582986. [PubMed] [Google Scholar]
- 23.Ogawa W, Miyazaki S. Diagnosis criteria for obesity and obesity disease. Health Eval Promot 2015;42:301-6 10.7143/jhep.42.301. [DOI] [Google Scholar]
- 24.Zhou BF. Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15:83-96.pmid:12046553. [PubMed] [Google Scholar]
- 25.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. GRADE Handbook 2013; Available at: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html.
- 26.Abbenhardt C, McTiernan A, Alfano CM, et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J Intern Med 2013;274:163-75. 10.1111/joim.12062 pmid:23432360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20:1628-38. 10.1038/oby.2011.76 pmid:21494229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050-60. 10.1001/jama.2013.280521 pmid:24240932. [DOI] [PubMed] [Google Scholar]
- 29.Ackermann RT, Liss DT, Finch EA, et al. A Randomized Comparative Effectiveness Trial for Preventing Type 2 Diabetes. Am J Public Health 2015;105:2328-34. 10.2105/AJPH.2015.302641 pmid:26378828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011;378:129-39. 10.1016/S0140-6736(11)60442-X pmid:21705068. [DOI] [PubMed] [Google Scholar]
- 31.Appel LJ, Champagne CM, Harsha DW, et al. Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003;289:2083-93.pmid:12709466. [DOI] [PubMed] [Google Scholar]
- 32.Elmer PJ, Obarzanek E, Vollmer WM, et al. PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med 2006;144:485-95. 10.7326/0003-4819-144-7-200604040-00007 pmid:16585662. [DOI] [PubMed] [Google Scholar]
- 33.Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation 2009;119:2026-31. 10.1161/CIRCULATIONAHA.108.809491 pmid:19349322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ard JD, Gower B, Hunter G, et al. Effects of Calorie Restriction in Obese Older Adults: The CROSSROADS Randomized Controlled Trial. J Gerontol A Biol Sci Med Sci 2016,. [epub ahead of print]. 10.1093/gerona/glw237. pmid:28003374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aveyard P, Lewis A, Tearne S, et al. Screening and brief intervention for obesity in primary care: a parallel, two-arm, randomised trial. Lancet 2016;388:2492-500. 10.1016/S0140-6736(16)31893-1 pmid:27789061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett GG, Warner ET, Glasgow RE, et al. Be Fit, Be Well Study Investigators. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med 2012;172:565-74. 10.1001/archinternmed.2012.1 pmid:22412073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson AS, Craigie AM, Caswell S, et al. The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ 2014;348:g1823 10.1136/bmj.g1823 pmid:24609919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blonk MC, Jacobs MA, Biesheuvel EH, Weeda-Mannak WL, Heine RJ. Influences on weight loss in type 2 diabetic patients: little long-term benefit from group behaviour therapy and exercise training. Diabet Med 1994;11:449-57. 10.1111/j.1464-5491.1994.tb00305.x pmid:8088122. [DOI] [PubMed] [Google Scholar]
- 39.Burke V, Mansour J, Beilin LJ, Mori TA. Long-term follow-up of participants in a health promotion program for treated hypertensives (ADAPT). Nutr Metab Cardiovasc Dis 2008;18:198-206. 10.1016/j.numecd.2006.10.004 pmid:17327140. [DOI] [PubMed] [Google Scholar]
- 40.Daumit GL, Dickerson FB, Wang NY, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013;368:1594-602. 10.1056/NEJMoa1214530 pmid:23517118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis BR, Blaufox MD, Oberman A, et al. Reduction in long-term antihypertensive medication requirements. Effects of weight reduction by dietary intervention in overweight persons with mild hypertension. Arch Intern Med 1993;153:1773-82. 10.1001/archinte.1993.00410150051005 pmid:8333814. [DOI] [PubMed] [Google Scholar]
- 42.de Vos BC, Runhaar J, Bierma-Zeinstra SMA. Effectiveness of a tailor-made weight loss intervention in primary care. Eur J Nutr 2014;53:95-104. 10.1007/s00394-013-0505-y pmid:23429925. [DOI] [PubMed] [Google Scholar]
- 43.Runhaar J, van Middelkoop M, Reijman M, et al. Prevention of knee osteoarthritis in overweight females: the first preventive randomized controlled trial in osteoarthritis. Am J Med 2015;128:888-895.e4. 10.1016/j.amjmed.2015.03.006 pmid:25818496. [DOI] [PubMed] [Google Scholar]
- 44.de Vos BC, Runhaar J, van Middelkoop M, Krul M, Bierma-Zeinstra SM. Long-term effects of a randomized, controlled, tailor-made weight-loss intervention in primary care on the health and lifestyle of overweight and obese women. Am J Clin Nutr 2016;104:33-40. 10.3945/ajcn.116.133512 pmid:27305950. [DOI] [PubMed] [Google Scholar]
- 45.de Waard F, Ramlau R, Mulders Y, de Vries T, van Waveren S. A feasibility study on weight reduction in obese postmenopausal breast cancer patients. Eur J Cancer Prev 1993;2:233-8. 10.1097/00008469-199305000-00007 pmid:8490542. [DOI] [PubMed] [Google Scholar]
- 46. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866-75. 10.1016/S2213-8587(15)00291-0 pmid:26377054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care 2005;28:888-94. 10.2337/diacare.28.4.888 pmid:15793191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uusitupa M, Peltonen M, Lindström J, et al. Finnish Diabetes Prevention Study Group. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study--secondary analysis of the randomized trial. PLoS One 2009;4:e5656 10.1371/journal.pone.0005656 pmid:19479072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindström J, Peltonen M, Eriksson JG, et al. Finnish Diabetes Prevention Study (DPS). Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013;56:284-93. 10.1007/s00125-012-2752-5 pmid:23093136. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgibbon ML, Stolley MR, Schiffer L, Sharp LK, Singh V, Dyer A. Obesity reduction black intervention trial (ORBIT): 18-month results. Obesity (Silver Spring) 2010;18:2317-25. 10.1038/oby.2010.47 pmid:20300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabriel KK, Conroy MB, Schmid KK, et al. The impact of weight and fat mass loss and increased physical activity on physical function in overweight, postmenopausal women: results from the Women on the Move Through Activity and Nutrition study. Menopause 2011;18:759-65. 10.1097/gme.0b013e31820acdcc pmid:21705864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol 2014;32:2231-9. 10.1200/JCO.2013.53.1517 pmid:24934783. [DOI] [PubMed] [Google Scholar]
- 53.Greaves C, Gillison F, Stathi A, et al. Waste the waist: a pilot randomised controlled trial of a primary care based intervention to support lifestyle change in people with high cardiovascular risk. Int J Behav Nutr Phys Act 2015;12:1 10.1186/s12966-014-0159-z. pmid:25592201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green CA, Yarborough BJH, Leo MC, et al. Weight maintenance following the STRIDE lifestyle intervention for individuals taking antipsychotic medications. Obesity (Silver Spring) 2015;23:1995-2001. 10.1002/oby.21205 pmid:26334929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heller SR, Clarke P, Daly H, et al. Group education for obese patients with type 2 diabetes: greater success at less cost. Diabet Med 1988;5:552-6. 10.1111/j.1464-5491.1988.tb01050.x pmid:2974778. [DOI] [PubMed] [Google Scholar]
- 56.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA 2003;289:1792-8. 10.1001/jama.289.14.1792 pmid:12684357. [DOI] [PubMed] [Google Scholar]
- 57.Horie NC, Serrao VT, Simon SS, et al. Cognitive Effects of Intentional Weight Loss in Elderly Obese Individuals With Mild Cognitive Impairment. J Clin Endocrinol Metab 2016;101:1104-12. 10.1210/jc.2015-2315 pmid:26713821. [DOI] [PubMed] [Google Scholar]
- 58.Hunt K, Wyke S, Gray CM, et al. A gender-sensitised weight loss and healthy living programme for overweight and obese men delivered by Scottish Premier League football clubs (FFIT): a pragmatic randomised controlled trial. Lancet 2014;383:1211-21. 10.1016/S0140-6736(13)62420-4 pmid:24457205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hydrie MZ, Basit A, Shera AS, Hussain A. Effect of intervention in subjects with high risk of diabetes mellitus in Pakistan. J Nutr Metab 2012;2012:867604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katula JA, Vitolins MZ, Morgan TM, et al. The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med 2013;44(Suppl 4):S324-32. 10.1016/j.amepre.2012.12.015 pmid:23498294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glechner A, Harreiter J, Gartlehner G, et al. Sex-specific differences in diabetes prevention: a systematic review and meta-analysis. Diabetologia 2015;58:242-54. 10.1007/s00125-014-3439-x pmid:25465437. [DOI] [PubMed] [Google Scholar]
- 62.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537-44. 10.2337/diacare.20.4.537 pmid:9096977. [DOI] [PubMed] [Google Scholar]
- 63.Wei X, Zou G, Gong W, et al. Cardiovascular disease risk reduction in rural China: a clustered randomized controlled trial in Zhejiang. Trials 2013;14:354 10.1186/1745-6215-14-354 pmid:24160442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Logue E, Sutton K, Jarjoura D, Smucker W, Baughman K, Capers C. Transtheoretical model-chronic disease care for obesity in primary care: a randomized trial. Obes Res 2005;13:917-27. 10.1038/oby.2005.106 pmid:15919846. [DOI] [PubMed] [Google Scholar]
- 65. Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145-54. 10.1056/NEJMoa1212914 pmid:23796131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wadden TA, Neiberg RH, Wing RR, et al. Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987-98. 10.1038/oby.2011.230 pmid:21779086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med 2013;173:113-21. 10.1001/2013.jamainternmed.987 pmid:23229846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mengham LH, Morris BF, Palmer CR, White AJS. Is intensive dietetic intervention effective for overweight patients with diabetes mellitus? A randomised controlled study in a general practice. Pract Diabetes Int 1999;16:5-8 10.1002/pdi.1960160107. [DOI] [Google Scholar]
- 69.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310:1263-73. 10.1001/jama.2013.277669 pmid:24065013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oldroyd JC, Unwin NC, White M, Mathers JC, Alberti KG. Randomised controlled trial evaluating lifestyle interventions in people with impaired glucose tolerance. Diabetes Res Clin Pract 2006;72:117-27. 10.1016/j.diabres.2005.09.018 pmid:16297488. [DOI] [PubMed] [Google Scholar]
- 71.O’Neil PM, Miller-Kovach K, Tuerk PW, et al. Randomized controlled trial of a nationally available weight control program tailored for adults with type 2 diabetes. Obesity (Silver Spring) 2016;24:2269-77. 10.1002/oby.21616 pmid:27804264. [DOI] [PubMed] [Google Scholar]
- 72.Patrick K, Calfas KJ, Norman GJ, et al. Outcomes of a 12-month web-based intervention for overweight and obese men. Ann Behav Med 2011;42:391-401. 10.1007/s12160-011-9296-7 pmid:21822750. [DOI] [PubMed] [Google Scholar]
- 73.Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health 2009;9:342 10.1186/1471-2458-9-342 pmid:19758428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perri MG, Limacher MC, von Castel-Roberts K, et al. Comparative effectiveness of three doses of weight-loss counseling: two-year findings from the rural LITE trial. Obesity (Silver Spring) 2014;22:2293-300. 10.1002/oby.20832 pmid:25376396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289-97. 10.1007/s00125-005-0097-z pmid:16391903. [DOI] [PubMed] [Google Scholar]
- 76.Rejeski WJ, Brubaker PH, Goff DC Jr, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med 2011;171:880-6. 10.1001/archinternmed.2010.522 pmid:21263080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rock CL, Flatt SW, Byers TE, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good health for You (ENERGY) Trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol 2015;33:3169-76. 10.1200/JCO.2015.61.1095 pmid:26282657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sedjo RL, Flatt SW, Byers T, et al. Impact of a behavioral weight loss intervention on comorbidities in overweight and obese breast cancer survivors. Support Care Cancer 2016;24:3285-93. 10.1007/s00520-016-3141-2 pmid:26945570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ross R, Lam M, Blair SN, et al. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Arch Intern Med 2012;172:414-24. 10.1001/archinternmed.2011.1972 pmid:22371872. [DOI] [PubMed] [Google Scholar]
- 80.Saito T, Watanabe M, Nishida J, et al. Zensharen Study for Prevention of Lifestyle Diseases Group. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352-60. 10.1001/archinternmed.2011.275 pmid:21824948. [DOI] [PubMed] [Google Scholar]
- 81.Shea MK, Houston DK, Nicklas BJ, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT Study. J Gerontol A Biol Sci Med Sci 2010;65A:519-25. 10.1093/gerona/glp217 pmid:20080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004;50:1501-10. 10.1002/art.20256 pmid:15146420. [DOI] [PubMed] [Google Scholar]
- 83.Shea MK, Nicklas BJ, Houston DK, et al. The effect of intentional weight loss on all-cause mortality in older adults: results of a randomized controlled weight-loss trial. Am J Clin Nutr 2011;94:839-46. 10.3945/ajcn.110.006379 pmid:21775558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kostis JB, Espeland MA, Appel L, Johnson KC, Pierce J, Wofford JL. Does withdrawal of antihypertensive medication increase the risk of cardiovascular events?Am J Cardiol 1998;82:1501-8. 10.1016/S0002-9149(98)00694-8 pmid:9874055. [DOI] [PubMed] [Google Scholar]
- 85.Whelton PK, Appel LJ, Espeland MA, et al. TONE Collaborative Research Group. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). JAMA 1998;279:839-46. 10.1001/jama.279.11.839 pmid:9515998. [DOI] [PubMed] [Google Scholar]
- 86.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007;334:885-8. 10.1136/bmj.39147.604896.55. pmid:17449506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. Arch Intern Med 1997;157:657-67. 10.1001/archinte.1997.00440270105009 pmid:9080920. [DOI] [PubMed] [Google Scholar]
- 88.Toobert DJ, Glasgow RE, Radcliffe JL. Physiologic and related behavioral outcomes from the Women’s Lifestyle Heart Trial. Ann Behav Med 2000;22:1-9. 10.1007/BF02895162 pmid:10892523. [DOI] [PubMed] [Google Scholar]
- 89.Uusitupa M, Laitinen J, Siitonen O, Vanninen E, Pyörälä K. The maintenance of improved metabolic control after intensified diet therapy in recent type 2 diabetes. Diabetes Res Clin Pract 1993;19:227-38. 10.1016/0168-8227(93)90118-O pmid:8319521. [DOI] [PubMed] [Google Scholar]
- 90.Vanninen E, Uusitupa M, Länsimies E, Siitonen O, Laitinen J. Effect of metabolic control on autonomic function in obese patients with newly diagnosed type 2 diabetes. Diabet Med 1993;10:66-73. 10.1111/j.1464-5491.1993.tb01999.x pmid:8435991. [DOI] [PubMed] [Google Scholar]
- 91.Vanninen E, Mustonen J, Vainio P, Länsimies E, Uusitupa M. Left ventricular function and dimensions in newly diagnosed non-insulin-dependent diabetes mellitus. Am J Cardiol 1992;70:371-8. 10.1016/0002-9149(92)90622-6 pmid:1632406. [DOI] [PubMed] [Google Scholar]
- 92.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218-29. 10.1056/NEJMoa1008234 pmid:21449785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011;365:1969-79. 10.1056/NEJMoa1109220 pmid:22082239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wing RR, West DS, Grady D, et al. Program to Reduce Incontinence by Diet and Exercise Group. Effect of weight loss on urinary incontinence in overweight and obese women: results at 12 and 18 months. J Urol 2010;184:1005-10. 10.1016/j.juro.2010.05.031 pmid:20643425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.West DS, Gorin AA, Subak LL, et al. Program to Reduce Incontinence by Diet and Exercise (PRIDE) Research Group. A motivation-focused weight loss maintenance program is an effective alternative to a skill-based approach. Int J Obes (Lond) 2011;35:259-69. 10.1038/ijo.2010.138 pmid:20680012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yardley L, Ware LJ, Smith ER, et al. Randomised controlled feasibility trial of a web-based weight management intervention with nurse support for obese patients in primary care. Int J Behav Nutr Phys Act 2014;11:67 10.1186/1479-5868-11-67 pmid:24886516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kwok CS, Pradhan A, Khan MA, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol 2014;173:20-8. 10.1016/j.ijcard.2014.02.026 pmid:24636546. [DOI] [PubMed] [Google Scholar]
- 98.Casagrande DS, Rosa DD, Umpierre D, Sarmento RA, Rodrigues CG, Schaan BD. Incidence of cancer following bariatric surgery: systematic review and meta-analysis. Obes Surg 2014;24:1499-509. 10.1007/s11695-014-1276-0 pmid:24817500. [DOI] [PubMed] [Google Scholar]
- 99.Hemkens LG, Contopoulos-Ioannidis DG, Ioannidis JPA. Agreement of treatment effects for mortality from routinely collected data and subsequent randomized trials: meta-epidemiological survey. BMJ 2016;352:i493 10.1136/bmj.i493 pmid:26858277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schwingshackl L, Dias S, Hoffmann G. Impact of long-term lifestyle programmes on weight loss and cardiovascular risk factors in overweight/obese participants: a systematic review and network meta-analysis. Syst Rev 2014;3:130 10.1186/2046-4053-3-130 pmid:25358395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015;3:968-79. 10.1016/S2213-8587(15)00367-8 pmid:26527511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.US Department of Health and Human Services, US Department of Agriculture. 2015-2020 Dietary guidelines for Americans December 2015; https://health.gov/dietaryguidelines/2015/guidelines/.
- 103.Nissen SE. U.S. dietary guidelines: an evidence-free zone. Ann Intern Med 2016;164:558-9. 10.7326/M16-0035 pmid:26783992. [DOI] [PubMed] [Google Scholar]
- 104.Ioannidis JPA, Evans SJW, Gøtzsche PC, et al. CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004;141:781-8. 10.7326/0003-4819-141-10-200411160-00009 pmid:15545678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix: Supplementary figures 1-11 and supplementary tables 1-6