Abstract
Purpose
Suberoylanilide hydroxamic acid (SAHA; vorinostat), a small molecule inhibitor of histone deacetylase (HDAC), attenuates signaling pathways known to confer trastuzumab resistance. A combination of SAHA and trastuzumab may be a promising strategy to improve the efficacy of trastuzumab against breast cancer. In this Phase I/II study, we evaluated the toxicity and response rate after treatment with SAHA and trastuzumab in patients with HER2 overexpressing metastatic breast cancer with trastuzumab-resistant progressive disease.
Methods
In Phase I, the SAHA dose was modified in cohorts of 3–6 patients to find the dose level at which 0 or 1 patients experienced a dose-limiting toxicity (DLT) during the first cycle of therapy. In the Phase II study, response to the recommended dose identified in Phase I was based on the Response Evaluation Criteria in Solid Tumors (RECIST). Overall survival and time to progression were also evaluated.
Results
The recommended dose was determined to be 200 mg twice a day on days 1–14 and IV trastuzumab 6 mg/kg on day 1 of a 21-day cycle (n= 6). The Phase II study (n=10) was terminated when the pre-planned efficacy evaluation found that none of the patients in the primary analysis set responded to combination SAHA and trastuzumab treatment.
Conclusions
In patients with HER2–positive metastatic breast cancer who had relapsed or progressed during trastuzumab therapy, we observed no DLTs with SAHA 200 mg twice daily combined with trastuzumab; however, there was insufficient statistical evidence that adding SAHA reversed trastuzumab resistance in these patients.
Keywords: histone diacetylase, toxicity, anticancer therapy, tumor, resistance
Introduction
Suberoylanilide hydroxamic acid (SAHA; NSC 701852; vorinostat) is a small molecule inhibitor of histone deacetylase (HDAC) that directly binds the enzyme’s active site in the presence of a zinc ion [1]. The action of HDACs on nucleosomal histones leads to tight coiling of chromatin and silencing of expression of various genes, including those implicated in the regulation of cell survival, proliferation, differentiation, and apoptosis [2]. HDACs also act as members of a protein complex to recruit transcription factors to gene promoter regions, including those of tumor suppressors, and HDACs also affect the acetylation status of specific cell cycle regulatory proteins [3]. Because aberrant HDAC activity has been implicated in a variety of cancers, several HDAC inhibitors have been developed as targeted anticancer therapeutics and are currently being evaluated in clinical trials. Trichostatin and butyric acid were among the first HDAC inhibitors to be administered to patients, but were found to be clinically unsuitable due to potency and formulation issues [4, 5]. Depsipeptide was originally selected for clinical study based on its antiproliferative effects; subsequently, it was discovered to be an antagonist of HDACs [6] and was the first HDAC inhibitor to demonstrate clinical efficacy [7]. Among the HDAC inhibitors currently in clinical trials, SAHA is the most potent HDAC inhibitor, targeting most human Class 1 and Class 2 enzymes [8, 9], and can be administered orally with excellent bioavailability. SAHA was the first HDAC inhibitor approved by the FDA for an oncologic indication in cutaneous T-cell lymphoma, with activity also seen in Hodgkin’s lymphoma and other hematologic malignancies [10]. Objective responses to SAHA monotherapy were not observed in a Phase II study in metastatic breast cancer resistant to conventional therapy, although 4 patients experienced stable disease [11].
Despite recent progress in our understanding of its genetic and molecular basis, advanced and metastatic breast cancer remains incurable [12]. Approximately 25% of breast cancers have amplification and over-expression of HER2/neu oncogene [13], which encodes a member of the epidermal growth factor (EGFR) family of tyrosine kinases [14]. HER2 positive breast cancer has a poor prognosis, making Her-2 a promising target for new therapies, including the monoclonal antibody trastuzumab. However, previous reports have indicated that responses to trastuzumab occur in only a minority (25–30%) of Her-2 overexpressing breast cancers [15, 16]; one potential mechanism of resistance to trastuzumab involves Her-2–independent increased activity of AKT [17–19].
HDAC inhibitors that are hydroxamic acid analogues, including SAHA, have been shown to induce p21WAF1 and p27KIP1, inhibit cell growth, and induce apoptosis of breast cancer cells [20–23]. Studies have also demonstrated that SAHA induces acetylation of hsp90, which inhibits the binding of hsp90 to ATP and destabilizes the chaperone complex with its target proteins, including Her-2, AKT, and c-Raf [22, 24], leading to ubiquitination and proteasomal degradation [22, 24]. Since SAHA attenuates the levels of pAKT and c-RAF-1, it may abrogate the signaling pathways known to confer trastuzumab resistance. Both SAHA and trastuzumab have also been shown to inhibit vascular endothelial growth factor levels and exert anti-angiogenic effects [25, 26]. Based on these findings, we and others have hypothesized that a combination of SAHA and trastuzumab may be a promising strategy to reverse trastuzumab resistance in trastuzumab pre-treated HER2 overexpressing breast cancers.
Therefore, the Phase I objective of the present study was to determine the recommended dose of SAHA used in combination with trastuzumab based on toxicity endpoints in patients with HER2 overexpressing metastatic breast cancer who had progressive disease after prior trastuzumab. The objective of the Phase II portion of the study was to evaluate response rate, overall survival, and time to progression after treatment with the Phase I-recommended dose of SAHA combined with trastuzumab.
Methods
Study design
This was a phase I/II study to determine a recommended dose of SAHA used in combination with trastuzumab and to evaluate the response rate and toxicity of the combination therapy in patients with HER2 overexpressing metastatic breast cancer who had progressive disease after prior treatment with trastuzumab (ClinicalTrials.gov identifier: NCT00258349).
Study participants
Patients with histologically confirmed HER2 overexpressing breast cancer, defined locally by immunohistochemical analysis (with 3+ indicating positive status), fluorescence in situ hybridization (with an amplification ratio ≥2.0 indicating positive status), or both. Efficacy analysis was performed only for those subjects with centrally confirmed HER2 overexpressing breast cancer. Evidence of measurable metastatic disease and/or chest wall recurrence were required for inclusion in the study. Patients may have received prior radiation therapy; however, the only site of measurable disease must not have been irradiated, except in the case of chest wall recurrence previously treated with adjuvant radiation therapy. Eligible patients must have had documented disease recurrence or progression while receiving trastuzumab or must have had relapsed within 3 months of completing the last dose of adjuvant trastuzumab or trastuzumab for metastatic disease. Patients must have had adequate organ and marrow function, defined as (1) CBC values (obtained within 14 days prior to registration) of ANC ≥1500/μL, platelet count ≥100,000/mm3, and hemoglobin ≥9 g/dL; and (2) prestudy chemistry values (obtained within 4 weeks prior to registration) of serum creatinine ≤1.5 g/dL, ALT ≤2×ULN, AST ≤2×ULN, and total bilirubin ≤1.5 mg/dL. Eligible patients must have had adequate cardiac function as defined by left ventricular ejection fraction less than or equal to the lower limit of institutional normal obtained within 6 weeks prior to registration, as assessed by an echocardiogram and nuclear scan and no evidence of PR prolongation or AV block on ECG performed within 4 weeks prior to registration.
CYP450 inducers or inhibitors must have been discontinued 1 week prior to initiating protocol treatment. Patients must not have had chemotherapy or radiotherapy within 3 weeks prior to entering the study. Patients must have recovered from all adverse events related to prior chemotherapy or radiation. Patients must not have been receiving any other investigational agents, nor might they have received any other investigational agents within 4 weeks prior to entering the study. Patients must not have had a history of untreated brain metastasis or brain metastasis currently undergoing radiation. Patients with brain metastasis representing the sole site of disease were not eligible. Patients with previously treated brain metastasis who had responded to brain radiotherapy and/or surgery and had continued in response were eligible, provided the brain was not the only site of measurable disease. Patients must not have had a history of allergic reactions attributed to compounds of similar chemical or biologic composition to SAHA or other agents used in study. Patients must not have had taken valproic acid, another histone deacetylase inhibitor, for at least 2 weeks prior to registration.
Patients who met inclusion criteria provided informed consent prior to enrollment.
Study treatment
In the Phase I portion of the trial, the SAHA dose was to be modified in cohorts of 3–6 patients based on the number of patients who experienced a dose-limiting toxicity (DLT) event at each dose level during the first cycle of therapy. If 0 or 1 patient out of 3 developed a DLT at a given SAHA dose, then 3 additional patients were to be treated at the same dose level. If 0 or 1 patient out of these 6 patients developed a DLT, this SAHA dose was to be determined to be the recommended dose for the Phase II study. If 2 or more patients developed a DLT at a given SAHA dose, the dose level was to be reduced. Specifically, the first 3–6 patients enrolled in the study were to be treated with 200 mg of SAHA orally twice a day (total 400 mg/day), on days 1–14 per 21-day cycle, in addition to IV trastuzumab (6 mg/kg) on day 1 per cycle (Arm A). If excessive toxicity was observed in Arm A, the dose was to be reduced in subsequent cohorts of 3–6 patients to 300 mg/day (100 mg AM, 200 mg PM) on days 1–14 (Arm B). If excessive toxicity was observed in Arm B, the dose was to be reduced again to 200 mg/day (100 mg twice a day) on days 1–14 (Arm C). If excessive toxicity was observed at this dose level, the study was to be closed to accrual.
Based on the Phase I study findings, the recommended dose of SAHA was administered orally twice a day, days 1–14 per 21-day cycle, in addition to IV trastuzumab (6 mg/kg) on day 1 in the Phase II study (Arm D). Patients continued on the Phase II protocol treatment until unacceptable toxicity or progression of disease.
Study outcomes
All adverse events were documented in both the Phase I and Phase II studies. Toxicity was assessed using the NCI Common Toxicity Criteria (CTC) Version 3.0. Patients were assessed weekly for DLTs, defined as: (1) excessive bone marrow function toxicity: Grade 3–4 thrombocytopenia and/or Grade 3–4 neutropenia lasting more than 7 days, Grade 3–4 febrile neutropenia, or Grade 4 anemia; (2) excessive GI toxicity: Grade 4 anorexia, Grade 4 diarrhea and/or vomiting not responding to maximal therapy or prophylaxis, Grade 3 diarrhea and/or vomiting persisting more than 24 hours despite maximum treatment, or any symptoms requiring sustained IV fluid replacement or hospitalization for IV fluid replacement (Grade 3 dehydration); (3) excessive general toxicity: Grade 3–4 fatigue, or fatigue, asthenia or malaise leading to a decrease in ECOG performance status to 3 or 4 (or 2 if the baseline performance status was 0).
In the Phase II study, treatment response was based on the Response Evaluation Criteria in Solid Tumors (RECIST). Tumor lesions at baseline were classified as measurable (accurately measured in at least one dimension as >20 mm with conventional techniques or as >10 mm with spiral CT scan) or non-measurable. Complete response (CR) was defined as the disappearance of all target lesions (maximum of 5 lesions per organ and 10 lesions total, selected based on size at baseline). Partial response (PR) was defined as at least a 30% decrease in the sum of the longest diameters of target lesions. CR or PR was confirmed by repeat assessments performed no less than 4 weeks after the criteria for response were first met. Progressive disease (PD) was defined as at least a 20% increase in the sum of the longest diameters of target lesions and stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. The best response rate was based on response of target and non-target tumors and duration of response. Overall survival, defined as the time from registration to death from any cause, and time to progression (appearance of new tumors) were also evaluated.
Statistical methods
Descriptive statistics were used to characterize patients at baseline. Best response rate was reported and its confidence interval was computed by the method of exact binomial confidence interval. The Kaplan-Meier method was used to estimate the overall survival and disease progression-free rates over time. Standard errors of the estimates were calculated using Greenwood’s formula. The confidence interval of the median overall survival and median time to progression were constructed using the method by Brookmeyer and Crowley.
For the Phase II study, a two-stage plan was employed to distinguish between a response rate (CR+PR) of 10%, which would be considered unworthy of further study, and a response rate of 30%, which would be considered worthy of further study. Adopting the optimal design of Simon et al [27], if 0 or 1 of the first 12 eligible patients (including the patients who were treated at the recommended Phase II SAHA dose level during the initial Phase I safety evaluation) showed a response, the study was stopped, with the conclusion that the regimen was not effective. If 2 or more patients responded, 23 additional eligible patients were accrued, for a total of 35. If 6 or more of these 35 patients responded, the regimen was considered worthy of further study. With this design, the study would be stopped early with probability of 66% if the true response rate was 10% and with a probability of 9% if the true response rate was 30%. Allowing up to 10% of patients to be false HER2 overexpressors based on the retrospective central review and up to 5% ineligibility rate for other reasons, a total of 35 patients were planned to be enrolled in the Phase II study, including the 6 patients from the Phase I study treated with the Phase II recommended SAHA dose.
Results
Phase I safety evaluation
The Phase I study was activated on August 23, 2006 at five institutions (Fox Chase Cancer Center, Albert Einstein College of Medicine/Montefiore Hospital, Vanderbilt University, University of Alabama Birmingham, and Johns Hopkins University). Six patients with confirmed Her-2–positive breast cancer were treated at the starting dose of SAHA 200 mg twice daily on days 1–14 combined with 6 mg/kg trastuzumab on day 1 every 21 days (Arm A). There were no DLTs at this dose level, eliminating the need to reduce the SAHA dose. The Arm A dose was determined to be the recommended dose for the Phase II study.
Phase II treatment response
The Phase II study was activated on June 28, 2007 and suspended on October 4, 2007 for the pre-planned efficacy evaluation. Due to a low response rate in this efficacy evaluation, the study was formally terminated on August 27, 2009. A total of 16 patients were enrolled in the Phase II study; all patients were from ECOG. One patient was ineligible because the measurement of lesions was completed after registration. Five patients were Her-2–negative from the ECOG central review; of these, 1 patient’s HER2 status from the central review was not available due to insufficient tissue. Therefore, 10 eligible patients were confirmed as HER2–positive by the central review and were included in the primary efficacy analysis of the Phase II study; this set of patients was named the primary analysis set. Efficacy analysis (response, overall survival, time to progression) included the primary analysis set (n=10). Toxicity was assessed in both the primary analysis set plus the 6 patients from the Phase I safety evaluation (N=16).
Patient characteristics
Table 1 presents the baseline characteristics of the primary analysis set (n=10). The median age was 54 years, ranging from 42 to 69 years. Three of the patients were pre-menopausal or younger than 50 years of age. Four patients had an ECOG performance status of 1 and 6 patients had a status of 0. At initial diagnosis, 2 patients were ER-positive and/or PR-positive. Four patients had 4 or 5 metastatic sites, and the other 6 patients had 1 to 3 metastatic sites.
Table 1.
Patient Characteristics (primary analysis set, n=10)
| Total (No.) | 10 |
|---|---|
| Median age (range, years) | 54 (42,69) |
| ECOG PS | |
| 0 | 4 (40%) |
| 1 | 6 (60%) |
| Pre-menopausal or <50 years of age | 3 (30%) |
| ER+ and/or PR+ | 2 (20%) |
| Involved sites (No.) | |
| 1 | 2 (20%) |
| 2 | 1 (10%) |
| 3 | 3 (30%) |
| 4 | 2 (20%) |
| 5 | 2 (20%) |
| Prior chemotherapy | |
| Yes | 9 (90%) |
| Missing | 1 (10%) |
| Prior hormonal therapy | |
| Yes | 2 (20%) |
| No | 7 (70%) |
| Missing | 1 (10%) |
| Prior immunotherapy | |
| Yes | 9 (90%) |
| Missing | 1 (10%) |
| Prior radiation therapy | |
| Yes | 6 (60%) |
| No | 3 (30%) |
| Missing | 1 (10%) |
| Prior surgery | |
| Yes | 9 (90%) |
| Missing | 1 (10%) |
Treatment compliance
Among the primary analysis set, 6 patients completed 2 cycles of treatment, 2 patients completed 3 cycles, 1 patient completed 7 cycles, and 1 patient completed only 1 cycle. Eight patients were off treatment due to disease progression; 1 was due to toxicity and 1 was due to patient withdrawal.
Toxicity
Table 2 summarizes counts of the observed toxicity for all 16 patients in the Phase I and II studies. One patient had Grade 3 decreased platelets and another patent had Grade 4 decreased platelets. Two patients experienced Grade 4 dyspnea. Ten patients experienced Grade 1 or 2 diarrhea without prior colostomy.
Table 2.
Adverse Events in All Study Patients (n=16)
| Grade
|
||||
|---|---|---|---|---|
| 1/2 | 3 | 4 | 5 | |
| Hemoglobin | 0 | - | - | - |
| Leukocytes | 3 | - | - | - |
| Neutrophils | 1 | - | - | - |
| Platelets | 2 | 1 | 1 | - |
| Hypertension | 1 | - | - | - |
| Hypotension | 1 | - | - | - |
| Fatigue | 6 | - | - | - |
| Insomnia | 1 | - | - | - |
| Weight loss | 3 | - | - | - |
| Alopecia | 1 | - | - | - |
| Nail changes | 1 | - | - | - |
| Rash: acne/acneiform | 1 | - | - | - |
| Anorexia | 5 | - | - | - |
| Constipation | 1 | - | - | - |
| Dehydration | 2 | - | - | - |
| Diarrhea w/o prior colostomy | 10 | - | - | - |
| Muco/stomatitis by exam, oral cavity | 1 | - | - | - |
| Nausea | 6 | - | - | - |
| Taste disturbance | 1 | - | - | - |
| Vomiting | 5 | - | - | - |
| Alkaline phosphatase | 1 | - | - | - |
| ALT, SGPT | 2 | - | - | - |
| AST, SGOT | 2 | - | - | - |
| Hypocalcemia | 1 | - | - | - |
| Creatinine | 5 | - | - | - |
| Hyperglycemia | 5 | - | - | - |
| Hypokalemia | 3 | - | - | - |
| Hyponatremia | 1 | - | - | - |
| Dizziness | 2 | - | - | - |
| Dyspnea | 1 | 2 | - | - |
| Pneumonitis/pulmonary infiltrates | 1 | - | - | - |
|
| ||||
| WORST DEGREE | 10 | 1 | 1 | - |
Best response rate
Of the 10 patients with HER2–positive disease in the primary analysis set, no patient (0%, 90% CI [0%, 26%]) had CR or PR, 1 (10%, 90% CI [0.5%, 39%]) patient had SD, 8 (80%) patients had PD, and 1 patient’s response was not evaluable due to non-protocol therapy prior to disease progression and no disease assessment after baseline. The patient with unknown HER2 status from the central review had PD. Among the 5 patients with centrally confirmed HER2–negative disease, 1 had a PR and the others had PD.
Overall survival
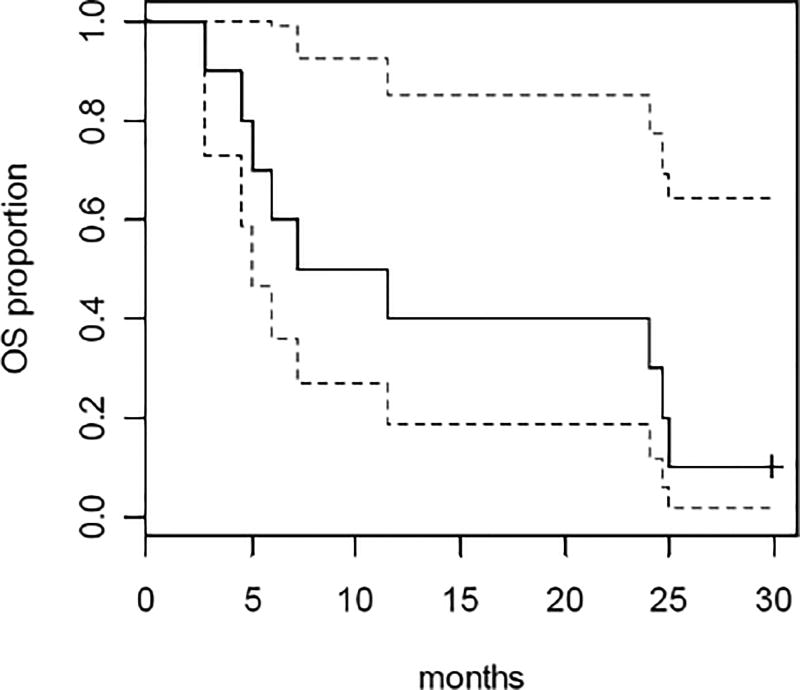
Among the primary analysis set of 10 patients, 9 (90%) died and 1 was alive after a follow-up of 30 months. Among all 16 patients treated, 2 were alive and 14 (87.5%) died. Figure 1 shows the Kaplan-Meier curve and 95% confidence interval for overall survival among the patients in the primary analysis set (n=10). The median survival time was 9.3 months (95% CI: 5.1, 24.7).
Figure 1.
Kaplan-Meier plot of overall survival with 95% confidence interval for the primary analysis set (n=10).
Time to progression
All 16 patients had documented disease progression. Figure 2 shows the Kaplan-Meier curve and 95% confidence interval for time to progression among the patients in the primary analysis set (n=10). The median time to progression was 1.5 months (95% CI: 1.3, 3.7).
Figure 2.
Kaplan-Meier plot of time to disease progression with 95% confidence interval for the primary analysis set (n=10).
Discussion
E1104 was a phase I/II study to determine the recommended dose of SAHA that could be used with minimal toxicity in combination with trastuzumab and to evaluate the response rate of combination therapy in patients with HER2 overexpressing metastatic breast cancer who had progressive disease after prior treatment with trastuzumab. In the Phase I study, the recommended dose of SAHA was determined to be 200 mg twice a day for days 1–14 of a 21-day cycle. The Phase II study of 16 patients treated with this combination therapy was terminated when the pre-planned efficacy evaluation suggested a low response rate. Among the 10 patients with centrally confirmed Her-2–positive breast cancer included in the primary analysis set, none responded to the treatment. Thus, there is insufficient statistical evidence that adding the HDAC inhibitor, SAHA, reverses trastuzumab resistance in these patients.
Similar to our study, another Phase I/II study of SAHA in combination with paclitaxel and bevacizumab found no dose-limiting toxicities at the recommended dose of SAHA 300 mg twice daily. For the primary efficacy analysis in 44 patients treated at with this SAHA dose, the objective response rate was 55% and SAHA was found to induce tubulin acetylation and Hsp 90 inhibition [28].
SAHA was also shown to reduce tamoxifen resistance in ER-positive breast cancer. In a Phase II study of 43 women with ER-positive breast cancer and progression while on tamoxifen, treatment with 400 mg of vorinostat daily for 3 weeks of a 4-week cycle plus 20 mg tamoxifen daily led to a 19% objective response rate, and 40% of patients experienced a response or stable disease after 24 weeks of treatment [29]. The authors also reported that HDAC2 expression in PBMC may be predictive of tamoxifen response and that histone deacetylation may be a useful pharmacodynamic marker for efficacy [29]. In our study, there were insufficient patient samples to perform similar correlative analyses.
More recently, preclinical work has shown that another HDAC inhibitor, SNDX-275 (entinostat), enhances the efficacy of trastuzumab in HER2 overexpressing breast cancer cell lines and exhibits the potential to overcome trastuzumab resistance [30]. SNDX-275 has also shown activity in metastatic hormone receptor-positive breast cancer in combination with an aromatase inhibitor and is currently being evaluated in a Phase III trial in this population [31]. There are no clinical data available currently with entinostat in HER2–positive breast cancer.
Conclusion
In this population of patients with HER2–positive breast cancer who had either relapsed or progressed during trastuzumab therapy (either alone or in combination with chemotherapy), we observed no dose-limiting toxicities with 200 mg twice daily for 14 days of a 21-day cycle combined with trastuzumab; however, there was insufficient statistical evidence that adding SAHA reversed trastuzumab resistance in these patients.
Acknowledgments
This study was supported by grant CA-23318, awarded by the National Cancer Institute, DHHS.
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180794, CA180820, CA189859, CA189851, CA180853, CA180855, CA180816, CA180795, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
“The project described was supported by Award Number P30 CA006927 (Richard Fisher, MD) and U10 CA027525 (Lori J. Goldstein, MD) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.”
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nature reviews. Genetics. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 3.Arts J, de Schepper S, Van Emelen K. Histone deacetylase inhibitors: From chromatin remodeling to experimental cancer therapeutics. Curr Med Chem. 2003;10:2343–2350. doi: 10.2174/0929867033456657. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert J, Baker SD, Bowling MK, Grochow L, Figg WD, Zabelina Y, Donehower RC, Carducci MA. A phase I dose escalation and bioavailability study of oral sodium phenylbutyrate in patients with refractory solid tumor malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:2292–2300. [PubMed] [Google Scholar]
- 5.Gore SD, Weng LJ, Figg WD, Zhai S, Donehower RC, Dover G, Grever MR, Griffin C, Grochow LB, Hawkins A, Burks K, Zabelena Y, Miller CB. Impact of prolonged infusions of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:963–970. [PubMed] [Google Scholar]
- 6.Kitagaki J, Shi G, Miyauchi S, Murakami S, Yang Y. Cyclic depsipeptides as potential cancer therapeutics. Anti-cancer drugs. 2015;26:259–271. doi: 10.1097/CAD.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 7.Piekarz RL, Robey R, Sandor V, Bakke S, Wilson WH, Dahmoush L, Kingma DM, Turner ML, Altemus R, Bates SE. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.V98.9.2865. [DOI] [PubMed] [Google Scholar]
- 8.Marks PA, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: Causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 9.Kelly WK, O'Connor OA, Marks PA. Histone deacetylase inhibitors: from target to clinical trials. Expert Opin Inv Drug. 2002;11:1695–1713. doi: 10.1517/13543784.11.12.1695. [DOI] [PubMed] [Google Scholar]
- 10.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luu TH, Morgan RJ, Leong L, Lim D, McNamara M, Portnow J, Frankel P, Smith DD, Doroshow JH, Wong C, Aparicio A, Gandara DR, Somlo G. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:7138–7142. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winer E, Morrow M, Osborne CK JH. Malignant tumors of the breast. In: Devita JVT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6. Lippincott-Raven Publishers; Philadelphia, PA: 2000. pp. 1651–1716. [Google Scholar]
- 13.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF. Studies of the Her-2/Neu Proto-Oncogene in Human-Breast and Ovarian-Cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 14.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. The EMBO journal. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England journal of medicine. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 17.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 18.Lu YH, Zi XL, Zhao YH, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer I. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 19.Kurokawa H, Arteaga CL. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:511S–515S. [PubMed] [Google Scholar]
- 20.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. J Natl Cancer I. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 21.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21(WAF1) expression and gene-associated histone acetylation. P Natl Acad Sci USA. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuino L, Bali P, Wittmann S, Donapaty S, Guo F, Yamaguchi H, Wang HG, Atadja P, Bhalla K. Histone deacetylase inhibitor LAQ824 down-regulates Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone B. Mol Cancer Ther. 2003;2:971–984. [PubMed] [Google Scholar]
- 23.Huang LL, Pardee AB. Suberoylanilide hydroxamic acid as a potential therapeutic agent for human breast cancer treatment. Mol Med. 2000;6:849–866. [PMC free article] [PubMed] [Google Scholar]
- 24.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 25.Deroanne CF, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, Blacher S, Verdin E, Foidart JM, Nusgens BV, Castronovo V. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21:427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 26.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumor biology - Herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 27.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled clinical trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 28.Ramaswamy B, Fiskus W, Cohen B, Pellegrino C, Hershman DL, Chuang E, Luu T, Somlo G, Goetz M, Swaby R, Shapiro CL, Stearns V, Christos P, Espinoza-Delgado I, Bhalla K, Sparano JA. Phase I-II study of vorinostat plus paclitaxel and bevacizumab in metastatic breast cancer: evidence for vorinostat-induced tubulin acetylation and Hsp90 inhibition in vivo. Breast cancer research and treatment. 2012;132:1063–1072. doi: 10.1007/s10549-011-1928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munster PN, Thurn KT, Thomas S, Raha P, Lacevic M, Miller A, Melisko M, Ismail-Khan R, Rugo H, Moasser M, Minton SE. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. British journal of cancer. 2011;104:1828–1835. doi: 10.1038/bjc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X, Wang S, Lee CK, Yang X, Liu B. HDAC inhibitor SNDX-275 enhances efficacy of trastuzumab in erbB2-overexpressing breast cancer cells and exhibits potential to overcome trastuzumab resistance. Cancer letters. 2011;307:72–79. doi: 10.1016/j.canlet.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Yardley DA, Ismail-Khan RR, Melichar B, Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee MJ, Trepel JB. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2128–2135. doi: 10.1200/JCO.2012.43.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]