Abstract
Caloric restriction (CR) is the only preventive intervention that has robust pro-longevity effects in experimental models. Various circulating hormones that regulate the state of negative energy balance may drive the multi-system beneficial effects of the CR phenomenon. Ghrelin, one such stomach-derived circulating peptide hormone stimulates food intake, promotes GH release and inhibits pro-inflammatory cytokines. We have recently demonstrated that ghrelin also reverses age-related thymic involution. Here, we report that chronic CR in aging mice results in reduction in body weight, and spleen size but remarkably, leads to a significant increase in the size and weight of stomach. The increased size of stomach was largely due to increased size of fundus (forestomach) and also smaller but statistically significant enlargement of antrum. The analysis of serial stomach sections revealed that chronic CR leads to a striking hypertrophy of lamina propria, stratum basale, stratum corneum and the stratified squamous epithelium of forestomach of the aged animals. We also report for the first time that chronic CR during aging significantly increases circulating ghrelin levels as well as total ghrelin production in the stomach and reverses age-related loss of ghrelin receptor expression in pituitary. Our data suggests that long-term CR-induced increased ghrelin production from hypertrophic stomach in mice may be an adaptive survival strategy in response to sustained negative energy balance that triggers heightened state of food seeking. Taken together, these data provide new insights into the underlying mechanism behind the salutary effects of chronic caloric restriction during aging process.
Keywords: Dietary restriction Nutrition, Immune system Ghrelin, GHS-R, Geriatrics CR mimetic, Inflammation Stomach Fundus Antrum Forestomach Aging Thymus Gastric bypass
Introduction
Reduction in calories below usual ad libitum intake without malnutrition retards aging and increases healthy life span in various animal models [29,30]. The underlying mechanisms responsible for the CR’s salutary effects on aging process are under intense investigations [4]. The chronic negative energy balance due to long-term CR elicits specific metabolic and neuroendocrine adaptive responses in animals [26]. It has been reported that elevated cortisol, decrease in sympathetic nervous system activity [15], reduced triiodothyronine, [18] and IGF-1 [24] are important neuroendocrine factors regulating the CR phenomenon. In response to sustained negative energy balance, CR promotes gluconeogenesis, improves insulin sensitivity and reduces glycolysis and fatty acid biosynthesis [3,30]. It is well recognized that rodents on CR diet display enhanced food-seeking behavior in an effort to make up for the reduction in caloric intake [27]. Ghrelin is the only known orexigenic peptide that circulates in high concentration and initiates food intake [16]. Ghrelin is predominantly expressed by the X/A-like cells of the stomach [19] but is also expressed in low levels in variety of other tissues [8,28]. During states of negative energy balance and fasting, ghrelin is released from the stomach into the circulation where it crosses the blood–brain barrier and impinges on feeding centers in hypothalamus to induce hunger [16]. In rodents, ghrelin administration stimulates feeding typically in less than 1 h of administration, and chronic ghrelin treatment induces sustained feeding leading to increased bodyweight and adiposity in a GH-independent manner [23]. Ghrelin also regulates hunger in humans as a pre-prandial rise in serum ghrelin levels induces feeding behavior [6]. Ghrelin mediates its orexigenic effects via regulation of leptin expression and the signaling of the NPY [1,2] AGRP and orexin pathways [14]. Ghrelin levels are elevated during fasting and signaling via ghrelin receptor (GHS-R) promotes memory and neurogenesis [7], immune function [8,10,11] and reduces pro-inflammatory cytokines by inhibiting NFkB activity [8,22,13]. One of the hallmarks of CR is reduction in inflammation [12] by regulating NFkB and associated reduction in age-related neurodegenerative changes [5]. Given ghrelin’s effects on key age-related processes, it has been hypothesized that reduction in ghrelin levels may be responsible for ‘anorexia of aging’, increase in low grade chronic inflammation, reduced T cell responses and reduction in muscle and bone mass due to reduced GH levels in humans [10]. Indeed, it was recently reported that older (30–56 years) lean women display lower levels of ghrelin compared to young (19–29 years) lean women [25] and CR in young women (18–24 years) could significantly increase the circulating ghrelin concentrations [31]. In the present study, we demonstrate for the first time that CR in aging mice promotes gastric-ghrelin production and this is related to a marked forestomach hypertrophy in these animals.
Materials and methods
Animals
The female CR (n = 20) mice and ad libitum (AL) fed (n = 20) 10–12-month-old C57/B6 mice were purchased from NIA-aging rodent colony. The calorie restriction was initiated at 14 weeks of age at 10% restriction, increased to 25% restriction at 15 weeks, and to 40% restriction at 16 weeks where it is maintained throughout the life of the animal. Mice were maintained on the CR diet during transit and sacrificed after 1-day rest.
Real-time PCR analysis
The pituitaries and hypothalamus was snap frozen in liquid nitrogen and total RNA was isolated and reverse transcribed and real-time PCR was performed as described previously [9,28].
Immunohistochemistry
The stomachs from AL and CR mice were flushed with cold PBS to remove the gastric contents. The fundus and antrum were dissected and weighed separately and snap frozen in liquid nitrogen. The whole stomachs were fixed in 4% buffered paraformaldehyde and embedded in paraffin and sectioned with 5 mm thickness as described previously [9,11].
The sections were stained with Meyer hematoxylin and eosin. The frozen sections were studied for ghrelin expression using immunoflourescence microscopy as described previously. Briefly, the anti-ghrelin polyclonal antibody (Phoenix Pharmaceuticals) was utilized for labeling. The frozen sections were fixed with cold ethanol and non-specific binding sites were blocked with protein blocking buffer. After overnight incubations with anti-rabbit ghrelin antibody, the sections were labeled with Alexa Fluor 488 (Invitrogen) and counter stained with DAPI (Invitrogen) to visualize the nuclei. Negative controls as obtained by occulting the primary antibody or by using an unrelated IgG displayed no specific labeling. Stomach sections from four AL and CR mice were used for staining and at least three serial sections were utilized for each labeling.
Ghrelin assay
The total immunoreactive ghrelin was measured in duplicate with an ELISA using a rabbit polyclonal antibody against full-length ghrelin based on manufacturer’s instructions (Phoenix Pharmaceuticals).
Results
Chronic caloric restriction in aging mice causes gastric hypertrophy
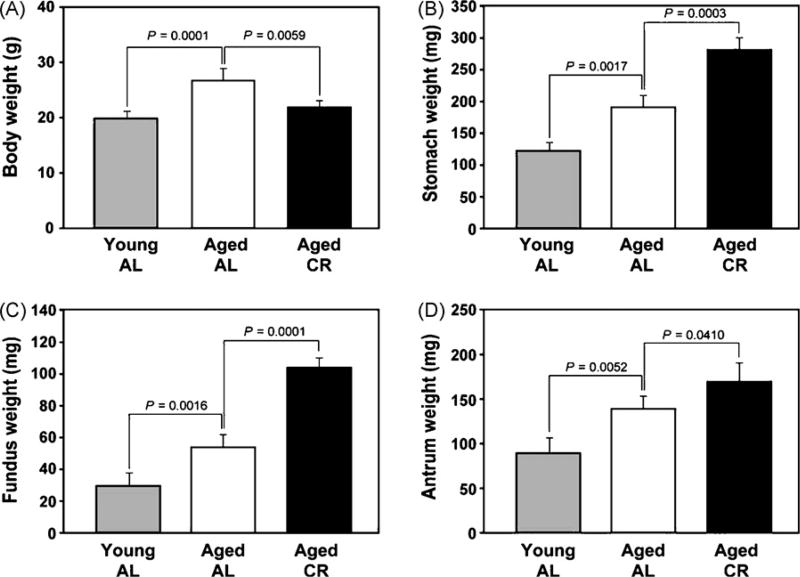
The examination of 10-month-old ad libitum fed (n = 10) and CR (n = 10) female mice revealed that long-term dietary restriction led to a significant reduction in the body weight (Fig. 1A) of mice as well as a proportional reduction in the spleen size (Fig. 2B). Interestingly, we observed that compared to AL fed animals, the stomach from CR mice was markedly enlarged (Fig. 1B) with a significant (p < 0.05) increase in the fundus (Fig. 1C) and antrum weight (Fig. 1D) after complete removal of gastric contents. Given a very striking phenotype of CR mice stomachs, we next confirmed our findings from a separate batch of 12 month AL fed and CR female mice from the NIA-aging rodent colony. The mice were kept on CR diet during the transit and were sacrificed within 48 h of arrival in our animal facility. Similar to 10-month-old mice from a previous batch we observed a marked increase in the size of the forestomach (Fig. 2A) of 12-month-old female mice on a CR diet.
Fig. 1.
Chronic CR decreases body weight and increases the weight of forestomach and antrum of aging mice. (A) Compared to ad libitum (AL) fed young mice (3 month of age) the 10-month-old aged mice (n = 10) had a significant increase in body weight and CR results in marked reduction in body weight of aging mice (10 month). CR in aging mice leads to increase weight of (B), stomach, (C) forestomach and antrum (D).
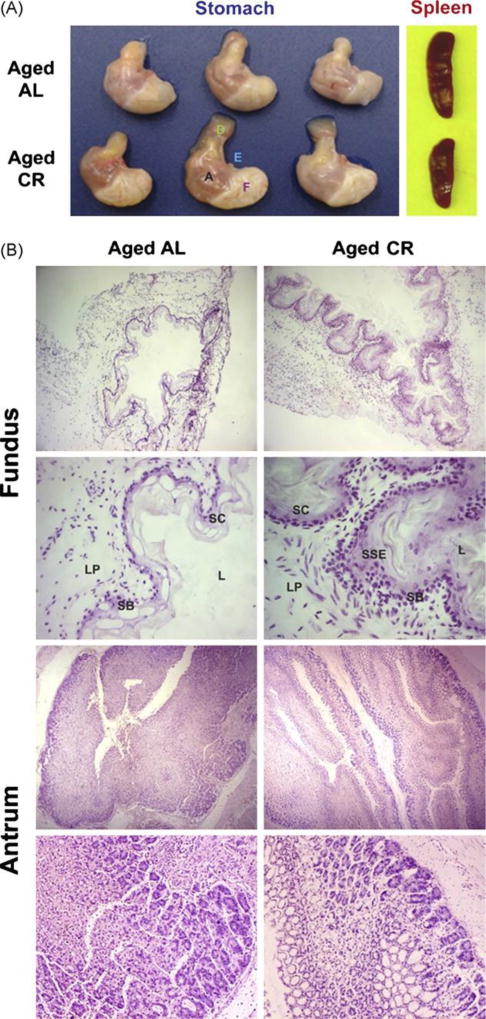
Fig. 2.
Chronic CR causes gastric hypertrophy in aging mice. (A) Representative image of stomach of CR (10 month) and AL fed old mice. There was a large increase in the size of forestomach (f) and antrum (a). The esophagus (e) and duodenum (d) of AL and CR mice did not display any change while spleen size was reduced in aging mice on dietary restriction. (B) Compared to AL fed mice, CR in aging mice resulted in a marked hypertrophy of lamina propria (LP), stratum basale (SB), stratum corneum (SC) and the stratified squamous epithelium (SSE) of forestomach of the aged animals while no significant difference could be detected in the antrum.
We next performed the histological analysis of the stomachs to determine if the increase in the stomach size was a result of specific cellular change or as a consequence of delayed gastric emptying or distension of stomach wall. Analysis of both frozen as well as paraffin embedded stomach sections revealed marked hypertrophy of all the cellular layers. Compare to AL fed mice; old CR animals displayed a marked hypertrophy of lamina propria, stratum basale, stratum corneum, stratified squamous epithelium and the muscularis layers (Fig. 2C). We did not observe any significant cellular changes in the antrum area of stomach.
Chronic CR-induced gastric hypertrophy leads to increased ghrelin production in aging mice
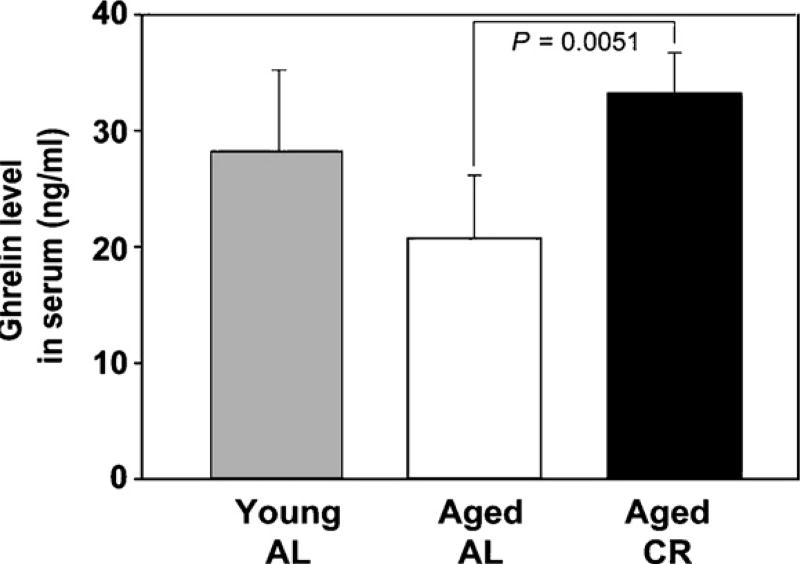
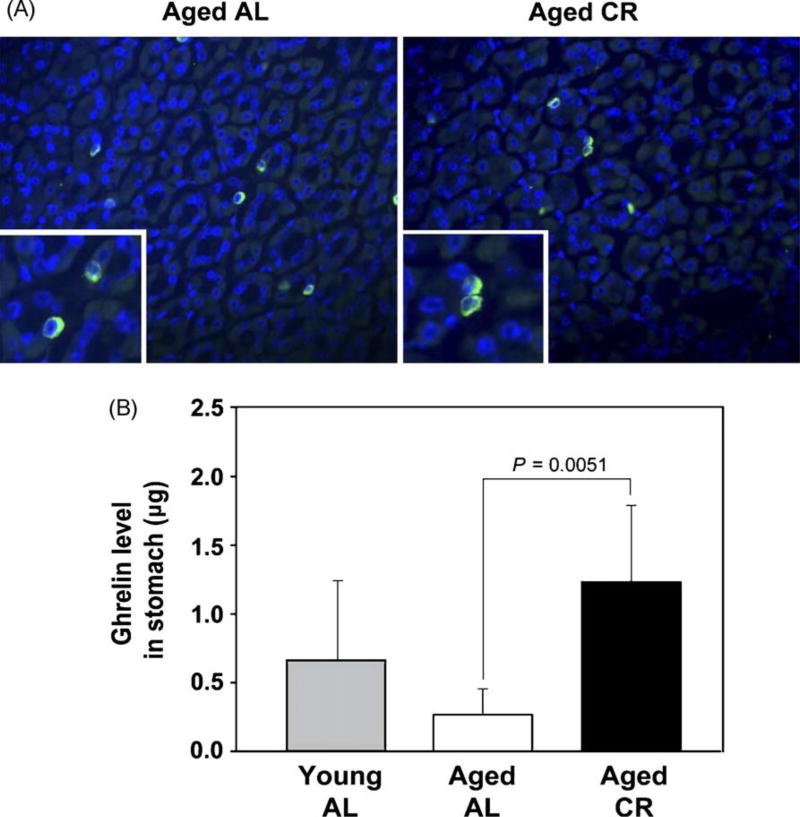
It has been hypothesized that sustained negative energy balance as a result of CR will elicit adaptive metabolic response that is driven by the balance of orexigenic and anorexigenic peptides [10]. Ghrelin is predominantly produced from enter-oendocrine cells in the stomach [19]. Given a marked hypertrophy of stomach in response to CR we next studied the peripheral and stomach-derived ghrelin in AL fed young (3 month) and aging (10–12 month) mice versus the aging female mice on a CR diet (10–12 month). We observed that compared to AL fed young mice, no significant difference in serum ghrelin levels was detected in the aging animals (p > 0.05). Interestingly, the aging mice on long-term CR exhibited a significant (p < 0.005) increase in serum ghrelin compared to the AL fed old mice (Fig. 3). Despite higher circulating ghrelin levels in CR mice, compared to the stomachs of aging mice we did not observe any increase in the number of ghrelin expressing cells in the stomach of CR animals (Fig. 4A). In order to quantify total ghrelin production from stomach, the protein lysates were prepared from the 3 and 12 month AL and 12 month CR mice and analyzed by ELISA. Total ghrelin production in stomach was found to be significantly higher in old CR animals compared to AL fed age-matched controls (Fig. 4B). These data suggest that despite a similar number of ghrelin producing cells in a given section, the overall increase in size of stomach CR mice results in an increase in ghrelin production from stomach.
Fig. 3.
Total ghrelin levels in aging mice on CR diet are significantly increased compared to AL fed age female mice. No significant differences in circulating ghrelin levels were detected in the young (3 month) and old animals (10 month).
Fig. 4.
Total ghrelin production from stomach is increased in aging upon CR. (A) The paraffin embedded stomach section were labeled with anti-rabbit ghrelin IgG, followed by specific Alexa Fluor 488 antibody. The nuclei were counterstained with DAPI. (B) The total ghrelin content in stomach was quantified by ELISA of protein lysates derived from stomachs of AL fed young (3 month), aging (10 month) and CR aging (10 month) female mice. Compared to AL aging mice, CR led to a significant increase in ghrelin production from stomach of aging mice.
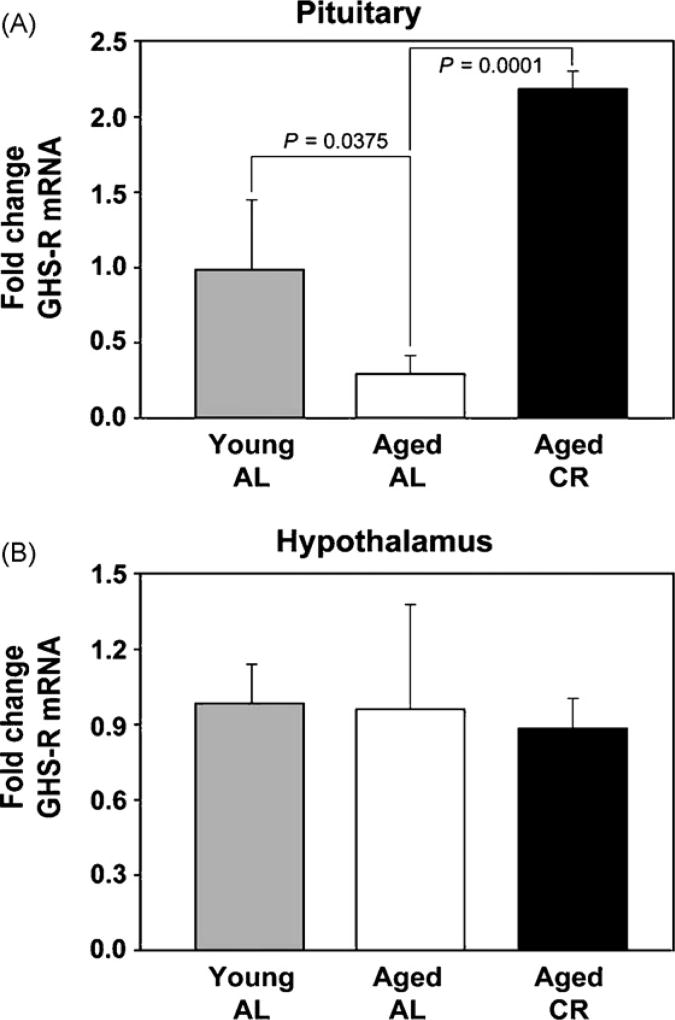
Chronic CR increases GHS-R expression in pituitary of aging mice
We next studied if increase in ghrelin post-CR in the aging mice is associated with changes in its receptor expression in the pituitary and hypothalamus. We demonstrate that compared to young animals, the pituitaries of 10–12-month-old aging mice displayed a significant decrease in GHS-R mRNA. Interestingly, chronic CR led to a marked increase in GHS-R expression in pituitary of the aging mice (Fig. 5A). We did not observe any change in GHS-R expression in the hypothalamus with age or post-CR (Fig. 5B).
Fig. 5.
Effect of chronic CR on age-related changes in GHS-R expression. (A) There is a significant decline in GHS-R mRNA expression in the pituitaries of 10-month-old mice that is completely restored to young levels upon chronic CR. (B) No changes in hypothalamic GHS-R expression were detected during aging and chronic CR did not alter this expression profile.
Discussion
Ghrelin, a stomach-derived orexigenic peptide has received considerable attention over the past years due to its ability to promote food intake, stimulate somatotropic axis, enhance immune function and increase memory and neurogenesis [16,19,8,10,11,7]. Given, aging is associated with deficits in each of these processes; ghrelin and ghrelin receptor mimetics have been proposed as possible target for drug development [10,21]. Caloric restriction is presently the only known intervention that results in robust life span extension effects in various species [17,29,30]. Pro-longevity effects of experimental intervention such as CR are difficult to be translated into clinical practice in humans due to challenges associated with adherence to strict dietary restriction regimens especially in current obesogenic environment [17,27]. Therefore, a major emphasis of ongoing research has been to unravel and harness the pathways activated by CR that could potentially result in future ‘anti-aging pill’ to mimic the CR’s salutary effects on aging [17]. In the present study, we demonstrate that the sustained negative energy balance without malnutrition in aging mice is associated with an increase in ghrelin production along with a marked hypertrophy of forestomach and enhanced hypophyseal GHS-R expression.
Chronic CR causes a reduction in body weight, loss of fat mass and a proportional decrease in the size of internal organs [17,27]. We observed that long-term CR in the aging mice resulted in a significant reduction of body weight with lower subcutaneous and ovarian fat (data not shown) and a decrease in the size of spleen. However, in CR mice we observed an unexpected and a large increase in the size of the forestomach as well as increased weight of fundus and antrum. This finding was first observed in the stomachs of 10-month-old CR mice (n = 10), and further confirmed in separate batch of 12-month-old mice on long-term CR (n = 10). We next demonstrated that this CR-induced increase in size of forestomach was not just a distension of the stomach wall, but is a marked hypertrophy of lamina propria, stratum basale, stratum corneum and the stratified squamous epithelium of forestomach of the aged animals. It is feasible that long-term CR is an adaptive response by the animal that is reflected in the gain of function of stomach to promote the nutrient utilization. Other than stomach, we did not observe any changes in the gross anatomy of the GI tract of CR aging mice.
Given, stomach is the major site of production of ‘hunger hormone’ ghrelin, we next hypothesized that CR during aging will result in increase in the circulating ghrelin levels. Similar to Sun et al. (2006), we did not observe any significant difference in peripheral ghrelin concentrations in young and old mice [28]. Interestingly, upon long-term CR a significant increase in ghrelin production was observed in the aging mice. Recently, it was shown that CR in young women results in increased ghrelin levels in the blood [20,25]. In young rats, moderate short-term CR has also been demonstrated to result in increased ghrelin production [2]. Our data demonstrates that chronic CR specifically up regulates GHS-R expression in the in the pituitary but does not affect GHS-R expression in the hypothalamus. A similar decline in GHS-R expression in pituitary has recently been reported to occur within 6 months of age in C57/B6 mice with no changes in hypothalamus [28]. Together, our current study demonstrates that long-term dietary restriction which is known to enhance healthy life span results in elevated circulating ghrelin levels and CR also reverses the age-related loss of GHS-R expression in the pituitary. We and others have demonstrated that ghrelin via GHS-R specific mechanism serves as a potent endogenous anti-inflammatory signal and inhibits age-related thymic involution [9,11]. Given that CR promotes longevity by reducing the incidence of various age-related inflammatory disorders [12,29,30], our present findings suggest that ghrelin which signals the state of negative energy balance manifested during CR may serve as a new class of CR-mimetic agents with potential therapeutic effects on various age-related degenerative disorders.
Acknowledgments
We would like to thank Drs. Donald K. Ingram, Barry Robert and Hans Rudolph-Berthoud at PBRC for helpful discussions and Ms. Cynthia Kloster for assistance with animal care and husbandry. This work was supported by the Pennington Biomedical Research Center and Foundations. CN was supported by fellowship from Howard Hughes Medical Institute (HHMI) and grant to LSU from HHMI through the Undergraduate Science Education Program. The present work utilized the facilities of the Genomics and CBB Core facilities supported by NIH Grant 1 P20 RR02/1945.
References
- 1.Akimoto-Takano S, Sakurai C, Kanai S, Hosoya H, Ohta M, Miyasaka K. Differences in the appetite-stimulating effect of orexin, neuropeptide Y and ghrelin among young, adult and old rats. Neuroendocrinology. 2005;82:256–63. doi: 10.1159/000092754. [DOI] [PubMed] [Google Scholar]
- 2.Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L, Guarnieri G. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology. 2003;124:1188–92. doi: 10.1016/s0016-5085(03)00281-6. [DOI] [PubMed] [Google Scholar]
- 3.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103:7901–5. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med. 2007;13:64–71. doi: 10.1016/j.molmed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–81. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 7.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 8.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixit VD, Sridaran R, Edmonsond MA, Taub D, Thompson WE. Gonadotropin-releasing hormone attenuates pregnancy-associated thymic involution and modulates the expression of antiproliferative gene product prohibitin. Endocrinology. 2003;144:1496–505. doi: 10.1210/en.2002-220955. [DOI] [PubMed] [Google Scholar]
- 10.Dixit VD, Taub D. Ghrelin and immunity: a young player in an old field. Exp Geron. 2005;40:900–10. doi: 10.1016/j.exger.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Dixit VD, Yang H, Sun Y, Youm YH, Weeraratna AT, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. doi: 10.1172/JCI30248. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–20. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 14.Goto M, Arima H, Watanabe M, Hayashi M, Banno R, Sato I, et al. Ghrelin increases neuropeptide Y and agouti-related peptide gene expression in the arcuate nucleus in rat hypothalamic organotypic cultures. Endocrinology. 2006;147:5102–9. doi: 10.1210/en.2006-0104. [DOI] [PubMed] [Google Scholar]
- 15.Han ES, Evans TR, Shu JH, Lee S, Nelson JF. Food restriction enhances endogenous and corticotropin-induced plasma elevations of free but not total corticosterone throughout life in rats. J Gerontol A Biol Sci Med Sci. 2001;56:B391–7. doi: 10.1093/gerona/56.9.b391. [DOI] [PubMed] [Google Scholar]
- 16.Horvath TL, Diano S, Sotonyi P, Heiman M, Tschop M. Minireview: ghrelin and the regulation of energy balance— a hypothalamic perspective. Endocrinology. 2001;142:4163–9. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 17.Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, et al. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 18.Katzeff HL, Yang MU, Presta E, Leibel RL, Hirsch J, Van Itallie TB. Calorie restriction and iopanoic acid effects on thyroid hormone metabolism. Am J Clin Nutr. 1990;52:263–6. doi: 10.1093/ajcn/52.2.263. [DOI] [PubMed] [Google Scholar]
- 19.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 20.Leidy HJ, Dougherty KA, Frye BR, Duke KM, Williams NI. Twenty-four hour ghrelin is elevated after calorie restriction and exercise training in non-obese women. Obesity. 2007;15:446–55. doi: 10.1038/oby.2007.542. [DOI] [PubMed] [Google Scholar]
- 21.Leite-Moreira AF, Soares JB. Physiological, pathological and potential therapeutic roles of ghrelin. Drug Discov Today. 2007;12:276–88. doi: 10.1016/j.drudis.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–6. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 23.Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27:719–27. doi: 10.1210/er.2006-0028. [DOI] [PubMed] [Google Scholar]
- 24.Rocha JS, Bonkowski MS, de Franca LR, Bartke A. Effects of mild calorie restriction on reproduction, plasma parameters and hepatic gene expression in mice with altered GH/IGF-I axis. Mech Ageing Dev. 2007;128:317–31. doi: 10.1016/j.mad.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Schutte AE, Huisman HW, Schutte R, van Rooyen JM, Malan L, Malan NT. Aging influences the level and functions of fasting plasma ghrelin levels: the POWIRS-Study. Regul Pept. 2007;139:65–71. doi: 10.1016/j.regpep.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Speakman JR, Hambly C. Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J Nutr. 2007;137:1078–86. doi: 10.1093/jn/137.4.1078. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Garcia JM, Smith RG. Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology. 2007;148:1323–9. doi: 10.1210/en.2006-0782. [DOI] [PubMed] [Google Scholar]
- 29.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1-year of age: effect on life span and spontaneous cancer incidence. Science. 1982;215:1415–8. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 30.Wolf G. Calorie restriction increases life span: a molecular mechanism. Nutr Rev. 2006;64:89–92. doi: 10.1301/nr.2006.feb.89-92. [DOI] [PubMed] [Google Scholar]
- 31.Yukawa M, Cummings DE, Matthys CC, Callahan HS, Frayo RS, Spiekerman CF, et al. Effect of aging on the response of ghrelin to acute weight loss. J Am Geriatr Soc. 2006;54:648–53. doi: 10.1111/j.1532-5415.2006.00689.x. [DOI] [PubMed] [Google Scholar]