Abstract
Advancing research in medicine and technology has benefitted the mankind immensely with its contribution toward an improved life quality and increased life expectancy. The inability of a human body to autoregenerate has resulted in an increased demand for newer and healthier tissues and organs. Therefore, the restoration of naturally replicated tissue components has become a subject of interest for the scientific community lately. There was felt an intense quest for promoting strategies that could restore tissue regeneration and fuel the field of regenerative medicine. It was then the role of platelets was accounted for its wound healing and regenerative effects. Consequently, the use of platelet concentrates to improve wound healing, and bone formation was explored, which was considered to be possible because platelets contain high quantities of growth factors which would be able to stimulate cell proliferation, matrix remodeling, and angiogenesis, thereby establishing a new era of research with the successful application of innovative medical therapies focused on healing damaged tissues or regenerate the affected organs.
KEYWORDS: Growth factors, platelets, tissue regeneration, wound healing
INTRODUCTION
The platelets are best known for their importance in clotting blood. However, platelets also contain hundreds of proteins called growth factors which are very important in the healing of injuries. First, the platelets were used for therapeutic purpose in transfusion medicine, wherein the platelet concentrates were originally used for the treatment and prevention of hemorrhage due to severe thrombopenia, which is often caused by medullar aplasia, acute leukemia, or significant blood loss during long-lasting surgery. The standard platelet concentrate for transfusion has been named plate-rich plasma (PRP) which classically contains 0.5 × 1011 platelets per unit.[1,2] PRP is plasma with many more platelets than what is typically found in blood. The concentration of platelets and thereby the concentration of growth factors can be 5–10 times greater (or richer) than usual. To develop a PRP preparation, blood must first be drawn from a patient. The platelets are separated from other blood cells, and their concentration is increased during a process called centrifugation. Then, the increased concentration of platelets when combined with the remaining blood will have a greater concentration of growth factors than whole blood.[1] This product is being used to encourage a brisk healing response in the surgical wounds across several specialties in particular dentistry, orthopedics, and dermatology.[3,4] As a concentrated source of blood plasma and autologous conditioned plasma, PRP contains several different growth factors and cytokines which can stimulate the effective healling of both soft and hard tissues. Although blood is mainly a liquid called plasma, it also contains small solid components red cells, white cells, and platelets which have been found to play a critical role in wound healing and hemostasis as well as in repairing the bone fracture.[2,5]
This review will highlight the innate properties of platelets including wound healing and bone remodeling as well as a brief description about the various therapeutic applications in other fields. This review is an attempt to summarize the research work updated by Dr. Anitua Eduardo since 2000 till 2016.
SEARCH STRATEGY
The methodology to update this review was based on secondary research involving library referencing from the year 2000 to 2017, through online search using keywords such as platelets, platelet concentrates, PRP, wound healing, bone remodeling, and angiogenesis.
DISCUSSION
ROLE OF PLATELETS IN WOUND HEALING
Platelets and macrophages are thought to be dominant regulatory cells in wound healing.[6,7] Activated platelets release locally acting growth factors that initiate division and migration of fibroblasts and formation of new capillaries. Wound healing has been found to be under the control of two processes, angiogenesis and collagen synthesis, which are the expected immediate host's reaction to any tissue injury. The normal wound healing process involves the activated fibroblasts at the wound healing site to divide and produce collagen along with intense capillary proliferation to provide nutrients for the increased cellular activity. Although the exact signaling mechanism still remains elusive, it is debated that the coagulation product itself would enhance the further coagulation. Platelets activated by thrombin release a mitogen (platelet-derived growth factor [PDGF]) for fibroblasts and smooth muscle cells (SMCs) and stimulate increased collagen synthesis by smooth muscle although the neutrophils are the predominant inflammatory cell in the traumatic central dead space of the wound from the 1st to 3rd day following injury. However, macrophages gradually replace the neutrophils 3–4 days after wounding and remain in the central dead space of the wound until repair is complete. Macrophages produce growth factors that stimulate neovascularization, fibroblast proliferation, and migration. It was Matras in 1972 who had first used platelets as sealants to promote homeostasis for surgical procedures. He also had demonstrated the importance of fibrin in reuniting human nerves with the aid of concentrated levels of fibrinogen and factor XIII. Gibble et al. in 1990, provided the initial evidence for the potentiality of this approach, and years later, Marx et al. using the technique of gradient density centrifugation produced PRP with the aim of enhancing the functionality of the bone grafts.[6,7] The autologous platelet products have high therapeutic potential and therefore can be used in various formulations and in various fields of medicine and tissue engineering. Several key biological factors have been found in PRP which are directly involved in tissue repair including insulin-like growth factor type I (IGF-I), Transforming Growth Factor Beta 1, PDGF, hepatocyte growth factor, vascular endothelial growth factor (VEGF), and basic fibroblastic growth factor among others.[8,9] PRP contributes to hemostasis by preventing blood loss at sites of vascular injury as they contain a large number of growth factors and cytokines that have a key role in bone regeneration and soft tissue maturation. In the past two decades, an increased understanding of the physiologic roles of platelets in wound healing after tissue injury has led to the idea of using platelets as therapeutic tools. Platelet concentrate is activated by way of thrombin generation with calcium which is a three-dimensional and biocompatible fibrin scaffold resulting in progressive release of a myriad of growth factors and proteins to the local environment thus contributing to the accelerated postoperative wound healing and tissue repair [Figure 1]. Eventually, autologous PRP as a novel therapeutic alternative has opened up new avenues in other fields including orthopedics, sport medicine, dentistry, periodontal surgery and plastic and maxillofacial surgery, where it creates biological safe environment for an effective and rapid wound healing and tissue repair or regeneration.[8,9]
Figure 1.
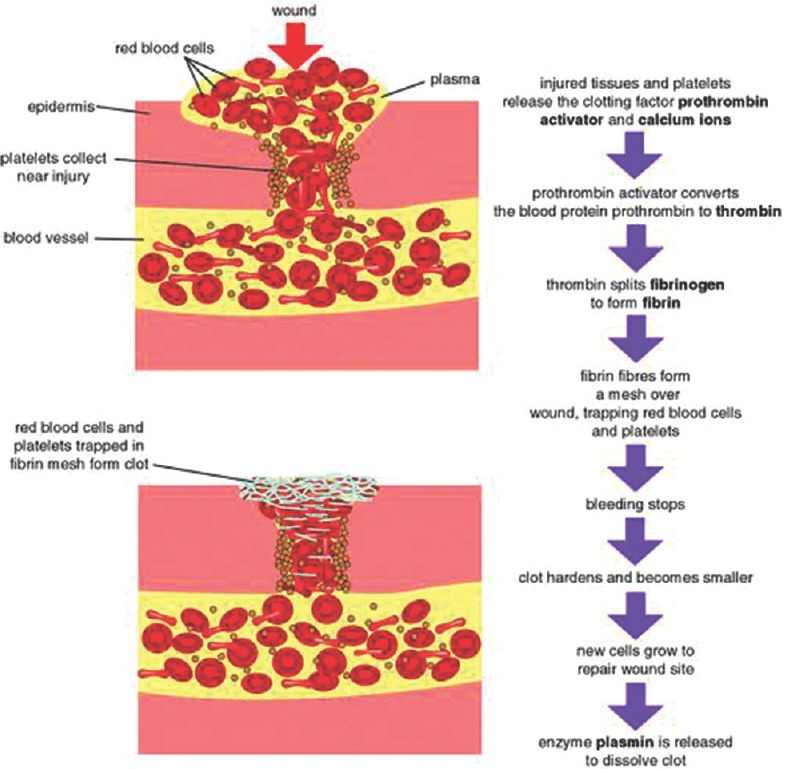
Mechanism of wound healing
Molecular control and signaling involved in wound healing process
As a result of hemostatic response to an external injury, restoration of the integrity of the vascular wall takes place which involves orchestrated proliferation and migration of SMCs, fibroblasts, and endothelial cells for which platelets, growth factors, and chemokines are known to contribute to regulate these processes. The migration and proliferation of SMCs in both the arterial and venous circulation is found to be regulated by PDGF which contributes effectively during hemostasis and tissue healing. Another important mediator in healing and remodeling is platelet-derived stromal cell-derived factor 1α (SDF-1α) which mediates CD34+ bone marrow-derived progenitor cells to the injury site and promote their differentiation into endothelial progenitor cells.[9] Since inhibition of SDF-1α binding to its receptor CXCR4 was shown to retard diabetic wound healing in experimental models by impairing cellular migration while concomitantly prolonging the inflammatory response supports the role of SDF-1α in tissue healing. In addition to angiogenic factors, platelets also store and secrete a number of tumor necrosis factor-α-related apoptosis regulators such as CD95, Apo2-L, and Apo3-L which can bring about apoptosis in other circulating cells as well as in antiapoptotic molecules.[10] Thus, the balance between proapoptotic and antiapoptotic molecules adequately promoting survival or eliminating the cells from the wound site is also crucial for regulation of wound healing. It can therefore be concluded that an in-depth understanding of platelets and their secretions in wound healing and tissue regeneration has led to applications of platelets in multiple fields especially in trauma management [Table 1].
Table 1.
Platelet protein classification and their biological role[1]

ROLE OF PLATELETS IN BONE REMODELING
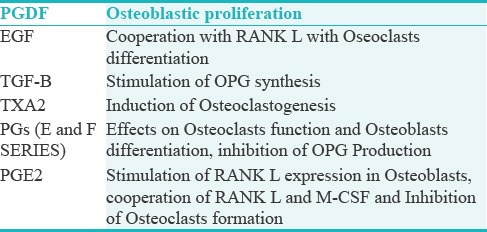
The effects of platelets on bone can be viewed within the frames of bone formation and resorption. Platelets act in both the processes. In acute conditions such as bone fractures, fracture site is surrounded by activated platelets and their aggregates. Degranulation of activated platelets leads to the release of several growth factors (e.g., PDGF, VEGF, IGF-1, IGF-2, EGF, and TGF-B).[11] Degranulation of activated platelets leads to the release of several growth factors (e.g; PDGF, VEGF, IGF-1, IGF-2, EGF and TGF-B), all contributing to the recruitment of osteogenic cells after tissue injury. Platelets release also contains mediators, which may be involved in the bone remodeling (e.g., thromboxane A2 and prostaglandins). The supportive effect of platelets on bone formation was reported in several in vitro and clinical studies where it is been reported that PDGFs promote bone formation by influencing cell proliferation, chemotaxis differentiation, and extracellular matrix synthesis. Some in vitro studies confirmed the contribution of platelets in osteoclastogenesis and bone resorption, but no exact mechanism has been proposed yet. The multidimensional effect of the platelet on bone resorption and bone formation represent a dilemma, as on the one side, there are growth factors enhancing bone formation such as PGDF; on the other side, there are EGF and TGF-B which are more likely to enhance the bone resorption. It is therefore concluded that platelets' effect on the bone is complicated because of the complex interactions between growth factors, inflammatory mediators, and cytokines. In fact, the main effect depends on the specific endothelial gene expression of osteogenesis and osteoclastogenesis. To be successful in bone regeneration and improving damaged bone healing, any substitute should be biologically compatible, nontoxic, and should provide scaffolding for angiogenesis [Table 2].[12]
Table 2.
Components of platelets release and their possible effect on bone[12]
ROLE OF PLATELETS AS BACTERIOSTATIC AGENTS
As it is learnt that growth factors are essentially involved in the regeneration process and are found to be helpful in stimulating cell proliferation (mitosis), cellular migration (chemotaxis), differentiation (morphogenic effect), angiogenesis, and the combination of several of these effects during the tissue regeneration period. In addition to their effective control over angiogenesis and proliferation, another important property of platelets is the bacteriostatic effect. This effect has been observed against Staphylococcus aureus and Escherichia coli bacteria,[7] Porphyromonas Gingivalis, and Actinobacillus actinomycetemcomitans.[13]
APPLICATIONS OF PLATELET CONCENTRATES IN ORAL AND MAXILLOFACIAL SURGERY
The clinical use of PRP may enable patients to achieve satisfactory wound healing involving both the hard and soft tissues with shorter recovery times, reduced levels of postoperative infection, edema, ecchymosis, and blood loss [Table 3].
Table 3.

NOVEL COMBINATIONS AND NEW APPLICATIONS OF PLATELET CONCENTRATES (PLATE-RICH PLASMA)
The roles played by platelets and growth factors have contributed immensely toward understanding their effectiveness in angiogenesis, chemotaxis, and cell proliferation. They are also found to be involved in controlling the synthesis and degradation of extracellular matrix and proteins by binding to the extracellular domain of a target growth factor receptor which in turn activates the intracellular translocation pathways. These findings support the use of different growth factors and cytokines as therapeutic molecules for the repair regeneration of a wide range of tissues [Table 4].
Table 4.
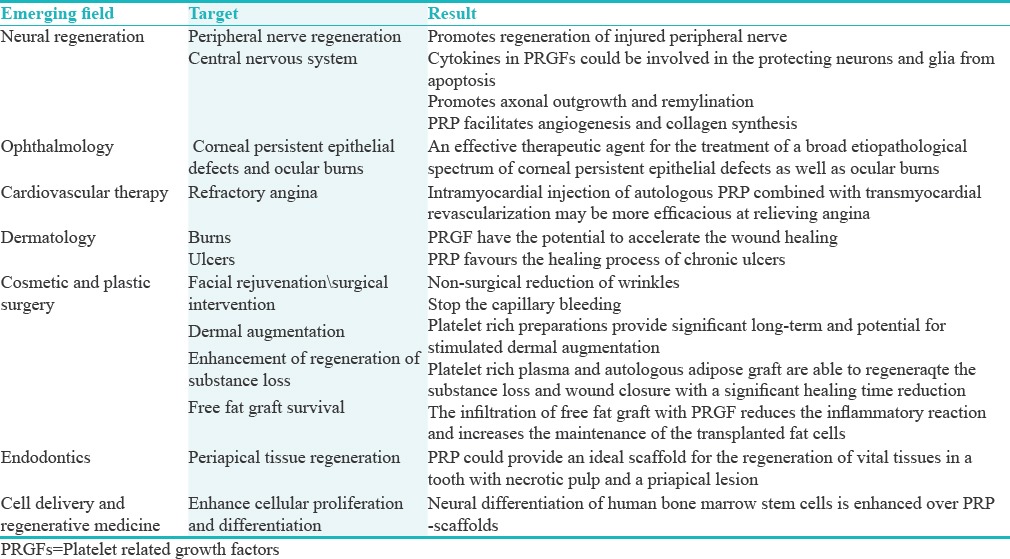
Platelets in combination with bone implants to facilitate the anchorage of the dental prosthesis
studies have investigated that titanium implants when coated with PDGFs before insertion into the host tissues resulted in improved quality of the bone around the implant. This resulted in sufficient osseointegration of the implant and their subsequent longevity which is attributed to the release of considerable growth factors and proteins including TGF-B1, PDGF, IGF, bone sialoproteins, thrombospondin, and osteonectin. Tissue-engineered bone regeneration showed strong correlation between osseointegration and injectable bone comprising of PRP with autogenous bone and mesenchymal stem cells (MSCs) when injected into the implant sites before installation of implant.[11]
Platelets concentrate for repair of the soft connective tissue
Both in vivo and in vitro studies have confirmed that these platelet-rich matrices are safe and effective in accelerating the tendon cell proliferation, stimulating the synthesis of type I collagen and promote neovascularization.[11]
Platelets concentrate for cosmetic surgery
Since the esthetic concerns have raised globally, the application of platelets and their concentrates are being used especially for the soft tissue and bony reconstructions encountered in facial plastic and reconstructive surgery. In these applications, their use has been associated with a decrease in operative time, necessity for drains and pressure dressings, and incidence of complications. Besides, its use is also documented in hair growth therapy where it promotes new hair follicles and strengthens the existing ones.[20]
Platelets concentrate in alveolar cleft
It has been found that in combination with allografts and xenografts, these concentrates have demonstrated effective bone remodeling which is contributed by the wound healing and angiogenic property. This combination in future could possibly become an alternative for autogenous bone graft which requires an additional surgery and carries the risk of donor-side morbidity.[5]
Combination of mesenchymal stem cell and plate-rich plasma
Combination of MSC and PRP was found to shorten the treatment time during distraction osteogenesis when injected at the surgical site consolidation phase by accelerating the bone regeneration safely and effectively.[21]
Platelets concentrate in combination with different bone matrices
There are several reviews and research studies where the use of allograft and xenograft has been suggested along with platelet concentrates which would give support to the matrix and its adaptation to the injured tissue. All needed is a suitable blood supply during the repair as the inadequate blood supply would result in decreased bone volume.[22]
CONCLUSION
Since PRP is a vehicle of mitogenic and chemotactic cytokines and growth factors, it shows to possess beneficial effects for several clinical applications and makes its use more attractive. The quintessential aim of using plasma rich in growth factors is based on the facts that such growth factors booster system will increase the local concentration of growth factors, thereby provide an effective cell-based fibrin scaffold activating the endogenous tissue regeneration mechanism and its intricate nature. However, quest of intense research is always encouraged to understand the intricacies of molecular mechanisms which control different biological cascades to explore novel therapeutic applications of platelets- and plasma-based technologies. The ability to understand all these challenges and pathways played by platelets and their concentrates will definitely avoid any ambiguity surrounding its clinical and therapeutic applicability so that its therapeutic benefits are extended worldwide for better treatment results.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGMENT
The work is dedicated to Yenepoya University and all the authors.
REFERENCES
- 1.Rodriguez IA, Growney Kalaf EA, Bowlin GL, Sell SA. Platelet-rich plasma in bone regeneration: Engineering the delivery for improved clinical efficacy. Biomed Res Int. 2014;2014:392398. doi: 10.1155/2014/392398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. Cell signaling pathways. Cell Signaling Biology: Module 2. 2012:1–130. [Google Scholar]
- 4.Carlson NE, Roach RB., Jr Platelet-rich plasma: Clinical applications in dentistry. J Am Dent Assoc. 2002;133:1383–6. doi: 10.14219/jada.archive.2002.0054. [DOI] [PubMed] [Google Scholar]
- 5.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: From the wound healing to bone regeneration. Immun Ageing. 2013;10:1–10. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anitua E, Alkhraisat MH, Orive G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J Control Release. 2012;157:29–38. doi: 10.1016/j.jconrel.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Anitua E, Prado R, Sanchez M, Orive G. Platelet-rich plasma: Preparation and formulation. Oper Tech Orthop. 2012;22:25–32. [Google Scholar]
- 8.Mazzacco I, Borzitu P, Gope R. Potential of endogenous regenerative technology for an in situ regenerative medicine. Adv Drug Deliv Rev. 2010;62:741–52. doi: 10.1016/j.addr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Zhu F, Zhang M, Zeng D, Luo D, Liu G, et al. Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs. 2013;197:103–13. doi: 10.1159/000342921. [DOI] [PubMed] [Google Scholar]
- 10.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G, et al. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–11. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 11.Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I, et al. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–34. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Sharif PS, Abdollahi M. The role of platelets in bone remodeling. Inflamm Allergy Drug Targets. 2010;9:393–9. doi: 10.2174/187152810793938044. [DOI] [PubMed] [Google Scholar]
- 13.Badade PS, Mahale SA, Panjwani AA, Vaidya PD, Warang AD. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J Dent Res. 2016;27:300–4. doi: 10.4103/0970-9290.186231. [DOI] [PubMed] [Google Scholar]
- 14.Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: A case series. J Biomed Sci. 2017;24:16. doi: 10.1186/s12929-017-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai LL, Huang YF, Chang YC. Treatment of bisphosphonate-related osteonecrosis of the jaw with platelet-rich fibrin. J Formos Med Assoc. 2016;115:585–6. doi: 10.1016/j.jfma.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Baslarli O, Tumer C, Ugur O, Vatankulu B. Evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. Med Oral Patol Oral Cir Bucal. 2015;20:e111–6. doi: 10.4317/medoral.19999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder CC, Scariot JS, Ribeiro JC, Deliberador TM, Giovanini AM. Platelet rich plasma (PRP) produces an atherofibrotic histophenotype during craniofacial bone repair due to changes of immunohistochemical expression of Erk1/2, p38α/β, adiponectin and elevated presence of cells exhibiting B-scavenger receptor (CD36+) Braz Dent J. 2016;27:243–54. doi: 10.1590/0103-6440201602450. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande RS, Yadav AO, Borle RM, Kala AV, Jajoo SN, Thakkar D, et al. Comparative efficacy of autologous alveolar bone grafting with autologous platelet-rich plasma and without platelet-rich plasma in cleft alveolus patients. J Cleft Lip Palate Craniofacial Anomalies. 2017;4:22–5. [Google Scholar]
- 19.Baniasadi B, Evrard L. Alveolar ridge preservation after tooth extraction with DFDBA and platelet concentrates: A Radiographic retrospective study. Open Dent J. 2017;11:99–108. doi: 10.2174/1874210601711010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30:157–71. doi: 10.1055/s-0034-1372423. [DOI] [PubMed] [Google Scholar]
- 21.Menezes DJ, Shibli JA, Gehrke SA, Beder AM, Sendyk WR. Effect of platelet-rich plasma in alveolar distraction osteogenesis: A controlled clinical trial. Br J Oral Maxillofac Surg. 2016;54:83–7. doi: 10.1016/j.bjoms.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Ueda M, Hibi H, Nagasaka T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: From basic research to clinical case study. Cell Transplant. 2004;13:343–55. doi: 10.3727/000000004783983909. [DOI] [PubMed] [Google Scholar]


