Abstract
Objective:
The aim of our study was to retrospectively validate a previously described rapid clinical score (RCS) in distinguishing tuberculous meningitis (TBM) from viral meningitis (VM) in people who are at increased risk of tuberculosis, as well as from cryptococcal meningitis (CM) in HIV-infected patients.
Methods:
We performed a retrospective study of patients admitted with a diagnosis of aseptic meningitis between January 2012 and December 2015, to a referral hospital for infectious diseases. The variables included in RCS were duration of symptoms before admission, neurological stage, cerebrospinal fluid (CSF) to blood glucose ratio, and CSF protein. We included in this retrospective study 31 patients with definite or probable TBM including 14 HIV-infected patients, 62 HIV-noninfected patients with VM, and 18 HIV-infected patients with CM.
Results:
The sensitivity of RCS to distinguish TBM from VM was 96.7%, with a specificity of 81.1% and the area under the receiver operating characteristic (ROC) curve was 0.949 (0.90–0.99). When all four criteria from the RCS were present, the specificity increased at 100%. In HIV-infected patients, the sensitivity and specificity of RCS in differentiating TBM from CM were 86.6% and 27.7%, respectively, and the area under the ROC curve was 0.669 (0.48–0.85).
Conclusion:
This easy-to-use RCS was found to be helpful in differentiating TBM from VM, with a better sensitivity than molecular amplification techniques and a relatively good specificity. However, the RCS was not useful to differentiate between TBM and CM in HIV-infected patients.
Keywords: Clinical score, diagnosis, tuberculous meningitis
INTRODUCTION
The outcome of tuberculous meningitis (TBM) is greatly influenced by the time to treatment initiation. Therefore, establishing a rapid diagnosis is crucial. A rapid clinical score (RCS) was found to have good sensitivity and specificity in differentiating TBM from viral meningitis (VM).[1]
The aim of our study was to retrospectively validate the RCS in distinguishing TBM from VM in people who are at increased risk of tuberculosis and evaluate its performance in differentiating TBM from cryptococcal meningitis (CM).
METHODS
We performed a retrospective study of patients admitted with a diagnosis of aseptic meningitis between January 2012 and December 2015, to a referral hospital for infectious diseases, from Bucharest, Romania. We reviewed medical records of all adults with clinical symptoms of meningitis (fever, headache, nausea/vomiting, neck stiffness), who had a clear appearance of cerebrospinal fluid (CSF) and abnormal CSF findings: pleocytosis ≥5 cells/mm3, the absence of bacteria either on Gram stain and/or in routine culture, and/or negative latex agglutination tests for bacterial antigens.
Patients were considered to have definite TBM if they had a microbiological confirmation of Mycobacterium tuberculosis from a CSF sample by either detection of acid-fast bacilli on CSF smear, positive culture for M. tuberculosis, and/or positive GeneXpert MTB/RIF (Cepheid, Sunnyvale, USA). Probable and possible TBM were defined according to a published consensus case definition and scoring system.[2]
We included patients with VM in HIV-noninfected patients. Patients were considered to have VM if bacterial and other noninfectious causes of meningitis were excluded, a viral cause was identified, or the outcome was favorable under antiviral or supportive treatment.
Diagnosis of CM in HIV-infected patients was based on positive India ink stain, culture, and/or cryptococcal antigen assay.
We excluded patients with possible TBM, prior antibiotic therapy, malignancy, and autoimmune disorders with CNS involvement.
We evaluated a previously described RCS that uses four variables: duration of symptoms before admission (DSBA), neurological stage according to the Medical Research Council (MRC) definitions, CSF to blood glucose ratio, and CSF protein to assess the probability of TBM in patients with clear CSF meningitis. Neurological stages were classified according to the MRC definitions as follows: Stage I - fully conscious and no focal deficits; Stage II - confusion, lethargy, focal neurological signs such as cranial nerve palsies; Stage III - stuporous or comatose, multiple cranial nerve palsies, or complete hemiparesis.[3]
The RCS confers 3 points for a DSBA ≥5 days or CSF/blood glucose ratio < 0.5, 2 points for neurological Stages II and III, and 1 point for CSF protein >100 mg/dl. A score of at least 6 points is suggestive of TBM with a sensitivity of 92% and a specificity of 94%.[1] We calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the cutoff of 6 and 9 points (all four criteria were present).
Statistical analysis
Sensitivity and specificity were calculated with CAT maker. The diagnostic value of the score was assessed by calculating the area under the receiver operating characteristics (ROCs) curves with SPSS version 19.0 (Statistical Package for the Social Science Inc., Chicago, IL, USA). The study was approved by the Hospital Ethics Committee.
RESULTS
We identified 31 patients with TBM, 62 patients with VM, and 18 patients with CM. Among patients with TBM, there were 21 (67.7%) patients with definite and 10 (32.3%) with probable TBM. HIV infection had been diagnosed in 14 (45.2%) patients with TBM. Seven (50%) HIV-infected patients were intravenous drug users. TBM diagnosis was confirmed by culture in 6 (19%) patients, GeneXpert MTB/RIF in 7 (23%) patients, and both culture and GeneXpert MTB/RIF in 8 (26%) patients.
We compared clinical, laboratory, and cerebral imaging findings from the 31 definite and probable TBM patients with those of the 62 patients with VM [Table 1].
Table 1.
Comparison of the clinical and laboratory characteristics in tuberculous and viral meningitis
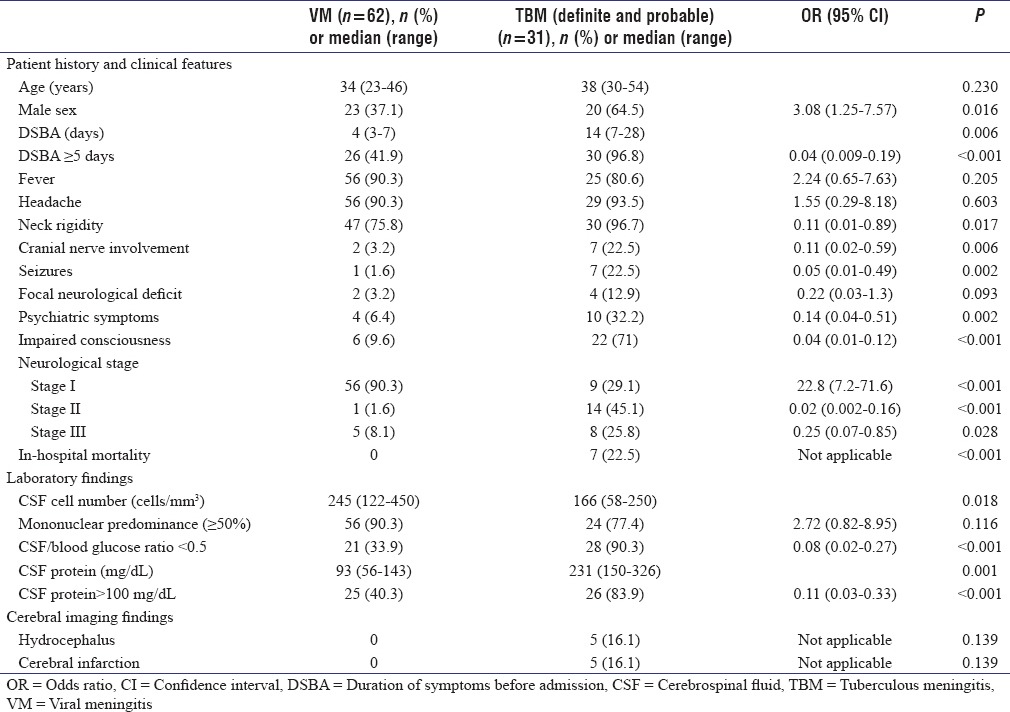
When comparing TBM with CM, a DSBA ≥5 days was noted in 30 (96.8%) patients with TBM versus 12 (67%) patients with CM (OR: 0.14 [95% CI: 0.01–1.36], P = 0.09); presence of neurological Stage II or III in 22 (71%) patients with TBM versus 14 (78%) patients with CM (OR: 1.27 [95% CI: 0.25–6.27], P = 1); CSF/blood glucose ratio <0.5 in 28 (90.3%) patients with TBM versus 13 (72%) patients with CM (OR: 0.2 1 [95% CI: 0.02–2.13], P = 0.35); CSF protein >100 mg/dl in 26 (83.9%) patients with TBM versus 7 (38.9%) patients with CM (OR: 0.11 [95% CI: 0.02–0.68], P = 0.02).
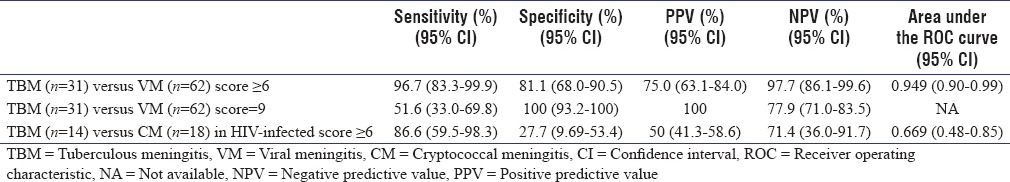
We calculated the sensitivity, specificity, PPV, and NPV for differentiating TBM from VM using a cutoff of at least 6 points or at least 9 points and from CM using a cutoff of at least 6 points [Table 2].
Table 2.
Diagnostic accuracy of rapid clinical score for the diagnosis of tuberculous meningitis
DISCUSSION
In this study, we aimed to validate a previously described RCS for TBM meningitis, in patients hospitalized during the four years that followed the time period when the original study was conducted. We could show a similar sensitivity of the RCS but a lower specificity.
In countries with a high prevalence of tuberculosis, differentiating TBM from other causes of acute lymphocytic meningitis might be an important issue. Since direct microscopic examination of CSF has a low sensitivity, results of culture are delayed, and molecular techniques continue to have a variable sensitivity; easy-to-use diagnostic scores might still play a role for identifying patients with TBM. The Xpert MTB/RIF which has been approved since 2010 for the diagnosis of pulmonary tuberculosis showed high specificity (99%) but relatively low sensitivity (55%–59%) in detecting M. tuberculosis in CSF; larger volumes of concentrated CSF with centrifugation increases the sensitivity of Xpert MTB/RIF to that of CSF culture (72%).[4,5,6] In addition, these tests may not be always available.
We found that the RCS had a modest specificity (81.1%) but a high sensitivity of 96.7% for differentiating TBM from VM. When all four criteria from the RCS were present, the specificity increased at 100%. The area under the ROC curve for TBM probabilities was similar in the original studied group and in the population on which RCS was described (0.949, 0.977, respectively).
Although in the population in which the RCS was originally developed, only 9% of TBM patients were HIV infected; in the present study, 45% of TBM patients were HIV infected. This might be associated with an increase among new HIV cases attributed to injecting drug use, from < 3% before 2010 to 19%–30% starting with 2011.[7] Thus, the score maintains a good sensitivity for identifying TBM regardless of HIV status.
Since persons living with HIV (PLHIV) are at increased risk for both TBM and CM, a RCS that would differentiate between the two conditions would be helpful in clinical practice. However, when applying the RCS in PLHIV for differentiating between TBM and CM, sensitivity was modest, while specificity was poor suggesting that the scoring system cannot be used for this purpose. Regarding CM and TBM in HIV patients, although several studies have identified clinical and laboratory features associated with TBM (fever, neck stiffness, altered consciousness, CSF neutrophil predominance, CSF pleocytosis, CSF protein >1 g/L) or with CM (nausea and vomiting, high CSF opening pressure, low CSF white blood cell count), these features are nonspecific and can be present in both conditions making an accurate differentiation between TBM and CM on this basis challenging.[8,9] However, CM diagnosis can easily be made using rapid and accessible tests that have been shown to have excellent sensitivities and specificities of 98%–99% when performed on both blood and CSF samples.[10]
The main limitations of our study are its retrospective nature and the small number of patients.
CONCLUSION
The RCS might be a useful tool in patients with high risk of TBM in differentiating TBM from VM. Although this RCS cannot be used to differentiate between TBM and CM in HIV-infected patients, there are other highly sensitive and easily accessible methods available for diagnosing CM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hristea A, Olaru ID, Baicus C, Moroti R, Arama V, Ion M. Clinical prediction rule for differentiating tuberculous from viral meningitis. Int J Tuberc Lung Dis. 2012;16:793–8. doi: 10.5588/ijtld.11.0687. [DOI] [PubMed] [Google Scholar]
- 2.Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10:803–12. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 3.Medical Research Council. Streptomycin treatment of tuberculous meningitis. Lancet. 1948;1:582–96. [PubMed] [Google Scholar]
- 4.Nhu NT, Heemskerk D, Thu do DA, Chau TT, Mai NT, Nghia HD, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52:226–33. doi: 10.1128/JCM.01834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pink F, Brown TJ, Kranzer K, Drobniewski F. Evaluation of Xpert MTB/RIF for detection of Mycobacterium tuberculosis in cerebrospinal fluid. J Clin Microbiol. 2016;54:809–11. doi: 10.1128/JCM.02806-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahr NC, Tugume L, Rajasingham R, Kiggundu R, Williams DA, Morawski B, et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert ® MTB/RIF of centrifuged CSF. Int J Tuberc Lung Dis. 2015;19:1209–15. doi: 10.5588/ijtld.15.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockholm: ECDC; 2016. European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2015. [Google Scholar]
- 8.Cohen DB, Zijlstra EE, Mukaka M, Reiss M, Kamphambale S, Scholing M, et al. Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop Med Int Health. 2010;15:910–7. doi: 10.1111/j.1365-3156.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- 9.Vidal JE, Peixoto de Miranda EJ, Gerhardt J, Croda M, Boulware DR. Is it possible to differentiate tuberculous and cryptococcal meningitis in HIV-infected patients using only clinical and basic cerebrospinal fluid characteristics? S Afr Med J. 2017;107:156–9. doi: 10.7196/SAMJ.2017.v107i2.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang HR, Fan LC, Rajbanshi B, Xu JF. Evaluation of a new cryptococcal antigen lateral flow immunoassay in serum, cerebrospinal fluid and urine for the diagnosis of cryptococcosis: A meta-analysis and systematic review. PLoS One. 2015;10:e0127117. doi: 10.1371/journal.pone.0127117. [DOI] [PMC free article] [PubMed] [Google Scholar]


