Abstract
Introduction:
In Guillain Barre syndrome (GBS), worsening of weakness or disability after initial period of recovery or stabilization is described as treatment-related fluctuations (TRF).
Aim:
This study aims to describe the clinical characteristics and outcome of six patients with GBS and TRF.
Patients and Methods:
Six patients with GBS fulfilling NINCDS criteria, evaluated at a tertiary care university hospital during 2008–2017, were diagnosed to have TRF. They form the basis of this report.
Results:
All patients were men and their mean age was 40 years. At presentation, mean duration of illness was 15 days; the illness had plateaued in three and progressive in other three patients. Two of the four patients had variant GBS. Initially, five patients were treated with large volume plasmapheresis (LVPP) and one patient with methyl prednisolone. At 17–28 days after disease onset, three patients developed new neurologic deficits (bilateral facial paresis in two; paralytic ileus in one). Other three patients with worsening of limb weakness (medical research council sum score of >5) and disability (Hughes disability grade by ≥1) fulfilled Kleyweg's criteria for TRF. All the six patients were treated with the completion of five cycles or additional cycles of LVPP.
Conclusion:
Awareness about TRF is essential for correct diagnosis and management of patients with GBS.
Keywords: Chronic inflammatory demyelinating polyneuropathy, Guillain Barre syndrome, large volume plasmapheresis, treatment-related fluctuations
INTRODUCTION
The spectrum of acquired inflammatory demyelinating neuropathies includes Guillain Barre syndrome (GBS), subacute inflammatory demyelinating polyradiculoneuropathy (SIDP), and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). GBS is an acute immune-mediated monophasic illness. Limb weakness and cranial palsies evolve over a period of few days to 4 weeks.[1] This is followed by a plateau of few days to several weeks. Recovery begins 2–4 weeks after cessation of progression.[2] Further, recurrences are rare, seen in about 2%–5% of the patients.[3]
On the contrary, CIDP is characterized by the evolution of weakness over a period of two or more months, remission, and relapses.[4] In addition, a group of patients with a progressive phase of four to eight weeks and a monophasic course have been described as SIDP.[5,6] These differences in the clinical profile are probably related to the longer duration of aberrant immune response that involves autoreactive T-cells, macrophages, and autoantibodies, and consequently, the duration of immune-mediated nerve damage in SIDP and CIDP as compared to GBS.[7] Similarly, the relapse in CIDP is probably related to reactivation of aberrant immune response resulting in recurrence of nerve damage and consequently limb weakness and other symptoms.
After the introduction of intravenous immunoglobulin (IVIG) and large volume plasmapheresis (LVPP), few investigators have reported worsening of weakness after the onset of improvement or a plateau phase.[8,9,10] These happen very early in the course of illness and have been called as “Treatment-related fluctuations (TRF)” which are different from relapses and CIDP.[9,10] TRF may either be improvement in disability score of at least one grade or in Medical Research Council (MRC) sum score of more than five points within 4 weeks, followed by a reduction in the MRC sum score of more than five points or a worsening in functional disability score of at least one grade. It can be stabilization of the clinical course for more than a week followed by a worsening of more than five points on the MRC sum score or at least one grade of the functional score.[11] Improvement, stable period, and worsening should be documented at least on two subsequent examinations, with an interval of three to 7 days, by the same investigator.[11] Ruts et al. opined that the worsening in neurological status occurs after completion of treatment and within few months after onset of illness.[12] The Kleyweg's criteria defined TRF within 4 weeks of disease onset whereas Ruts criteria. (2005) mentions that TRF occurs after completion of treatment and within few months after disease onset (ADO).[11,12] The occurrence of TRF is reported to be around 8%–16%, but rates can be as high as 30% to 70%.[8,9,10,13,14] TRF comprises of worsening of limb weakness as measured by MRC sum score or Hughes disability (HD) grade by five points or one grade, respectively. We report our observations with respect to worsening of patient status after onset of recovery or plateau phase.
PATIENTS AND METHODS
Six patients with GBS and its variants fulfilling NINCDS (1990) criteria were assessed prospectively during the period 2008–2017.[2] All the patients underwent detailed clinical examination and disability grading during hospital stay and follow-up. History of antecedent events, sensory symptoms, progress of motor weakness, cranial nerve involvement, autonomic involvement, and respiratory involvement was noted. Muscle power grading was done as per MRC grade.
MRC sum score was calculated as the sum of muscle power (MRC grade) of bilateral arm abductors, elbow flexors, wrist extensors, hip flexors, knee extensors, and foot dorsiflexors.[11] The total MRC sum score ranges from 0 to 60. The score is the sum of the MRC score of 6 muscles on both sides, each muscle graded from 0 to 5. HD grading was done at initial evaluation, peak of illness, during hospital stay, discharge, and subsequently during follow-up.[15] All patients were treated with five cycles of LVPP; one patient had also received steroids.
RESULTS
Mean age at presentation was 40 years (range: 18–72 years). All were men. Patients presented at a mean duration of 15 days to hospital (range: 4–40 days ADO). The mean onset to peak duration was 9.1 days (range: 5–15 days). All of them had predominant lower limb weakness. In upper limbs, distal weakness was more severe than proximal in five cases; in lower limbs, similar grade of muscle weakness was seen in both proximal and distal muscle in three cases. Patient details of stabilization/improvement and worsening and recovery are depicted in Tables 1 and 2, respectively.
Table 1.
Patient details of stabilization/improvement
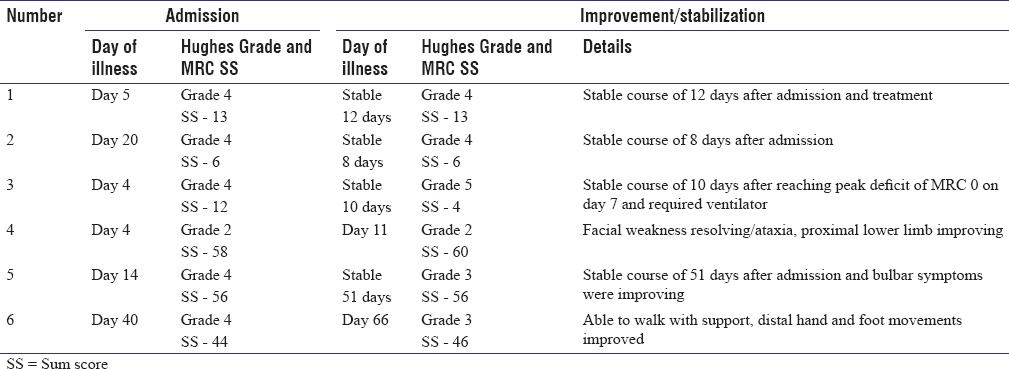
Table 2.
Patient details of worsening: Treatment related fluctuation and Recovery
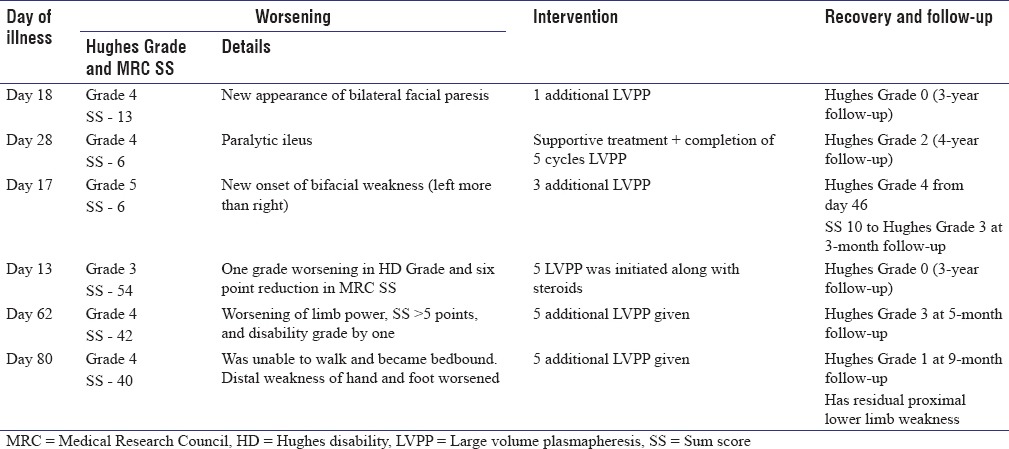
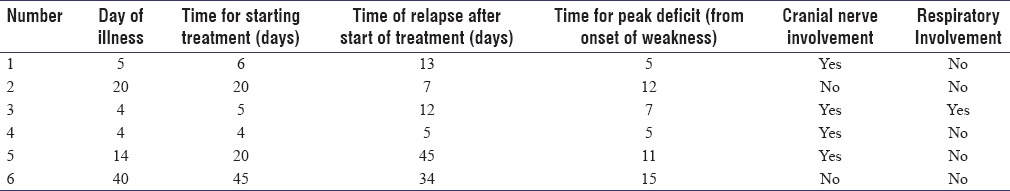
At admission, three patients had plateaued, and three were progressing in illness. Mean time of starting treatment from onset of illness was 16.7 days. Mean time of relapse after starting treatment was 19.3 days (range 5–45 days). Two of them had preceding antecedent event (febrile illness and diarrhea). Four of them had cranial nerve involvement at presentation (Facial nerve palsy in four and bulbar palsy in one). Only one of them had respiratory involvement. One had blood pressure fluctuations as autonomic involvement. Two had variant forms of GBS (GBS/Miller-Fischer Overlap syndrome). Cerebrospinal fluid (CSF) protein ranged between 87 and 217 mg % (mean: 155.1 mg %).
Clinical characteristics of TRF are shown in Table 3.
Table 3.
Clinical characters of treatment-related fluctuation
Patient 1
A 20-year-old young man presented with progressive areflexic quadriparesis on the 5th day of illness. His MRC sum score was 13 and HD Grade was 4 at admission. His conduction study revealed pure motor demyelinating neuropathy. The patient was treated with five cycles of LVPP. HD Grade remained as 4. On day 18 of illness, after completion of LVPP, he developed new onset asymmetric lower motor type of facial palsy. The patient was given one cycle of additional LVPP and he subsequently made gradual improvement to HD Grade 2 at 3 months and Grade 0 at the end of 3-year follow-up. Interestingly, the patient had past history of GBS in his childhood.
Patient 2
A 52-year-old male presented with acute areflexic quadriparesis on day 20 of illness. His MRC sum score was 6 and HD Grade was 4. He was in plateau stage for 8 days. His conduction studies revealed pure motor demyelinating neuropathy. He was treated with three cycles of LVPP. On day 28 of illness (after 16 days of stable course), he developed paralytic ileus. His serum potassium was normal, and his X-ray abdomen showed dilated bowel loops with fluid levels. He was evaluated by general surgeons who excluded surgical causes. He was initiated on parenteral feeding, treated conservatively, and subsequently improved over the next 4 days. Subsequently, the remaining two cycles of LVPP were continued, and the patient stabilized. He remained to be in HD Grade 4 at 3-month follow-up and had incomplete recovery (foot drop) with HD Grade 2 at the end of 4-year follow-up.
Patient 3
An 18-year-old boy presented with pain and paresthesias of proximal lower limb followed by upper limbs and mild breathing difficulty of 4 days' duration. His MRC sum score was 12 and HD Grade was 4. He progressively worsened over the next 3 days to limb power of 0/5, MRC sum score 0 and required ventilator support. His conductions were suggestive of pure motor axonal neuropathy. He was treated with five LVPP and gradually started improving and MRC sum score became 4 with HD still 5 by day 12 of illness.
On day 17 of disease onset (four days after fifth LVPP), he developed asymmetric bifacial weakness despite his limb power showing improvement (MRC sum score of 6 and HD Grade 5). He was given additional three cycles of LVPP plasmapheresis, and he gradually showed minimal improvement in limb power with HD Grade 4 and was extubated on day 46. At follow-up, 3 months later, his sum score was 36 and Hughes Grade was 3.
Patient 4
A 28-year-old young man presented on day four of illness with a stable plateau phase of two days. He had minimal limb weakness, mild gait ataxia (cerebellar type) and was ambulant. His nerve conductions showed evidence of motor sensory demyelinating and axonal neuropathy with sural sparing pattern. His HD Grade was 2 and MRC sum score was 58. With a clinical diagnosis of GB variant, he was treated with injectable methyl prednisolone. By day 11, his facial weakness resolved, ataxia and proximal weakness improved, and he had MRC sum score of 60. However, on day 13, he worsened in HD Grade from 2 to 3. His MRC sum score was 54. The worsening persisted for 4 days. LVPP was initiated along with pulse methylprednisolone, and he significantly improved 4 days later (MRC sum score was 60 and HD was 3). He improved in facial weakness, was able to walk, although ataxia persisted during discharge and had HD Grade 2 at 3 months, Grade 1 at 1 year, and Grade 0 at the end of 3 years.
Patient 5
A 72-year-old male presented with 2 weeks history of progressive tingling paresthesia of upper limbs, areflexic quadriparesis, and bulbar weakness of three days' duration. He had bilateral lower motor facial weakness, binocular mild abduction restriction, and limb incoordination (cerebellar type). His admission MRC sum score was 56 and HD Grade was 4. He was diagnosed as GBS variant and started on LVPP. He started showing improvement in limb power, walked with two-person support, 3 days after first LVPP and his bulbar symptoms started gradually improving. He developed hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion and developed altered sensorium and seizures 2 weeks after admission. After a stable period of 7 days after completion of fifth LVPP, his lower limb weakness worsened, and he became bedbound with difficulty in flexing limbs in bed. His MRC sum score became 42 and HD was 4. Because of worsening in MRC sum score and HD Grade around 62 days ADO, possibility of TRF was considered and five more LVPP cycles were given. After two cycles of repeat LVPP, he could move limbs in bed and facial weakness started improving. His MRI showed enhancement of lumbosacral roots and serial CSF protein done in view of diagnostic uncertainty was 270 mg% on day 28 with five cells,422 mg% on day 46 with 15 cells, 239 mg% with six cells on day 52 of illness, and increased to 546 mg% with three cells on day 62 after worsening on day 62 after worsening.
His conduction studies revealed motor and sensory demyelinating polyneuropathy with sural sparing pattern. He showed gradual improvement and could walk with walker support at the end of 5-month follow-up.
Patient 6
A 50-year-old male presented with tingling paresthesia of soles and progressive weakness of limbs of 40 days' duration. His disease onset to peak of illness interval was 15 days and had plateaued for 25 days at presentation. He had poliomyelitis in childhood with deformed atrophic and short left lower limb but could walk independently and could work as driver before the illness onset. On examination, he had deformed left lower limb with contracture, had distal upper limb weakness, distal more than proximal weakness of lower limbs, impaired proprioception and sluggish to absent reflexes, and was wheelchair bound. His MRC sum score was 44 and HD Grade was 4. His conduction studies revealed demyelinating and axonal motor and sensory neuropathy and his CSF protein was elevated (98 mg %). As he had not improved 40 days after onset of illness and 25 days of plateau phase, he was treated with LVPP and subsequently discharged. The patient improved gradually after 15 days of completion of five cycles of LVPP. He had improvement in his distal upper and lower limb weakness, could button unbutton, feed himself, could dorsiflex his right foot, and was ambulant with crutches support (MRC sum score was 46 and HD was 3). However, he presented again on day 80 of disease onset (day 34 after start of treatment) with worsening of ten days' duration, had paresthesia of both hands, his distal upper limb and ankle power worsened to around 0–1 power, and became bedbound. He was diagnosed as TRF, and LVPP was initiated again. After 2 weeks, he reported dramatic improvement of distal upper limb and lower limb weakness and could walk with support (HD Grade was 4 and MRC sum score was 40). He showed steady improvement and is independent for ambulation at almost 8½ months follow-up.
DISCUSSION
In the present study, six patients demonstrated the appearance of new neurologic deficits or worsening of clinical status after a plateau phase (four patients) or onset of recovery (two patients). Four of them had GBS like presentation and two patients had GBS variant presentation (patient no. 4 and 5). One patient also developed respiratory failure and required ventilatory assistance. Nerve conduction studies demonstrated pure motor demyelinating neuropathy in two patients (patient no. 1 and 2), pure motor axonal neuropathy in one patient (patient no. 3), motor sensory demyelinating neuropathy with axonopathy in two patients (patient no. 4 and 6), and motor sensory demyelinating neuropathy in one patient (patient no. 5). Patients were treated with five cycles of LVPP (patient no. 2 and 4), additional 1–3 LVPPs (patient no. 1, 3, and 5), and one (patient no. 4) received methyl prednisolone additionally.
The worsening of neurological deficits was seen at a mean duration of 36 days after the plateau phase or onset of recovery. It resulted in decrease in more than 5 point of MRC sum score and of HD Grade by one in patient no. 4, 5, and 6. Among our six patients, only three (patient no. 4, 5, and 6) fulfill Kleyweg's and Rut's criteria for TRF.[11,12] Patient no. 4 had worsening of one HD Grade at <three days after onset of recovery, and he improved after 4 days after initiating LVPP.
In three other patients (patient no. 1, 2, and 3), worsening comprised of appearance of new neurologic deficits: bilateral facial paresis in patient no. 1 and 3; paralytic ileus in patient no. 2. As all other causes of paralytic ileus had been excluded, paralytic ileus was considered to be secondary to involvement of autonomic nervous system.[16] Although dysautonomia is very common in GBS, seen in up to two-thirds of the patients, pseudo-obstruction of intestine is rarely reported as a presenting symptom of GBS.[17] We postulate that paralytic ileus manifesting after a long plateau phase of illness and a week of stable course of illness after initiation of treatment is rather a transient neurological fluctuation. New neurologic deficits were seen after a plateau phase (patient 1 and 2) or onset of recovery (patient 3). LVPP and IVIG, the standard treatment for patients with GBS, are also used to treat patients with TRF. Therefore, these three patients with new neurologic deficits were also treated with additional cycles of LVPP which resulted in improvement.
Three of our patients (patient no. 1–3) do not fulfill the criteria for TRF in GBS as the worsening comprised of appearance of facial paresis in two patients and of paralytic ileus in one patient; these features would not result in change of MRC sum score or HD Grade. Fluctuations in neurological deficits were demonstrable, and it did cause concern regarding treatment strategies. The appearance of new neurologic deficits also points toward inadequate treatment or continued disease activity. Patient no. 4 was recovering for only 2 days when new neurologic deficits appeared. LVVP was initiated promptly without waiting for further worsening over the ensuing days. In patient no. 6, there was definite improvement in distal limb weakness. Yet, there was no change in his MRC score. This is because of MRC sum score is calculated from muscle power at different joints, and mild improvement in muscle power of one or two muscles may not result in the required 5-point change in MRC sum score. Similarly, HD Grade places greater importance over lower limb weakness as compared to upper limb weakness. Therefore, Kleyweg's and Rut's criteria based on MRC sum score and HD Grade may be restrictive for the diagnosis of TRF. Our patients no. 1–3 may represent “TRF in evolution” or milder cases of TRF.
Patients in the present study had certain unique characteristics such as the presence of cranial nerve palsy, autonomic dysfunction, severe and global pattern of weakness, raised CSF protein, electrophysiological evidence of pure motor and motor sensory neuropathy, infrequent antecedent illness, and rarely respiratory distress. In the Dutch GBS trial study, none of the 16 GBS-TRF patients among the 172 patients with GBS had antecedent gastrointestinal illness, distal weakness, acute motor neuropathy or anti-GM1 antibodies whereas pure motor neuropathy was seen in three of our cases.[18] Furthermore, our patients had distal predominant weakness in upper limb and global (proximal and distal) weakness of lower limb as the pattern of weakness. Our patients with TRF did have cranial palsy at presentation and as a manifestation of TRF. Two patients had variant GBS in that they had additional cerebellar signs. As per literature, TRF is not seen in those with pure motor neuropathy or distal weakness.[18] Hence, it is possible that our series of GBS patients have different etiopathogenesis, predisposition, and risk factors for TRF. Further, cytomegalovirus infection, longer onset to nadir, protracted disease course indicating a prolonged immune attack, longer interval between onset of illness and treatment, associated medical comorbidities, and early treatment have all been proposed to be risk factors for TRF, although controversial.[8,14,18]
Two pathogenic mechanisms for TRF proposed include the presence of ongoing immune activation and initiation of therapy early in course of illness.[18] Visser et al. also reported that patients with TRF were found to have longer onset to peak time compared to those without fluctuations indicating ongoing/prolonged immune attack.[18] Among our six patients, four patients had presented within second week of illness; all six had onset to nadir duration < 2 weeks. In five patients, treatment was initiated within three weeks of illness. Yet, four patients worsened within one-two weeks of initiation of treatment. IVIG and LVPP only temporarily suppress effects of disease activity.
Therefore, initiation of therapy early in the course or for a shorter time may reduce antibody and immune complex levels for a short time. In the present study, LVPP was started at < 1 week after onset of symptoms in three patients. A randomized multicenter study of GBS in pediatric population also found that early relapses or secondary fluctuations in disability score of ≥1 point were common in those treated with 2-day regime of IVIGs compared to those with 5-day regimen.[19] Thus, ongoing immune activation reflected in longer onset to nadir time and initiation of therapy early in the course appears to play a role in the pathogenesis of TRF.
Treatment of GBS with IVIG or plasmapheresis appears to have been derived empirically.[20,21] Standard recommended dosage of IVIG or cycles of LVPP is sufficient in majority of patients. However, patients with TRF suggest that the standard dose of treatment may be inadequate in them. The time required to remove or dose required to block all the antibodies or immune complexes remains unclear. Once the patient becomes bedridden with 0/5 muscle power and respiratory failure, there is difficulty in assessing further progression. It is possible that the initial nerve damage may be demyelinating in nature; continued immune attack may lead on to axonopathy changes. This may contribute for delayed or incomplete recovery along with prolonged Intensive Care Unit stay. Therefore, treatment may have to be tailor-made for duration of enhanced immune response and consequent nerve damage. This may help in curtailing the time interval of continued nerve damage and consequently for early or more complete recovery. Thus, there is a need for biomarkers to assess the ongoing immune activity in GBS.
CONCLUSION
Despite causing concern, in our patients, worsening was milder with only one-half of them fulfilling Kleyweg's criteria for TRF (1991).[11] Our patients also improved with additional immunomodulatory treatment. Cranial nerve palsy, autonomic dysfunction, severe and distal predominant upper limb weakness and global pattern of weakness of lower limbs, raised CSF protein, pure motor and motor sensory conduction abnormalities, infrequent antecedent illness, and rarely respiratory distress characterize our series of TRF.
Identifying even “milder” TRF may be important. TRF raises the question of adequacy of currently administered standard treatment of five cycles of LVPP. Prospective studies would help in understanding the natural history and response to timely treatment in GBS and inflammatory polyneuropathy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Newswanger DL, Warren CR. Guillain-Barré syndrome. Am Fam Physician. 2004;69:2405–10. [PubMed] [Google Scholar]
- 2.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27 Supp:S21–4. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 3.Kuitwaard K, van Koningsveld R, Ruts L, Jacobs BC, van Doorn PA. Recurrent Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2009;80:56–9. doi: 10.1136/jnnp.2008.156463. [DOI] [PubMed] [Google Scholar]
- 4.Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Report from an Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41:617–8. [PubMed] [Google Scholar]
- 5.Hughes RA. The spectrum of acquired demyelinating polyradiculoneuropathy. Acta Neurol Belg. 1994;94:128–32. [PubMed] [Google Scholar]
- 6.Oh SJ, Kurokawa K, de Almeida DF, Ryan HF, Jr, Claussen GC. Subacute inflammatory demyelinating polyneuropathy. Neurology. 2003;61:1507–12. doi: 10.1212/01.wnl.0000096166.28131.4c. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann HC, Meyer Zu, Horste G, Kieseier BC, Hartung HP. Pathogenesis and treatment of immune-mediated neuropathies. Ther Adv Neurol Disord. 2009;2:261–81. doi: 10.1177/1756285609104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ropper AE, Albert JW, Addison R. Limited relapse in Guillain-Barré syndrome after plasma exchange. Arch Neurol. 1988;45:314–5. doi: 10.1001/archneur.1988.00520270096026. [DOI] [PubMed] [Google Scholar]
- 9.Kleyweg RP, van der Meché FG. Treatment related fluctuations in Guillain-Barré syndrome after high-dose immunoglobulins or plasma-exchange. J Neurol Neurosurg Psychiatry. 1991;54:957–60. doi: 10.1136/jnnp.54.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterman PO, Fagius J, Säfwenberg J, Wikström B. Early relapse of acute inflammatory polyradiculoneuropathy after successful treatment with plasma exchange. Acta Neurol Scand. 1988;77:273–7. doi: 10.1111/j.1600-0404.1988.tb05909.x. [DOI] [PubMed] [Google Scholar]
- 11.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103–9. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 12.Ruts L, van Koningsveld R, van Doorn PA. Distinguishing acute-onset CIDP from Guillain-Barré syndrome with treatment related fluctuations. Neurology. 2005;65:138–40. doi: 10.1212/01.wnl.0000167549.09664.b8. [DOI] [PubMed] [Google Scholar]
- 13.Irani DN, Cornblath DR, Chaudhry V, Borel C, Hanley DF. Relapse in Guillain-Barré syndrome after treatment with human immune globulin. Neurology. 1993;43:872–5. doi: 10.1212/wnl.43.5.872. [DOI] [PubMed] [Google Scholar]
- 14.Romano JG, Rotta FT, Potter P, Rosenfeld V, Santibanez R, Rocha B, et al. Relapses in the Guillain-Barré syndrome after treatment with intravenous immune globulin or plasma exchange. Muscle Nerve. 1998;21:1327–30. doi: 10.1002/(sici)1097-4598(199810)21:10<1327::aid-mus14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978;7(2):750–3. doi: 10.1016/s0140-6736(78)92644-2. [DOI] [PubMed] [Google Scholar]
- 16.Burns TM, Lawn ND, Low PA, Camilleri M, Wijdicks EF. Adynamic ileus in severe Guillain-Barré syndrome. Muscle Nerve. 2001;24:963–5. doi: 10.1002/mus.1095. [DOI] [PubMed] [Google Scholar]
- 17.Man BL, Fu YP. Intestinal pseudo-obstruction as a presenting symptom of Guillain-Barré syndrome. BMJ Case Rep 2014. 2014 doi: 10.1136/bcr-2014-205155. pii: Bcr2014205155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser LH, van der Meché FG, Meulstee J, van Doorn PA. Risk factors for treatment related clinical fluctuations in Guillain-Barré syndrome. Dutch Guillain-Barré study group. J Neurol Neurosurg Psychiatry. 1998;64:242–4. doi: 10.1136/jnnp.64.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korinthenberg R, Schessl J, Kirschner J, Mönting JS. Intravenously administered immunoglobulin in the treatment of childhood Guillain-Barré syndrome: A randomized trial. Pediatrics. 2005;116:8–14. doi: 10.1542/peds.2004-1324. [DOI] [PubMed] [Google Scholar]
- 20.Appropriate number of plasma exchanges in Guillain-Barré syndrome. The French Cooperative Group on Plasma Exchange in Guillain-Barré Syndrome. Ann Neurol. 1997;41:298–306. doi: 10.1002/ana.410410304. [DOI] [PubMed] [Google Scholar]
- 21.Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: A systematic review. Brain. 2007;130(Pt 9):2245–57. doi: 10.1093/brain/awm004. [DOI] [PubMed] [Google Scholar]


