Abstract
By definition, a brain abscess is an intraparenchymal collection of pus. Nocardia shows to have a special tropism for the neural tissue. Solitary abscess represents the most common manifestation in the central nervous system, accounting for 1%–2% of all cerebral abscesses. In this report, we present a case of primary multiple brain abscesses due to Nocardia farcinica in an immune competent patient. Early diagnosis and surgical intervention is significant for the patient.
Keywords: Actinomycete, brain abscess, magnetic resonance imaging brain, Nocardia trimethoprim-sulfamethoxazole
INTRODUCTION
Nocardiosis is a localized or disseminated infection caused by a soil-borne aerobic actinomycete, most commonly introduced through the respiratory tract. The pulmonary event in humans may be self-limited and transient or subclinical, or it may progress to an acute, subacute, or chronic process mimicking tuberculous or mycotic infection or malignancy. Hematogeneous dissemination spreads particularly to the nervous system and skeletal soft-tissue structures.[1] Nocardia shows to have a special tropism for the neural tissue. Solitary abscess represents the most common manifestation in the central nervous system (CNS), accounting for 1%–2% of all cerebral abscesses.[2]
CASE REPORT
History and presentation
A 75-year-old diabetic male patient presented to the emergency department of our hospital with a complaint of acute onset rapidly progressive weakness of right lower limb and upper limb for 3 days. The patient initially developed numbness in the right lower limbs for 3 days. The patient also developed dizziness, progressive headache and hoarseness of voice. No fever was noted in the past 3 months before admission. His medical history was unremarkable. He was immunocompetent, his diabetes was well controlled (HbA1c-6.2) and without a history of surgery and steroid abuse. His social history included intermittent alcohol consumption without smoking. He was alert and responsive on admission. Physical examination revealed clear lung sound without rales or wheezing. The heartbeat was regular without any murmur. There was no tenderness or rebound tenderness in the abdomen. Neurological examination revealed right-sided hemiparesis (power-1/5) with extensor plantar reflex on the right side. There were no other symptoms such as fever, neck stiffness, photophobia, papilledema, or other abnormalities. Routine blood tests were within normal limits except for erythrocyte sedimentation rate, which was 41 mm/h. Random blood sugar level on admission was 142 mg/dl. Human immunodeficiency virus (HIV) antibody, hepatitis B surface antigen and hepatitis C antibody were all negative. Chest and abdomen radiographs were normal.
Imaging
Urgent magnetic resonance imaging (MRI) brain plain was done which revealed focal rounded lesion in the cerebral parenchyma measuring 3.2 cm × 2.8 cm in left high frontoparietal lobe. The lesion shows hypointense signal on T1 and hyper-intensive signal on T2-weighted images with thin wall. The posterior aspect of the lesion shows irregular margins. The lesion is located at gray-white interface and shows perilesional edema and mass effect. There is abnormality restricted diffusion in the lesion mainly involving the peripheral aspect [Figure 1].
Figure 1.
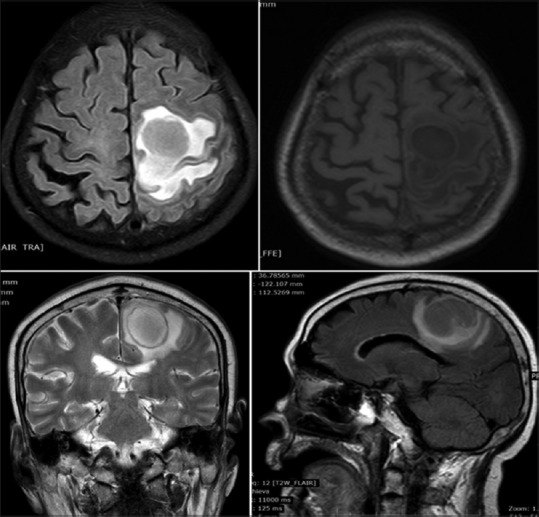
Focal rounded lesion in the cerebral parenchyma measuring 3.2 cm × 2.8 cm in the left high frontoparietal lobe. The lesion shows hypointense signal on T1 and hyperintensive signal on T2 weighted images with thin wall
Operation and histopathology
The patient was shifted from emergency to the operation theater to perform urgent left frontoparietal osteoplastic craniotomy and drainage and excision of left frontoparietal lobe abscess on the same day. Operative findings of the lesion suggested frank, yellowish pus formed in left frontoparietal lobe region with surrounding abnormal inflamed issue. Gram-stain examination of brain abscess revealed Gram-positive, unevenly stained, thin, filamentous branching bacteria in the background of polymorphonuclear leukocytes (suggestive of Nocardia) [Figure 2]. Modified Ziehl Neelsen staining of brain abscess using 1% sulfuric acid revealed; thin, filamentous, branching partially acid-fast bacteria in a background of many polymorphonuclear leukocytes, also suggestive of Nocardia [Figure 3]. The growth of pus culture on brain heart infusion blood agar showed buff colored dry, cerebriform colonies after 03 days of aerobic incubation at 37°C [Figure 4]. The growth was confirmed by Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDITOF-MS) as Nocardia farcinica.
Figure 2.
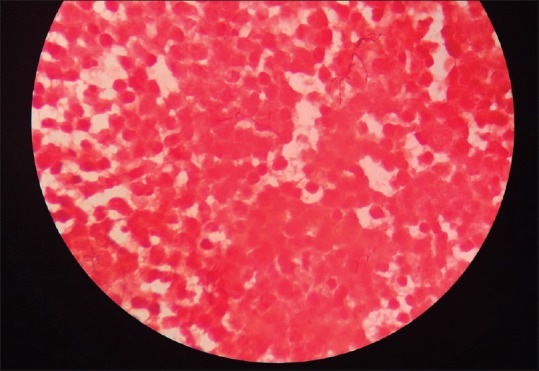
Gram-stain examination of brain abscess (×1000) - showing Gram-positive, unevenly stained, thin, filamentous branching bacteria in the background of polymorphonuclear leukocytes suggestive of Nocardia
Figure 3.
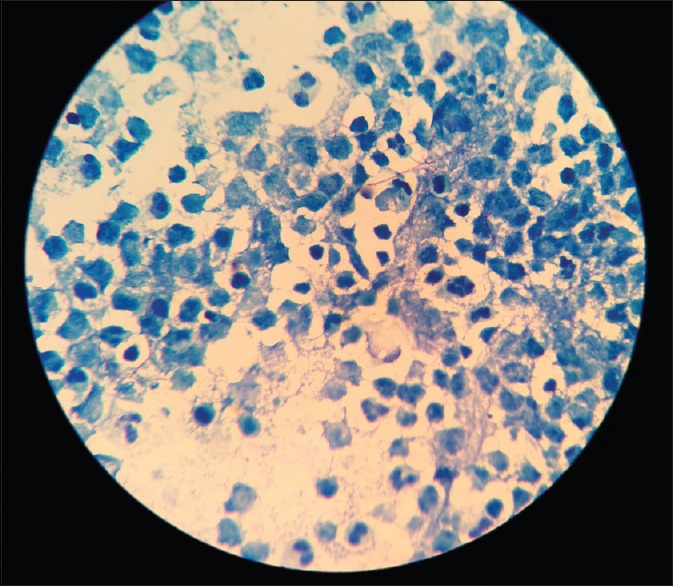
Modified Ziehl Neelsen staining of brain abscess using 1% sulfuric acid (×1000) - showing thin, filamentous, branching partially acid-fast bacteria in a background of many polymorphonuclear leukocytes suggestive of Nocardia
Figure 4.
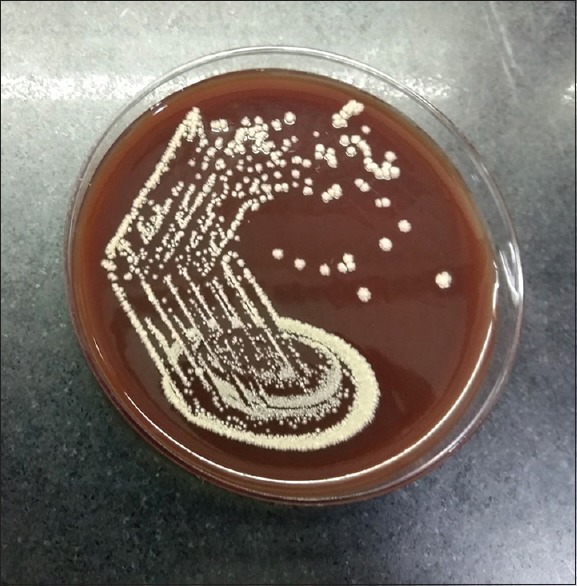
Growth of Nocardia farcinica on brain heart infusion blood agar - showing buff colored dry, cerebriform colonies after 3 days of aerobic incubation at 37°C
Postoperative course
There were no new neurological deficits, postoperatively. His right hemiparesis improved significantly with muscle power of 4/5. The patient was given ceftriaxone sodium and trimethoprim-sulfamethoxazole (TMP-SMX) during hospital course. Linezolid and TMP-SMX were used to treat the patient after surgery. Subsequently, the patient recovered well and was discharged in stable condition after 1 month. Repeat MRI brain revealed significant improvement in the perilesional edema [Figure 5]. No neurological deficits were demonstrated at further follow-up.
Figure 5.
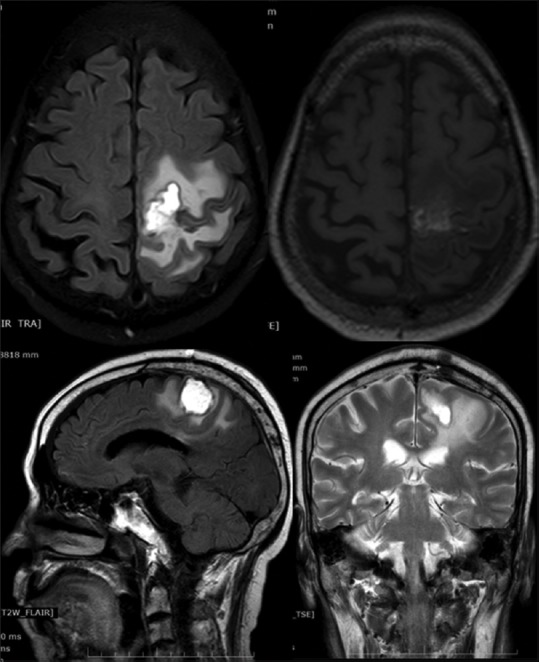
Postoperative repeat magnetic resonance imaging brain revealed significant improvement in the perilesional edema
DISCUSSION AND LITERATURE REVIEW
By definition, a brain abscess is an intraparenchymal collection of pus. The incidence of brain abscesses is ~8% of intracranial masses in developing countries, whereas in the West the incidence is ~1%–2%.[3,4,5] Brain abscesses arise from a contiguous focus of infection, a direct implantation due to trauma, or a hematogeneous spread from a remote site.[6,7] It was reported that the direct spread from surgery, trauma, meningitis, frontal sinusitis, and dental caries was seen in 50% of patients and that the hematogeneous spread from congenital or other heart diseases, lung and abdominal abscesses was found in 32% of patients. However, 18% had abscesses of an unknown origin.[7]
Nocardia accounts for as little as 1% to 2% of all brain abscesses, and it is a rare cause of brain abscess, particularly in immunocompetent host.[2] Nocardia species are Gram-positive, aerobic, branching filamentous bacteria belonging to Actinomycetales, which can be found in the soil and dust throughout the world.[8] Three main species cause humans infection, including N asteroides, N brasiliensis, and N caviae. N asteroides is the most commonly isolated. Here, we are reporting a brain abscess caused by the rarer species, i.e., N farcicina. Nocardia infection commonly arise in the immunocompromised states including organ transplants, leukemia, HIV, steroid abuse, and autoimmune disease.[9]
The most common predisposing factors are corticosteroid use (54% of patients) such as in this case and organ transplantation (25%). The concerned patient did not have any of these predisposing factors. The nocardial pathogen can spread to the CNS hematogenously or through inhalation. The involvement of the CNS was found in nearly half of all disseminated nocardial infections in the literature.[10,11]
In this report, we present a case of primary multiple brain abscesses due to Nocardia farcinica in an immune competent patient. CNS norcardial infections may manifest as single abscess, multiple brain or spinal cord lesions, diffuse cerebral inflammation, and meningitis mimicking neoplasms, vasculitis, and stroke.[12,13,14] The mortality rates estimated for Nocardia brain abscess are 55% and 20% in immune compromised and immune-competent patients, respectively; however, these rates rise for multiple abscesses and if the infection is due to the subspecies N. farcinica.[12,15,16,17] Nocardial brain abscesses are not common and Nocardiosis in immune competent individuals is even less frequent, being the infection usually observed in immune compromised hosts.[1,12,15,18]
For diagnosis of a Nocardia brain abscess, it is important to carry out the brain images and surgical interventions. Brain computed tomography, demonstrating a hypodense, enhancing lesion with surrounding edema, is sensitive for discovery and localization of the lesion; however, it is not sufficient for diagnosis. On brain MRI, multiple concentric rims are seen in abscesses due to Nocardia. Because it occasionally occurs that necrotic metastasis and brain tumors are wrongly recognized by imaging study, it is required to get a proper specimen through surgical intervention for staining, culture and identification. Besides, to prevent treatment delay, early surgical intervention is also required.[19]
Nocardial brain abscesses present as characteristic hyperenhanced multiloculated ring lesions. Perilesional edematous changes also might be present. It is sometimes difficult to differentiate a brain abscess from intracranial metastatic malignancy on regular MRI.[2] However, diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) map could be very helpful in the differential diagnosis, with brain abscess showing the characteristic homogeneously heperintense lesions on DWI and hypointense lesion on ADC. The restricted brownian motion of water molecules in the organized purulent milieu of microorganisms, macromolecules, and inflammatory cells contributes to the signal of restricted diffusion on DWI.[20] Our patient displayed similar imaging characteristics.
To treat a nocardial brain abscess, craniotomy with evacuation of the abscess, as well as collection of a specimen for culture to further assess drug sensitivity, is essential for successful treatment. Antibiotics are necessary and as an adjunct therapy after surgery. Sulfonamides are the drug of choice, based on empirical data. Given the high rate of relapse and the characteristic resistance pattern, treatment should be aggressive and continued for months, with antibiotic treatment being adjusted according to the drug sensitivity test.[20] A 12-month course of therapy is recommended for the treatment of nocardial brain abscesses. TMP-SMX, ceftriaxone, amikacin, and minocycline are used for nocardiosis. TMP-SMX is currently accepted as the first-line treatment for nocardiosis.[21] In our case, we gave ceftriaxone sodium for 1 month according to drug sensitive test of pus and then prescribed TMP-SMX to the patient for 1 year after surgery.
CONCLUSION
Nocardia species are Gram-positive, aerobic, nonmotile, urease, and catalase positive bacilli that form branching hyphae. The majority of patients with these infections are immunocompromised, but infection could occur without any predisposing factors as well. Diagnosis and treatment of nocardial brain abscess continue to challenge clinicians. It is important for the clinicians to be familiar with the characteristics of nocardial infections so that an early presumptive diagnosis can be made while awaiting confirmatory results of culture. Early diagnosis and surgical intervention is significant for the patient. Hence, MALDITOF-MS can serve as a rapid and accurate identification tool which can replace sequencing in a clinical Microbiology laboratory.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891–903. doi: 10.1093/clinids/22.6.891. [DOI] [PubMed] [Google Scholar]
- 2.Lin YJ, Yang KY, Ho JT, Lee TC, Wang HC, Su FW, et al. Nocardial brain abscess. J Clin Neurosci. 2010;17:250–3. doi: 10.1016/j.jocn.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Bernardini GL. Diagnosis and management of brain abscess and subdural empyema. Curr Neurol Neurosci Rep. 2004;4:448–56. doi: 10.1007/s11910-004-0067-8. [DOI] [PubMed] [Google Scholar]
- 4.Loftus CM, Osenbach RK, Biller J. Diagnosis and management of brain abscess. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. 2nd ed. Vol. 3. New York: McGraw-Hill; 1996. pp. 3285–98. [Google Scholar]
- 5.Sharma BS, Gupta SK, Khosla VK. Current concepts in the management of pyogenic brain abscess. Neurol India. 2000;48:105–11. [PubMed] [Google Scholar]
- 6.Harris LF, Maccubbin DA, Triplett JN, Jr, Haws FP. Brain abscess: Recent experience at a community hospital. South Med J. 1985;78:704–7. doi: 10.1097/00007611-198506000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Dohrmann PJ, Elrick WL. Observations on brain abscess. Review of 28 cases. Med J Aust. 1982;2:81–3. doi: 10.5694/j.1326-5377.1982.tb124234.x. [DOI] [PubMed] [Google Scholar]
- 8.Lai CC, Lee LN, Teng LJ, Wu MS, Tsai JC, Hsueh PR, et al. Disseminated Nocardia farcinica infection in a uraemia patient with idiopathic thrombocytopenia purpura receiving steroid therapy. J Med Microbiol. 2005;54:1107–10. doi: 10.1099/jmm.0.46084-0. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Lee KL, Lee DM, Jeong JH, Moon SM, Seo YH, et al. Nocardia brain abscess in an immunocompetent patient. Infect Chemother. 2014;46:45–9. doi: 10.3947/ic.2014.46.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anagnostou T, Arvanitis M, Kourkoumpetis TK, Desalermos A, Carneiro HA, Mylonakis E, et al. Nocardiosis of the central nervous system: Experience from a general hospital and review of 84 cases from the literature. Medicine (Baltimore) 2014;93:19–32. doi: 10.1097/MD.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaman BL, Beaman L. Nocardia species: Host-parasite relationships. Clin Microbiol Rev. 1994;7:213–64. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy KJ, Chung KH, Bowden FJ, Mews PJ, Pik JH, Fuller J, et al. A cluster of nocardial brain abscesses. Surg Neurol. 2007;68:43–9. doi: 10.1016/j.surneu.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 13.Kumar VA, Augustine D, Panikar D, Nandakumar A, Dinesh KR, Karim S, et al. Nocardia farcinica brain abscess: Epidemiology, pathophysiology, and literature review. Surg Infect (Larchmt) 2014;15:640–6. doi: 10.1089/sur.2012.205. [DOI] [PubMed] [Google Scholar]
- 14.Nandhagopal R, Al-Muharrmi Z, Balkhair A. Nocardia brain abscess. QJM. 2014;107:1041–2. doi: 10.1093/qjmed/hcu088. [DOI] [PubMed] [Google Scholar]
- 15.Alijani N, Mahmoudzadeh S, Hedayat Yaghoobi M, Geramishoar M, Jafari S. Multiple brain abscesses due to Nocardia in an immunocompetent patient. Arch Iran Med. 2013;16:192–4. [PubMed] [Google Scholar]
- 16.Mamelak AN, Obana WG, Flaherty JF, Rosenblum ML. Nocardial brain abscess: Treatment strategies and factors influencing outcome. Neurosurgery. 1994;35:622–31. doi: 10.1227/00006123-199410000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Al Tawfiq JA, Mayman T, Memish ZA. Nocardia abscessus brain abscess in an immunocompetent host. J Infect Public Health. 2013;6:158–61. doi: 10.1016/j.jiph.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Lederman ER, Crum NF. A case series and focused review of nocardiosis: Clinical and microbiologic aspects. Medicine (Baltimore) 2004;83:300–13. doi: 10.1097/01.md.0000141100.30871.39. [DOI] [PubMed] [Google Scholar]
- 19.Menkü A, Kurtsoy A, Tucer B, Yildiz O, Akdemir H. Nocardia brain abscess mimicking brain tumour in immunocompetent patients: Report of two cases and review of the literature. Acta Neurochir (Wien) 2004;146:411–4. doi: 10.1007/s00701-004-0215-6. [DOI] [PubMed] [Google Scholar]
- 20.Bose BB. Diagnosis and treatment of nocardial brain abscess. Neurosurg Q. 2002;12:182–93. [Google Scholar]
- 21.Lee GY, Daniel RT, Brophy BP, Reilly PL. Surgical treatment of nocardial brain abscesses. Neurosurgery. 2002;51:668–71. [PubMed] [Google Scholar]


