Abstract
Progressive loss of heart rate variability (HRV) and complexity are associated with increased risk of mortality in patients with cardiovascular disease and are a candidate marker for patients at risk of sudden cardiac death. HRV is influenced by the cardiac autonomic nervous system (ANS), although it is unclear which arm of the ANS (sympathetic or parasympathetic) needs to be perturbed to increase the complexity of HRV. In this case–control study, we have analyzed the relation between modulation of vagus nerve stimulation (VNS) and changes in complexity of HRV as a function of states of vigilance. We hypothesize that VNS – being a preferential activator of the parasympathetic system – will decrease the heart rate (HR) and increase the complexity of HRV maximum during sleep. The electrocardiogram (EKG) obtained from a 37-year-old, right-handed male with known intractable partial epilepsy and left therapeutic VNS was analyzed during wakefulness and sleep with VNS ON and OFF states. Age-matched control EKG was obtained from five participants (three with intractable epilepsy and two without epilepsy) that had no VNS implant. The study demonstrated the following: (1) VNS increased the complexity of HRV during sleep and decreased it during wakefulness. (2) An increase in parasympathetic tone is associated with increased complexity of HRV even in the presence of decreased HR. These results need to be replicated in a larger cohort before developing patterned stimulation using VNS to stabilize cardiac dysautonomia and prevent fatal arrhythmias.
Keywords: Approximate entropy, epilepsy, heart rate variability complexity, sample entropy, vagus nerve stimulation
INTRODUCTION
The temporal fluctuations in heart rate (HR), often called as heart rate variability (HRV), is a practical and reproducible tool to monitor cardiac autonomic function.[1] Progressive loss of HRV complexity is associated with increased risk of mortality in patients with cardiovascular disease and is a candidate marker for patients at risk of sudden cardiac death.[2,3] In epilepsy, abnormalities in HRV are associated with intractable epilepsy – a cohort with the highest risk of sudden unexpected death in epilepsy (SUDEP).[4] In spite of its significance in predictive risk assessment, the relation between sympathetic–parasympathetic imbalance and alteration in the complexity of HRV is unclear. How does modulation of vagal nerve alter the complexity of HRV? Understanding the relation is of prime importance as vagus nerve stimulation (VNS) is explored as a therapy against fatal arrhythmia in chronic cardiac conditions such as congestive heart failure.[5] In this single case study, we have analyzed the relation between modulation of VNS and changes in the complexity of HRV as a function of states of vigilance (SOV). SOV – defined as sleep and wakefulness – influences the activation of the autonomic nervous system (ANS) which is reflected in changes in HR and HRV. Studies with healthy participants have demonstrated a progressive increase in parasympathetic modulation as early stages of sleep (NREM N1-2) transitioned to late stages (NREM N3-4). We hypothesize that VNS – being a preferential activator of the parasympathetic system – will decrease the HR and increase the complexity of HRV maximum during sleep.
METHODS
A case–control study design was performed retrospectively involving one case and five controls. A 37-year-old, right-handed male (case) with known intractable partial temporal lobe epilepsy was referred to our Level-IV epilepsy center for evaluation of his recurrent episodes of dizziness, loss of consciousness, and falls. His seizures started at the age of 3, followed by meningoencephalitis, and by adolescence he continued to have complex partial seizures that were refractory to multiple antiseizure medications. Seizure semiology includes experiential feeling followed by manual automatism and behavioral arrest. Following scalp electroencephalogram (EEG), magnetic resonance imaging (MRI) brain, and neuropsychological investigations, he underwent left anterior temporal lobectomy by the age of 16. Postsurgery, he was seizure free for 4 months, but since then, his complex partial seizures returned and increased to the point that it disabled him. Before resective surgery, the seizure frequency ranged between 1 and 2 every week that decreased to 3 and 4 every month. There was no diurnal preference in seizure, and most of his seizures were at daytime. His antiepileptic medications include topiramate 100 mg twice a day, clobazam 10 mg once a day, oxcarbazepine XR 600 mg once a day, and brivaracetam 50 mg once a day. Six months before referral to our hospital, he underwent left vagal nerve stimulator implantation to control his intractable seizures. Unfortunately, the surgical procedure was complicated by left vocal cord paralysis that contributed to his hoarseness of voice. The recurrent episodes of dizziness and syncope succeeded the VNS implantation, and the potential differential diagnosis included aberrant activation of cardiac parasympathetic system contributing to bradycardia and subclinical seizures. He underwent video-EEG investigation for 3 days at our center, with the VNS being turned OFF for the 1st day and turned ON at the preadmission settings (VNS 0.25 mA, 20 Hz pulse width 250 μs, ON time 30 s, and OFF time 5 min) for the remaining days. A single-lead electrocardiogram (EKG) was recorded simultaneously with the EEG. Three epochs (each lasting 10–15 min) were selected during resting wakefulness with eyes open and during Stage II sleep (identified by delta slowing, sleep spindles, and K-complex) with VNS ON and VNS OFF. The EKG was visually screened, and artifact-free epochs were selected. During admission, he had a 12-lead EKG that was reported normal.
Controls
Three patients with intractable partial epilepsy but without VNS implant were referred to our epilepsy center for presurgical video EEG monitoring. We have identified them as positive controls as they have intractable epilepsy. Two additional patients who have undergone diagnostic video EEG-EKG investigation for clarification of dizzy spells were identified as negative controls. None of the control patients had VNS implant. Details of the controls are as follows:
Patient 1 (positive control 1) is a 33-year-old, right-handed man with a lifelong history of complex partial seizures (frequency 2–3/week) and occasional secondary generalized seizures (1 every year). He had a history of febrile seizures in his childhood, and his MRI brain was positive for left hippocampal sclerosis. He has failed over five antiepileptic medications and at present was on levetiracetam 2 g twice daily, lacosamide 150 mg twice daily, and lamotrigine 200 mg twice daily. Patient 2 (positive control 2) is a 39-year-old, left-handed woman with perinatal stroke and lifelong simple and complex partial seizures (frequency 1–2/day). She had a history of status epilepticus in the past. Her medications included carbamazepine 800 mg twice daily, gabapentin 600 mg three times daily, lacosamide 200 mg twice daily, topiramate 100 mg twice daily, and clobazam 20 mg daily. Patient 3 (positive control 3) is a 28-year-old, right-handed woman with known posttraumatic epilepsy since age 9. She had weekly complex partial seizures (2–4/week) and frequent generalized seizures (1–2/week). Her MRI brain showed right frontal encephalomalacia. Her medications included Depakote 1500 mg twice daily, carbamazepine 300 mg twice daily, levetiracetam 1500 mg twice daily, and clonazepam 1.5 mg twice daily. Patient 4 (negative control 1) is a 31-year-old, right-handed woman with spells of visual disturbances, dizziness, and passing out spells. EEG confirmed psychogenic nonepileptic spells. She was on topiramate 50 mg twice daily. Patient 5 (negative control 2) is a 19-year-old, right-handed male with episodes of visual blurring followed by passing out and subsequent waking up with urinary incontinence. EEG confirmed psychogenic nonepileptic spells, and he was not on any medication. None of the controls had any history of cardiac disease, and EKG was normal.
Lead I-EKG (adhesive electrodes placed below the clavicles of either side) sampled at 512 Hz was analyzed using Kubios HRV software version 2.1.[6] HRV was analyzed in the time and frequency domains and also by the use of complexity measures. In the time domain, parameters computed include mean HR, mean of RR interval (mean RR), standard deviation (SD) of RR intervals (SDNN), and square root of the mean squared differences between successive RR intervals (RMSSD). In the frequency domain, the following parameters were analyzed – low-frequency (LF) power (0.04–0.15 Hz), high-frequency (HF) power (0.15–0.4 Hz), and LF/HF ratio. Parasympathetic activity is expressed in RMSSD and HF power.[7] For complexity measure analysis, we have used approximate entropy and sample entropy.
Nonlinear dynamical analysis to estimate the complexity changes has gathered a lot of interest in the medical community. HRV was performed using sample entropy (SampEn) and approximate entropy (ApEn).[8,9,10] SampEn measures the likelihood of runs of patterns that are close and will remain close for subsequent incremental comparisons. ApEn was developed by Pincus and is based on the principle that a sequence is regular if a subsequence and an expansion of the subsequence are similar.[9] Both these methods have been used extensively in cardiac literature as nonlinear measures of HRV.[3,10]
One important aspect to note while using these complexity measures is the setting of parameters for computing these measures. Two parameters “m” (the length of pattern to compare for closeness) and “r” (a tolerance factor) need to be chosen carefully after due experimentation.[11] In the current experiment, values of “m” =1 and 2 and “r” =0.2, 0.5, and 0.7 times the SD of the input data were analyzed. Hence, any of these parameters can be used for this particular analysis.
A single-factor analysis of variance (ANOVA) with Tukey's test for pairwise comparison of the means between the case and controls was performed. Figure 1 shows the block diagram of the proposed analysis mechanism that summarizes the detailed description given in the method section.
Figure 1.
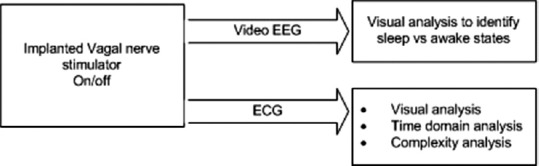
Block diagram showing the proposed analysis mechanism
RESULTS
Linear measures of heart rate variability
The mean HR during wakefulness with VNS ON and OFF was 71.39 and 69.6 per minute (P = 0.77), respectively, while during Stage II sleep with ON and OFF it was 57.98 and 62.13 per minute (P = 0.39), respectively. The corresponding mean RR intervals during similar epochs of wakefulness with VNS ON and OFF were 869.33 and 1237 ms (P = 0.02) while during sleep with ON and OFF the values were 1400 and 967.1 ms (P = 0.002). Therefore, although the differences in HR with VNS ON and OFF as a function of SOV were nonsignificant, the RR interval was significantly prolonged with VNS ON during sleep state. Following activation of VNS, the parasympathetic tone (as expressed by RMSSD and HF derived from entire EKG) was highest during sleep than wakefulness (P = 0.005).
Nonlinear measures of heart rate variability
From the HRV datasets, the consecutive values for 200 s are chosen from 50 random locations from each of the participant and the complexity values are calculated. Thus, we generate 50 complexity values for each participant. These 50 values are samples that represent the original populations. Using these sample values, interval plots for 95% confidence of the means are plotted as shown in Figures 2-5.
Figure 2.
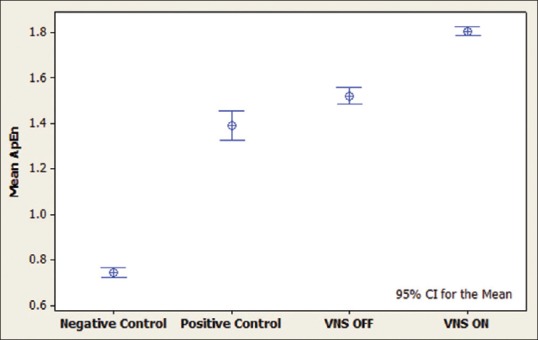
Interval plot for approximate entropy measurements during sleep. It can be seen that vagus nerve stimulation activation in the case has increased the complexity value. Positive controls include patients with intractable epilepsy, negative controls are age-matched patients without epilepsy and the case is a patient with intractable epilepsy and vagus nerve stimulation implanted
Figure 5.
Interval plot for sample entropy during awake state. It can be seen that vagus nerve stimulation activation in the case has decreased the complexity value. Positive controls include patients with intractable epilepsy, negative controls are age-matched patients without epilepsy and the case is a patient with intractable epilepsy and vagus nerve stimulation implanted
Figure 3.
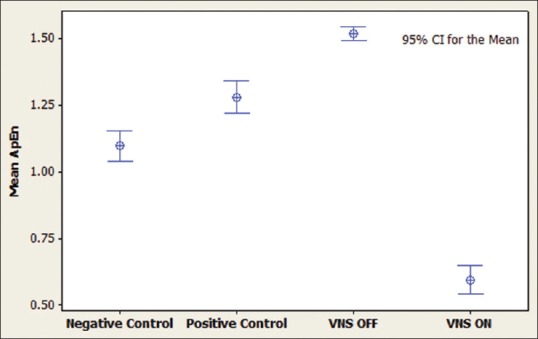
Interval plot for approximate entropy during awake state. It can be seen that vagus nerve stimulation activation in the case has decreased the complexity value. Positive controls include patients with intractable epilepsy, negative controls are age-matched patients without epilepsy and the case is a patient with intractable epilepsy and vagus nerve stimulation implanted
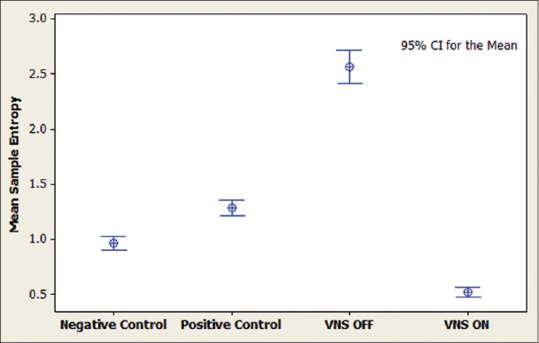
Figure 4.
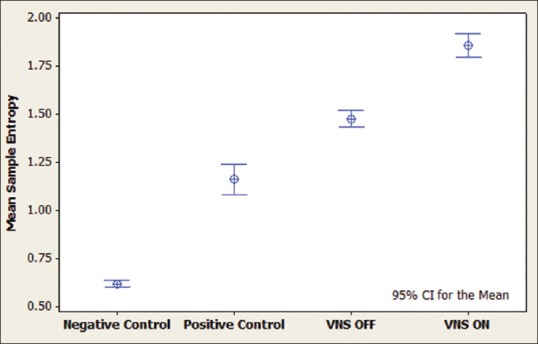
Interval plot for sample entropy during sleep. It can be seen that vagus nerve stimulation activation in the case has increased the complexity value. Positive controls include patients with intractable epilepsy, negative controls are age-matched patients without epilepsy and the case is a patient with intractable epilepsy and vagus nerve stimulation implanted
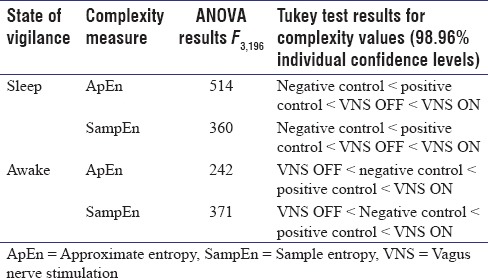
It can be seen that the activation of VNS during sleep increased the complexity in HRV (both ApEn and SampEn) significantly, while during wakefulness, they were decreased. A single-factor ANOVA with Tukey's test for pairwise comparison of the means was also done for verification, and the results are shown in Table 1.
Table 1.
Results for ANOVA and Tukey's honest significant difference tests
DISCUSSION
HR and variability in HR are influenced by the interaction between the cardiac ANS, respiration, and baroreflex mechanisms.[1] Mapping the relation between autonomic imbalances altering the complexity of HRV might improve our understanding and help in developing therapies that can manipulate the sympathetic or parasympathetic axis to increase the complexity of HRV. This study is an effort toward understanding the relation between cardiac ANS and complexity of HRV. The present study demonstrates the following:
An increase in parasympathetic tone is associated with increased complexity of HRV
Activation of VNS alters the complexity of HRV differently during sleep and wakefulness. During sleep, the complexity of HRV was increased, while at wakefulness, it was decreased by VNS [Figures 2-5].
SUDEP is the major cause of mortality in individuals with intractable epilepsy, and most of the deaths occur at night during sleep.[4] Few studies have demonstrated the association of decreased HRV with SUDEP, although the results are inconsistent.[7,12] VNS has been used over a decade to treat intractable epilepsy and has been shown to be partially effective in reducing seizures. However, the effectiveness of VNS in reducing SUDEP remains inconclusive. Studying the relation between complexity in HRV, SUDEP, and VNS treatment might offer an explanation for the inconsistency of these results.
In epilepsy, the rationale for selecting left and not right vagal nerve for stimulation is based on the conventional wisdom that left vagus nerve preferentially innervates the atrioventricular node while the right vagus nerve innervates the sinoatrial node (cardiac pacemaker region).[13] Hence, activation of left VNS would have significantly less cardiac side effects. However, reported studies with cardiac side effects following activation of left vagal nerve challenge this conventional wisdom.[14,15] Our case adds to the growing literature that left VNS can modulate cardiac autonomic system.
A single case study limits the observations and the results need to be replicated in a large cohort. However, the preliminary results are exciting and provide an insight into how VNS modulation alters the complexity of HRV as a function of sleep and wakefulness.
In summary, this case–control study demonstrated the ability of VNS to increase the complexity of HRV by preferential modulation of the parasympathetic system, and the effects are more pronounced during sleep. A practical application of this study would be to devise a system that can monitor the complexity values and dynamically control the application of VNS, thus automating the process of VNS application. It is envisaged that this would be a pioneering technique in the prevention of SUDEP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, Montano N, et al. Heart rate variability in normal and pathological sleep. Front Physiol. 2013;4:294. doi: 10.3389/fphys.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimeno-Blanes FJ, Blanco-Velasco M, Barquero-Pérez Ó, García-Alberola A, Rojo-Álvarez JL. Sudden cardiac risk stratification with electrocardiographic indices – A review on computational processing, technology transfer, and scientific evidence. Front Physiol. 2016;7:82. doi: 10.3389/fphys.2016.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau S, Haueisen J, Schukat-Talamazzini EG, Voss A, Goernig M, Leder U, et al. Low HRV entropy is strongly associated with myocardial infarction. Biomed Tech (Berl) 2006;51:186–9. doi: 10.1515/BMT.2006.033. [DOI] [PubMed] [Google Scholar]
- 4.Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: Epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15:1075–88. doi: 10.1016/S1474-4422(16)30158-2. [DOI] [PubMed] [Google Scholar]
- 5.Das UN. Vagal nerve stimulation in prevention and management of coronary heart disease. World J Cardiol. 2011;3:105–10. doi: 10.4330/wjc.v3.i4.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV – Heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113:210–20. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Lotufo PA, Valiengo L, Benseñor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53:272–82. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- 8.Nithin N, Balasubramanian K, Dey S. A new complexity measure for time series analysis and classification. Eur Phys J Spec Top. 2013;222:847–60. [Google Scholar]
- 9.Pincus SM, Viscarello RR. Approximate entropy: A regularity measure for fetal heart rate analysis. Obstet Gynecol. 1992;79:249–55. [PubMed] [Google Scholar]
- 10.Kim SG, Yum MK. Decreased RR interval complexity and loss of circadian rhythm in patients with congestive heart failure. Jpn Circ J. 2000;64:39–45. doi: 10.1253/jcj.64.39. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian K, Nagaraj N. Aging and cardiovascular complexity: Effect of the length of RR tachograms. Peer J. 2016;4:e2755. doi: 10.7717/peerj.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeppesen J, Fuglsang-Frederiksen A, Brugada R, Pedersen B, Rubboli G, Johansen P, et al. Heart rate variability analysis indicates preictal parasympathetic overdrive preceding seizure-induced cardiac dysrhythmias leading to sudden unexpected death in a patient with epilepsy. Epilepsia. 2014;55:e67–71. doi: 10.1111/epi.12614. [DOI] [PubMed] [Google Scholar]
- 13.McGregor A, Wheless J, Baumgartner J, Bettis D. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia. 2005;46:91–6. doi: 10.1111/j.0013-9580.2005.16404.x. [DOI] [PubMed] [Google Scholar]
- 14.Tatum WO 4th, Moore DB, Stecker MM, Baltuch GH, French JA, Ferreira JA, et al. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology. 1999;52:1267–9. doi: 10.1212/wnl.52.6.1267. [DOI] [PubMed] [Google Scholar]
- 15.Matheny RG, Shaar CJ. Vagus nerve stimulation as a method to temporarily slow or arrest the heart. Ann Thorac Surg. 1997;63:S28–9. doi: 10.1016/s0003-4975(97)00423-2. [DOI] [PubMed] [Google Scholar]


