Sir,
Myasthenia gravis (MG) is immunological disease of neuromuscular junction due to circulating antibodies. In generalized MG, 85% of patients are positive for acetylcholine receptor (AChR) antibodies, while 8%–10% of patients are positive for anti-MuSK antibodies.[1] Treatment of MG consists of symptomatic therapy with acetylcholinesterase inhibitors and immunotherapy such as corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, plasmapheresis, and intravenous immunoglobulin (IVIG).[2] Between 10% and 15% of patients remain refractory to such treatment. Rituximab is newer immunosuppressive drug used recently for the treatment of refractory MG.[1]
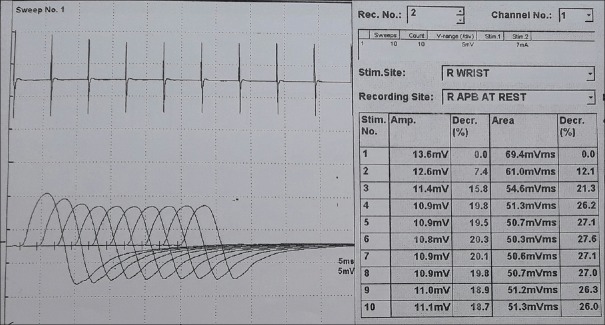
A 56-year-old male patient presented to a city hospital with weakness of facial muscles, drooping of eyelids, and slurring of speech since August, 2016. There, he was diagnosed to have MG with AChR antibody test positive (titer - 30.8). Repetitive nerve stimulation test (RNST) showed significant decrement in amplitudes [Figure 1]. Thymoma was not detected on contrast-enhanced computed tomography scan of thorax. He was started with pyridostigmine 30 mg twice daily dose, subsequently increased to 60 mg 4 times daily due to symptom aggravation. After 10 days, he was given IVIG (27 g/day) for 5 days at some other center, possibly due to rapid deterioration and apprehending that he may go into myasthenic crisis. Mycophenolate 500 mg daily and prednisolone 10 mg daily were started. After 1 month, due to progression of symptoms, 5 cycles of plasmapheresis were given. He showed significant improvement in symptoms for 4 days, after which symptoms reappeared.
Figure 1.
Repetitive nerve stimulation test of abductor pollicis brevis showing decremental response
Due to worsening of symptoms, he was referred to our center with severe bulbar weakness and difficulty in swallowing and speaking. AChR antibody titer was 19.60 nmol/L. Symptoms were suggestive of acute aggravation of myasthenia which was refractory to usual immunosuppressive treatment. Knowing that steroids may worsen symptoms during crisis and as the patient was rapidly deteriorating, dose of prednisolone was not increased. Hence, it was decided to treat him with rituximab. He was started on rituximab 500 mg intravenous infusion over 4 h on 2 days. Another dose of rituximab 500 mg IV infusion was repeated after 15 days for 2 more days. He showed improvement of symptoms within 4 days after 1st infusion of rituximab. Mycophenolate which was started 2 months ago was tapered off within 1 month because of sustained improvement after rituximab infusion. He was discharged with advice to take pyridostigmine 30 mg 4 times daily. After 15 days follow-up, he showed improvement in symptoms. On follow-up in February, he showed further improvement with negative RNST and AChR titer of 12. Till date, after 7 months of 1st infusion of rituximab, the patient is having sustained improvement.
Rituximab is a monoclonal anti-CD20 antibody that causes destruction of B cells by three mechanisms: antibody-dependent cellular cytotoxicity,[3] complement-dependent cytotoxicity,[4] and apoptosis.[4]
In the recent study, MuSK-positive patients of MG treated with rituximab achieved higher rates of disease remission even after 1st cycle of rituximab infusion and did not require repeated infusions whereas AChR-positive patients did not achieve complete remissions even after 2–3 cycles of rituximab.[5] However, in our case, the patient was AChR positive and achieved remission within 7 days of starting 1st infusion of rituximab and maintained improved clinical status till date.
Rituximab can cause early and complete disease remission in AChR-positive refractory MG patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Silvestri NJ, Wolfe GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis. 2014;15:167–78. doi: 10.1097/CND.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 2.Sathasivam S. Steroids and immunosuppressant drugs in myasthenia gravis. Nat Clin Pract Neurol. 2008;4:317–27. doi: 10.1038/ncpneuro0810. [DOI] [PubMed] [Google Scholar]
- 3.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 4.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 5.Díaz-Manera J, Martínez-Hernández E, Querol L, Klooster R, Rojas-García R, Suárez-Calvet X, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78:189–93. doi: 10.1212/WNL.0b013e3182407982. [DOI] [PubMed] [Google Scholar]