Abstract
Background
The surgical Apgar score (SAS) is a 10-point scale using the lowest heart rate, lowest mean arterial pressure, and estimated blood loss (EBL) during surgery to predict postoperative outcomes. The SAS has not yet been validated in liver transplantation patients, because typical blood loss usually exceeds the highest EBL category. Our primary aim was to develop a modified SAS for liver transplant (SAS-LT) by replacing the EBL parameter with volume of red cells transfused. We hypothesized that the SAS-LT would predict death or severe complication within 30 days of transplant with similar accuracy to current scoring systems.
Methods
A retrospective cohort of consecutive liver transplantations from July 2007 to November 2013 was used to develop the SAS-LT. The predictive ability of SAS-LT for early postoperative outcomes was compared with Model for End-stage Liver Disease, Sequential Organ Failure Assessment, and Acute Physiology and Chronic Health Evaluation III scores using multivariable logistic regression and receiver operating characteristic analysis.
Results
Of 628 transplants, death or serious perioperative morbidity occurred in 105 (16.7%). The SAS-LT (receiver operating characteristic area under the curve [AUC], 0.57) had similar predictive ability to Acute Physiology and Chronic Health Evaluation III, model for end-stage liver disease, and Sequential Organ Failure Assessment scores (0.57, 0.56, and 0.61, respectively).
Seventy-nine (12.6%) patients were discharged from the ICU in 24 hours or less. These patients’ SAS-LT scores were significantly higher than those with a longer stay (7.0 vs 6.2, P < 0.01). The AUC on multivariable modeling remained predictive of early ICU discharge (AUC, 0.67).
Conclusions
The SAS-LT utilized simple intraoperative metrics to predict early morbidity and mortality after liver transplant with similar accuracy to other scoring systems at an earlier postoperative time point.
Liver transplant (LT) is the only curative treatment option for patients with end-stage liver disease, with more than 6000 performed annually in the United States.1 Liver transplantation is a major surgery for patients who are potentially highly compromised by both liver disease and comorbid conditions and is associated with a high risk of postoperative complications and readmission.2,3 Post-LT patients are frequently monitored in intensive care units, although the necessity of this as a routine practice is controversial, and a fast-track approach to selected patients is becoming more widely practiced.4-7 The ability to stratify post-LT patients’ risk of postoperative complications before operating room exit would be of benefit in both the identification of high-risk patients and the fast-tracking of low-risk patients.
Currently, patient selection for liver transplantation is based on the model for end-stage liver disease (MELD) score. Although the MELD score is a good predictor of pretransplant mortality, it has not been shown to be a strong predictor of posttransplant outcomes.8 Other scoring systems to predict posttransplant outcomes have been developed, but none have been widely used in direct clinical practice.9-11
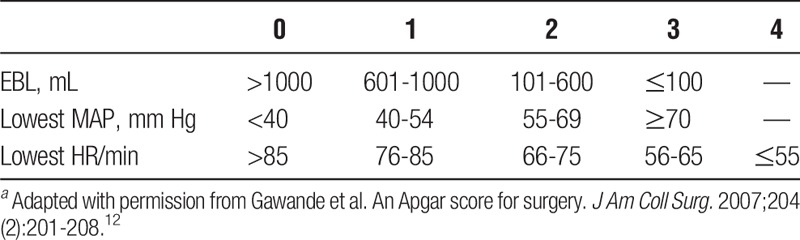
Although scoring systems have been developed for outcomes based on preoperative variables, to our knowledge, no scoring system for liver transplants has been developed using strictly intraoperative variables. The surgical Apgar score (SAS) is a 10-point scale first described in 2007 by Gawande et al,12 as a simple means of grading patients after general or vascular operations using 3 intraoperative variables: lowest heart rate (HR), lowest mean arterial pressure (MAP), and estimated blood loss (EBL) (Table 1). It has since been validated as predictive of early postoperative outcomes in a number of surgeries,13-18 including abdominal6,19 and vascular operations,12,16 but has not been studied specifically in the liver transplant population. One reason may be that the EBL category in liver transplantation frequently meets or exceeds the maximal score in the SAS (>1000 mL).
TABLE 1.
The original SASa
Blood loss in LT may be difficult to estimate, and providers often use transfusion requirement as a surrogate. Miki et al20 have described a procedure-specific adjustment to the SAS, which resulted in improved predictive ability in gastrectomy patients. We therefore hypothesized that a modification of the SAS, substituting appropriate categories of volume of intraoperative blood transfused in place of EBL, could be developed for the liver transplant population and would be a predictor of short-term outcomes. Such a score may be of clinical utility in guiding decisions regarding posttransplant fast-tracking or intensive care assignment.
MATERIALS AND METHODS
Data Collection
After receiving approval from the Mayo Clinic Institutional Review Board as minimal risk (ID 14-003485), data was collected from 628 consecutive liver transplantations performed in 613 adult patients older than 17 years between July 2007 and November 2013. Only patients who had provided prior authorization for the use of their medical records for research were included in the study. Retransplantations within 30 days and combined heart/liver transplants were excluded as were transplants in which intraoperative mortality occurred. The primary surgical approach was the piggyback technique with caval interposition. Venovenous bypass was used only when caval interposition was not feasible. Anesthesia was provided by a dedicated liver transplant anesthesia team using a volatile-based technique. Advanced invasive monitoring including the placement of a pulmonary artery catheter was routine. Laboratory support was provided by an in-OR “stat” laboratory and included the use of viscoelastic testing. At the conclusion of surgery, patients were transferred to the intensive care unit for further management.
Preoperative, intraoperative, and postoperative variables were collected, including demographic, comorbidity, hemodynamic, and transfusion data. Mortality and major morbidity within 30 days of transplant were identified. Major morbidities were new diagnoses of myocardial infarction, cardiac arrest, stroke, pulmonary embolism, and respiratory failure requiring reintubation, renal failure requiring hemodialysis, sepsis, and seizures.
Patient data were obtained and confirmed from the electronic medical record using an institution-based query-building tool (Data Discovery and Query Builder21) and from the institution’s prospectively maintained liver transplant database. Intraoperative hemodynamic and fluid management data were abstracted from the anesthesia record (Anesthesia Information Management System [PICIS ChartPlus, Wakefield, MA]) in 10-minute nonoverlapping intervals as described by Hyder et al.22 Additional demographic, comorbidity, and outcome data were collected by electronic text and International Statistical Classification of Diseases and Related Health Problems-9 code search of patient notes using Data Discovery and Query Builder followed by manual review. In the case of conflicting information, the discharge summary (for non-intraoperative variables) and anesthesia record (for intraoperative variables) took precedence. A confirmed physician diagnosis of each outcome variable (eg, myocardial infarction) was required; however, severity was not differentiated. MELD, Sequential Organ Failure Assessment (SOFA), and Acute Physiology and Chronic Health Evaluation III (APACHE 3) scores were calculated based on preoperative, intraoperative, and intensive care data.23-25 All data were collected using Microsoft Excel and JMP software (Microsoft Corporation, 2010, and SAS Institute Inc., 2012, respectively).
Statistical Analysis
Development of SAS-LT
A SAS for liver transplant (SAS-LT) was developed using lowest intraoperative HR, lowest MAP, and transfusion volume. Lowest HR and MAP were categorized based on the cutpoints specified by the SAS.12 Transfusion volume was analyzed as a continuous variable, and also categorically with cutpoints established after reviewing receiver operating characteristic (ROC) curves. Blood transfusion volume was divided into quartiles, and a score from 0 to 3 was assigned according to descending quartiles with adjustment of volumes to nearest 1000-mL value.
Outcome Prediction
The classificatory ability of the SAS-LT, SOFA, APACHE 3, and MELD scores for death or severe complications were compared by ROC analysis. The associations of scoring systems with death or severe complications were analyzed separately for dichotomous outcomes using multivariable logistic regression. Additional ROC analysis was also performed using the SAS-LT components as continuous variables both combined and individually. Area under the curve (AUC) estimates for individual SAS-LT components were obtained by logistic regression models. Two-tailed P values of 0.05 or less were considered statistically significant, and findings were summarized using point estimates and 95% confidence intervals.
RESULTS
Six hundred thirteen patients underwent 628 liver transplants during the study period. Two hundred thirty-four (37.3%) were female patients. Combined liver-kidney transplants comprised 58 (9.2%). Median MELD was 18.9 (interquartile range [IQR], 11.5-28.7). Patient demographics and donor data are shown in Tables 2 and 3.
TABLE 2.
Patient characteristics
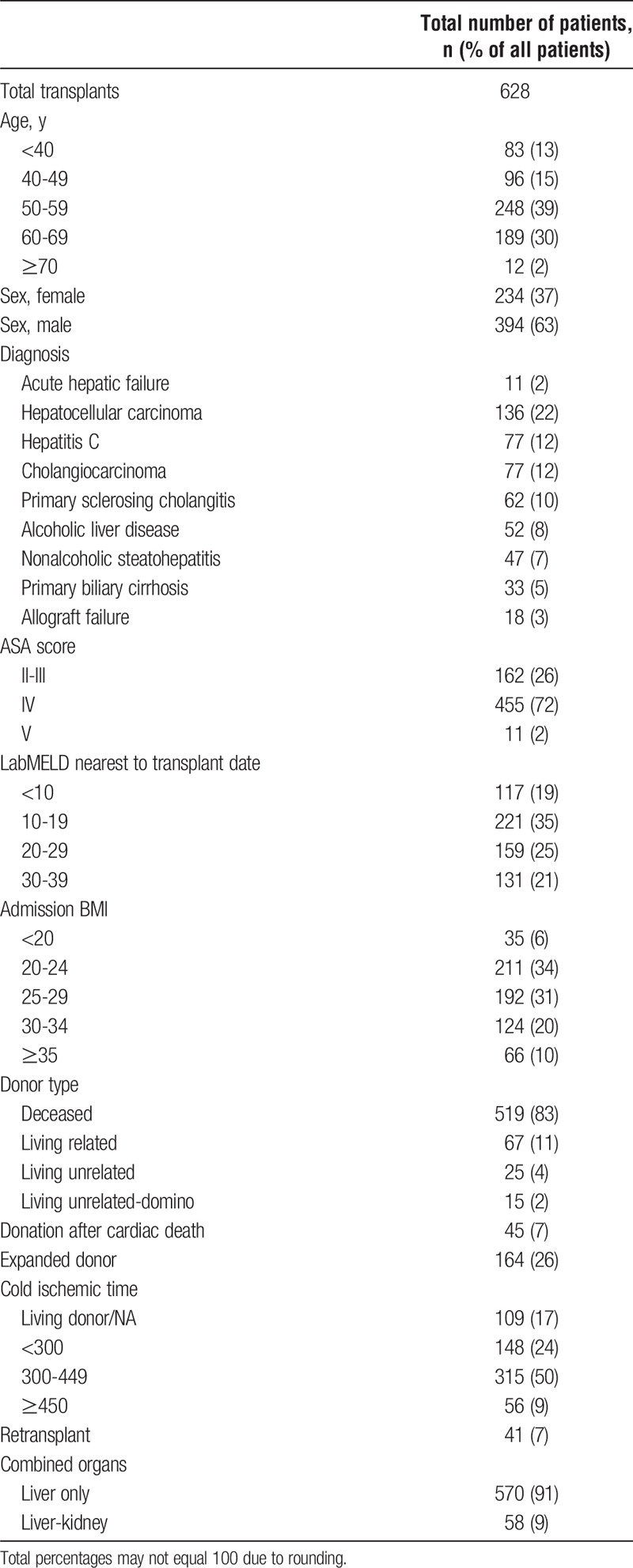
TABLE 3.
Patient comorbidities, n (%)

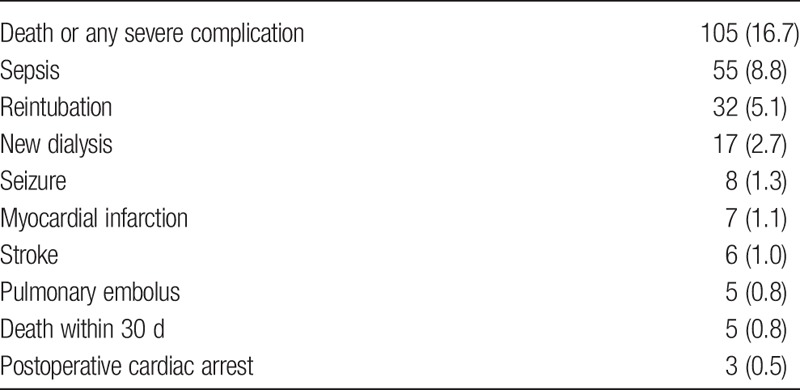
Death or any severe complication occurred after 105 (16.7%) procedures, mortality within 30 days occurred in 5 (0.8%) cases. The frequencies of complications are shown in Table 4.
TABLE 4.
Complications, n (%)
The final SAS-LT scoring system is shown in Table 5. The mean SAS-LT score was 6.3 (SD, 1.5). The SAS-LT AUC for death or severe complications was 0.57 (IQR, 0.51-0.63) (P = 0.020) (Table 6). Additional ROC analysis using the SAS-LT components showed that RBC transfusion was the single strongest predictive component (AUC, 0.61; P <0.001) compared with HR and blood pressure (AUC, 0.51, 0.58; P = 0.826, 0.012, respectively) (Table 6). The APACHE 3, MELD, and SOFA score AUCs were 0.57, 0.56, and 0.62 (P = 0.024, 0.059, <0.001, respectively).
TABLE 5.
Modified SAS for liver transplant, SAS-LT
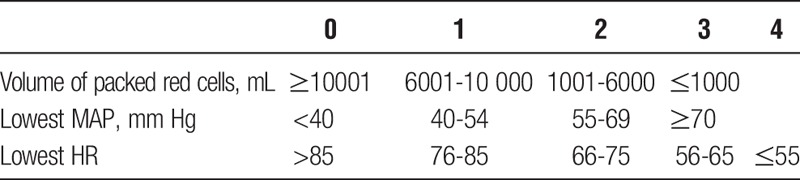
TABLE 6.
Association of MELD, SOFA, APACHE 3, and surgical Apgar components with death or severe complicationsa
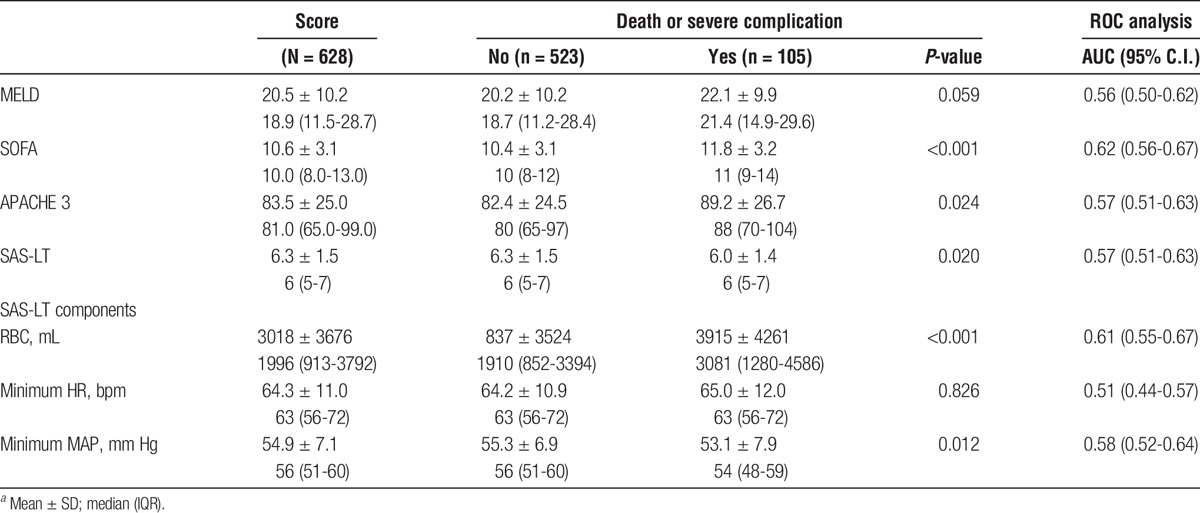
Finally, we evaluated the relationship between SAS-LT and ICU discharge within 24 hours. Seventy-nine (12.6%) patients were discharged from the ICU in ≤24 hours. SAS-LT for these patients was significantly higher than those with a longer stay (7.0 vs 6.2, P < 0.01). SAS-LT alone had an AUC of 0.64 (0.58-0.70). After multivariable analysis including SAS-LT, age, MELD, preoperative ICU stay, cold ischemia time, and use of expanded donor criteria, the AUC was 0.67 (0.61-0.73).
DISCUSSION
In this study, we found that the SAS can be adapted for liver transplantation and that the adapted score (SAS-LT) is associated with both major perioperative morbidity and mortality or early ICU discharge according to the score. When compared with other scoring systems, the SAS-LT had similar discriminant abilities to MELD and APACHE 3 for mortality and major morbidity, while the SOFA was the most discriminant.
The SAS-LT has some advantages over other scoring systems. The data points are easily obtained, easily calculated, and immediately available at the end of surgery for postoperative decision-making. They do not require sophisticated monitoring or elaborate calculations.12 The SAS-LT is tailored to a specific patient population (those with end-stage liver disease meeting transplant criteria) and specific surgery (orthotopic liver transplant). The volume of transfused red cells is a more objective measurement than EBL and may be an appropriate variable for applying the SAS to other surgeries for which blood loss may be extensive or difficult to quantify.
Because the SAS-LT can be obtained in the immediate postoperative phase, the score is available to clinicians earlier than the APACHE 3 or SOFA scores, which use the worst values obtained in the first 24 hours of ICU care.24,25 Many hospitals use fast-tracking systems, in which patients’ care processes are streamlined based on acuity.7 A higher SAS-LT may indicate better candidacy for a fast-tracking protocol, whether in the ICU or a step-down unit, although it should not supersede clinician judgment. As the SAS-LT had an AUC of 0.64 in this case, it may be a better predictor of fast-track eligibility than it is of morbidity or mortality. However, this would need to be confirmed with a prospective trial.
When we evaluated the components of SAS-LT individually, we found that blood transfusion was the strongest individual component followed by minimum BP; minimum HR showed no significant association with outcomes. In liver transplantation, many studies have demonstrated an association of increased blood transfusion requirements with poor outcomes. Cywinski et al26 found that mortality related to red cell transfusion peaked within 2 weeks of liver transplant. Other studies have also shown a strong association with blood transfusion requirement and early morbidity or mortality.27,28 In fact, volume of RBCs transfused alone performed better than the SAS-LT according to AUC analysis (0.61 vs 0.57). Amount of RBCs transfused could be further studied as an independent predictor of postoperative outcomes.
Previous studies have identified low intraoperative BP as associated with adverse outcome.29-31 The lack of association between lowest HR and outcome is in contrast to the findings of Gawande et al in formulating the SAS. The reason for this is unclear—it may be related to the deranged cardiovascular physiology associated with liver dysfunction or to the frequent use of beta blockade in this patient population for management of portal hypertension. In addition, although we did not explicitly collect data on surgical technique. During the study period, the primary surgical technique was the piggyback technique with caval interposition; venovenous bypass was only used when this could not be performed (<5% of cases). The alterations in vital signs could have been related to caval manipulation or other surgical maneuvers. That the HR component of the SAS-LT was ineffective does raise the possibility that substitution of a different intraoperative variable or variables may improve SAS-LT; several cardiovascular parameters including mean pulmonary artery pressure, cardiac output, central venous pressure, and blood pressure lability have previously been linked to outcome.19,20,30
The adaptation of SAS required us to revise the blood transfusion categories from the original. Although the categories are appropriate for our transfusion practice over the period studied, it should be noted that there is a wide range of transfusion practices reported amongst liver transplant programs with similar outcomes.26 It is therefore possible that SAS-LT it is not generalizable to other institutions. However, the methodology we used to identify the blood transfusion categories could be used to develop a customized score if this were the case. Also, blood transfusion requirements during liver transplantation have been declining over time, so it is possible that the transfusion categories may require adjustment should this trend continue.32,33
The main limitations of this study are its retrospective nature and reliance on single institution data. Before using SAS-LT in clinical decision-making, prospective validation should be undertaken. Given the potential for institution-specific practices to influence perioperative variables, especially in relation to transfusion, multicenter validation should also be pursued.
All scoring systems in this study had AUCs less than 0.63, which may not be high enough to be clinically meaningful. Further study into other intraoperative variables as predictors of liver transplant outcomes is warranted. Based on our findings, volume of RBCs transfused should be studied as an independent predictor of outcomes. Central venous pressure, mean pulmonary artery pressure, cardiac output, and blood pressure lability have been shown to correlate with postoperative outcomes and may also be appropriate parameters for an intraoperative scoring system.29,31,34 Change in hemoglobin and vasopressor requirements could also be investigated as possible predictors. Furthermore, emerging techniques, such as transesophageal echocardiography and thromboelastography, are becoming more commonly used and may provide further insight into intraoperative factors affecting outcomes.35 Because the electronic medical record system is more widely adopted, there may be opportunity to develop more sophisticated and accurate calculators that incorporate both preoperative and intraoperative data that may result in improved prediction of postoperative morbidity and mortality.
CONCLUSIONS
The SAS-LT is an easily calculated score specific to liver transplant patients. It has an association with perioperative outcomes and may provide useful information to assist in allocating postliver transplant resources.
ACKNOWLEDGMENTS
The authors would like to thank Melissa Passe, RRT, Verlin (Wayne) Weber, RRT, and Alberto Marquez, RRT, for their help with data collection. The authors would also like to thank Jane Fasbender for her assistance with the Mayo Clinic liver transplant database.
Footnotes
Published online 6 October, 2017.
The authors received a grant from the Mayo Clinic Department of Anesthesiology & Perioperative Medicine to fund the statistical work.
The authors declare no conflicts of interest in this work.
A.P. contributed to the article’s conception and design, data collection and interpretation, and drafting and revising of the article. J.F. contributed to the article’s conception and design, data interpretation, and drafting and revising of the article. A.S. contributed to the article’s conception and design, data collection and interpretation, and revising of the article. D.S. contributed to the article’s conception and design, data analysis and interpretation, and revising of the article.
REFERENCES
- 1.Kim WR, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant. 2014;14(Suppl 1):69–96. [DOI] [PubMed] [Google Scholar]
- 2.Paterno F, Wilson GC, Wima K, et al. Hospital utilization and consequences of readmissions after liver transplantation. Surgery. 2014;156:871–878. [DOI] [PubMed] [Google Scholar]
- 3.Yataco M, Cowell A, David W, et al. Predictors and impacts of hospital readmissions following liver transplantation. Ann Hepatol. 2016;15:356–362. [DOI] [PubMed] [Google Scholar]
- 4.Aniskevich S, Pai SL. Fast track anesthesia for liver transplantation: review of the current practice. World J Hepatol. 2015;7:2303–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shum S, Tanzola R, McMullen M, et al. How well are prebooked surgical step-down units utilized? J Clin Anesth. 2013;25:202–208. [DOI] [PubMed] [Google Scholar]
- 6.Sobol JB, Gershengorn HB, Wunsch H, et al. The surgical Apgar score is strongly associated with intensive care unit admission after high-risk intraabdominal surgery. Anesth Analg. 2013;117:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taner CB, Willingham DL, Bulatao IG, et al. Is a mandatory intensive care unit stay needed after liver transplantation? Feasibility of fast-tracking to the surgical ward after liver transplantation. Liver Transpl. 2012;18:361–369. [DOI] [PubMed] [Google Scholar]
- 8.Klein KB, Stafinski TD, Menon D. Predicting survival after liver transplantation based on pre-transplant MELD score: a systematic review of the literature. PLoS One. 2013;8:e80661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutkowski P, Oberkofler CE, Slankamenac K, et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745–753; discussion 753. [DOI] [PubMed] [Google Scholar]
- 10.Halldorson JB, Bakthavatsalam R, Fix O, et al. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318–326. [DOI] [PubMed] [Google Scholar]
- 11.Rana A, Jie T, Porubsky M, et al. The survival outcomes following liver transplantation (SOFT) score: validation with contemporaneous data and stratification of high-risk cohorts. Clin Transplant. 2013;27:627–632. [DOI] [PubMed] [Google Scholar]
- 12.Gawande AA, Kwaan MR, Regenbogen SE, et al. An Apgar score for surgery. J Am Coll Surg. 2007;204:201–208. [DOI] [PubMed] [Google Scholar]
- 13.Haynes AB, Regenbogen SE, Weiser TG, et al. Surgical outcome measurement for a global patient population: validation of the surgical Apgar score in 8 countries. Surgery. 2011;149:519–524. [DOI] [PubMed] [Google Scholar]
- 14.Prasad SM, Ferreria M, Berry AM, et al. Surgical Apgar outcome score: perioperative risk assessment for radical cystectomy. J Urol. 2009;181:1046–1052; discussion 1052–1043. [DOI] [PubMed] [Google Scholar]
- 15.Regenbogen SE, Ehrenfeld JM, Lipsitz SR, et al. Utility of the surgical Apgar score: validation in 4119 patients. Arch Surg. 2009;144:30–36; discussion 37. [DOI] [PubMed] [Google Scholar]
- 16.Regenbogen SE, Lancaster RT, Lipsitz SR, et al. Does the surgical Apgar score measure intraoperative performance? Ann Surg. 2008;248:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds PQ, Sanders NW, Schildcrout JS, et al. Expansion of the surgical Apgar score across all surgical subspecialties as a means to predict postoperative mortality. Anesthesiology. 2011;114:1305–1312. [DOI] [PubMed] [Google Scholar]
- 18.Ou CY, Hsu SY, Huang JH, et al. Surgical Apgar score in patients undergoing lumbar fusion for degenerative spine diseases. Clin Neurol Neurosurg. 2017;152:63–67. [DOI] [PubMed] [Google Scholar]
- 19.Regenbogen SE, Bordeianou L, Hutter MM, et al. The intraoperative surgical Apgar score predicts postdischarge complications after colon and rectal resection. Surgery. 2010;148:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki Y, Tokunaga M, Tanizawa Y, et al. Perioperative risk assessment for gastrectomy by surgical Apgar score. Ann Surg Oncol. 2014;21:2601–2607. [DOI] [PubMed] [Google Scholar]
- 21.Alsara A, Warner DO, Li G, et al. Derivation and validation of automated electronic search strategies to identify pertinent risk factors for postoperative acute lung injury. Mayo Clin Proc. 2011;86:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyder JA, Kor DJ, Cima RR, et al. How to improve the performance of intraoperative risk models: an example with vital signs using the surgical Apgar score. Anesth Analg. 2013;117:1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 24.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. [DOI] [PubMed] [Google Scholar]
- 26.Cywinski JB, Alster JM, Miller C, et al. Prediction of intraoperative transfusion requirements during orthotopic liver transplantation and the influence on postoperative patient survival. Anesth Analg. 2014;118:428–437. [DOI] [PubMed] [Google Scholar]
- 27.Ramos E, Dalmau A, Sabate A, et al. Intraoperative red blood cell transfusion in liver transplantation: influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003;9:1320–1327. [DOI] [PubMed] [Google Scholar]
- 28.Rana A, Petrowsky H, Hong JC, et al. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. J Am Coll Surg. 2013;216:902–907. [DOI] [PubMed] [Google Scholar]
- 29.De Maria S, Jr, Nurnberg J, Lin HM, et al. Association of intraoperative blood pressure instability with adverse outcomes after liver transplantation. Minerva Anestesiol. 2013;79:604–616. [PubMed] [Google Scholar]
- 30.Nasraway SA, Klein RD, Spanier TB, et al. Hemodynamic correlates of outcome in patients undergoing orthotopic liver transplantation. Evidence for early postoperative myocardial depression. Chest. 1995;107:218–224. [DOI] [PubMed] [Google Scholar]
- 31.Reich DL, Wood RK, Jr, Emre S, et al. Association of intraoperative hypotension and pulmonary hypertension with adverse outcomes after orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2003;17:699–702. [DOI] [PubMed] [Google Scholar]
- 32.Coelho GR, Feitosa Neto BA, de G Teixeira CC, et al. Single-center transfusion rate for 555 consecutive liver transplantations: impact of two eras. Transplant Proc. 2013;45:3305–3309. [DOI] [PubMed] [Google Scholar]
- 33.Findlay JY, Long TR, Joyner MJ, et al. Changes in transfusion practice over time in adult patients undergoing liver transplantation. J Cardiothorac Vasc Anesth. 2013;27:41–45. [DOI] [PubMed] [Google Scholar]
- 34.Chung HS, Jung DH, Park CS. Intraoperative predictors of short-term mortality in living donor liver transplantation due to acute liver failure. Transplant Proc. 2013;45:236–240. [DOI] [PubMed] [Google Scholar]
- 35.Schumann R, Mandell MS, Mercaldo N, et al. Anesthesia for liver transplantation in United States academic centers: intraoperative practice. J Clin Anesth. 2013;25:542–550. [DOI] [PubMed] [Google Scholar]


