Abstract
To explore the association of the X-ray repair cross-complementing gene 1 (XRCC1) codon 399 single-nucleotide polymorphism (SNP) with acute radiation dermatitis and oral mucositis in nasopharyngeal carcinoma (NPC) patients treated by intensity-modulated radiation therapy (IMRT).
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used to detect the SNP of the XRCC1 codon 399 in 114 NPC patients before radiotherapy.
The risk of patients with the Arg/Arg genotype suffering from acute radiation dermatitis Grade ≥2 was higher than the other 2 genotypes (P = .014, 95% CI: 1.182–4.582). No significant difference was observed in the degree of acute radiation oral mucositis injury among the patients with different genotypes (P = .449, 95% CI: 0.691–2.304). Multivariate analysis showed that N stage and genotype were significantly associated with acute radiation dermatitis of Grade ≥2 (OR = 3.221, P < .001, 95% CI: 1.669–6.216, OR = 2.860, P = .006, 95% CI: 1.354–6.043). T stage and smoking status were significantly associated with acute radiation oral mucositis with Grade ≥2 (OR = 2.508, P = .001, 95% CI: 1.427–4.408, OR = 6.355, P < .001, 95% CI: 2.533–15.841).
The XRCC1 codon 399 genotype in NPC could be an important predicting factor in the risk of acute radiation dermatitis during IMRT.
Keywords: nasopharyngeal carcinoma, oral mucositis, radiation dermatitis, XRCC1 codon 399 polymorphism
1. Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common types of cancer that occurs in South China, and its morbidity ranks first among the malignant tumors of the head and neck region and ranks 11th among all malignant tumors.[1] Radiotherapy is considered as the most important primary treatment for NPC.[2] The efficacy increases with the use of high-intensity radiotherapy, but the risk of radiation injury also increases. The skin and oral mucosa are the most commonly affected sites and significant acute reactions are observed at these sites.[3,4] Due to those reasons some patients might drop the option of radiotherapy, affecting their prognosis. Thus, there is a need for exploring the factors associated with acute dermatitis and oral mucositis caused by radiotherapy and currently this becomes one of the hotspot of research for NPC patients undergoing radiotherapy.
The reaction of normal tissues to radiotherapy is complex and involves complicated biological processes that are influenced by many external and intrinsic factors such as age, gender, staging, pathological type, etc.[5–7] Normal tissues of different individuals show different radioreactions after radiotherapy with similar clinical dosage.[8] This difference in radiosensitivity may be related, at least in part, to the intrinsic sensitivity determined by individual genetic factors.[7–9] Physicians have realized that the intrinsic nature of radiosensitivity differences might be associated with the differences in biological reaction of interrelated genes to radiation. The gene mutations, gene polymorphisms, and epigenetic modifications related to the biological effects of radiations may cause radiosensitivity differences.[6,10] DNA is the key target of ionizing radiation. The direct effect of radiation and the indirect effect of free radicals generated by ionization lead to DNA injury. Whereas, differences in DNA repair functions can result in radiosensitivity differences among different individuals.[6] The X-ray repair cross-complementing gene 1 (XRCC1) was the first gene proved to influence the sensitivity of cells to ionizing radiation in mammals.[11] XRCC1 can affect the repair activity of DNA, and it is the key protein in the base repair pathway, which repairs DNA injury caused by ionizing radiation.[12–14] Genetic mutations occur in XRCC1 in malignant tumors, and single-nucleotide polymorphism (SNP) arises as a common and important genetic variation type. The existence of polymorphism changes the structure of coding amino acids, and influences the structure and function of the repair process.[15] In vitro studies have proved that the sensitivity to radiotherapy was affected after XRCC1 mutation.[16] Previous clinical studies also indicated that SNPs may act as potential biomarkers for predicting the radiosensitivity of malignant and normal tissues.[9,13,17] In the XRCC1 gene coding region, the 3 most common SNPs are Arg194Trp (exon 6), Arg280His (exon 9), and Arg399Gln (exon 10).[18] Among them, the most possible one that influences cellular DNA repair is Arg399Gln.[19] Although few studies have demonstrated associations between gene polymorphism, DNA repair function, and individual sensitivity,[20] other studies have indicated associations between sensitivity to radiotherapy and genetic changes.[9,13,16,17] There are a few reports on the association between SNP of XRCC1 and radiotherapy sensitivity as well as their related toxicities and side effects,[12,13,17,21,22] but limited data are available in NPC patients. Especially, the association between the XRCC1 codon 399 SNP and radiation injury still remains controversial.[12,13,17,22–24]
Thus, in this study, after the detection of the XRCC1 codon 399 SNP in locally advanced NPC patients planned to receive radiotherapy at our hospital, we analyzed the occurrence of conditions such as acute radiation dermatitis and oral mucositis in patients with different genotypes. In addition, we explored the correlation between the SNPs and acute radiation dermatitis and oral mucositis. Optimizing therapy is important to improve the quality of life of the patients.
2. Materials and methods
2.1. Patients
Between August 1, 2013 and July 31, 2015, 114 NPC patients who were initially treated at the Oncology Department of The Fourth Affiliated Hospital of Guangxi Medical University were enrolled.
The inclusion criteria were: patients 18 to 75 years of age who were diagnosed with NPC by histological examination, no distant metastasis at diagnosis, and the clinical stage III/IVa according to the 7th AJCC/UICC staging system; at least one measurable nasopharynx + neck lesion on magnetic resonance imaging (MRI); radiotherapy model was a whole-course intensity-modulated radiation therapy (IMRT); performance status (PS) ≤2 or Karnofsky ≥70; and normal skin and oral mucosa before the first radiotherapy fraction.
The exclusion criteria were: no pathological diagnosis; with relapse or metastasis; severe comorbidities; dermatosis or autoimmune disease; incomplete treatment regimen; interrupted follow-up; or missing or incomplete data.
The project was approved by the Ethics Committee of the hospital. All the enrolled patients had been informed regarding the protocol and have signed the informed consent. Intravenous blood (2 mL) was drawn into EDTA anticoagulative tubes before concurrent chemoradiotherapy. After standing still, the blood clot was taken out to extract DNA, and the DNA was stored at −80°C. The information of all the enrolled patients was recorded and followed up until 3 months after radiotherapy.
2.2. Therapy method
2.2.1. Chemoradiotherapy regimen
The patients initially received induction chemotherapy for 2 to 3 cycles (paclitaxel 135 mg/m2 on the first day, cisplatinum 75 mg/m2 on the first day). After 3 weeks, concurrent chemoradiotherapy was performed. All patients received radical IMRT, and the range of therapy covered from the skull base to the clavicle. Computed tomography (CT) simulation positioning was used in all patients, and the target delineation was performed by CT and MRI fusion. According to the ICRU 50 (International Commission on Radiation Units and Measurements) and Report No. 62, radiotherapy target was delineated, as well as contiguous threatening of organs such as brainstem and spinal cord. Radiotherapy was conducted using a 6-MV X-ray from Elekta precise linear accelerator (Version 10.0.28; Varian Medical Systems, Palo Alto, CA). The radiotherapy dosage to the nasopharynx and neck in the presence of positive lymph node 70 to 76 Gy. The radiotherapy dose of CTV1 and CTV2 were 60 to 66 Gy and 54 to 60 Gy, respectively. All radiotherapies were performed once daily, 5 times/week. In the concurrent chemoradiotherapy session, single cisplatinum regimen for 3 weeks was applied (cisplatinum 75 mg/m2).
2.2.2. XRCC1 genotyping detection
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used to detect the genotypes. The primers were synthesized by Sangon Biotech. Polymerase chain reaction (PCR) amplification included DNA fragments of the XRCC1 codon 399 polymorphic site.
The primer was 5′-TCCTCCACCTTGTGCTTTCT-3′. The PCR reaction system included 25 μL of PCR mixture solution, 0.1 μg of template DNA, 0.4 μmol/L primers, 0.1 mmol/L dNTP, 1.5 mmol/L MgCl2, 1.0 U Taq polymerase, and 1× reaction buffer. The PCR conditions included 95°C predenaturation for 5 minutes, followed by 95°C for 30 seconds, 61°C for 30 seconds, and 72°C for 45 seconds, for a total of 35 cycles, followed by extension for 10 minutes at 72°C. The PCR products of codon 399 (5 μL) and the restriction endonuclease PvuII or NciI (5 μL) were incubated overnight at 37°C. PCR products (6 μL) were taken and added into 2.0% agarose gel wells. Fifty base pairs of DNA Ladder Marker (5 μL) was added in the first well as standard reference. The gel was placed in l× Trihydroxyaminomethane + boric acid + EDTA electrophoresis buffer, and horizontal electrophoresis was performed under 120 V for 40 minutes. After electrophoresis, the agarose gel was taken out, placed under UV and photographed. The result of genetic typing was recorded. In the presence of a mutation in the XRCC1 codon 399, the cleavage site is lost and cannot be recognized by NciI, and only 1 fragment with 517 bp is generated. The digestion site exists in the wild-type allele; after being digested by restriction endonuclease NciI, 2 fragments (384 and 133 bp) are generated. Heterozygote generates 3 fragments (517, 384, and 133 bp).[25] Ten percent of all samples were selected randomly and delivered to Sangon Biotech to sequence the PCR product to validate the PCR-RFLP results.
2.3. Evaluation of radiation injury
Acute radiation injury is defined as injury appearing from the initial day of radiotherapy until 3 months after the end of radiotherapy. In the process of patients’ radiotherapy, the radiation injuries to the skin and oral mucosa were observed and recorded 3 times daily. At the 1st and 3rd months after radiotherapy, the evaluation was performed again. Upper neck was selected as the observing area in patients with acute radiation dermatitis. The severity was determined by specialists according to the acute radiation injury grading standard from the Radiation Therapy Oncology Group (RTOG). The occurrence rate of oral mucositis was graded according to the RTOG grading standard[26] and verbal rating scales (VRS).
2.4. Statistical analysis
Data were analyzed using SPSS 19.0 (IBM, Armonk, NY). Goodness-of-fit Chi-square test was applied to confirm whether each genotype complied with the Hardy–Weinberg equilibrium. Univariate logistic regression analysis was applied to calculate the association between acute radiation dermatitis at different degrees, clinical factors, and genotype frequency. The factors with statistical significance were entered into a multivariate logistic regression model to obtain odds ratio (OR) and 95% confidence interval (95% CI). P < .05 was considered to be statistically significant.
3. Results
3.1. Patient enrollment
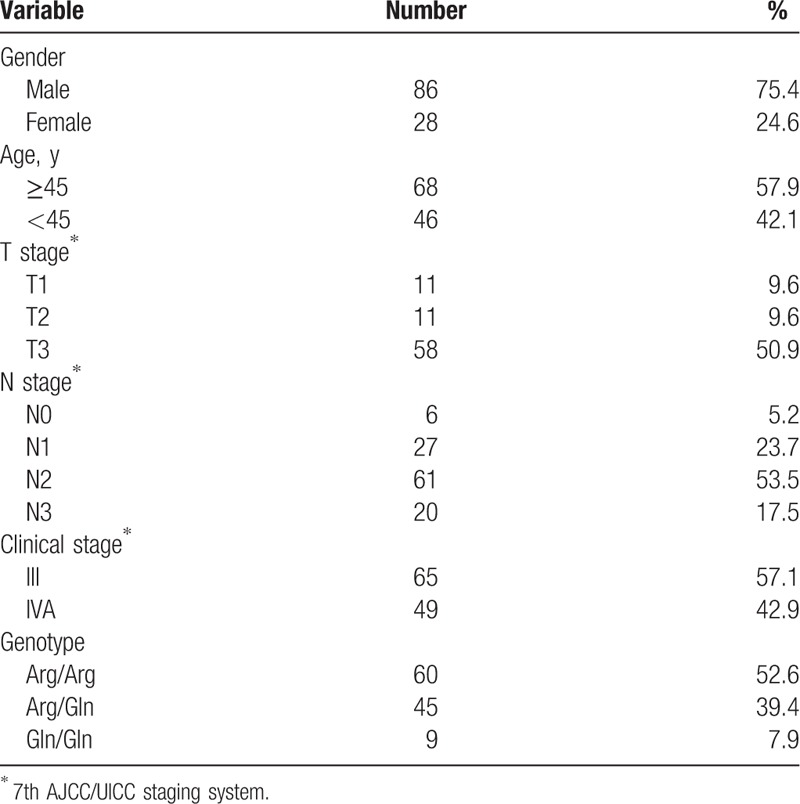
The number of enrolled patients in the initial experiment was 131, and 17 patients (14.9%) were excluded as complete follow-up information could not be obtained. Finally, 114 patients were enrolled in the study. There were 86 males and 28 females, with ratio of 3.1:1. The age range was 18 to 71 years, with a median age of 46 years. The clinical characteristics of all patients are presented in Table 1.
Table 1.
Baseline characteristics of the NPC patients.
3.2. Genotype distribution of the XRCC1 codon 399
XRCC1 codon 399 genotypes in the 114 patients are shown in Table 1. Electrophoresis is shown in Fig. 1. The genotypic distribution met the population genetics of the Hardy–Weinberg equilibrium (P > .1). There was no association between the XRCC1 codon 399 genotypes and smoking (P = .445).
Figure 1.
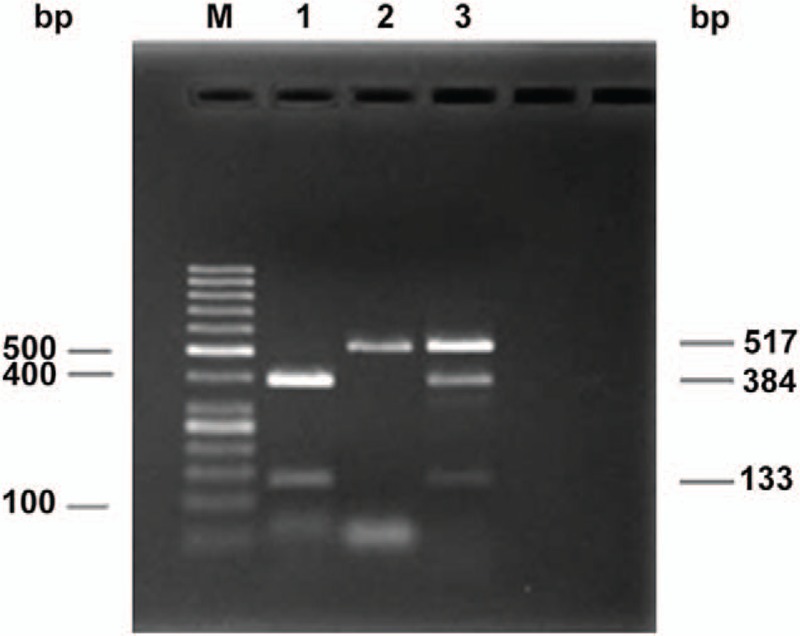
Agarose gel electrophoretogram of XRCC1 genotypes using PCR-RFLP. M: DNA marker. The 3 fragments of the XRCC1 codon 399 digested by the NciI restriction enzyme are 517, 384, and 133 bp, respectively. 1, Arg/Arg homozygote; 2, Gln/Gln homozygote; 3, Arg/Gln heterozygote.
3.3. Acute radiation injury association with the XRCC1 codon 399 genotypes in locally advanced NPC
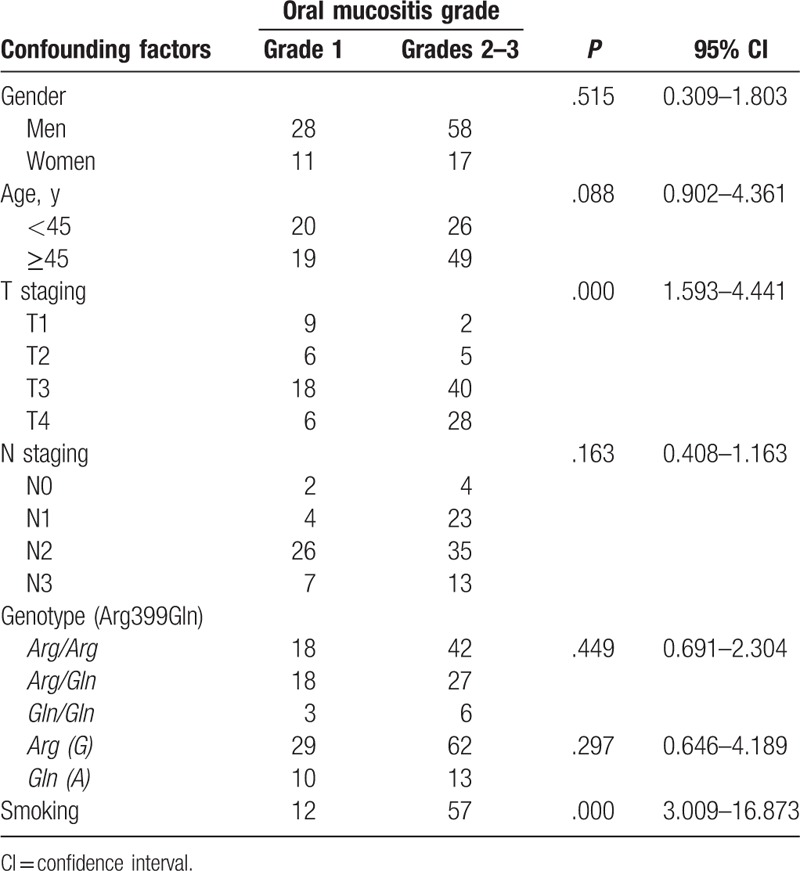
All patients with locally advanced NPC showed acute radiation dermatitis and oral mucositis at different degrees, but no radiation related adverse reactions Grade ≥4 were observed (Table 2). There were 28 patients (24.6%) with the Arg/Arg genotype who showed Grade ≥2 acute radiation dermatitis, compared with 13 (11.4%) and 1 (0.9%) patients with the Arg/Gln and Gln/Gln genotypes, respectively (Table 2). The risk of patients with the Arg/Arg genotype of suffering from Grade ≥2 acute radiation dermatitis was higher than for the other 2 genotypes (P = .014, 95% CI: 1.182–4.582) (Table 2). The risk of the wild-type allele Arg (G) suffering from Grade ≥2 acute radiation dermatitis appeared higher than that of the mutant type allele Gln (A) (P = .031, 95% CI: 1.122–11.312) (Table 2). In the locally advanced NPC patients with different genotypes, the injury degree of acute radiation oral mucositis showed no significant difference (P = .449, 95% CI: 0.691–2.304) (Table 3). The number of Grade ≥2 acute radiation oral mucositis occurring in the Arg/Arg, Arg/Gln, and Gln/Gln genotypes were 42 (36.8%), 27 (23.7%), and 6 (5.3%), respectively (Table 3).
Table 2.
Association of the clinical parameters including the XRCC1 codon 399 SNP and radiation dermatitis in patients with locally advanced nasopharyngeal carcinoma.
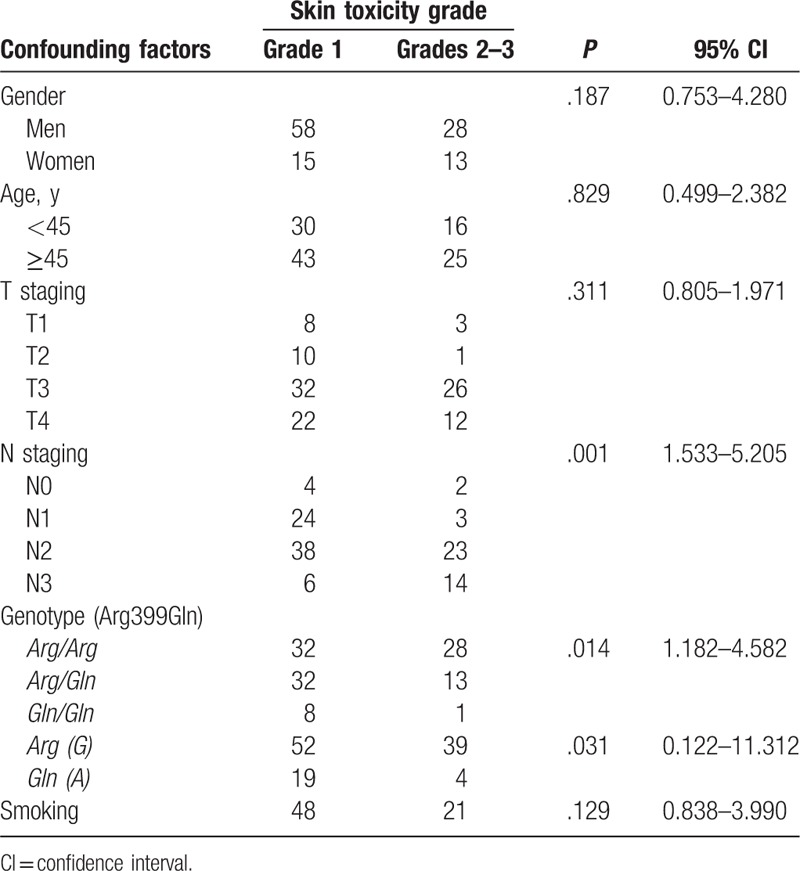
Table 3.
Association of the clinical parameters including the XRCC1 codon 399 SNP and radiation-induced oral mucositis in patients with locally advanced nasopharyngeal carcinoma.
3.4. Correlation of the XRCC1 codon 399 SNP and clinical parameters with acute radiation injury of locally advanced NPC
Logistic regression analysis indicated that XRCC1 codon 399 and N stage were associated with Grade ≥2 acute radiation dermatitis, while T stage and smoking status were associated with Grade ≥2 acute radiation with oral mucositis (Table 4). Further nonconditional logistic multivariate regression model fitting showed that in acute radiation dermatitis, the order of relative risk was N stage > genotype, suggesting that when each N grade was increased, the risk of occurrence of Grade ≥2 acute radiation dermatitis was increased by 3.221-fold (P < .001, 95% CI: 1.669–6.216) (Table 5); the risk of wild homozygous type Arg/Arg was increased by 2.860-fold when compared with Arg/Gln and Gln/Gln (P = .006, 95% CI: 1.354–6.043) (Table 5). For Grade ≥2 acute radioactive oral mucosa, the order of relative risk was smoking status >T stage, suggesting that when each T grade was increased, the risk of occurrence of Grade ≥2 acute radiation oral mucositis was increased by 2.508-fold (P = .001, 95% CI: 1.427–4.408) (Table 4). The risk of smoking increased by 6.355-fold the occurrence of Grade ≥2 acute radiation oral mucositis (P < .001, 95% CI: 2.533–15.841) (Table 4).
Table 4.
Logistic regression analysis of factors of acute radiation-induced oral mucositis in patients with locally advanced nasopharyngeal carcinoma.

Table 5.
Logistic regression analysis of factors of acute radiation dermatitis in patients with locally advanced nasopharyngeal carcinoma.

4. Discussion
In this study, we analyzed the association between the XRCC1 codon 399 SNP and acute radiation dermatitis as well as oral mucositis in 114 NPC patients who received radical IMRT therapy, and the related risk factors were evaluated by logistic regression analysis. The results indicated that the patients with different genotypes showed significant differences in acute radiation dermatitis reaction. Patients with XRCC1 codon 399 Arg/Arg had higher risk of Grade ≥2 acute radiation dermatitis compared with patients with the other genotypes. Multivariate logistic regression analysis indicated that the genotype was a risk factor for acute radiation dermatitis. The risk of wild-type Arg/Arg was increased by 2.860-fold compared with Arg/Gln and Gln/Gln in the occurrence of Grade ≥2 acute radiation dermatitis. Furthermore, it could protect the radiated normal tissues, which was in line with previous studies.[21,24] Alsbeih et al[12] also observed the relationship between XRCC1 codon 399 SNP and radioactive skin injury in patients who received radiotherapy for head and neck cancer. They also believed that Arg allele could increase the risk of radioactive skin injury. Although this study focused on the advantage of radioactive injury, the acute and terminal stages of adverse reactions acted upon the molecules and cellular pathways in different mechanisms, wherein it might include some other genes.[27] Li et al[24] also observed the relationship between XRCC1 codon 399 SNPs and radiosensitivity in NPC patients who received radical radiotherapy, but they thought that heterozygous Arg/Gln patients were more likely to suffer from severe acute skin injury (OR = 2.65). Zhai et al[5] found no association between XRCC1 codon 399 SNP and NPC patients with acute radiation dermatitis. The results from a study by Zhai et al[5] are different from those by Li et al[24] and this difference might be correlated with the aspect that some patients in the later study received 3-dimensional conformal radiotherapy (3D-CRT) and some received IMRT. Furthermore, different radiotherapies showed diverse influences in the predictive value of different SNPs.[23,28–30] Another possible reason for the inconsistency of the conclusion is that Li et al[24] defined radiation dermatitis Grade ≥3 as positive result according to CTCAE 3.0 Toxicity classification standard. On the other hand, in our study, we defined radiation dermatitis Grade ≥2 as positive result according to the RTOG toxicity classification standard. Normally, radiation dermatitis whose degree was Grade <2 is considered as an acceptable injury degree, which does not influence further radiotherapy, but the skin injury Grade ≥2 is considered as severe side-effect, and part of the patients need to interrupt the therapy and be treated actively, which is in line with the opinions of Li and Zheng[31] and Raabe et al.[13] Thus, we defined skin injury Grade ≥2 as positive result and Grade <2 as negative result. Other possible reasons for the inconsistencies with other studies include that all the patients with NPC were in locally advanced stage, and all of them received mainstream radical IMRT in this study. This not only proved the unification of therapy indices, but also had common clinical significance. Another reason includes that the reaction of individual to ionizing radiation is synthetically affected by various genes and polymorphism sites. Many polymorphism sites often exist in a candidate gene, and the analysis of a single site could achieve different conclusions.[10,21,27] This is because the change in one polymorphic site is not enough to change the clinical radiosensitivity, since the combination of various polymorphic sites in various genes determines the complexity of individual radiosensitivity.[20] Additional studies that comprehensively analyze panels of polymorphisms thought to be involved in radiosensitivity are necessary. Nine previous studies about the XRCC1 codon 399 SNP and radioactive skin injury were included in a meta-analysis.[13,21,23,24,28–30,32,33] The forest plot (Fig. 2) showed that there was no statistically significant correlation between them. Thus, we cannot bring out specific answer about the association observed in the present study.
Figure 2.
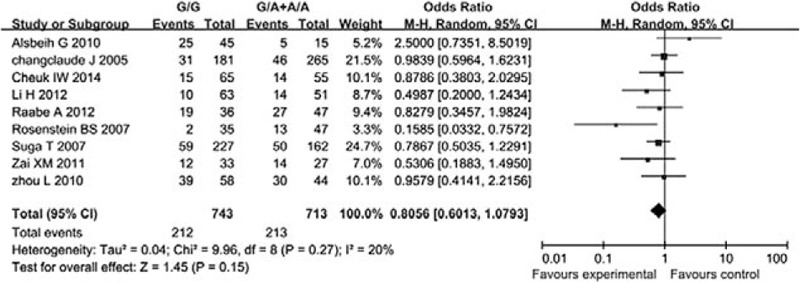
Relationship between the XRCC1 codon 399 SNP and radiation dermatitis: a pooled analysis of studies by forest plot. P > .05; no statistically significant correlations were found.
Our study also proved that N stage and genotype were risk factors for acute radiation dermatitis, and smoking and T stage for acute radioactive mucositis. Practically, as the N stage and T stage increase, in order to control the tumor better, the target volume of radiation dosage locally in the neck or oropharynx will be increased, which further changes the relationship between dosage-volume and radiation injury, and similar results have been observed by Raabe et al,[13] Bentzen et al,[34] and Musha et al.[35] Furthermore, it has been reported that the risk of acute severe oral mucosal injury in smoking patients is 6.355-fold higher than that of nonsmoking patients. The reason behind this is that the toxic substances such as phenols in tobacco can stimulate oral mucosa. The increased temperature in the oral cavity when smoking causes burning in the contact segment of oral cavity. Smoking can also influence the local blood circulation and humoral immunity. Furthermore, the proliferation of epithelial cells in the oral mucosa of patients receiving radiotherapy is poor, which may lead to more severe injuries caused by radiotherapy. It has also been proved that the epidermal growth factor levels in the saliva from smokers are lower than those from nonsmokers. The epidermal growth factor could promote wound healing which means that the repair of oral mucosal injury is slow, which more easily caused severe oral mucositis.[36]
In conclusion, our study confirmed an association between XRCC1 codon 399 genotype and the risk of acute radiation dermatitis in locally advanced NPC patients. However, due to the small sample size, analysis of additional genes in combination with various biomarkers was not possible. In the future, multicenter studies with a larger sample size should be performed to explore the value of genetic factors on predicting radiosensitivity. This further provides more powerful theory to support individualized radiotherapy based on molecular markers.
Footnotes
Abbreviations: 3D-CRT = 3-dimensional conformal radiotherapy, CI = confidence interval, CT = computed tomography, IMRT = intensity-modulated radiation therapy, MRI = magnetic resonance imaging, NPC = nasopharyngeal carcinoma, OR = odds ratio, PCR = polymerase chain reaction, PCR-RLFP = polymerase chain reaction-restriction fragment length polymorphism, PS = performance status, RTOG = Radiation Therapy Oncology Group, SNP = single-nucleotide polymorphism, VRS = verbal rating scales, XRCC1 = X-ray repair cross-complementing gene 1.
Funding: This study was supported by the Guangxi Zhuang Autonomous Region Health Department (no. Z2014392).
The authors have no conflicts of interest to disclose.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer—success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 2014;110:377–84. [DOI] [PubMed] [Google Scholar]
- [3].Ozdemir S, Akin M, Coban Y, et al. Acute toxicity in nasopharyngeal carcinoma patients treated with IMRT/VMAT. Asian Pac J Cancer Prev 2015;16:1897–900. [DOI] [PubMed] [Google Scholar]
- [4].Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 2014;110:398–403. [DOI] [PubMed] [Google Scholar]
- [5].Zhai XM, Hu QC, Gu K, et al. Significance of XRCC1 codon399 polymorphisms in Chinese patients with locally advanced nasopharyngeal carcinoma treated with radiation therapy. Asia Pac J Clin Oncol 2016;12:e125–32. [DOI] [PubMed] [Google Scholar]
- [6].Bentzen SM, Overgaard J. Patient-to-patient variability in the expression of radiation-induced normal tissue injury. Semin Radiat Oncol 1994;4:68–80. [DOI] [PubMed] [Google Scholar]
- [7].Werbrouck J, De Ruyck K, Duprez F, et al. Acute normal tissue reactions in head-and-neck cancer patients treated with IMRT: influence of dose and association with genetic polymorphisms in DNA DSB repair genes. Int J Radiat Oncol Biol Phys 2009;73:1187–95. [DOI] [PubMed] [Google Scholar]
- [8].Liu S, Zou S, Zhao J. Association between polymorphism of XRCC1 gene and susceptibility to esophageal carcinoma. Shi Yong Zhong Liu Za Zhi 2013;3:253–60. [Google Scholar]
- [9].Popanda O, Marquardt JU, Chang-Claude J, et al. Genetic variation in normal tissue toxicity induced by ionizing radiation. Mutat Res 2009;667:58–69. [DOI] [PubMed] [Google Scholar]
- [10].Lehmann BD, McCubrey JA, Jefferson HS, et al. A dominant role for p53-dependent cellular senescence in radiosensitization of human prostate cancer cells. Cell Cycle 2007;6:595–605. [DOI] [PubMed] [Google Scholar]
- [11].Lunn RM, Langlois RG, Hsieh LL, et al. XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res 1999;59:2557–61. [PubMed] [Google Scholar]
- [12].Alsbeih G, Al-Harbi N, Al-Hadyan K, et al. Association between normal tissue complications after radiotherapy and polymorphic variations in TGFB1 and XRCC1 genes. Radiat Res 2010;173:505–11. [DOI] [PubMed] [Google Scholar]
- [13].Raabe A, Derda K, Reuther S, et al. Association of single nucleotide polymorphisms in the genes ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with risk of severe erythema after breast conserving radiotherapy. Radiat Oncol 2012;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect 2003;111:1843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ladiges WC. Mouse models of XRCC1 DNA repair polymorphisms and cancer. Oncogene 2006;25:1612–9. [DOI] [PubMed] [Google Scholar]
- [16].Cornetta T, Festa F, Testa A, et al. DNA damage repair and genetic polymorphisms: assessment of individual sensitivity and repair capacity. Int J Radiat Oncol Biol Phys 2006;66:537–45. [DOI] [PubMed] [Google Scholar]
- [17].Churchill ME, Peak JG, Peak MJ. Repair of near-visible- and blue-light-induced DNA single-strand breaks by the CHO cell lines AA8 and EM9. Photochem Photobiol 1991;54:639–44. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Y, Luo Z, Yang L, et al. The association between four SNPs of X-ray repair cross complementing protein 1 and the sensitivity to radiotherapy in patients with esophageal squamous cell carcinoma. Oncol Lett 2016;11:3508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu HP, Zeng XY, Qiu XQ, et al. Polymorphisms in the DNA repair gene XRCC1 and susceptibility to hu man lung cancer. J Guangxi Med Univ 2006;23:355–8. [Google Scholar]
- [20].Stur E, Agostini LP, Garcia FM, et al. Prognostic significance of head and neck squamous cell carcinoma repair gene polymorphism. Genet Mol Res 2015;14:12446–54. [DOI] [PubMed] [Google Scholar]
- [21].Zschenker O, Raabe A, Boeckelmann IK, et al. Association of single nucleotide polymorphisms in ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with clinical and cellular radiosensitivity. Radiother Oncol 2010;97:26–32. [DOI] [PubMed] [Google Scholar]
- [22].Langsenlehner T, Renner W, Gerger A, et al. Association between single nucleotide polymorphisms in the gene for XRCC1 and radiation-induced late toxicity in prostate cancer patients. Radiother Oncol 2011;98:387–93. [DOI] [PubMed] [Google Scholar]
- [23].Chang-Claude J, Popanda O, Tan XL, et al. Association between polymorphisms in the DNA repair genes, XRCC1, APE1, and XPD and acute side effects of radiotherapy in breast cancer patients. Clin Cancer Res 2005;11:4802–9. [DOI] [PubMed] [Google Scholar]
- [24].Li H, You Y, Lin C, et al. XRCC1 codon 399Gln polymorphism is associated with radiotherapy-induced acute dermatitis and mucositis in nasopharyngeal carcinoma patients. Radiat Oncol 2013;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Duell EJ, Wiencke JK, Cheng TJ, et al. Polymorphisms in the DNA repair genes XRCC1 and ERCC2 and biomarkers of DNA damage in human blood mononuclear cells. Carcinogenesis 2000;21:965–71. [DOI] [PubMed] [Google Scholar]
- [26].Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6. [DOI] [PubMed] [Google Scholar]
- [27].Andreassen CN, Alsner J, Overgaard J. Does variability in normal tissue reactions after radiotherapy have a genetic basis—where and how to look for it? Radiother Oncol 2002;64:131–40. [DOI] [PubMed] [Google Scholar]
- [28].Giotopoulos G, Symonds RP, Foweraker K, et al. The late radiotherapy normal tissue injury phenotypes of telangiectasia, fibrosis and atrophy in breast cancer patients have distinct genotype-dependent causes. Br J Cancer 2007;96:1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Suga T, Ishikawa A, Kohda M, et al. Haplotype-based analysis of genes associated with risk of adverse skin reactions after radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys 2007;69:685–93. [DOI] [PubMed] [Google Scholar]
- [30].Cheuk IW, Yip SP, Kwong DL, et al. Association of XRCC1 and XRCC3 gene haplotypes with the development of radiation-induced fibrosis in patients with nasopharyngeal carcinoma. Mol Clin Oncol 2014;2:553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li XD, Zheng XY. Investigation on the related factors for oral mucositis caused by radiotherapy in nasopharyngeal carcinoma patients with intensity modulated radiotherapy. Acta Universitatis Medicinalis Nanjing 2011;43:134–7. [Google Scholar]
- [32].Zhou L, Xia J, Li H, et al. Association of XRCC1 variants with acute skin reaction after radiotherapy in breast cancer patients. Cancer Biother Radiopharm 2010;25:681–5. [DOI] [PubMed] [Google Scholar]
- [33].Zhai XM, Wang JP, Gu K, et al. The clinical relevance between single nucleotide polymorphisms of XRCC1 Codon399 and chronic irradiation-induced injury of normal tissue in patients with nasopharyngeal carcinoma. Pract J Cancer 2011;26:596–9. [Google Scholar]
- [34].Bentzen SM, Parliament M, Deasy JO, et al. Biomarkers and surrogate endpoints for normal-tissue effects of radiation therapy: the importance of dose-volume effects. Int J Radiat Oncol Biol Phys 2010;76(3 suppl):S145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Musha A, Shimada H, Shirai K, et al. Prediction of acute radiation mucositis using an oral mucosal dose surface model in carbon ion radiotherapy for head and neck tumors. PLoS ONE 2015;10:e0141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Epstein JB, Gorsky M, Guglietta A, et al. The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer 2000;89:2258–65. [DOI] [PubMed] [Google Scholar]


