Supplemental Digital Content is available in the text
Keywords: antibody, death, fluoroquinolone, serious arrhythmia, ventricular arrhythmia
Abstract
Background:
The association between oral fluoroquinolones (FQs) usage and risk of severe arrhythmia-related events (ventricular arrhythmias and sudden cardiac death) remains controversial. Therefore we aimed to quantify this association and to evaluate the effects of FQs on adverse cardiovascular (CV) outcomes.
Methods:
We retrieved data from the Cochrane Collaboration, PubMed, and China National Knowledge Infrastructure (CNKI) databases until August 2017. The studies that reported relative risk (RR) estimates with 95% confidence intervals (CIs) for the associations of interest were included. Data were extracted from the eligible articles, and we used a random effects model to calculate the effect estimates.
Results:
Of the 16 studies that were included, 7 studies included serious arrhythmias, 3 studies included CV death, and 11 studies included all-cause death. The pooled RRs of FQs use were: 2.29 (95% CI: 1.20–4.36, P = .01) for serious arrhythmias; 1.60 (95% CI: 1.17–2.20, P = .004) for CV death; and 1.02 (95% CI: 0.76–1.37, P = .92) for all-cause death. The RRs associated with serious arrhythmias were 6.27 for gatifloxacin, 4.20 for moxifloxacin, 1.73 for ciprofloxacin, and 1.41 for levofloxacin. Current FQs users showed an increased risk of serious arrhythmias in the subgroup analysis. Treatment with FQs is associated with an absolute risk increase of 160 additional sudden deaths or ventricular arrhythmias, and 43 additional CV deaths per 1 million treatment courses.
Conclusion:
The use of FQs could increase the risk of serious arrhythmias and CV death but not increase or all-cause death. Moreover, moxifloxacin and levofloxacin showed a higher risk of serious arrhythmias.
1. Introduction
Fluoroquinolones (FQs) are a class of antibiotics widely used in the treatment of common bacterial infections in patients. Although FQs are well tolerated, with a broad spectrum of antibacterial activity and high oral bioavailability,[1] their cardiovascular (CV) toxicity has been largely questioned. Some types of FQs have been reported from a number of studies to elicit arrhythmia-related cardiac effects, including QT interval prolongation, torsades de pointes (TdP), ventricular tachycardia, ventricular fibrillation, and sudden cardiac death (SCD).[2,3] Owing to higher incidences of adverse cardiac events including SCD, the FQs sparfloxacin and gatifloxacin were sequentially removed from the US and European markets in 1999 and 2001, respectively.[4]
Although the adverse effect of FQ-induced arrhythmia had been raised by some studies.[5–9] Results from studies were conflicting. Randomized trials in healthy patients found the effect of FQs on prolonged QT interval.[10–12] Interesting, no significant relationship was found between incidences of adverse cardiac events and prolongation of the QT interval, which possible limited its small simple size.[13,14] Notable, inconsistent results were also found in large population-based studies in real world.[7–9,15,16] A cohort studies of Medicaid patients in Canada reported increased risk of ventricular tachyarrhythmia (VTA) and CV death associated with moxifloxacin and levofloxacin. However, a binational cohort study from Denmark and Sweden showed no relationship between FQs use and the risk VTA.[15] In another large cohort study of the Tennessee Medicaid program, ciprofloxacin was also not associated with increased risks of CV death and all-cause death.[16] Given this argument, an assessment of the risks and benefits of FQs use should be warranted to guide clinical treatment decisions. Thus, we conducted a meta-analysis to evaluate the associations between FQs and adverse CV outcomes.
2. Materials and methods
2.1. Literature search
We systematically searched the Cochrane Collaboration, PubMed, and CNKI (China National Knowledge Infrastructure) databases through August 2017 for studies published in any language using the following text and key words in combination, both as medical subject headings terms and text words: moxifloxacin, levofloxacin, ciprofloxacin, fluoroquinolones, cardiac, cardiovascular, death, mortality, ventricular tachycardia, ventricular arrhythmia, torsades de pointes, sudden cardiac death, and cardiac arrest. Further manual retrieval was performed using reference lists from the relevant original and review to identify other potentially relevant articles.[6–9,15–17]
2.2. Study selection
Studies were considered eligible if they fulfilled the following criteria: reported the relative risk (RR) and the corresponding 95% confidence intervals (CIs) or provided data to calculate them; were designed as RCTs (randomized controlled trials), cohort studies or case–control studies; assessed primary outcomes including serious arrhythmias, CV death, and all-cause death; and were independent. Studies with insufficient data (e.g., case report, no relevant outcome, compared CV risk between FQs and macrolides and reported only the prevalence of arrhythmia in FQs users) were excluded from analysis. For multiple publications/reports using the same data, we chose the estimates from the most informative or recent studies.
2.3. Outcome measures
The primary study outcome was serious arrhythmias, which were defined by the International Classification of Diseases 10th revision (ICD-10) codes, as ventricular tachycardia, ventricular fibrillation, TdP, ventricular flutter, cardiac arrest, and SCD. The secondary outcome was CV death. Our data indicated that FQs showed a proarrhythmic effect because of the increasing incidence of CV death. Moreover, further analysis of all-cause death was performed to examine whether the risk for CV death would be counterbalanced by the survival benefit of antiinfection by FQs.
2.4. Data extraction and quality assessment
Two researchers (XL and JM) independently assessed the eligibility of the literature according to the aforementioned inclusion criteria. All discrepancies were resolved through discussion or by a third researcher (KH) as necessary. For each study, the basic characteristics were extracted, including the first author, publication year, geographical location, participants (sex, mean age, and sample size), study type, follow-up duration, outcome events, FQs categories, adjusted covariates, and RRs with 95% CIs.
The methodological quality of the RCTs was assessed independently by 2 researchers using the Cochrane Collaboration's Risk of Bias tool, which scores each study for the following: randomization (sequence generation and allocation concealment), blinding (participant and outcome assessors), incomplete outcome data, selective outcome reporting, and other risks of bias. Each parameter was graded as a high, low, or unclear risk of bias.[18] The observational studies were assessed with the Newcastle-Ottawa Scale (NOS) method, with scores ranging from 0 to 9 points. Studies were regarded to be low-quality or high-quality if their NOS scores were <6 or ≥6 points, respectively.[19]
2.5. Statistical analysis and risk of bias assessment
The RRs were used as the common risk estimates, and the odds ratio was deemed to be equivalent to the RR.[20] The effect measures were transformed to their natural logarithm (logRR), and the standard error (SElogRR) was calculated from the corresponding 95% CIs. Summary RRs were estimated by pooling the study-specific estimates using the random effects models to take into account the between-study heterogeneity. To assess the heterogeneity of RRs across studies, the I2 (95% CI) statistic was calculated with the following interpretation: low heterogeneity, defined as I2 < 50%; moderate heterogeneity, defined as I2 50% to 75%; and high heterogeneity, defined as I2 > 75%.[19] Moreover, subgroup analyses were carried out when appropriate. Sensitivity analysis was performed to assess the effects of the selected study quality. Possible publication bias was assessed using the Egger test.[21] Since Egger linear regression method had stronger statistical and discriminatory powers than other Begg method and Macaskill method for detecting publication bias.[22] All statistical analyses were performed using the Review Manager (RevMan) software (version 5.30, Nordic Cochrane Center, Rigshospitalet, Denmark) and Stata software (version 12.0, Stata Corp LP, College Station, TX). A P-value <.05 was considered statistically significant. In addition, we calculated the absolute risk difference as risk per 1,000,000 treatment courses: (RR − 1) × I0, where RR indicates pooled RRs and I0 was the crude rate among users of without FQs group. On the basis of population-based cohort studies, I0 was calculated by weighting the sample size of each study.
2.6. Ethics approval
The ethical approval was not necessary in this study because of the meta-analysis study design.
3. Results
3.1. Literature search
As shown in Fig. 1, a total of 2833 studies were retrieved in our initial database search. After the removal of duplicates and other studies with inadequate information on the FQs, the 154 remaining studies were reviewed in more detail. Of these, 114 studies were excluded because of no relevant outcomes, and another 25 studies were eliminated because they did not provide enough data to calculate the RR. Finally, 16 studies (5 cohort studies, 3 case–control studies, and 8 RCTs) were included in this meta-analysis.[6–9,15,16,23–32]
Figure 1.
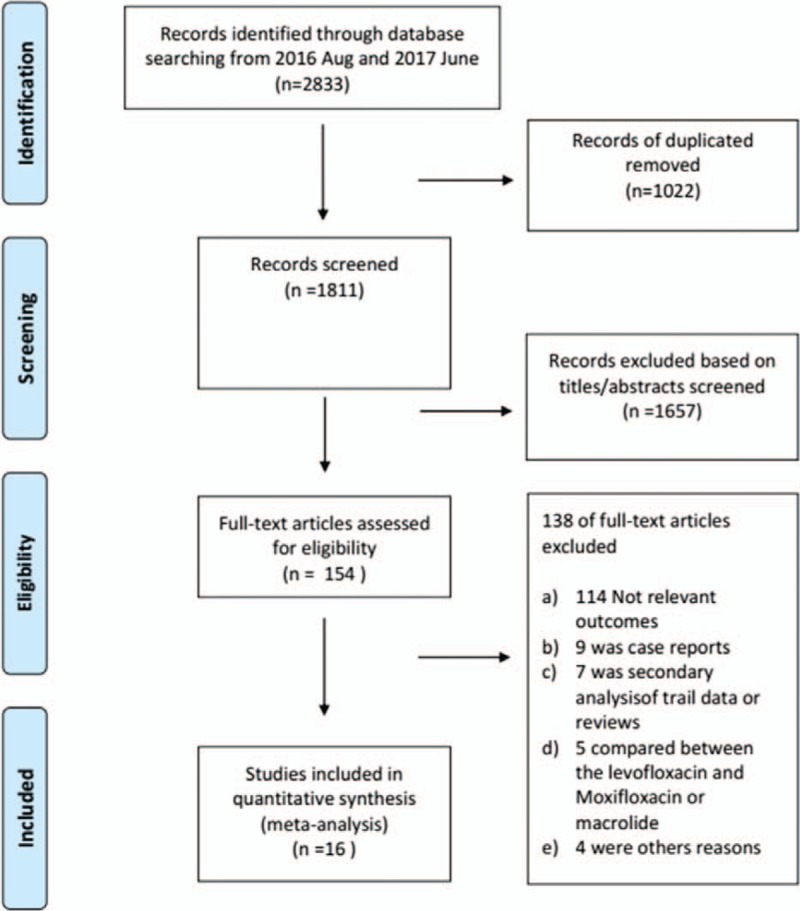
Flow diagram of study selection.
3.2. Study characteristics and quality
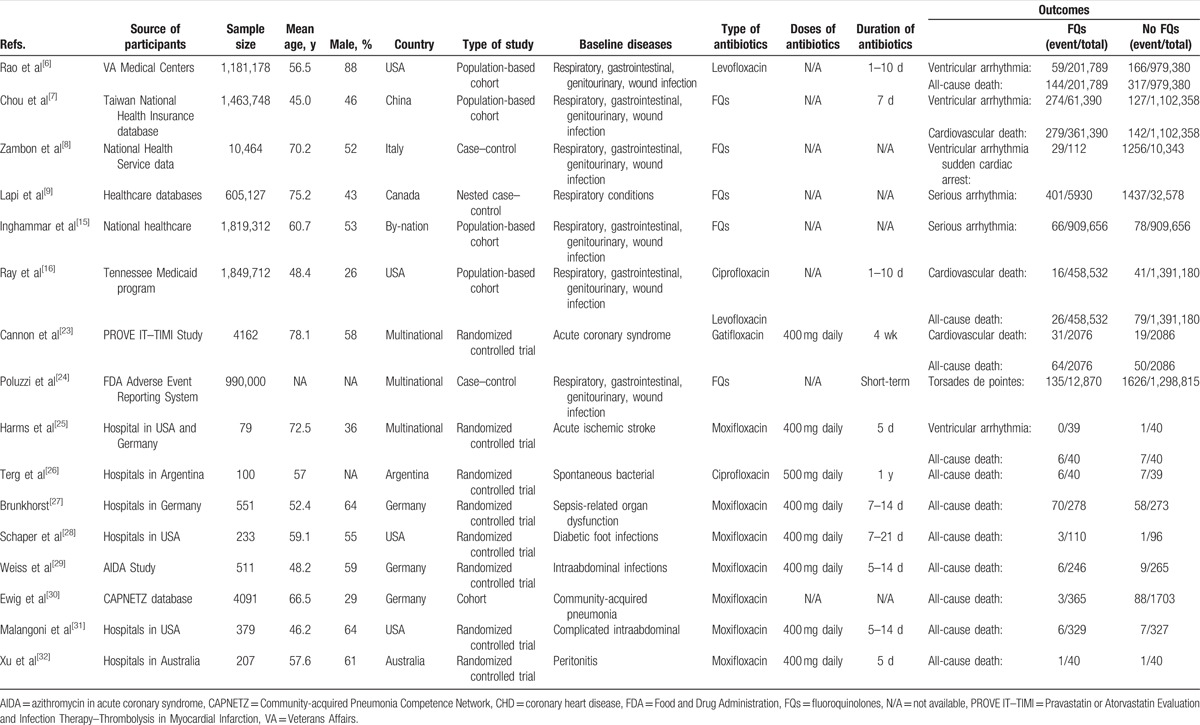
The detailed characteristics of the included studies are presented in Table 1. Overall, these studies were published from 2005 to 2016. The sample size of included studies varied from 79 to 1,849,712, with a total of 6,139,004 participants (54.6% women). The duration of FQs use across the studies varied from 1 day to 14 months, and the mean age ranged from 45 to 76 years. Data on daily FQs doses were provided in 8 RCTs but in none of the observational studies. As shown in Table S1 (for cohort), Table S2 (for case–control), and Fig. S1 (for RCT), the reporting quality of the included articles was globally acceptable. All observational studies obtained an NOS of ≥6 points. The RCT methodological quality was typically good.
Table 1.
Main characteristics of the studies (n = 16) included in the meta-analysis.
3.3. Risk of serious arrhythmias
A total of 7 studies (1 RCT, 3 cohort studies, and 3 case–control studies) reported risk estimates for serious arrhythmias.[6–9,15,24,25] As shown in Fig. 2, FQs treatment was significantly associated with an increased risk of serious arrhythmias (RR 2.29, 95% CI: 1.20–4.36, P = .01). There was a significant heterogeneity of RRs across the included studies (I2 = 95%, P < .001). Egger test (P = .54) showed no evidence of publication bias (Fig. 3). As compared with no FQs use, FQs treatment was associated with an estimated 160 additional serious arrhythmia per 1,000,000 courses.
Figure 2.
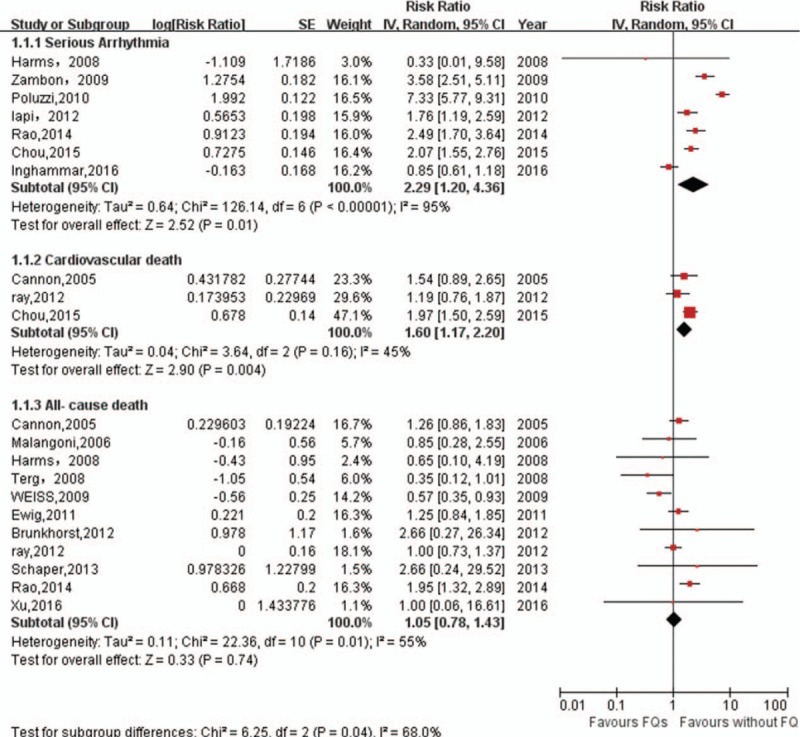
Meta-analysis of RR of serious arrhythmia, cardiovascular death, and all-cause death associated with FQs compared to no FQs use. CI = confidence interval, FQs = fluoroquinolones, IV = inverse of the variance, RR = relative risks, SE = standard error.
Figure 3.
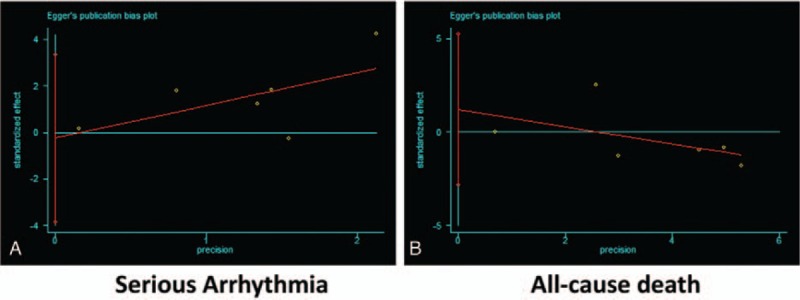
Egger test showing the public bias of association of cardiovascular risk with FQs. (A) Serious arrhythmia. (B) All-cause death. FQs = fluoroquinolones.
In the subgroup analysis of FQs type, gatifloxacin (RR 6.27, 95% CI: 3.11–12.66; P < .001), moxifloxacin (RR 4.20, 95% CI: 1.91–9.27; P < .001), and levofloxacin (RR 1.41, 95% CI: 1.16–1.70; P < .001) showed an increased risk of serious arrhythmias, whereas ciprofloxacin showed a pooled RR of 1.73 (95% CI: 0.89–3.37; P = .1) (Table S3). In addition, further subgroup analysis was performed to evaluate the effect of time with application of FQs. Both current and former FQs users showed an increased the risk of serious arrhythmias (Table S3).
3.4. Risk of CV death
Only 3 studies (1 RCTs and 2 cohort studies) reported an association between FQs and CV death.[7,16,23] The pooled results showed that FQs were associated with an increased risk of CV death (RR 1.60, 95% CI: 1.17–2.20; P = .004) with no significant heterogeneity (I2 = 45%, P = .16) (Fig. 2), although relatively few studies were included in this outcome (Fig. 2). Compared with controls, the absolute risk difference was 43 cases of serious arrhythmia per 1,000,000 courses of FQs.
3.5. Risk of all-cause death
Eleven studies (8 RCTs, 3 cohort studies) were included for the outcome of any-cause death.[6,16,23,25–32] Overall, treatment with FQs was not associated with an increased risk of all-cause death (1.02, 95% CI: 0.76–1.37, P = .92) with moderate heterogeneity (I2 = 56%, P < .05) (Fig. 2). There was no statistical evidence of publication bias (Egger test, P = .26) (Fig. 3). The results were still non-significant in the subgroup analyses (Table S3).
3.6. Sensitivity analysis
Specifically, exclusion of 1 study at a time in turn did not affect the pooled RRs for the aforementioned associations. When we repeated our meta-analyses with a fixed-effects model,[33] none of the initial significant associations were substantially influenced (data not shown).
4. Discussion
In the present study, we demonstrated that FQs treatment could increase the risk of serious arrhythmias and CV death but not all-cause death because our results showed pooled RRs of 2.29 for serious arrhythmias, 1.60 for CV death, and 1.02 for all-cause death in subjects with FQs use. The increased risk of serious arrhythmias was seen in both current and former users of FQs. In the subgroup analysis, gatifloxacin, moxifloxacin, and levofloxacin had a higher risk of serious arrhythmias. Sensitivity analysis indicated that these results were stable.
Several studies have investigated the association between FQs and serious arrhythmias, but the results remain controversial.[6,8,15,24] Consistent with our results, an increased risk of serious arrhythmias is supported by studies from healthy individuals and patients as well as in vitro experiments. In studies with healthy individuals, FQs prolonged corrected Q-T intervals from 0 to 17.8 milliseconds.[10] Several RCTs with 1000 patients also reported events of serious arrhythmias (1 case of TdP from the application of levofloxacin and 1 case of sustained VT from the application of moxifloxacin), although the event rate was too small to draw meaningful conclusions.[13,14,17,34] Moreover, pharmacovigilance reports from the USA prescription data and observational studies also show evidence for the side-effect of arrhythmia.[35]
There is a question raised by our results regarding why FQs are associated with an increased risk for CV death but not all-cause death. A few reasonable interpretations have been suggested. One possible reason is that an increased risk of CV death might be partly offset by the survival benefit of anti-infection by FQs. In addition to antimicrobial properties, FQs are known to exert modulatory activity on immune responses to microbial infection. They can reduce the production of proinflammatory cytokines and antiinflammatory cytokines, which may provide additional benefits in the treatment of infections that are independent of their antibacterial properties.[36,37] Thus, they may reduce the risk of all-cause death. However, because of the small number of studies included in this meta-analysis, these assumptions need further verification.
How FQs increase the risk of serious arrhythmias remains unclear. The evidence from experimental studies supports that FQs molecules block cardiac rapid delayed rectifier potassium channels though interactions with the S6 aromatic amino acid residues of their subunits.[38] Thereby, the action potential duration is prolonged, which predisposes to early after-depolarizations and eventually leads to a vulnerability to TdP.[39] In addition, it can be speculated that FQs might exacerbate the risk of serious arrhythmias in patients with QT interval prolongation-related concomitant risk factors, such as electrolyte disturbance, hypothyroidism, and concurrent use of antiarrhythmic agents.[40] Moreover, acute infection may intrinsically play a synergistic effect on the arrhythmia risk associated with FQs. Numerous laboratory studies have demonstrated that proinflammatory cytokines can facilitate arrhythmia directly through affecting cardiac electrophysiology.[41] Therefore, further experimental studies are required to elucidate the mechanisms underlying the association between FQs and arrhythmia.
Notably, we observed an increased risk of serious arrhythmias with gatifloxacin, moxifloxacin, and levofloxacin, but not with ciprofloxacin. Consistent with our results, the available current mechanistic data comparing individual FQs suggested that gatifloxacin, moxifloxacin, and levofloxacin had a high potency for Ikr inhibition, and thus, higher potential for QT prolongation and proarrhythmic properties. For example, Abo-Salem et al[42] previously reported the serum concentrations of moxifloxacin and levofloxacin for Ikr inhibition is 2 and 15 times higher than that of ciprofloxacin, respectively. Additionally, pharmacovigilance reports also showed that TdP incidence was more prevalent in the moxifloxacin and levofloxacin users compared to ciprofloxacin users.[3] For example, in the FDA's adverse-event reporting system from 2004 to 2008, ciprofloxacin was least frequently reported to induce TdP among antibacterial agents. A total of 230 cases of TdP were found to be related to the administration of antibacterial agents, whereas only 35 cases were associated with the use of ciprofloxacin. Of note, the absence of an association between ciprofloxacin and CV risk in our study suggests ciprofloxacin exhibits relative cardiac safety. This is consistent with the current opinion that ciprofloxacin has limited proarrhythmic liability.[43]
Another factor may confound the association between FQs and CV risk. The patients taking FQs might have more serious disease or CV risk than the patients not taking antibiotics. Therefore, the increased risk of CV observed in our study may be related to the acute infection itself rather than FQs use. However, the risk of serious arrhythmias was similar in subjects with current or former FQs use, which indicated that baseline differences between the groups did not significantly influence the results. In addition, in another meta-analysis involving 33 studies revealed that there was no increased risk of SCD in the subgroup of individuals taking penicillin or amoxicillin compared with those not taking antibiotics.[19] However, the study from Inghammar et al[15] included in our meta-analysis showed no increased risk of CV because the majority (82.6%) of subjects included in Inghammar's study received ciprofloxacin treatment and, therefore, a confounding factor due to infection might be excluded.
Recently, the U.S. Food and Drug Administration revised the Boxed Warning to address the serious safety issues of FQs, including its CV risk. Although the incident rate of fatal adverse cardiac events is low, considering the widespread use of FQs, the risks and benefits of antibacterial therapies should be taken into consideration. Our results suggest that moxifloxacin and levofloxacin but not ciprofloxacin increase the risk of serious arrhythmias. Clinicians may prefer not to prescribe moxifloxacin and levofloxacin when other antibiotic choices are available for patients. Moreover, patients with preexisting risk factors that increase their vulnerability to life-threatening arrhythmia should be paid special attention, such as patients with hyperlipidemia, severe heart disease, current use of another QT interval-prolonging drug, a family history of long QT syndrome and a history of drug-induced TdP.[40] For patients with these concomitant risk factors, alternative drugs may be considered, or it would be recommended to perform additional monitoring when the use of FQs is necessary.
4.1. Study limitations
Several limitations need to be considered when interpreting the findings of this meta-analysis. First, a substantial heterogeneity across the studies was found in the outcomes (serious arrhythmias and all-cause death). Although this heterogeneity may be partially attributable to the differences in study design, analysis strategy, participant characteristics, and the duration, doses, and type of FQs used across the studies, similar RRs were consistently obtained in all stratified analyses, suggesting that heterogeneity might not substantially affect the results. Second, it is widely appreciated that sex is an independent risk factor for cardiac arrhythmia, including TdP.[36] However, the scarcity of data precluded us from performing a subgroup analysis based on sex and other variables that may also influence outcomes. Third, the limited data on the doses of FQs used in the observational studies makes it difficult to assess whether differences in doses would be a source of the heterogeneity. Fourth, given the relatively limited number of studies included in this analysis, further larger RCTs are warranted to confirm these findings.
5. Conclusions
The findings of our meta-analysis demonstrate that FQs could increase the risk of serious arrhythmias and CV death; however, FQs do not increase the risks of all-cause death. Moreover, moxifloxacin and levofloxacin are associated with a higher risk of serious arrhythmias. Further studies are required to evaluate the CV safety of FQs. Treatment with FQs is associated with an absolute risk increase of 160 additional SCDs or VTA, and 43 additional CV deaths per 1 million treatment courses.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, CNKI = China National Knowledge Infrastructure, CV = cardiovascular, FQs = fluoroquinolones, RCT = randomized controlled trial, RR = relative risk, SCD = sudden cardiac death, TdP = torsades de pointes, VTA = ventricular tachyarrhythmia.
XL and JM contributed equally to this work.
Funding: The authors wish to acknowledge support from the National Natural Science Foundation of China (8153000545).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Mehlhorn AJ, Brown DA. Safety concerns with fluoroquinolones. Ann Pharmacother 2007;41:1859–66. [DOI] [PubMed] [Google Scholar]
- [2].Anderson ME, Mazur A, Yang T, et al. Potassium current antagonist properties and proarrhythmic consequences of quinolone antibiotics. J Pharmacol Exp Ther 2001;296:806–10. [PubMed] [Google Scholar]
- [3].Frothingham R. Rates of torsades de pointes associated with ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Pharmacotherapy 2001;21:1468–72. [DOI] [PubMed] [Google Scholar]
- [4].Owens RJ, Bhavnani SM, Ambrose PG. Assessment of pharmacokinetic-pharmacodynamic target attainment of gemifloxacin against Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2005;51:45–9. [DOI] [PubMed] [Google Scholar]
- [5].Corrao G, Botteri E, Bagnardi V, et al. Generating signals of drug-adverse effects from prescription databases and application to the risk of arrhythmia associated with antibacterials. Pharmacoepidemiol Drug Saf 2005;14:31–40. [DOI] [PubMed] [Google Scholar]
- [6].Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med 2014;12:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and beta-lactam/beta-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis 2015;60:566–77. [DOI] [PubMed] [Google Scholar]
- [8].Zambon A, Polo FH, Contiero P, et al. Effect of macrolide and fluoroquinolone antibacterials on the risk of ventricular arrhythmia and cardiac arrest: an observational study in Italy using case-control, case-crossover and case-time-control designs. Drug Saf 2009;32:159–67. [DOI] [PubMed] [Google Scholar]
- [9].Lapi F, Wilchesky M, Kezouh A, et al. Fluoroquinolones and the risk of serious arrhythmia: a population-based study. Clin Infect Dis 2012;55:1457–65. [DOI] [PubMed] [Google Scholar]
- [10].Noel GJ, Natarajan J, Chien S, et al. Effects of three fluoroquinolones on QT interval in healthy adults after single doses. Clin Pharmacol Ther 2003;73:292–303. [DOI] [PubMed] [Google Scholar]
- [11].Noel DGJ, Goodman DDB, Chien S, et al. Measuring the effects of supratherapeutic doses of levofloxacin on healthy volunteers using four methods of QT correction and periodic and continuous ECG recordings. J Clin Pharmacol 2004;44:464–73. [DOI] [PubMed] [Google Scholar]
- [12].Tsikouris JP, Peeters MJ, Cox CD, et al. Effects of three fluoroquinolones on QT analysis after standard treatment courses. Ann Noninvasive Electrocardiol 2006;11:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Makaryus AN, Byrns K, Makaryus MN, et al. Effect of ciprofloxacin and levofloxacin on the QT interval: is this a significant “clinical” event? South Med J 2006;99:52–6. [DOI] [PubMed] [Google Scholar]
- [14].Lipsky BA, Miller B, Schwartz R, et al. Sparfloxacin versus ciprofloxacin for the treatment of community-acquired, complicated skin and skin-structure infections. Clin Ther 1999;21:675–90. [DOI] [PubMed] [Google Scholar]
- [15].Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ 2016;352:i843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mehrzad R, Barza M. Weighing the adverse cardiac effects of fluoroquinolones: a risk perspective. J Clin Pharmacol 2015;55:1198–206. [DOI] [PubMed] [Google Scholar]
- [18].Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2015;387:537–44. [DOI] [PubMed] [Google Scholar]
- [19].Cheng YJ, Nie XY, Chen XM, et al. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol 2015;66:2173–84. [DOI] [PubMed] [Google Scholar]
- [20].Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- [21].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol 2005;15:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cannon CP, Braunwald E, McCabe CH, et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med 2005;352:1646–54. [DOI] [PubMed] [Google Scholar]
- [24].Poluzzi E, Raschi E, Motola D, et al. Antimicrobials and the risk of torsades de pointes. Drug Saf 2012;33:303–14. [DOI] [PubMed] [Google Scholar]
- [25].Harms H, Prass K, Meisel C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE 2008;3:e2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Terg R, Fassio E, Guevara M, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: a randomized, placebo-controlled study. J Hepatol 2008;48:774–9. [DOI] [PubMed] [Google Scholar]
- [27].Brunkhorst FM. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis. JAMA 2012;307:2390–9. [DOI] [PubMed] [Google Scholar]
- [28].Schaper NC, Dryden M, Kujath P, et al. Efficacy and safety of IV/PO moxifloxacin and IV piperacillin/tazobactam followed by PO amoxicillin/clavulanic acid in the treatment of diabetic foot infections: results of the RELIEF study. Infection 2013;41:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weiss G, Reimnitz P, Hampel B, et al. Moxifloxacin for the treatment of patients with complicated intra-abdominal infections (the AIDA Study). J Chemother 2009;21:170–80. [DOI] [PubMed] [Google Scholar]
- [30].Ewig S, Hecker H, Suttorp N, et al. Moxifloxacin monotherapy versus β-lactam mono- or combination therapy in hospitalized patients with community-acquired pneumonia. J Infect 2011;62:218–25. [DOI] [PubMed] [Google Scholar]
- [31].Malangoni MA, Song J, Herrington J, et al. Randomized controlled trial of moxifloxacin compared with piperacillin-tazobactam and amoxicillin-clavulanate for the treatment of complicated intra-abdominal infections. Ann Surg 2006;244:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu R, Yang Z, Qu Z, et al. Intraperitoneal vancomycin plus either oral moxifloxacin or intraperitoneal ceftazidime for the treatment of peritoneal dialysis-related peritonitis: a randomized controlled pilot study. Am J Kidney Dis 2017;70:30–7. [DOI] [PubMed] [Google Scholar]
- [33].Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org. Assessed July 22, 2017. [Google Scholar]
- [34].Morganroth J, Dimarco JP, Anzueto A, et al. A randomized trial comparing the cardiac rhythm safety of moxifloxacin vs levofloxacin in elderly patients hospitalized with community-acquired pneumonia. Chest 2005;128:3398–406. [DOI] [PubMed] [Google Scholar]
- [35].Clark DW, Layton D, Wilton LV, et al. Profiles of hepatic and dysrhythmic cardiovascular events following use of fluoroquinolone antibacterials: experience from large cohorts from the Drug Safety Research Unit Prescription-Event Monitoring database. Drug Saf 2001;24:1143–54. [DOI] [PubMed] [Google Scholar]
- [36].Beisswenger C, Honecker A, Kamyschnikow A, et al. Moxifloxacin modulates inflammation during murine pneumonia. Respir Res 2014;15:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Muller-Redetzky HC, Wienhold SM, Berg J, et al. Moxifloxacin is not anti-inflammatory in experimental pneumococcal pneumonia. J Antimicrob Chemother 2015;70:830–40. [DOI] [PubMed] [Google Scholar]
- [38].Alexandrou AJ, Duncan RS, Sullivan A, et al. Mechanism of hERG K+ channel blockade by the fluoroquinolone antibiotic moxifloxacin. Br J Pharmacol 2006;147:905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Milberg P, Hilker E, Ramtin S, et al. Proarrhythmia as a class effect of quinolones: increased dispersion of repolarization and triangulation of action potential predict torsades de pointes. J Cardiovasc Electrophysiol 2007;18:647–54. [DOI] [PubMed] [Google Scholar]
- [40].Amankwa K. Torsades de pointes associated with fluoroquinolones: importance of concomitant risk factors. Clin Pharmacol Ther 2004;75:242–7. [DOI] [PubMed] [Google Scholar]
- [41].Lazzerini PE, Capecchi PL, Acampa M, et al. Arrhythmic risk in rheumatoid arthritis: the driving role of systemic inflammation. Autoimmun Rev 2014;13:936–44. [DOI] [PubMed] [Google Scholar]
- [42].Abo-Salem E, Fowler JC, Attari M, et al. Antibiotic-induced cardiac arrhythmias. Cardiovasc Ther 2014;32:19–25. [DOI] [PubMed] [Google Scholar]
- [43].Owens RJ, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006;43:1603–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


