Abstract
The prognosis of glioblastoma (GBM), a major subtype of grade IV glioma, is rather poor nowadays. The efficiency of chemotherapy serving as the adjunct to radiotherapy (RT) for treating GBM is still controversial. In this study, we aim to investigate the overall survival (OS) and progression-free survival (PFS) in patients with newly diagnosed GBM received RT plus chemotherapy or with RT alone.
Literatures were searched from the PubMed, Embase, and Cochrane Library between January 2001 and June 2015. Study selection was conducted based on the following criteria: randomized clinical trial (RCT) of adjuvant RT plus chemotherapy versus RT alone; studies comparing OS and/or PFS; and studies including cases medically confirmed of newly diagnosed GBM.
Five RCTs (1655 patients) were eligible in this study. The meta-analysis showed a significant improvement in OS of patients treated with RT plus oral chemotherapy compared with that of RT alone (hazard ratio 0.70; 95% confidence interval, 0.56–0.88, P = .002).
Adjuvant chemotherapy confers a survival benefit in patients newly diagnosed with GBM.
Keywords: chemotherapy, glioblastoma, overall survival, radiotherapy
1. Introduction
Malignant gliomas, including the most common subtype glioblastoma multiforme (GBM), are notorious primary brain tumors in adults.[1] Nowadays, the prognosis of GBM, the major subtype of grade IV glioma accounting for approximately 60% of primary brain tumors worldwide,[2] remains dismal despite the advances in treatment. The median survival is generally <1 year after diagnosis, and the 2-year survival rate is only 5% to 10%.[3–5]
Currently, the treatment of newly diagnosed GBM is highly depending on surgical resection, radiotherapy (RT), and chemotherapy.[4] Nowadays, the efficiency of chemotherapy, given as the adjunct to RT or before RT,[6] is still controversial.[7–13] Several systematic reviews[6,14–16] have been carried out to provide reliable evidences for the aggressive chemotherapy combined with RT in newly diagnosed GBM. In a literature[16] based on 12 randomized controlled trials (RCTs), chemotherapy induced a small survival benefit in the 2-year survival compared with that of RT alone. In addition, Zhang et al[14] indicated that adjuvant chemotherapy played a beneficial role in the treatment of anaplastic glioma. Nevertheless, chemotherapy using procarbazine, lomustine, and vincristine (PCV) regime plus RT did not prolong the survival of patients with anaplastic oligodendroglioma and anaplastic oligoastrocytoma compared with the RT alone.[17,18] These lead us to compare the efficiency of adjuvant chemotherapy plus RT versus RT in the treatment of GBM.
To date, most of the studies focus on the combination of nitrosoureas-based traditional chemotherapy and RT, or temozolomide (TMZ) chemotherapy combined with RT. For example, TMZ, a novel oral alkylating agent commonly used worldwide, has been reported to show antitumor activity for the treatment of newly diagnosed malignant gliomas.[19–22] Stupp et al[6] reported the combination of RT and TMZ increased the median survival (14.6 vs 12.1 months, P < .001) of GBM patients with acceptable toxicity compared with RT alone. Besides, in the European Organization for Research on Treatment Cancer (EORTC) 26,981 trial, RT with concurrent and adjuvant TMZ was set as the standard treatment for adult patients with GBM. Moreover, Yin et al[15] demonstrated a 41% reduction in the risk of death in GBM patients received combined RT/TMZ (hazard ratio [HR] 0.59; 95% confidence interval [CI], 0.48–0.72, P < .001) with acceptable side effects. Some studies[23,24] confirmed that other oral chemotherapy such as difluromethylornithine (DFMO) and marimastat (MT) were also reported as adjuvant chemotherapy for the treatment of GBM multiforme.
Nowadays, rare studies have been carried out to evaluate the overall survival (OS) of patients with newly diagnosed GBM after receiving oral chemotherapy drugs. In this study, we focus on the outcome of oral chemotherapy using different chemotherapy drugs plus RT versus RT alone for newly diagnosed GBM. We aim to answer whether oral chemotherapy plus RT contributed to the improvement in the OS and progression-free survival (PFS) in the patients with newly diagnosed GBM compared with RT alone.
2. Materials and methods
2.1. Eligibility criteria
Literature search was performed by 2 authors (Wang Z and Song Y) independently from the PubMed, Cochrane Library, and EMBASE bibliographic databases from January 2001 to July 2015. Only trials properly randomized were included in the meta-analysis. Terms used in the literature search were as follows: “glioma” or “glioblastoma” or “malignant glioma” or “glioblastoma multiforme” and “radiotherapy” or “radiation therapy” or “chemotherapy” or “temozolamide” or “temozolomide” and “Temodar”. The reference lists of relevant studies were also checked for additional trials. Studies included in the meta-analysis should meet all the following criteria: patients should be newly diagnosed and histologically confirmed GBM or gliosarcoma; RCTs comparing simultaneous adjuvant chemotherapy plus radiotherapy versus radiotherapy alone; reported the HR and the corresponding 95% CI for OS and PFS. The language of publications was limited to Chinese and English. This study was approved by the ethics committee of Norman Bethune First Hospital.
2.2. Data extraction
The following data were extracted from each included study: first author, year of publication, country of research, age range of patients, number of patients (with/without chemotherapy), study design, median survival, HR of OS, and adverse events (AEs). We tried to contact the authors for the missing data required for our meta-analysis. In cases of any disagreement, a deep discussion was held among all investigators.
Time-to-event data (e.g., OS and PFS) were analyzed using HR. In cases of HR values not reported, the value was estimated by the method described by Tierney et al.[25]
2.3. Quality assessment
The bias risk was evaluated using the domain-based Cochrane Collaboration's tool as previously described.[26] Risk of selection bias, performance bias, detection bias, attrition bias, and reporting bias were classified as “low”, “high”, or “unclear” (Fig. 1).
Figure 1.
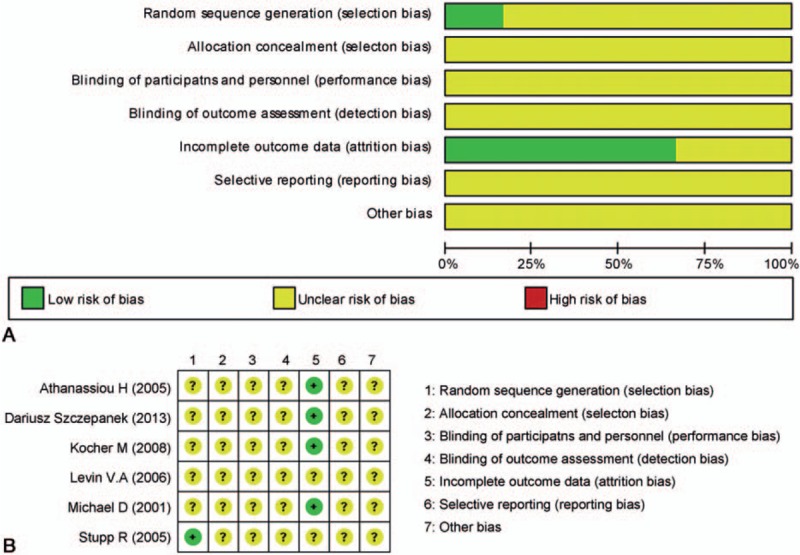
Risk of bias graph (A) and risk of bias summary (B).
2.4. Statistical analysis
The heterogeneity between studies was assessed by the χ2-based Q test and I[2]-statistics. The heterogeneity was assessed by the χ2 test based Q-statistics, and the degree of heterogeneity was estimated with the I2-statistic. In the presence of P < .10 or the I2-statistic >50%, the random effects model (DerSimonian–Laird method) was used. Otherwise, a fixed-effects model (Mantel–Haenszel method) was accepted. Sensitivity analysis was performed by recalculating the pooled statistics after omitting each study. Statistical analyses were performed using the software Review Manager 5.3 (Cochrane Collaboration, Oxford, England).
3. Results
3.1. Study characteristics
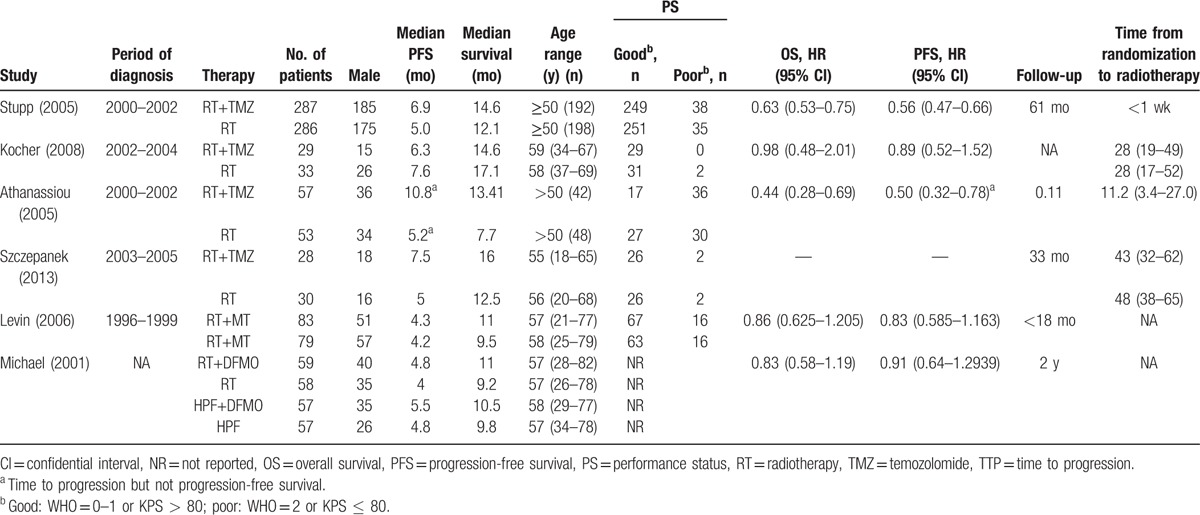
The flow chart of the study selection procedure is shown in Fig. 2. Initially, 7 RCTs including 1655 patients were included. The characteristics of these studies are summarized in Table 1. The studies were published from 2001 to 2013, and the sample sizes ranged from 58 to 573. Two studies[6,27] published in 2005 and 2009 used the same data from the same study design, and only one study was included. One study[28] was excluded as the HR for neither OS nor PFS cannot be extracted. Eventually, 5 studies were included in this meta-analysis.
Figure 2.
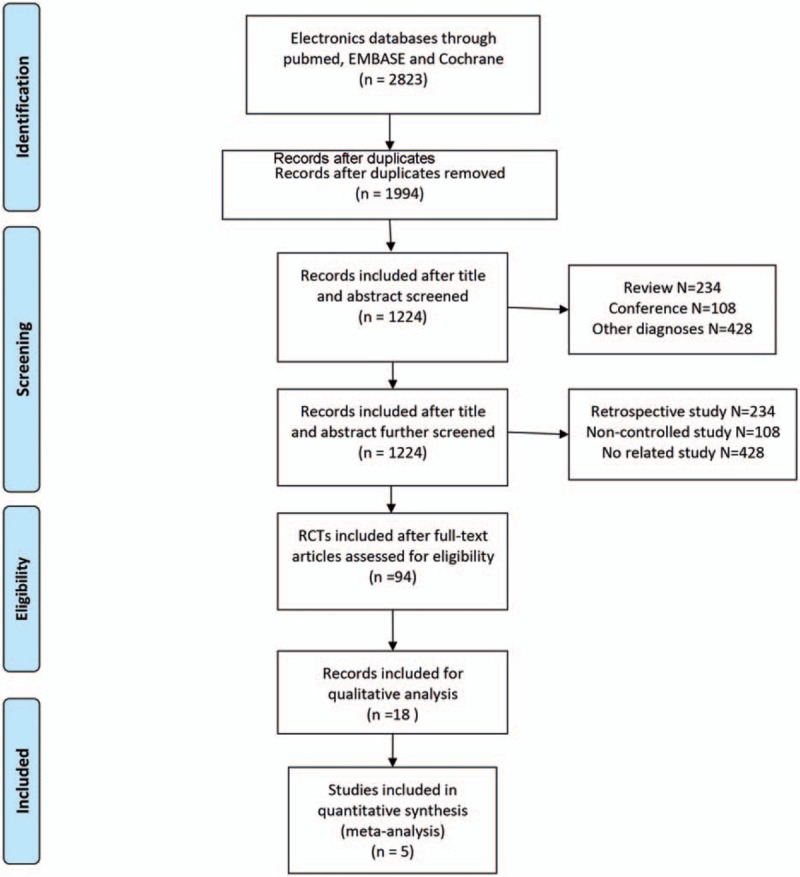
Flow diagram of literature retrieval and screening.
Table 1.
Statistical information and characteristics of included studies.
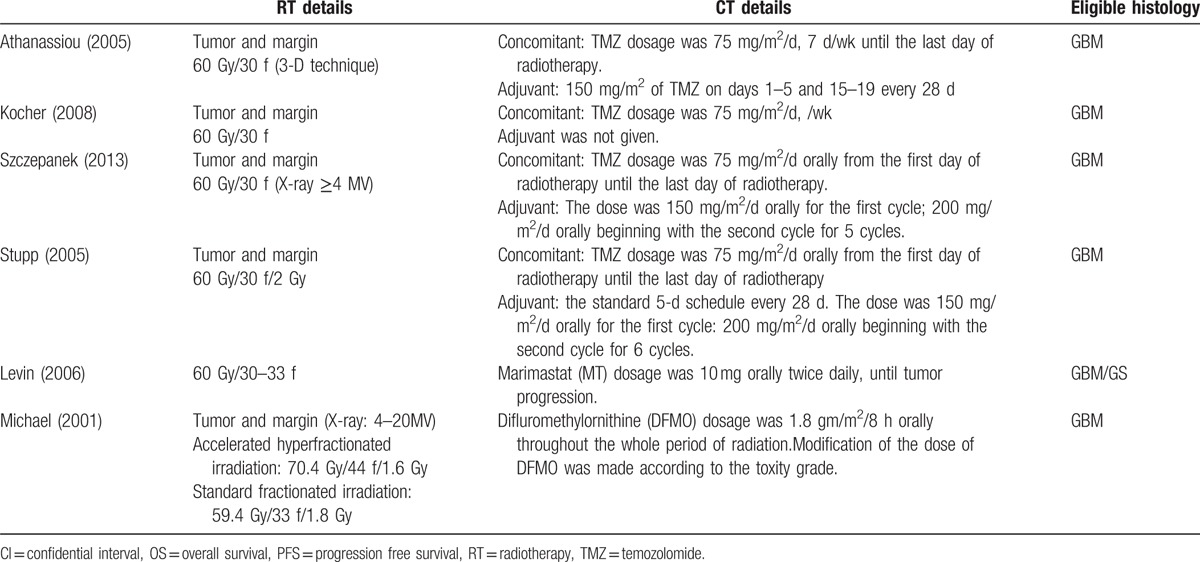
Among the included studies, 2 TMZ schedules were used: a concomitant schedule (75 mg/m2 orally from the first day of radiotherapy until the last day of radiotherapy) was used in 3 studies,[6,29,30] and an adjuvant schedule (150 mg/m2/d orally for the first cycle; 200 mg/m2/d orally beginning with the second cycle) was used in 2 studies.[6,29] In the primary treatment, a standard radiation schedule (60 Gy) was utilized. An accelerated hyperfractionated irradiation course of 70.4 Gy was used in the 4-arm trial.[23] In this study, we performed a single comparison between TMZ and standard radiotherapy (Table 2).
Table 2.
Adjuvant therapy.
3.2. Overall survival
As shown in Fig. 3A, chemotherapy and radiotherapy were associated with a significant improvement in OS (HR 0.70; 95% CI, 0.56–0.88, P < .001) compared with that of RT. Besides, the risk of death was reduced by 30% in those received combination of chemotherapy and radiotherapy (Fig. 3A).
Figure 3.
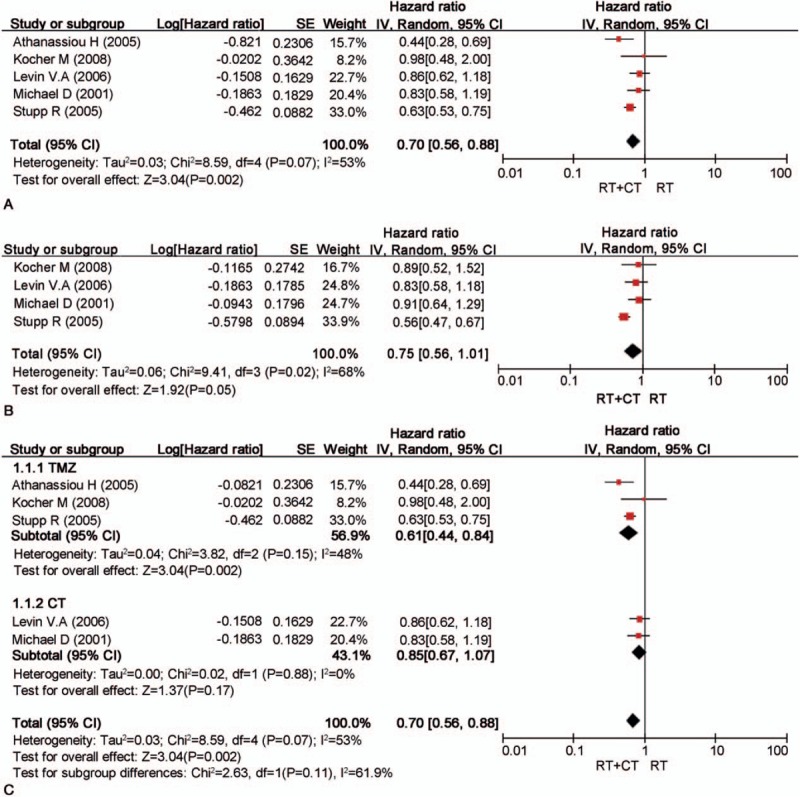
Meta-analyses result of survival rate (A), progression-free survival (B), subgroup analysis of OS (C).
3.3. Additional analysis of survival rate
All RCTs reported the 6-month survival rate with no heterogeneity (P = .28, I2 = 21%). Thus, the fixed-effect model was used, and significant differences were observed between the combination of chemotherapy and radiotherapy and the radiotherapy group (RR 1.07; 95% CI, 1.01–1.13, P = .02, I2 = 21%). Heterogeneity was observed in the 12-month surival rate of all RCTs (P = .02, I2 = 61%). On this basis, the random-effects model was used, which showed significant differences between the combined therapy and the radiotherapy alone (RR 1.23; 95% CI, 0.97–1.54, P = .08, I2 = 61%). All RCTs reported the 18-month survival rate, and no heterogeneity was identified (P = .38, I2 = 6%). Therefore, the fixed-effects model was used, which revealed statistical difference between the combined group and the radiotherapy group (RR 1.87; 95% CI, 1.51–2.33, P < .001, I2 = 6%).
3.4. Progression-free survival
The meta-analysis indicated a pooled HR of 0.75 (95% CI, 0.56–1.01; P = .05; Fig. 3B). This indicated that RT plus chemotherapy benefited to the PFS of patients compared with those received RT alone. However, such fact should be interpreted carefully as the data were limited.
According to the therapy model, we divided the 5 RCTs into 2 subgroups, which focused on the patients with TMZ and those with other oral drugs, respectively. Subgroup analysis by the regimen of TMZ identified a significant association between TMZ combined with RT and RT alone (HR 0.61; 95% CI, 0.44–0.84, P < .002), whereas the association of the other subgroups was not significant (Fig. 3C).
3.5. Adverse events
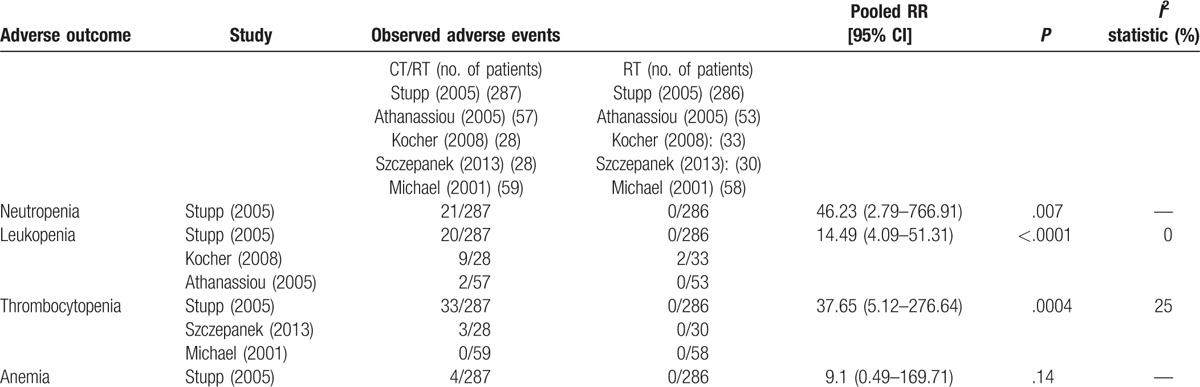
Safety data were reported in 5 studies,[6,22,23,31,32] and the hematological adverse events (HAEs) were the major safety concerns (Table 3). An increased risk of grade 3 to 4 HAEs was identified in the combined groups, and most of these toxicities could be managed by delaying the chemotherapy schedule or through reducing the drug dose. Some patients showed nonhematological toxicities after receiving combined therapy, including gastrointestinal toxicity (e.g., nausea and vomiting), neurologic toxicity (e.g., cognitive or mood change or seizures), liver enzyme elevation, cutaneous adverse events, thromboembolic events, and fatigue.
Table 3.
A toxicity (Grade 3 or greater) comparison between the CT and RT groups.
4. Discussion
High-grade glioma (HGG), the most common malignant primary brain tumor, accounts for 80% of all gliomas in the United States.[31] To date, the efficiency of oral chemotherapy drugs, given as the adjunct to radiotherapy or before radiotherapy, is still controversial. Our meta-analysis indicates a clear survival advantage for combined RT/oral chemotherapy compared with RT alone among newly diagnosed GBM patients.
In this meta-analysis, primary analysis indicated a clear improvement in OS and PFS for patients received combined therapy. When taking the study of Athanassiou et al[29] into consideration, the combined therapy induced obvious survival benefits (HR 0.70; 95% CI, 0.56–0.88; P = .0002) with a high heterogeneity (I2 = 53%), whereas, if the study was omitted, the survival benefits were comparatively weaker (HR 0.75; 95% CI = 0.61–0.92; P = .005), but the heterogeneity was low (I2 = 37%). The high heterogeneity was mainly associated with the elder age and the poor Karnofsky performance status (KPS). According to the previous study, advanced age and poor KPS are important adverse prognostic factors for malignant gliomas,[32] whereas TMZ combined with radiotherapy could produce a significant survival benefit. In addition, Yin et al[15] confirmed that combined RT/TMZ conferred a clear survival benefit in elder GBM patients (HR 0.59; 95% CI = 0.48–0.72; P < .0001), and the high heterogeneity might also cause by the treatment design, the number of patients, and skills of neurosurgeons.
Four studies included in this meta-analysis reported the outcomes of PFS. When taking the study by Stupp et al [6] into consideration, high heterogeneity (I2 = 68%) and clear survival benefit (HR 0.75; 95% CI, 0.56–1.01, P = 0.05) were obtained. The high heterogeneity may be related to the high weight and statistical differences.
Chemotherapy plays an important role in the treatment of malignant gliomas. Studies[33,34] confirmed that cancer cells in anaplastic glioma patient with the 1p and 19q codeletion were particularly sensitive to PCV chemotherapy. Nevertheless, the treatment outcome of the PCV regimen for GBM is not satisfactory. Recently, besides TMZ, many novel oral drugs (e.g., DFMO and MT) have been used for the treatment of GBM. For example, DFMO (a polyamine inhibitor) is well tolerated by oral administration. In a previous study, Prados et al[23] confirmed the efficiency of DFMO in patients of recurrent malignant glioma when combining with BCNU and other polyamine inhibitors. However, no benefits were seen with DFMO as a radosesitizer.[23] To explain this, we speculate that it may be related to the effects of DFMO due to the function of the depletion of polyamines rather than the direct cytotoxic effects. Besides, some other reasons may be responsible for it, such as the treatment design and patient characteristics. MT is an oral low molecular weight inhibitor of the matrix metalloproteases (MMPs) which is considered as a potential therapy target for gliomas as it is upregulated in malignant gliomas and correlated with malignant progression.[35–37] Up to now, the activity of MT in GBM patients has been proved by several studies.[23] Some randomized trials with MT therapy, including Levin VA, resulted in negative results.[38] For instance, MT combined with TMZ contributed to an increase in the PFS rate at 6 months than TMZ alone.[39,40] Taken together, these findings partly suggested DFMO and MT may contribute to the response of chemotherapy or/and radiotherapy in GBM patients.
Glioma stem cells might be the main reason for the resistance of malignant glioma to the standard treatment.[41] Radioresistance in glioma stem cells is mainly associated with the activation of DNA-damage response pathways,[42] whereas the chemoresistance may partly from the overexpression of O-6-methylguanine-DNA methyltransferase (MGMT).[43–45] MGMT, an important prognostic marker, has been considered as a predictive marker in response to TMZ in patients with newly diagnosed GBM. In an EORTC/NCIC phase III trial,[46] the methylated MGMT promoter region was related to a survival benefit associated with TMZ among GBM patients. Stupp et al[6] testified that MGMT promoter methylation was the strongest prognostic factor for survival (HR 0.49; 95% CI, 0.32–0.76, P = .001). Furthermore, the methylation was regarded as the strong prognostic relevance in malignant gliomas, irrespective of chemotherapy or radiotherapy.[47] On the contrary, some studies reported that MGMT promoter methylation status alone was deficient to provide evidence about the sensitivity of grade III glioma to alkylating agents[48,49] as MGMT protein expression was also regulated by other independent factors, such as MGMT mRNA expression.[50] In future, further studies are needed to investigate the correlation between MGMT promoter methylation and the treatment outcome.
Indeed, there are limitations in this study. First, the number of eligible studies and patients included is not large. Second, there are differences in the study design in the included trials. For example, the schedules of radiotherapy and the timing of chemotherapy are not totally consistent. Third, some data[24,29,30] are estimated from the survival curves as the HRs and 95% CIs are not presented, which may lead to bias. Fourth, we just focus on the effectiveness of chemotherapy combined with radiotherapy, and the adverse effects and complications are not analyzed. This might exaggerate the benefits of chemotherapy combined with radiotherapy. Finally, our results might be influenced by the potential publication bias.
In conclusion, oral chemotherapy combined with radiotherapy contributes to the survival in patients with newly diagnosed GBM. In future, more studies, especially RCTs, should be designed to study the efficiency in the treatment of the newly diagnosed GBM.
Acknowledgments
We appreciate the work of editors and anonymous reviewers.
Footnotes
Abbreviations: AEs = adverse events, CI = confidence interval, DFMO = difluromethylornithine, EORTC = European Organization for Research on Treatment Cancer, GBM = glioblastoma, HAEs = hematological adverse events, HGG = high-grade glioma, HR = hazard ratio, KPS = Karnofsky performance status, MGMT = O-6-methylguanine-DNA methyltransferase, MMPs = matrix metalloproteases, MT = marimastat, OS = overall survival, PCV = procarbazine, lomustine, and vincristine, PFS = progression-free survival, RCT = randomized clinical trial, RT = radiotherapy, TMZ = temozolomide.
ZW and GY equally contributed to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Fisher PG, Buffler PA. Malignant gliomas in 2005: where to GO from here? JAMA 2005;293:615–7. [DOI] [PubMed] [Google Scholar]
- [2].Reardon DA, Rich JN, Friedman HS, et al. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol 2006;24:1253–65. [DOI] [PubMed] [Google Scholar]
- [3].Kortmann RD, Jeremic B, Weller M, et al. Radiochemotherapy of malignant glioma in adults. Clinical experiences. Strahlenther Onkol 2003;179:219–32. [DOI] [PubMed] [Google Scholar]
- [4].DeAngelis LM. Brain tumors. N Engl J Med 2001;344:114–23. [DOI] [PubMed] [Google Scholar]
- [5].Mahaley MS, Jr, Mettlin C, Natarajan N, et al. National survey of patterns of care for brain-tumor patients. J Neurosurg 1989;71:826–36. [DOI] [PubMed] [Google Scholar]
- [6].Stupp R, Mason WP, van den Bent MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [7].Chang CH, Horton J, Schoenfeld D. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer 1983;52:997–1007. [DOI] [PubMed] [Google Scholar]
- [8].Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep 1983;67:121–32. [PubMed] [Google Scholar]
- [9].Deutsch M, Green SB, Strike TA, et al. Results of a randomized trial comparing BCNU plus radiotherapy, streptozotocin plus radiotherapy, BCNU plus hyperfractionated radiotherapy, and BCNU following misonidazole plus radiotherapy in the postoperative treatment of malignant glioma. Int J Radiat Oncol Biol Phys 1989;16:1389–96. [DOI] [PubMed] [Google Scholar]
- [10].Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg 1989;71:1–9. [DOI] [PubMed] [Google Scholar]
- [11].Shapiro WR. Chemotherapy of malignant gliomas: studies of the BTCG. Rev Neurol (Paris) 1992;148:428–34. [PubMed] [Google Scholar]
- [12].Grossman SA, O’Neill A, Grunnet M, et al. Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncology Group Trial 2394. J Clin Oncol 2003;21:1485–91. [DOI] [PubMed] [Google Scholar]
- [13].Stupp RHM. Perry MC, et al. Recent developments in the management of malignant glioma. ASCO 2003 Educational Book. Alexandria, VA: American Society of Clinical Oncology; 2003. 779–88. [Google Scholar]
- [14].Zhang L, Wu X, Xu T, et al. Chemotherapy plus radiotherapy versus radiotherapy alone in patients with anaplastic glioma: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2013;139:719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yin AA, Zhang LH, Cheng JX, et al. Radiotherapy plus concurrent or sequential temozolomide for glioblastoma in the elderly: a meta-analysis. PloS One 2013;8:e74242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002;359:1011–8. [DOI] [PubMed] [Google Scholar]
- [17].Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol 2006;24:2707–14. [DOI] [PubMed] [Google Scholar]
- [18].Van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol 2006;24:2715–22. [DOI] [PubMed] [Google Scholar]
- [19].Friedman HS, McLendon RE, Kerby T, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. J Clin Oncol 1998;16:3851–7. [DOI] [PubMed] [Google Scholar]
- [20].Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol 1999;17:2762–71. [DOI] [PubMed] [Google Scholar]
- [21].Brada M, Hoang-Xuan K, Rampling R, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol 2001;12:259–66. [DOI] [PubMed] [Google Scholar]
- [22].Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 2002;20:1375–82. [DOI] [PubMed] [Google Scholar]
- [23].Levin VA, Phuphanich S, Yung WK, et al. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J Neurooncol 2006;78:295–302. [DOI] [PubMed] [Google Scholar]
- [24].Prados MD, Wara WM, Sneed PK, et al. Phase III trial of accelerated hyperfractionation with or without difluromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2001;49:71–7. [DOI] [PubMed] [Google Scholar]
- [25].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Higgins JP GS, et al. Cochrane Handbook for Systematic Reviews of Interventions; 2013. Available at: http://handbook.cochrane.org/. Accessed January 5, 2013. [Google Scholar]
- [27].Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. [DOI] [PubMed] [Google Scholar]
- [28].Szczepanek D, Marchel A, Moskala M, et al. Efficacy of concomitant and adjuvant temozolomide in glioblastoma treatment. A multicentre randomized study. Neurol Neurochir Pol 2013;47:101–8. [DOI] [PubMed] [Google Scholar]
- [29].Athanassiou H, Synodinou M, Maragoudakis E, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol 2005;23:2372–7. [DOI] [PubMed] [Google Scholar]
- [30].Kocher M, Frommolt P, Borberg SK, et al. Randomized study of postoperative radiotherapy and simultaneous temozolomide without adjuvant chemotherapy for glioblastoma. Strahlenther Onkol 2008;184:572–9. [DOI] [PubMed] [Google Scholar]
- [31].Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 2012;14(Suppl. 5): v1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 1993;85:704–10. [DOI] [PubMed] [Google Scholar]
- [33].Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 1998;90:1473–9. [DOI] [PubMed] [Google Scholar]
- [34].Ino Y, Betensky RA, Zlatescu MC, et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 2001;7:839–45. [PubMed] [Google Scholar]
- [35].Rao JS, Yamamoto M, Mohaman S, et al. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis 1996;14:12–8. [DOI] [PubMed] [Google Scholar]
- [36].Sawaya RE, Yamamoto M, Gokaslan ZL, et al. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis 1996;14:35–42. [DOI] [PubMed] [Google Scholar]
- [37].Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 2003;3:489–501. [DOI] [PubMed] [Google Scholar]
- [38].King J, Zhao J, Clingan P, et al. Randomised double blind placebo control study of adjuvant treatment with the metalloproteinase inhibitor, Marimastat in patients with inoperable colorectal hepatic metastases: significant survival advantage in patients with musculoskeletal side-effects. Anticancer Res 2003;23:639–45. [PubMed] [Google Scholar]
- [39].Osoba D, Brada M, Yung WK, et al. Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme. J Clin Oncol 2000;18:1481–91. [DOI] [PubMed] [Google Scholar]
- [40].Groves MD, Puduvalli VK, Hess KR, et al. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol 2002;20:1383–8. [DOI] [PubMed] [Google Scholar]
- [41].Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359:492–507. [DOI] [PubMed] [Google Scholar]
- [42].Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756–60. [DOI] [PubMed] [Google Scholar]
- [43].Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5:275–84. [DOI] [PubMed] [Google Scholar]
- [44].Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 2006;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Salmaggi A, Boiardi A, Gelati M, et al. Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype. Glia 2006;54:850–60. [DOI] [PubMed] [Google Scholar]
- [46].Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl ‘J Med 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
- [47].Suri V, Jha P, Sharma MC, et al. O6-methylguanine DNA methyltransferase gene promoter methylation in high-grade gliomas: a review of current status. Neurol India 2011;59:229–35. [DOI] [PubMed] [Google Scholar]
- [48].Brell M, Tortosa A, Verger E, et al. Prognostic significance of O6-methylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin Cancer Res 2005;11:5167–74. [DOI] [PubMed] [Google Scholar]
- [49].Siker ML, Chakravarti A, Mehta MP. Should concomitant and adjuvant treatment with temozolomide be used as standard therapy in patients with anaplastic glioma? Crit Rev Oncol Hematol 2006;60:99–111. [DOI] [PubMed] [Google Scholar]
- [50].Kreth S, Thon N, Eigenbrod S, et al. O-methylguanine-DNA methyltransferase (MGMT) mRNA expression predicts outcome in malignant glioma independent of MGMT promoter methylation. PloS One 2011;6:e17156. [DOI] [PMC free article] [PubMed] [Google Scholar]


