Abstract
High viral load is an independent risk factor for development of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB). Antiviral therapy can reduce but not eliminate the risk of HCC. The aim of this study was to identify the risk factors for HCC development in CHB patients during antiviral therapy.
CHB patients with HBV DNA level ≥104 copies/mL, with or without compensated cirrhosis receiving adefovir were followed up every 6 months for 10 years (2004–2014). The primary endpoint was the development of HCC. The cumulative incidence and risk factors of HCC were evaluated by the Kaplan-Meier method and multivariate Cox proportional hazards models.
At baseline, 28 of the 120 patients (23.3%) were cirrhotic. One patient developed HCC within 1 year, and therefore 119 patients were analyzed. At the end-point of follow-up, 59.7% (71/119) patients achieved virological remission (VR). Overall, 16 patients developed HCC, giving a 10-year cumulative incidence of 15.73%. Multivariate analysis showed that cirrhosis at baseline and failure to achieve VR were significant risk factors for HCC. The 10-year incidence of HCC was significantly higher in cirrhotic than noncirrhotic patients (43.16% vs. 7.05%, P < .0001). For cirrhotic patients, the 10-year incidence of HCC was significantly higher in patients without VR than those with VR (62.24% vs. 27.78%, P = .0139).
Cirrhosis at baseline and failure to achieve VR during antiviral therapy were significant risk factors for HCC development in CHB patients. Effective viral suppression is necessary to reduce HCC development in cirrhotic CHB patients.
Keywords: antiviral therapy, chronic hepatitis B, hepatocellular carcinoma, liver cirrhosis
1. Introduction
More than 80% of hepatocellular carcinoma (HCC) cases occur in Eastern Asia, where the dominant risk factor is chronic hepatitis B virus (HBV) infection.[1] In China, it is estimated that about 74 million people are chronically infected with HBV,[2] and HCC is the second-leading cause of cancer mortality.[3]
Two large-scale population studies have shown that high HBV viral load is an independent risk factor for development of HCC.[4,5] Several studies have also confirmed that long-term nucleotide/nucleoside analog (NA) therapy could decrease the risk of HCC by preventing progression of cirrhosis.[6–8] However, HCC still develops in chronic hepatitis B (CHB) patients receiving antiviral therapy.[8–12]
The aim of this 10-year follow-up study was to identify risk factors in HCC development in patients with cirrhotic and noncirrhotic CHB receiving oral antiviral therapy.
2. Patients and methods
2.1. Study design and patient demographic
In this prospective study, patients with cirrhotic and noncirrhotic CHB were recruited at Beijing Friendship Hospital, Capital Medical University, China, between January 2004 and June 2004. The inclusion criteria were hepatitis B surface antigen (HBsAg) positivity for >6 months, HBV DNA level ≥104 copies/mL and consent to receive adefovir dipivoxil (ADV) at a dose of 10 mg/day. Patients were excluded from this study if they were coinfected with hepatitis D, hepatitis C, or human immunodeficiency virus; had other liver diseases, such as alcoholic liver diseases, autoimmune hepatitis, or primary biliary cholangitis; showed decompensation with history of ascites, varices bleeding, or hepatic encephalopathy; had HCC diagnosed at baseline or within 1 year of follow-up; had combined poorly controlled renal, cardiac, hematological, or pulmonary diseases; and had a history of any malignancy.
Written informed consent was obtained from all patients and the study was approved by the institutional ethical review board.
2.2. Follow-up
All patients were followed up every 6 months by monitoring platelets (PLT), HBsAg status, hepatitis B e antigen (HBeAg)/anti-HBe status, HBV DNA level, liver biochemistry, alpha-fetoprotein, and ultrasonography of the liver. Computer tomography (CT) and/or magnetic resonance imaging (MRI) and/or hepatic angiogram were performed if ultrasound showed suspicion of HCC.
Virological remission (VR) was defined as undetectable HBV DNA at the time of HCC development or the end of follow-up. Virological breakthrough was defined as a confirmed increase in HBV DNA level of >1 log10 copies/mL compared with the lowest HBV DNA level during therapy. Suboptimal virological response was defined as a decrease in HBV DNA of >1 log10 copies/mL, but HBV DNA remained detectable after at least 12 months of therapy in compliant patients. Absence of VR was defined as either virological breakthrough or suboptimal virological response.
The primary endpoint was the diagnosis of HCC during follow-up.
2.3. Diagnosis criteria of liver cirrhosis
Liver cirrhosis was clinically diagnosed based on the following criteria (modified from reference[13]): esophageal varices on endoscopy, exclusion of noncirrhotic portal hypertension; or 2 of the following 3 criteria: imaging studies (ultrasound, CT, or MRI) showing any of the following signs of cirrhosis: irregular liver surface, granular or nodular liver parenchyma, splenomegaly (spleen thickness >4.0 cm or >5 rib units); PLT count <100 × 109 cells/L, without other causes; serum albumin <35.0 g/L, or international normalized ratio >1.3, or prothrombin time prolonged >3 seconds.
2.4. Diagnosis criteria of HCC
According to HCC practice guidelines from the American Association for the Study of Liver Diseases,[14] HCC was diagnosed by the presence of a typical vascular pattern identified by triphasic CT scan or MRI; if typical findings of HCC were not observed by imaging, the diagnosis was confirmed by fine-needle aspiration biopsy followed by histological examination.
2.5. Statistical analysis
All data were analyzed with the SPSS V.19.0 (SPSS, Inc, an IBM Company, Chicago, IL). For continuous variables, the data were first tested for normal distribution by the Kolmogorov-Smirnov test. Mean values and standard deviation are presented if data were normally distributed; median values and range are presented if data were not normally distributed. Data were compared using the nonparametric Mann-Whitney U test. The χ2 or Fisher exact test was used for comparisons of categorical variables.
Univariable and multivariable Cox proportional hazards regression models were used to estimate the effect of different variables on the hazard of HCC occurrence. Hazard ratios with 95% confidence intervals and P values from the Wald test are presented. The cumulative probabilities of HCC occurrence in different subgroups were estimated by the Kaplan-Meier method and compared using the log-rank test. A P value of <.05 was considered to be statistically significant.
3. Results
3.1. Patient demographic
One hundred and twenty patients were enrolled in the study. During the 10-year follow-up, 1 patient developed HCC within 1 year, so 119 patients were ultimately analyzed. A flowchart of the study design is shown in Figure 1.
Figure 1.
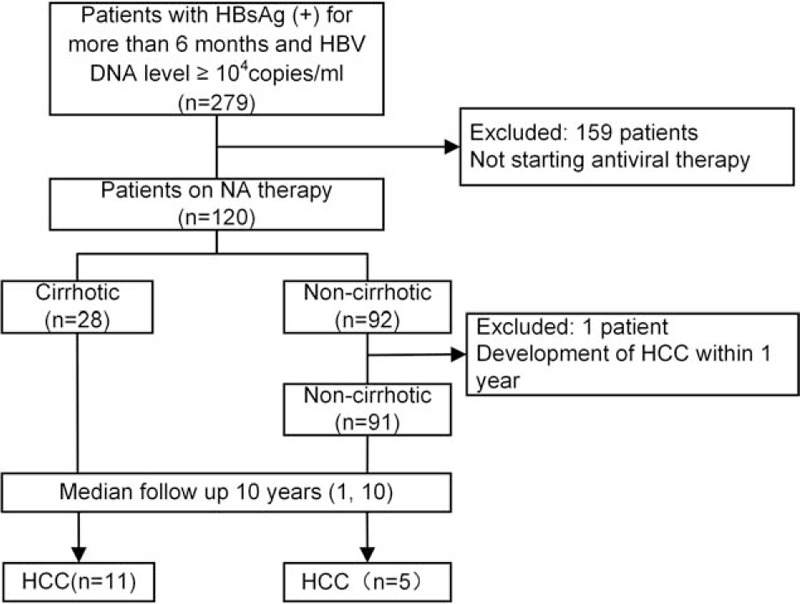
Flowchart of the study. HBsAg = HBV surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, NA = nucleos(t)ide analogs.
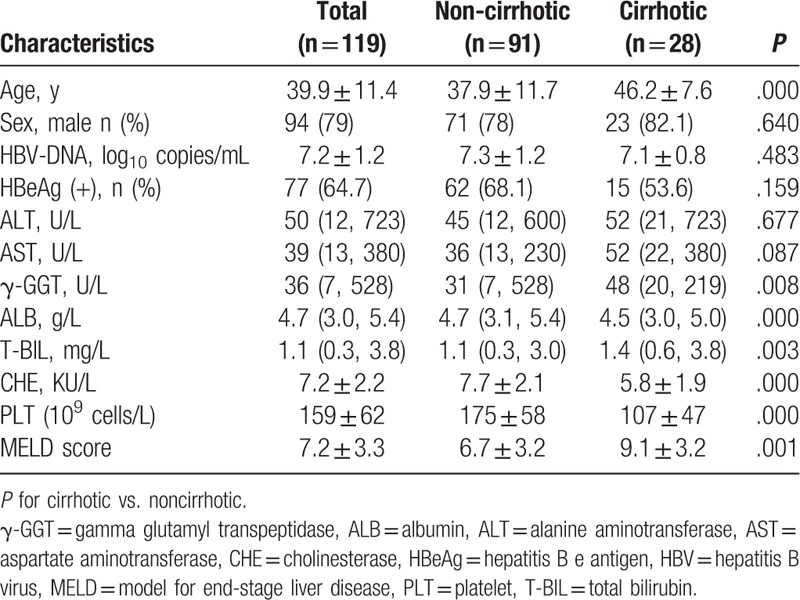
The baseline characteristics of the patients studied are shown in Table 1. In total, the mean age was 39.9 years, 94 (79.0%) were male, 77 (64.7%) were HBeAg-positive, and 28 (23.5%) were cirrhotic. Compared with non-cirrhotic patients, cirrhotic patients were older and had lower albumin (ALB), cholinesterase (CHE), and PLT, but higher gamma-glutamyltransferase (GGT), total bilirubin (T-BIL), and model for end stage liver disease (MELD) score. However, the variables of sex ratio, HBeAg status, HBV-DNA level, alanine transaminase, and aspartate transaminase between cirrhotic and noncirrhotic patients did not show significant differences.
Table 1.
Baseline characteristics of CHB patients.
Two patients were diagnosed with lung cancer and gastric cancer at 2.5 years and 3.0 years, respectively; 1 patient died in a traffic accident at 1.5 years, and another succumbed to pneumonia at 7.5 years; a further 31 of the initial 120 patients (25.8%) were lost to follow-up.
3.2. Efficacy of antiviral therapy
At the end-point of follow-up, 59.7% (71/119) patients achieved VR; 46.8% (36/77) of baseline HBeAg-positive patients lost HBeAg and of these, 24.7% (19/77) achieved HBeAg seroconversion; HBsAg became undetectable in 3 patients and a further 2 patients underwent HBsAg seroconversion.
3.3. Cumulative incidence and risk factors for development of HCC
During a median follow-up of 10 years, HCC was diagnosed in 16 (13.4%) of the 119 patients. In total, the 3-, 5-, 7-, and 10-year cumulative incidences of HCC were 6.15%, 9.03%, 11.17%, and 15.73%, respectively (Fig. 2).
Figure 2.
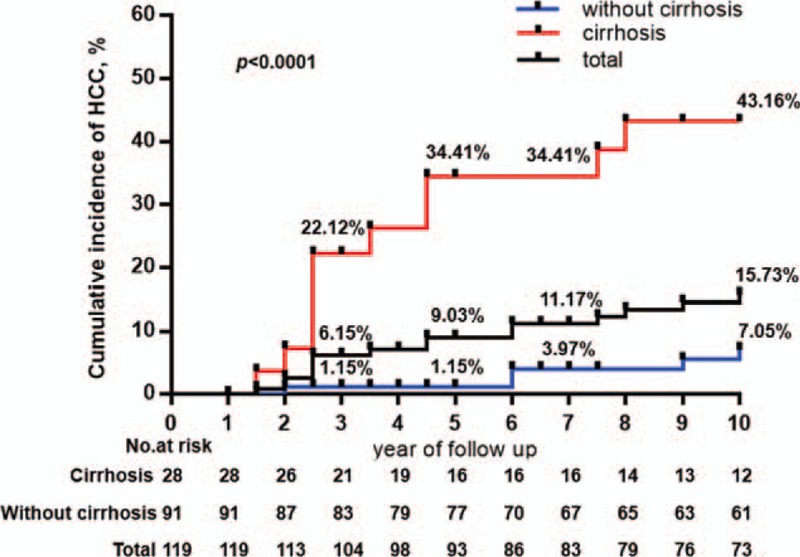
Cumulative risk for the development of HCC in all patients and in patients with and without cirrhosis. The cumulative incidence rate of HCC in patients with cirrhosis was significantly higher compared with non-cirrhotic patients (P < .001, log-rank test). HCC = hepatocellular carcinoma.
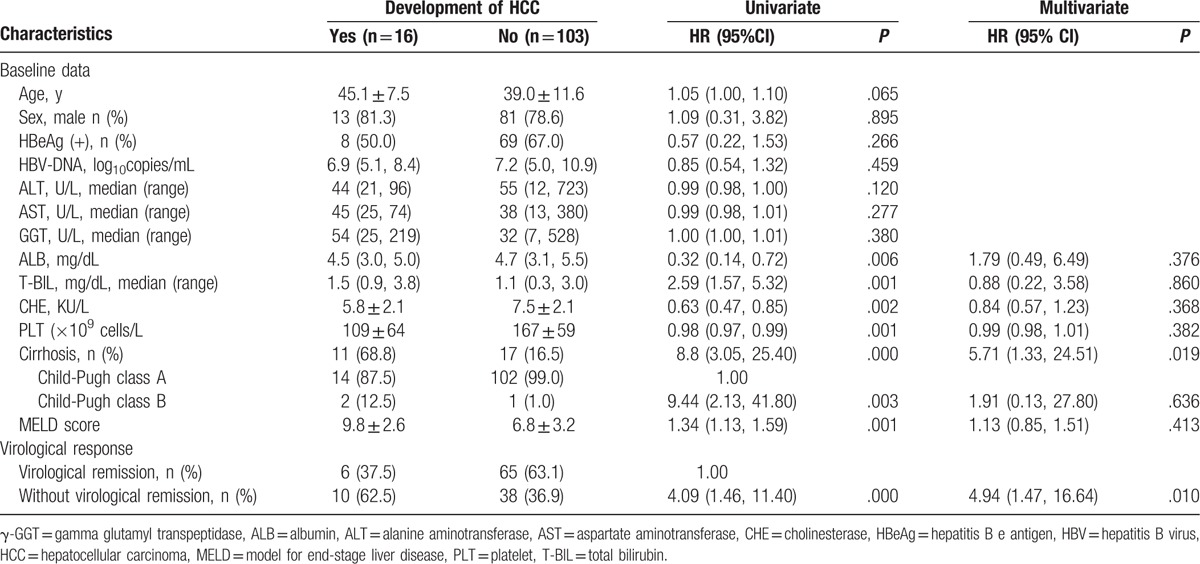
Univariate analyses showed the risk factors for development of HCC were liver cirrhosis, Child-Pugh class B, higher GGT, T-BIL, and MELD score, and lower ALB, CHE, and PLT, as well as failure to achieve VR (Table 2). Multivariate analyses showed that liver cirrhosis at baseline and failure to achieve VR during therapy were independent risk factors for HCC development (Table 2).
Table 2.
Univariate and multivariate analysis of risk factors for development of HCC.
Noticeably, the 3-, 5-, 7-, and 10-year incidences of HCC were significantly higher in patients with cirrhosis compared with noncirrhotic patients at baseline (22.12% vs. 1.15%, P < .0001; 34.41% vs. 1.15%, P < .0001; 34.41% vs. 3.97%, P < .0001; 43.16% vs. 7.05%, P < .0001, respectively) (Fig. 2).
3.4. Relationship between virological response and incidence of HCC
Patients were divided into VR and non-VR groups, based on treatment response to antiviral therapy. In total, the 3-, 5-, 7-, and 10-year incidences of HCC were significantly higher in patients without VR compared with those who achieved VR (16.39% vs. 0.00%, P = .0004; 19.09% vs. 2.94%, P = .0022; 26.44% vs. 2.94%, P = .0002; 26.44% vs. 9.13%, P = .0036, respectively) (Fig. 3A).
Figure 3.
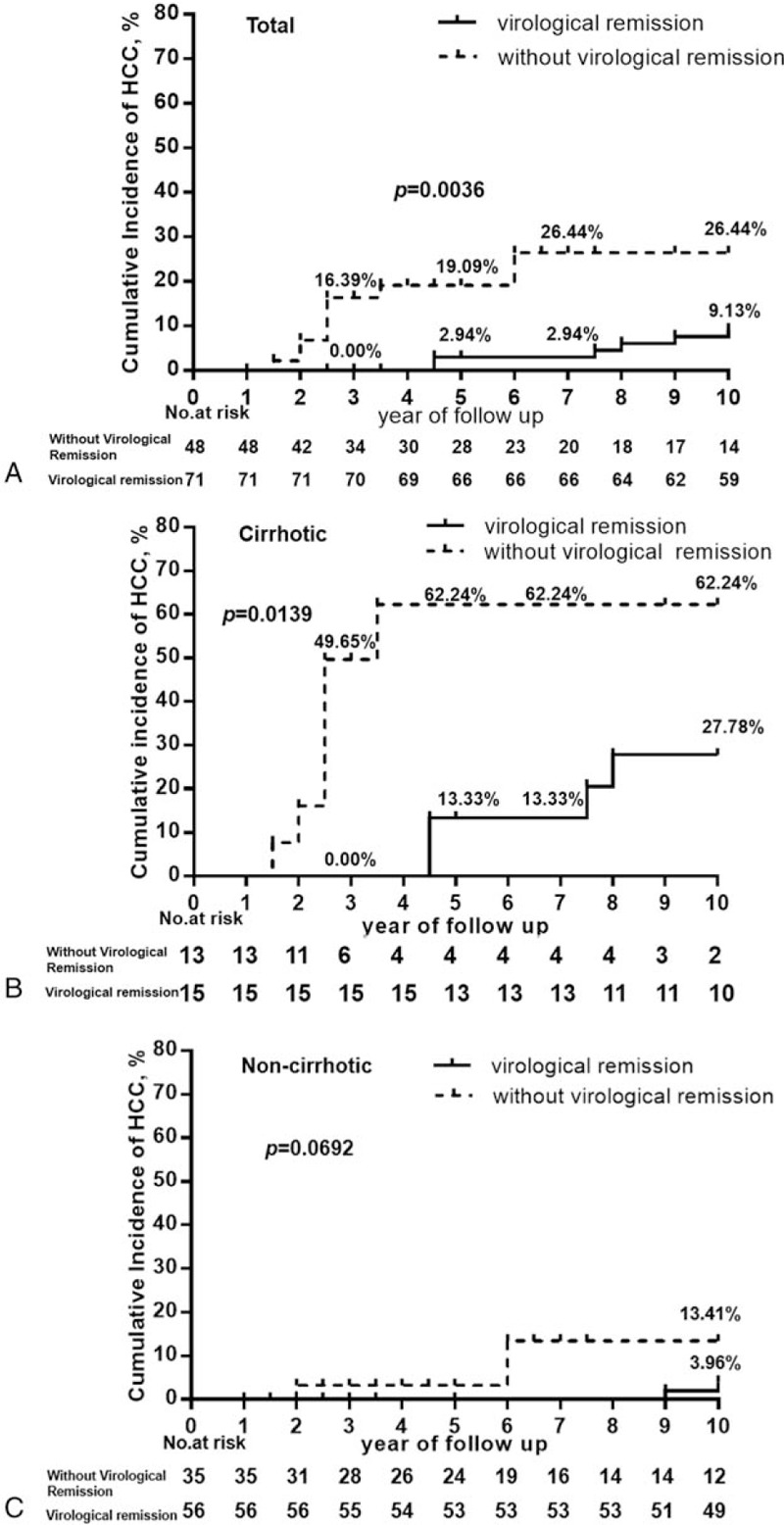
Cumulative risk for the development of HCC according to virological remission in total, cirrhotic and noncirrhotic patients. The cumulative incidence rate of HCC was significantly higher in patients without virological remission compared with patients who achieved virological remission in the total cohort (A) and in cirrhotic patients only (B). Among non-cirrhotic patients, the cumulative incidence of HCC in patients failing to achieve virological remission was higher compared with those attaining virological remission, but this difference did not achieve statistical significance. HCC = hepatocellular carcinoma (C).
As cirrhosis was an independent contributing factor to the incidence of HCC, the following analysis was performed. Patients were classified as cirrhotic and noncirrhotic, then the relationship between virological response and incidence of HCC was studied in these 2 groups. In patients with cirrhosis, the 3-, 5-, 7-, and 10-year incidences of HCC were significantly higher in patients who failed to achieve VR compared with patients with VR (49.65% vs. 0.00%, P = .0023; 62.24% vs. 13.33%, P = .0028; 62.24% vs. 13.33%, P = .0028; 62.24% vs. 27.78%, P = .0139, respectively) (Fig. 3B). Furthermore, the annual incidence of HCC in patients with cirrhosis who attained VR on NA therapy decreased to 2.8%.
Among noncirrhotic patients, 2 patients with VR were diagnosed with HCC during the 9th and 10th year, respectively. One patient without VR developed HCC during the second year and a further 2 patients without VR developed HCC during the 6th year. The 10-year incidence of HCC in patients without VR was higher than those with VR, but there was no statistically significant difference (13.41% vs. 3.96%, P = .0692) (Fig. 3C).
4. Discussion
This 10-year follow-up study of CHB patients shows that the risk factors for development of HCC in patients with chronic HBV infection receiving NA therapy were liver cirrhosis at baseline and failure to achieve VR at the time of HCC diagnosis.
In our study, univariate analysis showed that the baseline variables of lower ALB, CHE, PLT, higher T-BIL, cirrhosis at baseline, and absence of VR during NA therapy were risk factors for development of HCC. However, multivariate analysis showed that only liver cirrhosis and failure to achieve VR during NA therapy were independent risk factors for HCC. One explanation is that high T-BIL, along with low ALB, CHE, and PLT, were all associated with cirrhosis, so multivariate analysis did not show significant associations between these factors and the development of HCC.
Our finding is in line with previous reports that cirrhosis was found to be an important risk factor for HCC not only in CHB patients not receiving antiviral therapy,[15] but also in those receiving antiviral therapy.[8] Furthermore, a meta-analysis of pooled data from 49 studies of 6129 CHB patients with cirrhosis and 3359 CHB patients without cirrhosis revealed that the rate of HCC was 10-fold higher among patients with cirrhosis compared with those without cirrhosis (3 vs. 0.3 per 100-person year follow-up).[16] In our study, the 10-year cumulative incidence of HCC was nearly 6-fold higher in cirrhotic patients compared with noncirrhotic patients (43.16% vs. 7.05%). This further supports the recommendations for closer HCC surveillance in CHB patients with cirrhosis even they are on antiviral therapy.
In addition to liver cirrhosis, failure to achieve VR on NA therapy was an independent risk factor for development of HCC in CHB patients in the present study. For cirrhotic CHB patients, those achieving VR showed a significantly lower incidence of HCC compared with patients without VR. For CHB patients without cirrhosis, the 10-year cumulative incidence of HCC was higher in patients without VR than in patients who attained VR, but this was not statistically significant. One possible reason for the absence of significance is the small number of noncirrhotic CHB patients progressing to HCC development (2 and 3 patients with and without VR, respectively) during the study period.
The long-term effect of NA in lowering the risk of HCC development is still an issue under discussion. Studies involving CHB patients from Asia showed that lamivudine decreased the risk of HCC not only in patients with cirrhosis,[6] but also in noncirrhotic patients.[17] However, results of a Greek nationwide cohort study including 818 CHB patients, with or without cirrhosis, followed up for a median duration of 4.7 years, showed that VR on lamivudine monotherapy did not significantly affect the incidence of HCC in all patients or those with cirrhosis.[11] One possible reason for this contradictory finding may be differences in the patients’ baseline characteristics and duration of antiviral therapy.
Our study was a long-term study; the relationship between VR and the risk of HCC was evaluated in both cirrhotic and non-cirrhotic patients. Our study further confirmed that only effective treatment could decrease the risk of HCC in CHB patients. Furthermore, in a systematic review including 2 randomized and 19 cohort studies with 3881 treated and 534 untreated patients, HCC occurred less frequently in the treated group compared with untreated patients during a 46 (32–108) month period (2.8% and 6.4%, respectively; P = .003), and HCC developed less frequently in patients achieving VR compared with those with virological nonresponse or breakthrough (2.3% vs. 7.5%, P < .001).[12]
The limitations of the present study include the following. First, the patients did not undergo liver biopsy for the diagnosis of cirrhosis. Cirrhosis was clinically diagnosed with the presence of definite evidence. Therefore, the presence of cirrhosis may have been underestimated in the patient population. Second, drug resistance mutation analysis was not performed in patients who did not achieve VR. Third, according to current international CHB guidelines,[18] ADV is not recommended as the first-line NA treatment; thus, the patients in the present study may not represent the current population of CHB patients who have been treated with entecavir or tenofovir. However, the high resistance to ADV in the present study supports the view that we should pay more attention to virological response to NA therapy and drugs with potent antiviral activity and high-barrier to resistance should be the first choice for CHB patients.
In summary, cirrhosis at baseline and failure to achieve VR during antiviral therapy were significant risk factors for HCC development in CHB patients. Effective viral suppression is necessary to reduce HCC development in cirrhotic patients with CHB. We should strictly monitor the virological response to NA therapy in CHB patients, especially in those with cirrhosis.
Footnotes
Abbreviations: ADV = adefovir dipivoxil, ALB = albumin, CHB = chronic hepatitis B, CHE = cholinesterase, CT = computer tomography, GGT = gamma-glutamyl transferase, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HIV = human immunodeficiency virus, MELD = model for end-stage liver disease, MRI = magnetic resonance imaging, NA = nucleotide/nucleoside analog, PLT = platelet, T-BIL = total bilirubin, VR = virological remission.
XO and JJ share co-corresponding authorship.
Funding: This study was funded by the Project from Beijing Municipal Science and Technology Commission (D161100002716003,Z151100004015084), National Key Technologies R&D Program of China (2015BAI13B09).
The authors report no conflicts of interest.
References
- [1].El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- [2].Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546–55. [DOI] [PubMed] [Google Scholar]
- [3].Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65–73. [DOI] [PubMed] [Google Scholar]
- [5].Chan HL, Tse CH, Mo F, et al. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol 2008;26:177–82. [DOI] [PubMed] [Google Scholar]
- [6].Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–31. [DOI] [PubMed] [Google Scholar]
- [7].Wu C, Lin J, Ho HJ, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B—a nationwide cohort study. Gastroenterology 2014;147:143–51. [DOI] [PubMed] [Google Scholar]
- [8].Eun JR, Lee HJ, Kim TN, et al. Risk assessment for the development of hepatocellular carcinoma: according to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol 2010;53:118–25. [DOI] [PubMed] [Google Scholar]
- [9].Watanabe T, Tokumoto Y, Joko K, et al. Effects of long-term entecavir treatment on the incidence of hepatocellular carcinoma in chronic hepatitis B patients. Hepatol Int 2016;10:320–7. [DOI] [PubMed] [Google Scholar]
- [10].Orito E, Hasebe C, Kurosaki M, et al. Risk of hepatocellular carcinoma in cirrhotic hepatitis B virus patients during nucleoside/nucleotide analog therapy. Hepatol Res 2015;45:872–9. [DOI] [PubMed] [Google Scholar]
- [11].Papatheodoridis GV, Manolakopoulos S, Touloumi G, et al. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut 2011;60:1109–16. [DOI] [PubMed] [Google Scholar]
- [12].Papatheodoridis GV, Lampertico P, Manolakopoulos S, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol 2010;53:348–56. [DOI] [PubMed] [Google Scholar]
- [13].Suk KT, Baik SK, Yoon JH, et al. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol 2012;18:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36. [DOI] [PubMed] [Google Scholar]
- [15].Yuen M, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009;50:80–8. [DOI] [PubMed] [Google Scholar]
- [16].Singal AK, Salameh H, Kuo YF, et al. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharm Ther 2013;38:98–106. [DOI] [PubMed] [Google Scholar]
- [17].Yuen MF, Seto WK, Chow DH, et al. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther 2007;12:1295–303. [PubMed] [Google Scholar]
- [18].Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]


