Abstract
Background:
Recently, platelet-rich plasma (PRP) has been used as an alternative therapy for plantar fasciitis (PF) to reduce heel pain and improve functional restoration. We evaluated the current evidence concerning the efficacy and safety of PRP as a treatment for PF compared with the efficacy and safety of steroid treatments.
Methods:
Databases (PubMed, EMBASE, and The Cochrane Library) were searched from their establishment to January 30, 2017, for randomized controlled trials (RCTs) comparing PRP with steroid injections as treatments for PF. The Cochrane risk of bias (ROB) tool was used to assess the methodological quality. Outcome measurements were the visual analogue scale (VAS), Foot and Ankle Disability Index (FADI), American Orthopedic Foot and Ankle Society (AOFAS) scale, and the Roles and Maudsley score (RMS). The statistical analysis was performed with RevMan 5.3.5 software.
Results:
Nine RCTs (n = 430) were included in this meta-analysis. Significant differences in the VAS were not observed between the 2 groups after 4 [weighted mean difference (WMD) = 0.56, 95% confidence interval (95% CI): −1.10 to 2.23, P = .51, I2 = 89%] or 12 weeks of treatment (WMD = −0.49, 95% CI: −1.42 to 0.44, P = .30, I2 = 89%). However, PRP exhibited better efficacy than the steroid treatment after 24 weeks (WMD = −0.95, 95% CI: −1.80 to −0.11, P = .03, I2 = 85%). Moreover, no significant differences in the FADI, AOFAS, and RMS were observed between the 2 therapies (P > .05).
Conclusion:
Limited evidence supports the conclusion that PRP is superior to steroid treatments for long-term pain relief; however, significant differences were not observed between short and intermediate effects. Because of the small sample size and the limited number of high-quality RCTs, additional high-quality RCTs with larger sample sizes are required to validate this result.
Keywords: meta-analysis, plantar fasciitis, platelet-rich plasma, randomized controlled trials
1. Introduction
Plantar fasciitis (PF) is a common lesion that occurs in the heel, and approximately 11% to 15% of adult foot symptoms require professional care.[1,2] Pain is intensified by prolonged weight bearing, obesity, and gradually increased activity.[3,4] It is estimated that approximately 1 in 10 people experience heel pain at some point. Although PF occurs at all ages, the highest risk of occurrence of PF is 40 to 60 years of age, with no significant sex bias.[5] The diagnosis of PF is mainly based on the patient's history and clinical examination, and further investigation is rarely needed. In terms of treatment, various methods have also been used in the treatment of PF, including nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, and nondrug approaches, such as ice packs, shoe inserts, plantar fascia stretching exercises, extracorporeal shock wave therapy, and even surgical treatment.[6–8] It is reported that the symptoms will disappear after nonsurgical treatment in more than 80% of patients.[9] In 10% of patients, symptoms do not improve with conservative measures and further develop into chronic diseases.[10] In general, when these conservative treatments fail, injecting steroids is considered an option.[11] However, steroid injections are often not successful after 1 injection and can thus require multiple injections, which may be associated with potential complications, including plantar fascia rupture and fat pad atrophy.[12,13] Therefore, the study of alternative therapies is important.
A local injection of platelet-rich plasma (PRP) is an emerging therapy for ligament pathologies and recalcitrant tendons, including PF. PRP is prepared from autologous whole blood that contains an increased concentration of autologous platelets. In the clinic, PRP has been widely applied to various tissue injuries, such as osteoarthritis, muscle strain, bone healing, and tendon injury.[14–16] PRP has also been used as an effective treatment modality in sports medicine to rehabilitate disabled muscles.[17] However, all of these approaches have resulted in inconsistent treatment response rates in different clinical trials.
Recently, many studies have focused on the effectiveness of PRP as a treatment for PF; however, the results are inconsistent. In addition, the relationships between PRP and pain relief and improvements in functional restoration are unknown. Thus, we conducted a systematic review and meta-analysis to further analyze the effects of PRP on functional restoration and pain control in patients with PF.
2. Materials and methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria, we created a prospective protocol, including objectives, literature-search strategies, inclusion and exclusion criteria, outcome measurements, and methods of statistical analysis before commencing the study. The study was approved by the ethics committee of Guangdong Provincial Hospital of Chinese Medicine. This review is registered in Research Registry as reviewregistry260 (http://www.researchregistry.com/).
2.1. Data sources and search strategies
A systematic literature search of PubMed (1950–January 2017), EMBASE (1974–January 2017), and the Cochrane Library (January 2017) was performed. The following MeSH or Emtree terms and their combinations were searched in the title and abstract: “platelet-rich plasma,” “plasma, platelet-rich,” “platelet rich plasma,” “fasciitis, plantar,” “plantar fasciitis,” “heel spur syndrome,” “chronic plantar fasciitis,” “fasciitis, chronic plantar,” “plantar fasciitis, chronic,” and “fasciitis, plantar, chronic.” Only articles that were originally written in English were included, and unpublished trials were excluded. When multiple reports describing the same population were published, the most recent or complete report was used. Additional eligible studies were identified by searching the reference lists from primary articles and relevant reviews.
2.2. Inclusion criteria
-
(1)
The study compared PRP with a control (such as a corticosteroid, steroid, or glucocorticoid treatment) in patients who were diagnosed with PF.
-
(2)
The study was an RCT or prospective cohort study only.
-
(3)
The major outcomes included the visual analogue scale (VAS), Foot and Ankle Disability Index (FADI), American Orthopedic Foot and Ankle Society (AOFAS) scale, and the Roles and Maudsley Score (RMS).
2.3. Exclusion criteria
-
(1)
Articles were from the same institution, duplicated publications, or included the same data sets.
-
(2)
No outcomes of interest were reported.
-
(3)
Subjects had a traumatic disease, a history of surgical interventions, or systemic disorders such as rheumatoid arthritis.
-
(4)
Animal studies, case reports, and nonoriginal research (such as editorials, review articles, and letters to the editor).
2.4. Data extraction and analysis
Data were independently extracted by 2 authors (Yang and Cao). The demographic characteristics (first author, publication year, location, sample size, average age, male/female ratio, intervention, and study design) were extracted. All outcomes as mentioned above were extracted for meta-analysis.
2.5. Quality assessment
Two independent reviewers (Pan and Zeng) evaluated the methodological quality of the included studies using the risk of bias (ROB) tool provided by the Cochrane collaboration. The following 7 items were assessed in all included studies: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective reporting, and other bias. Each item was assigned a judgment of “a high risk of bias (HRB),” “an unclear risk,” or “a low risk of bias (LRB)” based on the data presented in the article.[18] Namely, the judgment was “high risk” if the item was reported incorrectly. The judgment was “low risk” if the item possessed sufficient and correct information. If the item possessed insufficient or unmentioned information, the judgment was “an unclear risk.” An “unclear risk” judgment was also assigned if the item was reported, but the ROB was unknown. Disagreements were addressed by obtaining a consensus between experienced reviewers (Han and Liu).
2.6. Data synthesis and analysis
The desired outcomes were VAS, FAID, AOFAS, and RMS. The follow-up times were divided into short periods (2–4 weeks), intermediate periods (4–24 weeks), and long periods (≥24 weeks) to investigate the efficacy of the treatments among trials of different durations. The statistical analysis was performed with RevMan 5.3.5 software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) by 2 reviewers (Han and Lin). The weighted mean difference (WMD) and relative risk (RR), both of which were reported with 95% confidence intervals (95% CIs), were adopted to analyze continuous variables and dichotomous data, respectively. The I2 value was used to estimate statistical heterogeneity. When I2 was >50%, heterogeneity was accepted and the randomized-effects model was adopted. Otherwise, the fixed-effects model was adopted. Publication bias was assessed using Egger test. A P value <.05 was considered statistically significant.
3. Results
3.1. Study selection
Ninety-five records were retrieved via database and manual searches. After thoroughly screening the titles and abstracts, 56 records were excluded. The full texts of the remaining 26 articles were assessed. Finally, 9 studies[19–27] including 416 participants met the inclusion criteria and were included in the meta-analysis (Fig. 1).
Figure 1.
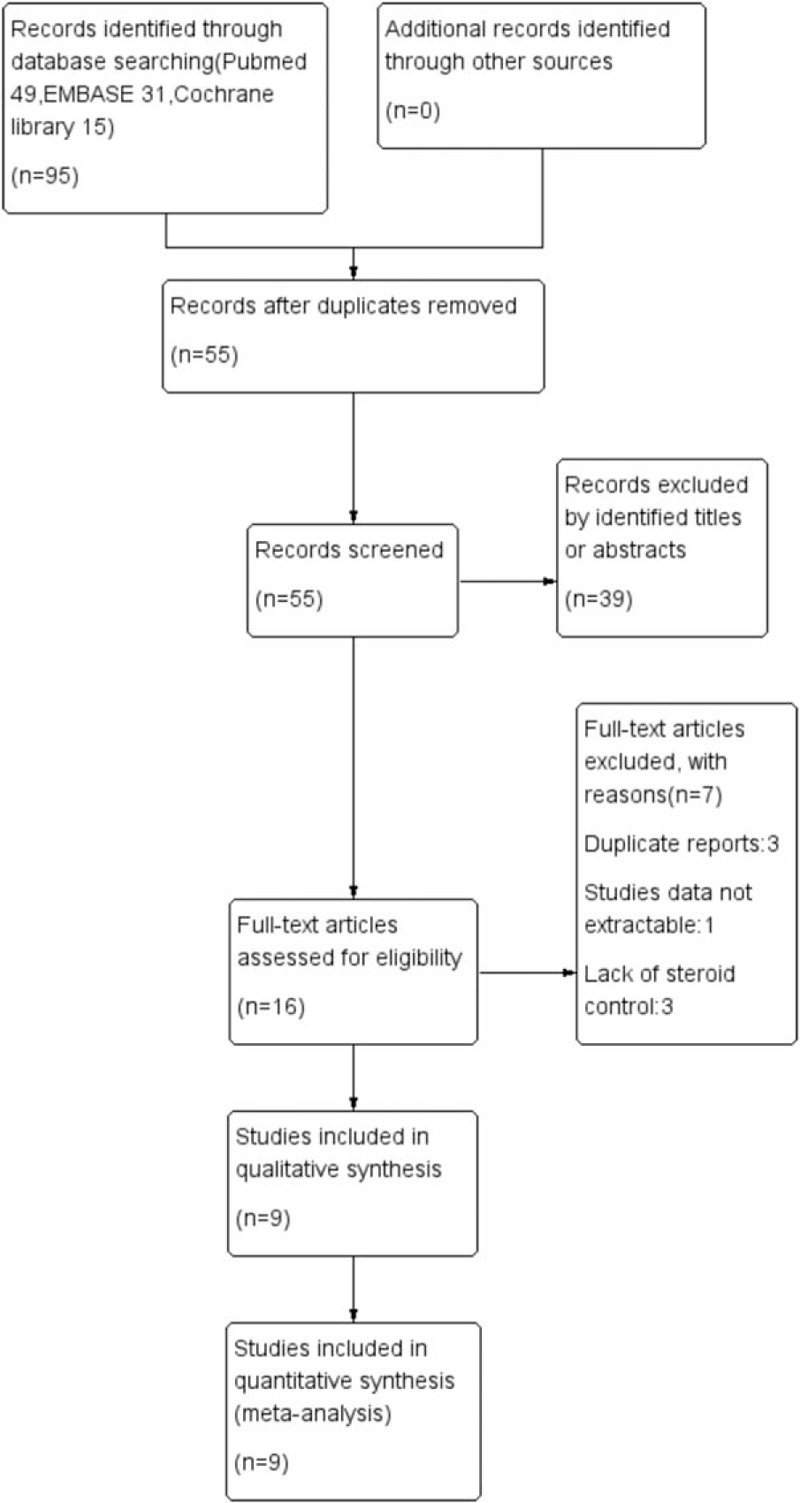
Flow diagram of studies identified, included, and excluded.
3.2. Characteristics of the included studies
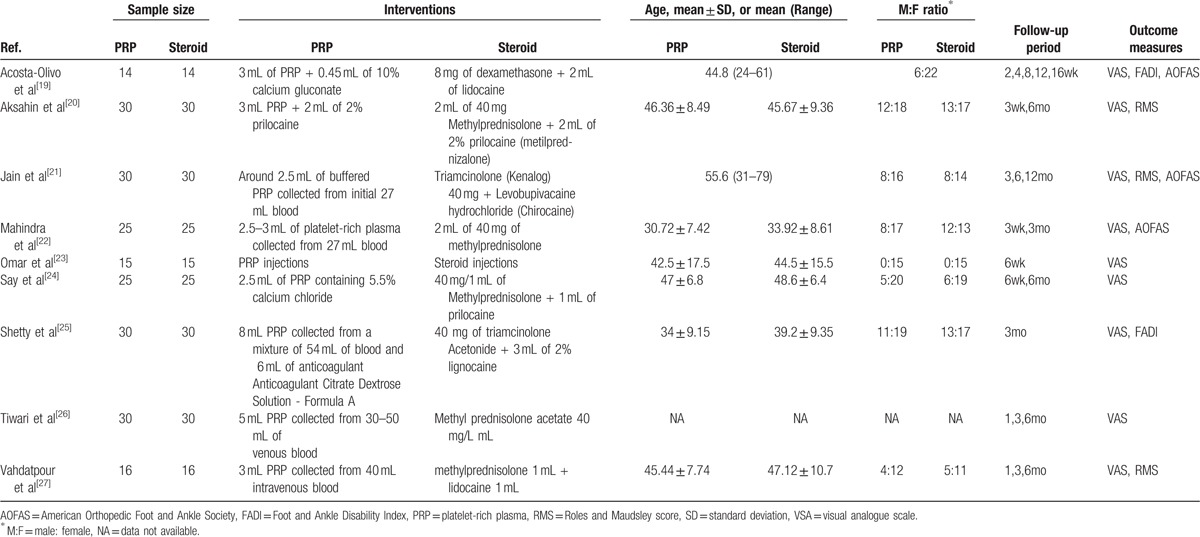
The characteristics of the included studies are listed in Table 1. Most injections were performed at the point of maximum tenderness in the heel. In all 9 trials,[19–27] subjects were treated with PRP in the experimental group. Therapies in the control group varied among the different studies. Control subjects were treated with dexamethasone in 1 study,[19] triamcinolone in 2 studies,[21,25] methylprednisolone in 5 studies,[20,22,24,26,27] and an unidentified steroid in 1 study.[23] A combination of local anesthetics, such as prilocaine or lidocaine, was applied in 6 trials,[19–21,24,25,27] whereas the remaining 3 studies[22,23,26] did not use this combination. Overall, 9 trials[19–27] described the detailed process used to produce PRP. Only the studies by Acosta-Olivo et al[19] and Say et al[24] reported the use of an activating agent to activate platelets. Most studies reported the use of anticoagulants during the production of PRP.
Table 1.
Characteristics of included studies.
3.3. Assessment of the ROB
We evaluated the methodological quality of all studies using the Cochrane ROB criteria. The assessment of ROB is shown in Figs. 2 and 3. Randomly generated sequences were judged as a LRB in 4 trials.[19,21,22,27] However, 2 trials[20,25] used inadequate randomization, leading to their categorization as a HRB. Other studies mentioned that the clinical trial was randomized, but did not report further details. Two trials[19,22] described adequate allocation concealment. Blinding of treating doctors, subjects, and outcome assessors were judged as a LRB in 2 trials,[19,22] 3 trials,[19,20,22] and 3 trials,[19,20,22] respectively. One trial[19] had a high risk of incomplete outcome data due to a lack of details regarding adverse events. Two trials[19,26] had an unclear risk of selective reporting bias. We did not identify other obvious sources of bias in the trials.
Figure 2.
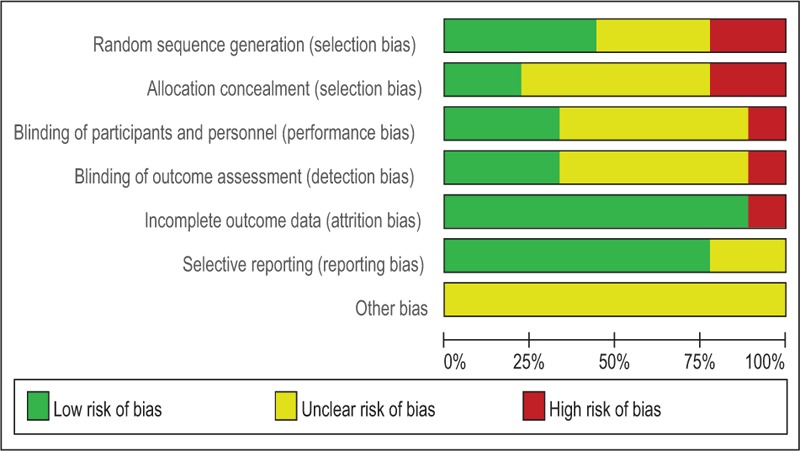
Risk of bias summary.
Figure 3.
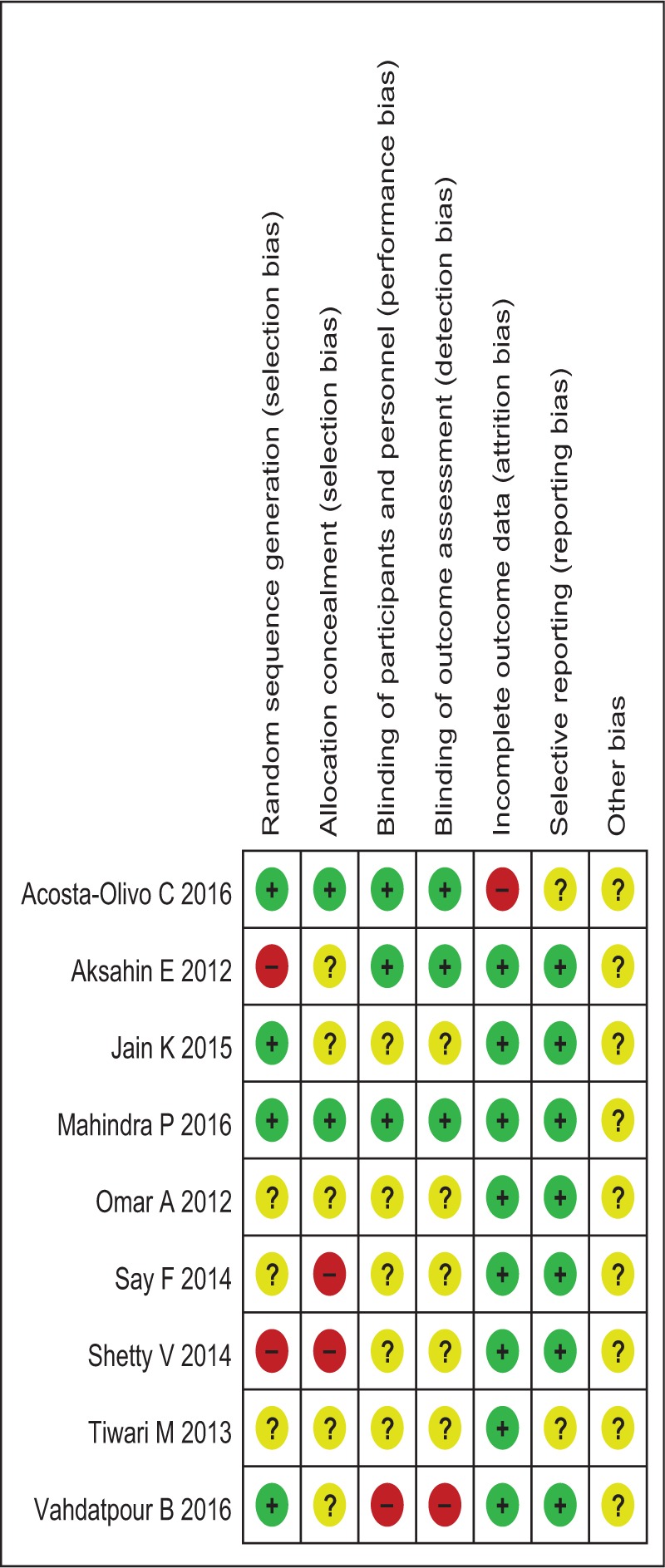
Risk of bias assessment.
3.4. VAS
All pooled analyses were conducted using a random effects model because of significant statistical heterogeneity. Nine trials (n = 430) provided the data for the VAS used to compare efficacy. As shown in Fig. 4, no significant differences in the visual analogue scale scores were observed between the 2 groups in the short term (WMD = 0.56, 95% CI: −1.10 to 2.23, P = .51, I2 = 89%) and intermediate term (WMD = −0.49,95% CI: −1.42 to 0.44, P = .30, I2 = 89%). However, PRP exhibited better long-term efficacy than steroid treatments (WMD = −0.95, 95% CI: −1.80 to −0.11, P = .03, I2 = 85%).
Figure 4.
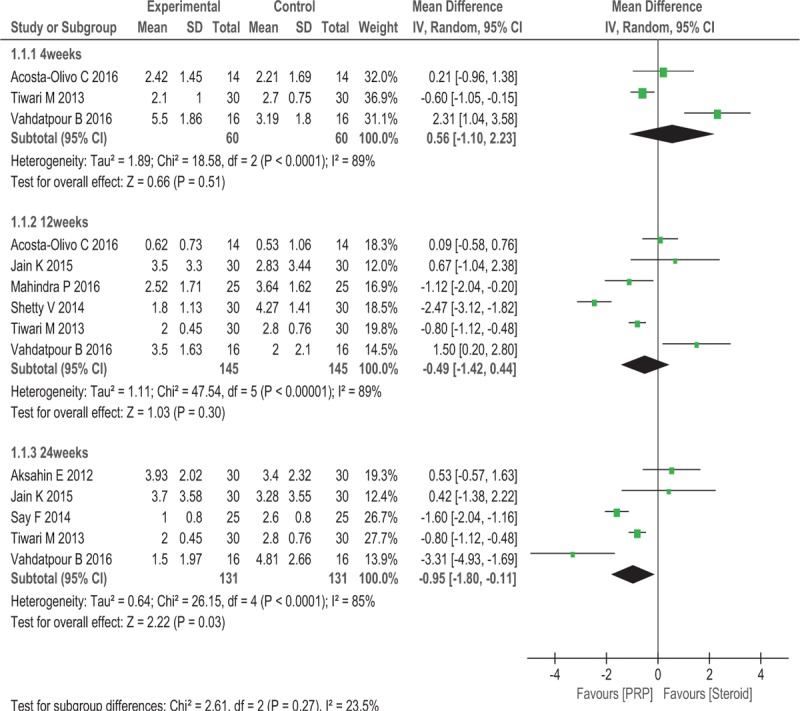
Forest plot of VAS when comparing PRP with steroid.
3.5. FADI
Two trials (n = 88) reported the FADI as the major outcome. No significant differences in the FADI were observed between the 2 groups after 12 weeks (WMD = 14.08, 95% CI: −11.57 to 39.73, P = .28, I2 = 99%; Fig. 5).
Figure 5.
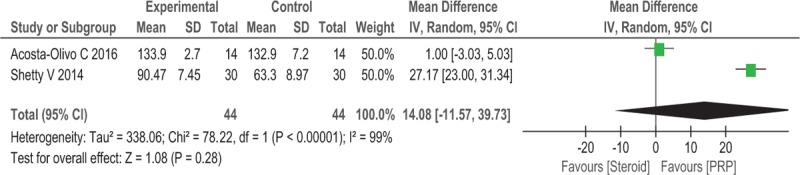
Forest plot of FADI when comparing PRP with steroid.
3.6. AOFAS Scale
Three trials (n = 138) reported the AOFAS scale score as the major outcome. No significant differences in the AOFAS scale scores were observed between the 2 groups after 12 weeks (WMD = 0.94, 95% CI: −5.99 to 7.86, P = .79, I2 = 81%; Fig. 6).
Figure 6.
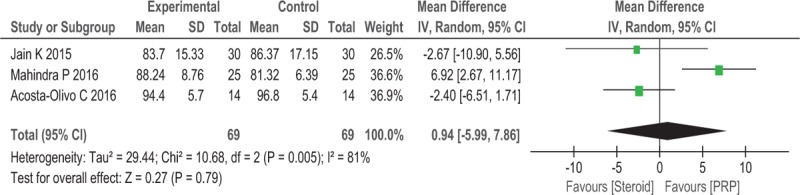
Forest plot of AOFAS when comparing PRP with steroid.
3.7. RMS
Two trials (n = 138) reported the RMS as the major outcome. No significant differences in the RMS were observed between the 2 groups after 6 months (RR = 1.75, 95% CI: 0.27–11.38, P = .56, I2 = 90%; Fig. 7).
Figure 7.
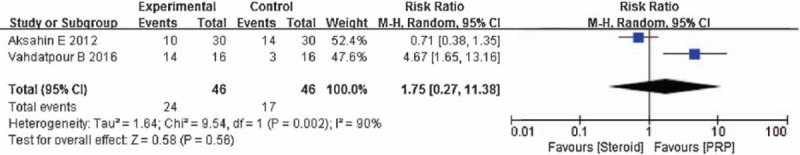
Forest plot of RMS when comparing PRP with steroid.
3.8. Sensitivity analysis
A sensitivity analysis was performed by individually removing each study to determine whether the pooled results changed. All results were stable, with the exception the AOFAS scale score. For the AOFAS scale score, the difference between groups was no longer statistically significant after 1 study was removed; in addition, the removal of another study from the AOFAS analysis reduced I2 to 0% and resulted in a lack of a significant difference between groups. On the basis of the results of the AOFAS analysis, PRP is equally effective for patients with PF.
4. Discussion
PF is common in general populations, particularly among overweight individuals and people who spend a lot of time standing, and can have a serious effect on a person's life and work. The etiology of PF is not fully understood and may be multifactorial.[28,29] Numerous therapies have been reported, but the available evidence supporting a preferred treatment is inadequate or even conflicting. Steroid injections are considered one of the first-line treatments and have been found to produce satisfactory short-term results by blocking the inflammatory response and improving local edema, swelling, pain, and foot function. Unfortunately, steroid injections have been reported to be related to abscesses, osteomyelitis, fat pad atrophy, and plantar fascia tears.[30,31]
PF is considered a degenerative tissue condition in many studies rather than original inflammation at the site of the plantar fascia at the tuberosity of the calcaneus.[4,32] Due to the small tear of the fascia that cannot heal, collagen denaturation occurs in these lesions. As the normal fascia and surrounding tissue are replaced by angiofibroblastic hyperplastic tissue, the lesion sites do not show inflammatory cell invasion, the histological features of chronic PF.[32,33] The cytokines and growth factors present in PRP may play an important role in the treatment of PF. PRP is rich in transforming growth factor, vascular endothelial growth factor, and platelet-derived growth factor. In addition, PRP also has some anti-inflammatory and pro-inflammatory cytokines and interleukins, such as interleukin 4, 8, 13, interferon-α, and tumor necrosis factor-α.[34] The combination of these growth and anti-inflammatory components is necessary to initiate the healing stages and to reverse the degenerative process at the base of the plantar fascia.[35] The plantar fascia is inaccessible to high concentrations of platelets and growth factors because of hypovascularity and hypocellularity; however, PRP injections enable delivery directly to the lesion site.[36] Platelets contain dense and alpha granules; alpha particles can release stored platelet-derived growth factors after platelet stimulation, and platelet-derived growth factors can promote angiogenesis and fiber repair. Therefore, local injection of PRP promotes the repair of the plantar fascia.[34]
Our primary concerns in treating PF are pain relief and functional improvement, which are also the main concerns of patients. In this meta-analysis, we did not observe a significant difference in pain relief between PRP and steroids in the short (2 to 4 weeks: WMD = 0.56, 95% CI: −1.10 to 2.23, P = .51, I2 = 89%) or intermediate term (4–24 weeks: WMD = −0.49, 95% CI: −1.42 to 0.44, P = .30, I2 = 89%). However, PRP displayed better long-term efficacy in relieving pain (≥24 weeks: WMD = −0.95, 95% CI: −1.80 to −0.11, P = .03, I2 = 85%). Recent studies have shown that PRP can increase collagen gene expression and production of vascular endothelial growth factor to promote tendon healing.[37] High concentrations of PRP can be effective in recruiting regenerative cells and increasing angiogenesis.[38] The presence of growth factors in the PRP allows the regenerative process to begin shortly after administration. This process may require a minimum time period to achieve its maximum clinical efficacy, which may explain the equivalent short-term effects of PRP and steroids and the improved long-term effects of PRP. Because the steroid group did not display differences in the FADI (WMD = 14.08, 95% CI: −11.57 to 39.73, P = .28, I2 = 99%), AOFAS (WMD = 0.94, 95% CI: −5.99 to 7.86, P = .79, I2 = 81%), or RMS (RR = 1.75, 95% CI: 0.27–11.38, P = .56, I2 = 90%), we concluded that PRP and steroids have similar effects on functional improvement.
5. Limitations
First, only 9 studies were included in our meta-analysis, including 416 patients; thus, the credibility for all outcomes may be limited by the small sample size. According to the ROB assessment, none of the included studies exhibited a LRB, while our study provides the latest evidence regarding the use of PRP as a treatment for PF. Additional RCTs with a LRB are required to increase the level of confidence in our results. Second, we should not ignore the heterogeneity between different studies. It may have been caused by a number of factors, including different treatment algorithms, clinical skills, and physician experience levels.[39] An experienced physician would be considerably more likely to administer an accurate injection with fewer adverse events.[39,40] Third, some of the reported results are subjective, such as the VAS score. Other objective changes included FADI, AOFAS, and RMS, which were not reported in all included studies. In addition, not all of the included studies mentioned all of the included studies did not mention the recurrence of PF after PRP treatment. As the longest assessment period in the included studies was 48 weeks after PRP administration,[21] we were unable to determine whether the issue reoccurred 1 year after PRP treatment. Despite some shortcomings, the current meta-analysis suggests that PF patients may benefit from PRP therapy in the long-term control of pain symptoms.
6. Conclusion
As shown in our meta-analysis, significant differences in short-term (2–4 weeks) and intermediate-term (4–8 weeks) pain relief were not observed. However, PRP had better long-term efficacy in relieving pain (≥24 weeks). In addition, no differences in functional improvement were observed between PRP and steroid treatments. Considering the long-term effectiveness of PRP, we recommend the use of PRP as the preferred treatment for PF. Additional high-quality RCTs with more patients and a uniform scoring standard are needed to confirm the effectiveness and safety of PRP and steroids as treatments for PF.
Footnotes
Abbreviations: AOFAS = American Orthopedic Foot and Ankle Society scale, CI = confidence intervals, FADI = Foot and Ankle Disability Index, HRB = high risk of bias, LRB = low risk of bias, NSAIDs = non-steroidal-anti-inflammatory drugs, PF = plantar fasciitis, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analysis, PRP = platelet-rich plasma, RMS = Roles and Maudsley Score, ROB = risk of bias, RR = relative risk, VAS = visual analogue scale, WMD = weighted mean difference.
W-YY, Y-HH, and X-WC contributed equally to this work.
Authorship: Conceived and designed the experiments: JL. Performed the experiments: WYY, YHH, and XWC. Analyzed the data: JKP, LFZ, JTL. Contributed reagents/materials/analysis tools: WYY, YHH, XWC, and JL. Wrote the paper: WYY, YHH, and XWC.
Funding/support: This study was funded by grants from the National Natural Science Foundation of China (Nos. 81473698 and 81273781), the Doctoral Fund of Ministry of Education of China (No. 20124425110004), the TCM Standardization Projects of State Administration of Traditional Chinese Medicine of China (Nos. SATCM-2015-BZ115 and SATCM-2015-BZ173), the Science and Technology Planning Project of Guangdong Province, China (No. 2011B031700027), the Project of Guangdong Provincial Department of Finance [No. (2014) 157], the Administration of Traditional Chinese Medicine of Guangdong Province (No. 20164020), and the Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (Nos. YK2013B2N19 and YN2015MS15).
The authors report no conflicts of interest.
References
- [1].Schwartz EN, Su J. Plantar fasciitis: a concise review. Perm J 2014;18:e105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cole C, Seto C, Gazewood J. Plantar fasciitis: evidence-based review of diagnosis and therapy. Am Fam Physician 2005;72:2237–42. [PubMed] [Google Scholar]
- [3].Irving DB, Cook JL, Menz HB. Factors associated with chronic plantar heel pain: a systematic review. J Sci Med Sport 2006;9:11–22. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- [4].Buchbinder R. Clinical practice. Plantar fasciitis. N Engl J Med 2004;350:2159–66. [DOI] [PubMed] [Google Scholar]
- [5].Taunton JE, Ryan MB, Clement DB, et al. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med 2002;36:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dastgir N. Extracorporeal shock wave therapy for treatment of plantar fasciitis. J Pak Med Assoc 2014;64:675–8. [PubMed] [Google Scholar]
- [7].Salvi AE. Targeting the plantar fascia for corticosteroid injection. J Foot Ankle Surg 2015;54:683–5. [DOI] [PubMed] [Google Scholar]
- [8].Johnson RE, Haas K, Lindow K, et al. Plantar fasciitis: what is the diagnosis and treatment? Orthop Nurs 2014;33:198–204. quiz 205–196. [DOI] [PubMed] [Google Scholar]
- [9].Porter MD, Shadbolt B. Intralesional corticosteroid injection versus extracorporeal shock wave therapy for plantar fasciopathy. Clin J Sport Med 2005;15:119–24. [DOI] [PubMed] [Google Scholar]
- [10].Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician 2011;84:676–82. [PubMed] [Google Scholar]
- [11].Podolsky R, Kalichman L. Taping for plantar fasciitis. J Back Musculoskelet Rehab 2015;28:1–6. [DOI] [PubMed] [Google Scholar]
- [12].Ogden JA, Alvarez RG, Marlow M. Shockwave therapy for chronic proximal plantar fasciitis: a meta-analysis. Foot Ankle Int 2002;23:301–8. [DOI] [PubMed] [Google Scholar]
- [13].Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg 2008;16:338–46. [DOI] [PubMed] [Google Scholar]
- [14].Wei LC, Lei GH, Sheng PY, et al. Efficacy of platelet-rich plasma combined with allograft bone in the management of displaced intra-articular calcaneal fractures: a prospective cohort study. J Orthop Res 2012;30:1570–6. [DOI] [PubMed] [Google Scholar]
- [15].Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med 2013;41:356–64. [DOI] [PubMed] [Google Scholar]
- [16].Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med 2014;42:610–8. [DOI] [PubMed] [Google Scholar]
- [17].Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004;62:489–96. [DOI] [PubMed] [Google Scholar]
- [18].Higgins JP, Altman DG, Gotzsche PC, et al. Cochrane Bias Methods, G. Cochrane Statistical Methods. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Acosta-Olivo C, Elizondo-Rodriguez J, Lopez-Cavazos R, et al. Plantar fasciitis. A comparison of treatment with intralesional steroids versus platelet-rich plasma (PRP). A randomized, blinded study. J Am Podiatr Med Assoc 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [20].Aksahin E, Dogruyol D, Yuksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg 2012;132:781–5. [DOI] [PubMed] [Google Scholar]
- [21].Jain K, Murphy PN, Clough TM. Platelet Rich Plasma Versus Corticosteroid Injection for Plantar Fasciitis: A Comparative Study. In: Foot (Edinburgh, Scotland), 2015, 235–237. [DOI] [PubMed] [Google Scholar]
- [22].Mahindra P, Yamin M, Selhi HS, et al. Chronic plantar fasciitis: effect of platelet-rich plasma, corticosteroid, and placebo. Orthopedics 2016;39:e285–9. [DOI] [PubMed] [Google Scholar]
- [23].Omar AS, Ibrahim ME, Ahmed AS, et al. Local injection of autologous platelet rich plasma and corticosteroid in treatment of lateral epicondylitis and plantar fasciitis: Randomized clinical trial. The Egyptian Rheumatologist 2012;34:43–9. [Google Scholar]
- [24].Say F, Gurler D, Inkaya E, et al. Comparison of platelet-rich plasma and steroid injection in the treatment of plantar fasciitis. Acta Orthop Traumatol Turc 2014;48:667–72. [DOI] [PubMed] [Google Scholar]
- [25].Shetty VD, Dhillon M, Hegde C, et al. A study to compare the efficacy of corticosteroid therapy with platelet-rich plasma therapy in recalcitrant plantar fasciitis: a preliminary report. J Foot Ankle Surg 2014;20:10–3. [DOI] [PubMed] [Google Scholar]
- [26].Tiwari M, Bhargava R. Platelet rich plasma therapy: a comparative effective therapy with promising results in plantar fasciitis. J Clin Orthop Trauma 2013;4:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vahdatpour B, Kianimehr L, Moradi A, et al. Beneficial effects of platelet-rich plasma on improvement of pain severity and physical disability in patients with plantar fasciitis: a randomized trial. Adv Biomed Res 2016;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Beeson P. Plantar fasciopathy: revisiting the risk factors. Foot Ankle Surg 2014;20:160–5. [DOI] [PubMed] [Google Scholar]
- [29].Kirkland P, Beeson P. Use of primary corticosteroid injection in the management of plantar fasciopathy: is it time to challenge existing practice? J Am Podiatr Med Assoc 2013;103:418–29. [DOI] [PubMed] [Google Scholar]
- [30].Buccilli TA, Jr, Hall HR, Solmen JD. Sterile abscess formation following a corticosteroid injection for the treatment of plantar fasciitis. J Foot Ankle Surg 2005;44:466–8. [DOI] [PubMed] [Google Scholar]
- [31].Acevedo JI, Beskin JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int 1998;19:91–7. [DOI] [PubMed] [Google Scholar]
- [32].Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc 2003;93:234–7. [DOI] [PubMed] [Google Scholar]
- [33].Barrett SJ, O’Malley R. Plantar fasciitis and other causes of heel pain. Am Fam Physician 1999;59:2200–6. [PubMed] [Google Scholar]
- [34].Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med 2003;33:381–94. [DOI] [PubMed] [Google Scholar]
- [35].Martinelli N, Marinozzi A, Carni S, et al. Platelet-rich plasma injections for chronic plantar fasciitis. Int Orthop 2013;37:839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fenwick SA, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis Res 2002;4:252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Anitua E, Pino A, Orive G. Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J Wound Care 2016;25:680–7. [DOI] [PubMed] [Google Scholar]
- [38].Wang JH, Nirmala X. Application of tendon stem/progenitor cells and platelet-rich plasma to treat tendon injuries. Oper Tech Orthop 2016;26:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chou LC, Liou TH, Kuan YC, et al. Autologous blood injection for treatment of lateral epicondylosis: a meta-analysis of randomized controlled trials. Phys Ther Sport 2016;18:68–73. [DOI] [PubMed] [Google Scholar]
- [40].Li Z, Yu A, Qi B, et al. Corticosteroid versus placebo injection for plantar fasciitis: a meta-analysis of randomized controlled trials. Exp Ther Med 2015;9:2263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


