Abstract
We retrospectively compared overall survival (OS) and progression-free survival (PFS) of patients with single (3–5 cm) hepatocellular carcinoma (HCC) with Barcelona Clinic Liver Cancer (BCLC) stage A treated by surgical resection (SR), radiofrequency ablation (RFA), or transarterial chemoembolization (TACE).
Of the 38,167 HCC patients registered between 2008 and 2010 at Korea Central Cancer Registry, National Cancer Center of South Korea, 13% patients were randomly abstracted, and 4596 patients could be analyzed. Of these 4596 patients, 337 patients with single 3 to 5 cm sized HCC with BCLC stage A were enrolled. OSs and PFSs among SR (n = 151), RFA (n = 36), and TACE groups (n = 150) were compared, respectively. Propensity score (PS) weighting was used to adjust differences among 3 groups.
Median follow-up duration was 45 months (range, 1–73 months). After PS weighting, the cumulative OS rates were significantly higher in the SR (P < .001) and RFA (P = .027) groups than in the TACE group, respectively, but not statistically different between SR and RFA groups (P = .116). The cumulative PFS rates were significantly higher in the SR (P < .001) and RFA (P < .001) groups than in the TACE group, respectively. TACE (hazard ratio [HR] 2.46, P < .001), serum albumin (HR 0.57, P = .002), and tumor size (HR 1.66, P = .001) were predictors for OS. TACE (HR 3.14, P < .001), serum bilirubin (HR 1.38, P = .020), and tumor size (HR 1.32, P = .024) were predictors for PFS.
RFA has better OS and PFS rates than TACE, and provides comparable survival outcomes compared with SR in single (3–5 cm) HCC with BCLC stage A.
Keywords: hepatocellular carcinoma, radiofrequency ablation, surgery, transarterial chemoembolization
1. Introduction
Hepatocellular carcinoma (HCC) accounts for at least 500,000 deaths annually worldwide.[1–3] As a curable option for HCC treatment, surgical resection (SR), radiofrequency ablation (RFA), or liver transplantation (LT) has been recommended.[4–7] These treatment methods are influenced by tumor size or number, liver function, or other variables,[4,7] and prognosis can be different based on the treatments chosen.[4,7] Considering these factors, Barcelona Clinical Liver Cancer (BCLC) staging system, which is mostly widely used, recommends SR as a treatment of choice for single (3–5 cm) HCC with BCLC stage A.[4,7] However, such a strategy remains to be debate because not all patients can be surgical candidates.
LT is also effective therapeutic option for single (3–5 cm) HCC, but due to the limited number of available liver donors, it cannot be frequently applied to this tumor. RFA has been the best therapeutic option for HCC patients who are not suitable for SR or LT, and it has been usually indicated to small (≤3 cm) HCC with <3 tumors.[7,8] However, multicenter study recently conducted for small (<2 cm) HCC in Italy showed that a 5-year survival rate of RFA was comparable to that of SR.[9] The other Western study also reported that RFA can be safely and effectively applied for small (<5 cm) HCC as a first-line therapy.[10] But, these studies did not show comparative survival outcomes between SR and RFA in single (3–5 cm) HCC with BCLC stage A. As a noncurative treatment, transarterial chemoembolization (TACE) can be also applied to single (3–5 cm) HCC.[4,7] Although several studies compared RFA and SR, they were limited to single center and follow-up duration was short.[11–16] To date, furthermore, there has been no robust conclusion based on a large-scaled comparative study or randomized controlled trial (RCT) in the literature about therapeutic effectiveness among SR, RFA, and TACE for single (3–5 cm) HCC with BCLC stage A.
Therefore, we conducted a nationwide cancer registry-based cohort study to evaluate therapeutic effectiveness of RFA compared with SR and TACE in patients with single (3–5 cm) HCC with BCLC stage A using the database of the Korea Central Cancer Registry (KCCR) in South Korea. To achieve this, we compared posttreatment overall survivals (OSs) and progression-free survivals (PFSs) among patients who underwent SR, RFA, and TACE, respectively. Moreover, we sought to identify predictors of posttreatment survival in these patients.
2. Patients and methods
2.1. Database sources and study population
The Ministry of Health and Welfare, South Korea developed a nationwide cancer registry called the Korea Central Cancer Registry (KCCR) in 1980. The International Classification of Disease 10th edition (ICD-10) coding system was used to select HCC patients from the KCCR registry using code C22.0 which defines HCC. From January 2008 to December 2010, the National Cancer Center (NCC) and Korean Liver Cancer Study group (KLCSG) exhaustively investigated the KCCR database abstracted using the random sample audit method. Briefly, during the 2008 to 2010 study period, 38,167 HCC patients were registered as KCCR records at 47 hospitals. Of these patients, 4962 (13%) patient records, which included an additional 3% with considering sample error, were randomly abstracted to avoid selection bias. Finally, 4596 patients with clinically available data on HCC tumor status were registered in the population of this study.
Flowsheet of the study population is shown in Fig. 1. In 2008 to 2010 registry, HCC patients with BCLC stage 0, B, C, D, or undetermined BCLC stage (n = 2836), multiple (≥2) tumors (n = 298), a tumor size of ≤3 cm or >5 cm (n = 1099), and those treated by other than RFA, TACE, or SR (n = 26) were excluded. Eventually, 337 patients were enrolled in this retrospective cohort study, and 36, and 150, and 151 received RFA, TACE, and SR, respectively (Fig. 1).
Figure 1.
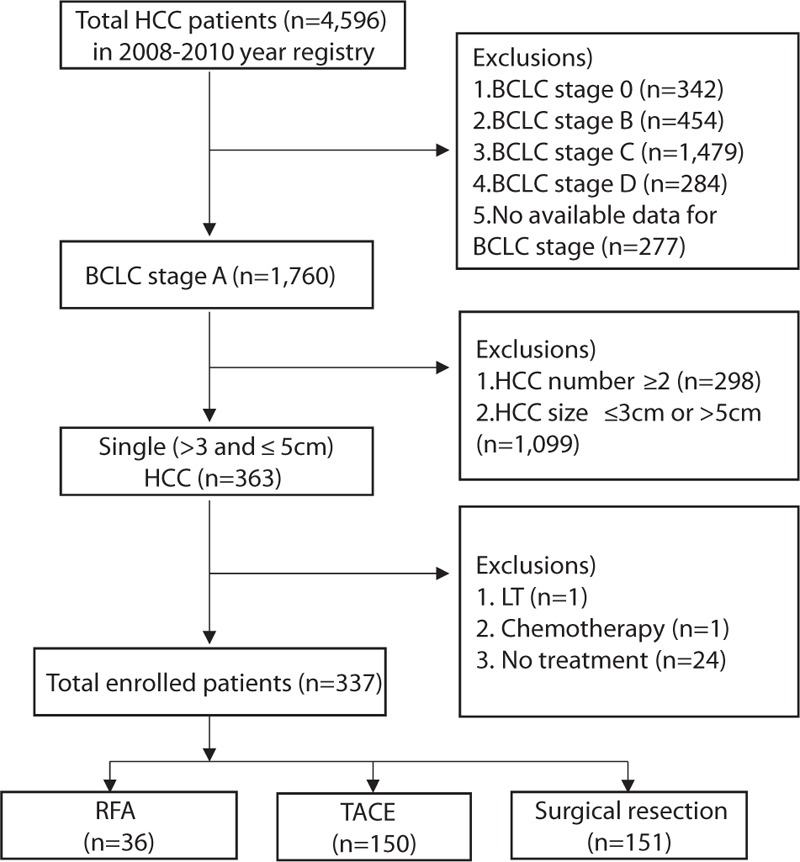
Study populations Of the 4596 patients in 2008 to 2010 registry, 337 patients were finally enrolled, and of these, 36 and 150 received RFA and TACE, respectively, and 151 underwent SR. RFA = radiofrequency ablation, SR = surgical resection, TACE = transarterial chemoembolization.
Mortality data for patients could be obtained from the Korean National Statistics Office (KNSO). Initial treatment dates and dates of disease progression were recruited from KCCR records. For OS and PFS analysis, follow-up durations were estimated from date of initial treatment to date of death and date of disease progression, respectively, or were calculated to December 31, 2013. The study was approved by the Institutional Review Board of Inha University Hospital, Incheon, South Korea (Approval number: INHAUH 2016-08-005).
2.2. Statistical analyses
The primary endpoint of this study was OS rate of patients with single (3–5 cm) HCC with BCLC stage A treated by RFA, SR, or TACE. The secondary endpoints were PFS rate of study subjects treated by RFA, SR, or TACE, and significant predictive factors of posttreatment survival.
Potential prognostic factors for OS or PFS were evaluated at the time of initial treatment for HCC. These factors included age, sex, HCC etiology, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, total bilirubin, prothrombin time (PT), serum sodium (Na), creatinine, Child-Turcotte-Pugh (CTP) class, model for end-stage liver disease (MELD) scores, MELD-sodium (MELD-Na) scores, tumor size and number, and alpha-fetoprotein (AFP).
The clinical characteristics of patients or HCCs were expressed as medians (ranges) or mean (standard deviation) for continuous variables, and numbers (percentages) for categorical variables. Differences between categorical or continuous variables were analyzed using the chi-square test (with post hoc Bonferroni) or the Kruskal–Wallis test (with post hoc Dunn's test). For multivariate analyses, adjust hazard ratios (HRs) and corresponding 95% confidence interval (CI) were estimated using Cox proportional hazards regression analysis to identify predictors of posttreatment OS or PFS. Kaplan–Meier analysis was used to estimate cumulative OS and PFS rates, and different groups were compared using the log-rank test.
Rigorous adjustment for differences in baseline characteristics of patients was performed using propensity score weighting of 3 types of treatments. The propensity score weighting were conducted with the R package Twang using its mnps function.[17–19] Briefly, propensity score is similar to the use of sampling weights in survey data analysis to account for unequal probabilities of inclusion in a study sample. Propensity scores and their associated weights were estimated with generalized boosted models, which utilize an automated, nonparametric machine learning technique that only requires the input of the pretreatment covariates one would like to balance among groups. Propensity score is similar to the use of sampling weights in survey data analysis to account for unequal probabilities of inclusion in a study sample. In addition, balance checking and survival analysis using propensity score weights were computed using weighted analyses. The clinical factors entered into the propensity score weighting were age, sex, albumin, total bilirubin, PT, CTP class, creatinine, Na, MELD score, MELD-Na, tumor size, AFP, and HCC etiology. Standardized differences were estimated for all baseline covariates before and after matching to assess pre-match imbalance and post-match balance. Standardized differences of <10.0% for a given covariate were defined as indicative of relatively small imbalance. For weighted data, P values are derived from Rao-Scott chi-square test, and ANOVA test.
To assess the impact of treatment type on OS or PFS after minimizing potential confounders, the comparative risks of OS or PFS rates were additionally adjusted for weighted data using the weighted Cox proportional hazards regression analysis. Statistical analysis was performed using the software package SAS 9.4 (SAS Institute, Inc, Cary, NC), R software version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) or SPSS v19.0 (IBM SPSS Inc., Chicago, IL). Two-sided P values of <0.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics
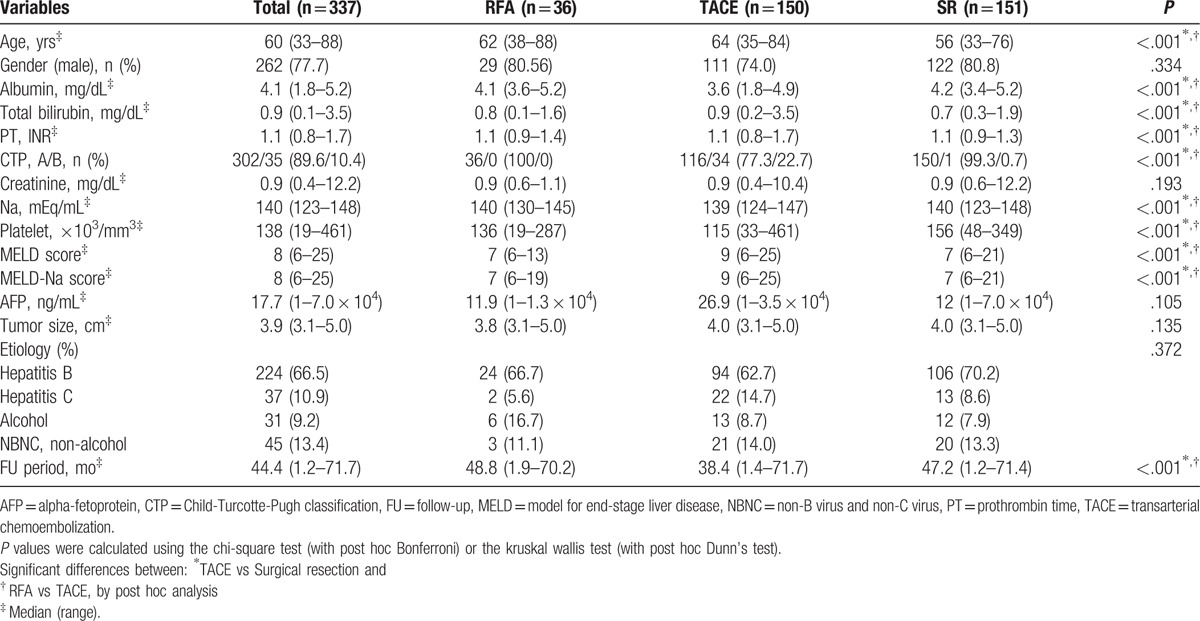
The baseline characteristics of patients are shown in Table 1. In total 337 patients, median age was 60 years (range, 33–88 years), median tumor size was 3.9 cm (range, 3.1–5.0 cm), and median follow-up duration was 44.4 months (range, 1.2–71.7 months). Between RFA and TACE treatment groups, albumin level (P < .001), frequency of CTP class A (P < .001), Na level (P < .001), platelet count (P < .001) were significantly greater for RFA treated patients, whereas age (P < .001), total bilirubin level (P < .001), PT (P < .001), MELD score (P < .001), and MELD-Na score (P < .001) were significantly higher for TACE treated patients. Between SR and TACE treatment groups, albumin level (P < .001), frequency of CTP class A (P < .001), Na level (P < .001), platelet count (P < .001) were significantly greater for surgery treated patients, whereas age (P < .001), total bilirubin level (P < .001), PT (P < .001), MELD score (P < .001), and MELD-Na score (P < .001) were significantly higher for TACE treated patients. Between RFA and SR treatment groups, significant difference could not be found (Table 1).
Table 1.
Baseline patient characteristics.
3.2. OS and PFS rates by treatment type
The 1-, 3-, and 5-year cumulative OSs of those that underwent RFA were significantly greater than those that received TACE (91.7%, 72.2%, and 53.3% vs 88.7%, 56.7%, and 36.7%, respectively, P = .044), but lower than those that received SR (vs 94.0%, 86.8%, and 79.0%, respectively, P = .021) (Fig. 2A). The 1-, 3-, and 5-year cumulative PFSs of those that underwent RFA were significantly greater than those that received TACE (75.0%, 72.2%, and 53.3% vs 40.0%, 21.3%, and 13.5%, respectively, P = .001), but lower than those that received SR (vs 80.1%, 60.9%, and 53.9%, respectively, P = .035) (Fig. 2B).
Figure 2.
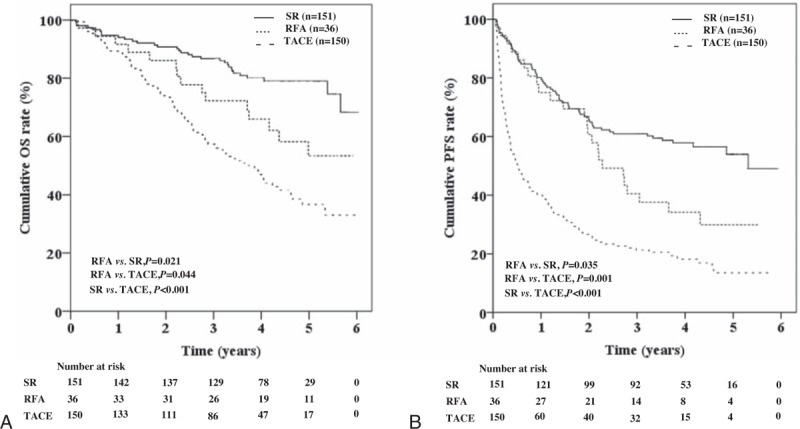
Cumulative overall survivals and progression free survivals by treatment type, The 1-, 3-, and 5-year cumulative OSs of patients that underwent RFA were significantly greater than those that received TACE (P = .044), but lower than those that received SR (P = .021) (A). The 1-, 3-, and 5-year cumulative PFSs of patients that received RFA were significantly greater than those that received TACE (P = .001), but lower than those that received SR (P = .035) (B). OS = overall survival, PFS = progression-free survival, RFA = radio frequency ablation, SR = surgical resection, TACE = transarterial chemoembolization.
3.3. Predictive factors of posttreatment OS and PFS
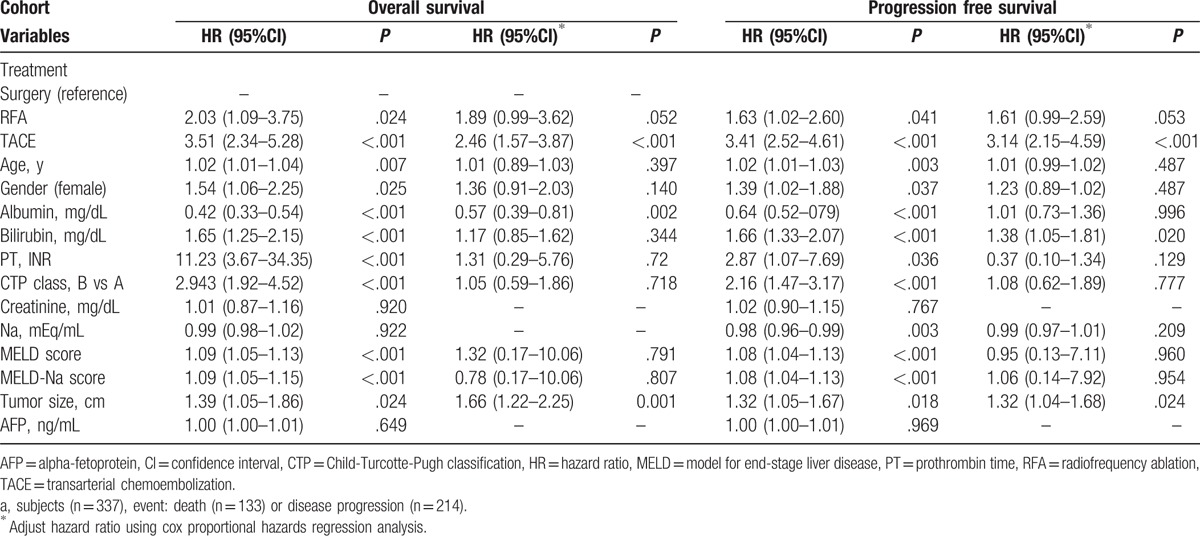
Multivariate analysis showed that TACE (hazard ratio [HR] 2.46, P < .001), serum albumin (HR 0.57, P = .002), and tumor size (HR 1.66, P = .001) were independent predictors for OS. TACE (HR 3.14, P < .001), serum bilirubin (HR 1.38, P = .020), and tumor size (HR 1.32, P = .024) were significant predictors for PFS (Table 2). RFA showed marginal significance in terms of OS (P = .052) and PFS (P = .053).
Table 2.
Significant predictive factors of posttreatment mortality.
3.4. OS and PFS rates by treatment type after propensity score weighting
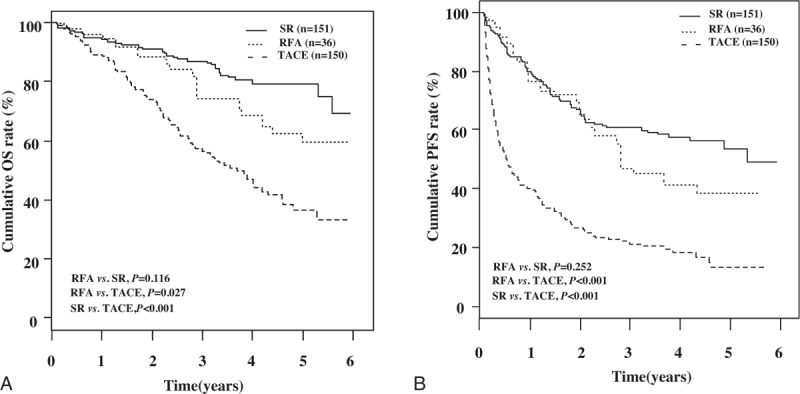
Patients’ characteristics after propensity score weighting for each treatment group are shown in Table 3. The cumulative OS rates were significantly higher in the SR (P < .001) and RFA (P = .027) group than in the TACE group, respectively (Fig. 3A). The cumulative OS rates were not statistically different between SR and RFA groups (P = .116) (Fig. 3A). In the weighted Cox proportional hazards regression analysis using weighted data, HR (95% CI) of posttreatment OS after TACE versus SR was 3.63 (2.40–5.51) (P < .001), and after RFA versus TACE was 0.49 (0.26–0.92) (P = .027). HR (95% CI) of posttreatment OS after RFA versus SR was 1.76 (0.87–3.57) (P = .116) (Table 4).
Table 3.
Comparison of patients’ characteristics after propensity score weighting.
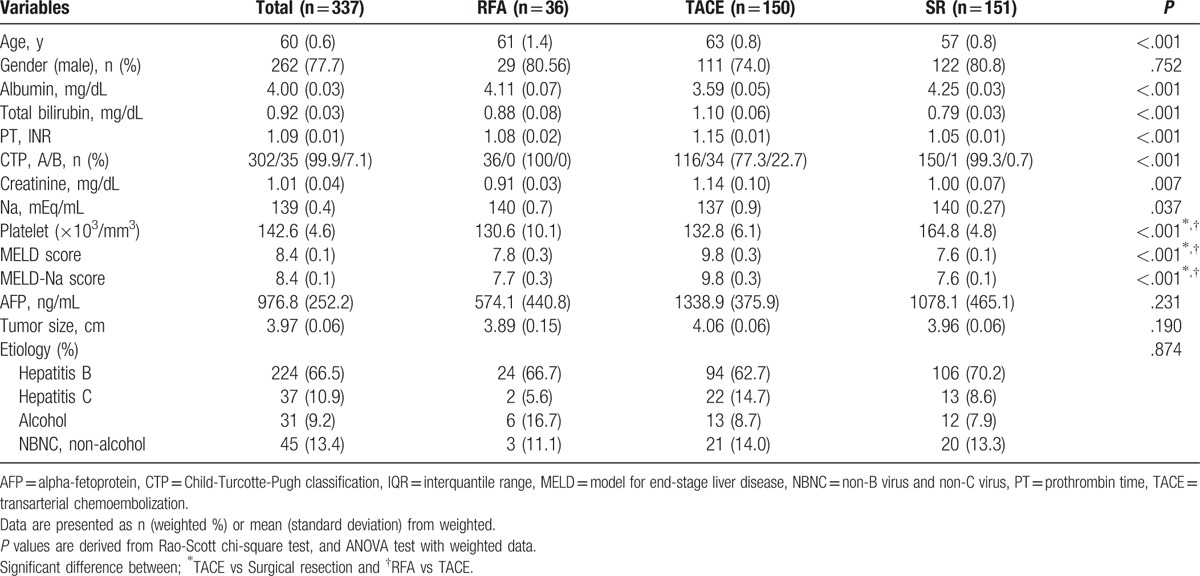
Figure 3.
Cumulative overall survivals and progression free survivals after propensity score weighting by treatment type The cumulative OS rates were significantly higher in the SR (P < .001) and RFA (P = .027) group than in the TACE group, respectively (A). The cumulative PFS rates were significantly higher in the SR (P < .001) and RFA (P < .001) group than in the TACE group, respectively (B). However, the cumulative OS and PFS rates of SR group showed no significant difference compared with those of RFA group (P = .116 and P = .252, respectively) (A and B). OS = overall survival, PFS = progression-free survival, RFA = radio frequency ablation, SR = surgical resection, TACE = transarterial chemoembolization.
Table 4.
OS and PFS by weighted Cox proportional hazards regression analysis.
The cumulative PFS rates were significantly higher in the SR (P < .001) and RFA (P < .001) group than in the TACE group, respectively, but there was no statistically different between SR and RFA group (P = .252) (Fig. 3B). In the weighted Cox proportional hazards regression analysis using weighted data, HR (95% CI) of posttreatment PFS after TACE versus SR was 3.54 (2.56–4.88) (P < .001), and after RFA versus TACE was 0.39 (0.23–0.64) (P < .001). HR (95% CI) of posttreatment OS after RFA versus SR was 1.36 (0.80–2.32) (P = .252) (Table 4).
3.5. Comparison of short-term (≤2 year) OS and PFS rates by treatment type
Short term (≤2 year) OSs and PFSs of patients were additionally analyzed, and the results were shown in Fig. 4. The cumulative OS (P = .409) and PFS rates (P = .647) were not significantly different between RFA and SR group (Fig. 4A and B, respectively). Even after propensity score weighting, the cumulative OS (P = .703) and PFS rates (P = .955) were also not significantly different between RFA and SR group (Fig. 4C and D, respectively).
Figure 4.
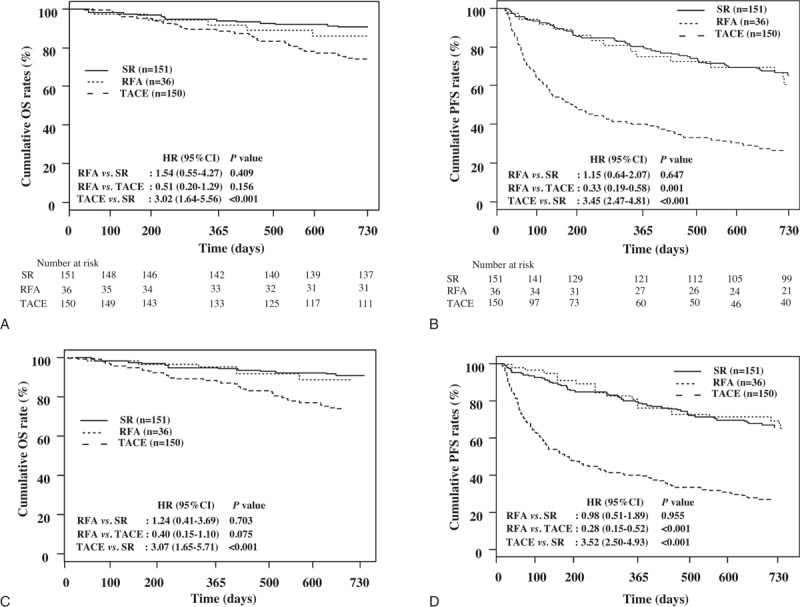
Comparison of short term (≤2 year) overall survivals and progression free survivals of patients by treatment type. The cumulative OS and PFS rates were not significantly different between RFA and SR group (A and B, respectively, P > .05), even after PS weighting (C and D, respectively, P > .05). The cumulative OS rates of RFA group were not significantly different compared with TACE group (A, P = .156), but after PS weighting, the cumulative OS rates tended to be higher in the RFA group than in the TACE group (C, P = .075). However, the cumulative PFS rates were significantly higher in the RFA group than in the TACE group (B, P = .001), even after PS weighting (D, P < .001). The cumulative OS rates and PFS rates were significantly higher in the SR group than in the TACE group, before (A and B, respectively, P-values for all <.001) and after PS weighting (C and D, P-values for all <.001). OS = overall survival, PFS = progression-free survival, RFA = radio frequency ablation, SR = surgical resection, TACE = transarterial chemoembolization.
The cumulative OS rates of RFA group were not significantly different compared with TACE group (Fig. 4A, P = .156), but after propensity score weighting, the cumulative OS rates tended to be higher in the RFA group than in the TACE group (Fig. 4C, P = .075). However, the cumulative PFS rates were significantly higher in the RFA group than in the TACE group (Fig. 4B, P = .001), even after propensity score weighting (Fig. 4D, P < .001). The cumulative OS rates and PFS rates were significantly higher in the SR group than in the TACE group, before (Fig. 4A and B, respectively, P values for all <.001) and after propensity score weighting (Fig. 4C and D, P values for all <.001).
4. Discussion
This comparative study showed that after propensity score weighting method, posttreatment OS and PFS were not statistically different between RFA and SR, respectively. Especially, posttreatment short term (≤2 year) OS and PFS of RFA were comparable to that of SR. This study is unique because this is the first large-scaled comparative study based on nationwide database to compare OS and PFS among RFA, TACE, and SR in patients with single (3–5 cm) HCC of BCLC stage A. In addition, efforts were made to minimize the effects of potential confounders associated with patients’ enrollment by using a random sample audit of the nationwide KCCR database, multivariate analysis, and propensity-score weighting method.
Based on the BCLC staging system, SR has been considered as treatment of choice for single (3–5 cm) HCC of BCLC stage A.[7] Recent retrospective studies reported that RFA was safely applied to HCCs >3 cm,[10,20–22] but these studies did not compare posttreatment patients’ survival between RFA and SR in these tumors, or enrolled multiple HCCs unlike to the present study. The other previous studies also compared RFA with SR in small (<5 cm) HCC with showing comparable outcomes between 2 treatments.[11–16] However, they were single center studies, and follow-up duration was short as <2–3 years. In addition, small tumors <3 cm[12–16] or multiple tumors[12,15] were also included. Of these, only 1 study reported that SR has better OS than RFA,[15] but RFA group had significantly more multiple tumors and showed worse liver function than SR group. Furthermore, subgroup analysis for single (>3 cm) HCC in the previous study[12] showed no significant difference in OS between 2 groups although the number of patients was small in each group. However, after propensity score weighting in the present study, OSs and PFSs were not statistically different between RFA and SR in single (3–5 cm) HCC with BCLC stage A. These results suggested that RFA may provide comparable survival benefits in patients with single (3–5 cm) HCC with BCLC stage A. However, due to retrospective study design and small number of RFA in the present study, large-scaled prospective RCTs are warranted to validate these outcomes.
In the previous study,[14] RFA is an effective first-line therapy for HCC up to 5 cm, and the OSs at 3-years after RFA treatment were 71.4%, which was similar to 72.2% of the present study. The other retrospective study[13] showed that OSs of RFA and SR were also similar. Moreover, the present study adjusted differences in clinical data of the 3 types of treatment using propensity score weighting. Especially, posttreatment early (<2 year) OSs and PFSs in the present study were similar between SR and RFA, regardless of propensity score weighting. In terms of predisposing factors for patient's survival, multivariate analysis in the present study showed that TACE was negative factor for OS and PFS after treatment. Based on these results, it is evident that RFA or SR has better survival benefits compared with TACE, suggesting that TACE should be not be recommended as first-line therapy for single (3–5 cm) HCC with BCLC stage A. Although the number of RFA was relatively small, RFA was not significantly associated with poor OS and PFS compared to SR in the present study. These suggest that it may be reasonable to recommend RFA in selected patients, such as those with poor liver function, medical comorbidities, or centrally located HCC as an alternative therapy for single (3–5 cm) HCC with BCLC stage A if SR or LT cannot be applied. However, the present study could not reveal the safety and additional treatment types after initial treatment due to the lack of these clinical data in the KCCR database.
This study has some limitations. First, inherent selection bias cannot be completely avoided in this study due to non-RCT. Second, we could not analyze survival outcomes of patients who underwent LT because only 1 patient received LT during the study period. Limited number of liver donor may affect the case of LT. Third, our study results may not be representative of the entire world because this study was conducted in single country, South Korea. Therefore, multinational prospective RCTs are warranted to raise the level of evidence of the study results. Fourth, unfortunately, we could not analyze survival outcomes of patients who were treated with nexavar. This may be resulted by medical insurance system of our country, which did not allow the use of nexavar in this tumor during that time.
In conclusion, this nationwide cancer registry-based cohort study shows that RFA has better OS and PFS rates than TACE, respectively, but similar OS and PFS rates compared with SR, respectively, for single 3 to 5 cm sized HCC with BCLC stage A. These findings suggest that RFA can be preferentially recommended compared with TACE for non-surgical candidates. Moreover, we expect that our findings will provide clinically useful information for treatment decision-making for single (3–5 cm) HCC. However, well-designed prospective RCTs need to be performed to validate our results in the future.
Acknowledgments
The authors thank Korea Central Cancer Registry (KCCR) and the Korean Liver Cancer Study Group (KLCSG). The statistical consultation was supported by Department of Biostatistics of the Catholic Research Coordinating Center, Catholic Medical Center, South Korea.
Footnotes
Abbreviations: AFP = alpha-fetoprotein (AFP), ALT = alanine aminotransferase, AST = aspartate aminotransferase, BCLC = Barcelona Clinic Liver Cancer, CT = confidence interval, CTP = Child-Turcotte-Pugh, HCC = hepatocellular carcinoma, HR = hazard ratio, KCCR = Korea Central Cancer Registry, KLCSG = Korean Liver Cancer Study group, KNSO = Korea National Statistics Office, LT = liver transplantation, MELD = model for end-stage liver disease, MELD-Na = MELD-sodium, Na = sodium, NCC = National Cancer Center, OS = overall survival, PFS = progression-free survival, PT = prothrombin time, RCT = randomized controlled trial, RFA = radiofrequency ablation, SR = surgical resection, TACE = transarterial chemoembolization.
Author Contributions: SHL and YJJ were responsible for the concept and design of the study, the acquisition, analysis, and interpretation of the data, and drafting of the manuscript. JWL helped with interpretation of data.
This work was supported by WCSL (World Class Smart Lab) research grant directed by Inha University. Data were provided by the Korean Central Cancer Registry, Ministry of Health and Welfare, South Korea, and the Korean Liver Cancer Study Group (KLCSG).
All authors have no conflict of interest to declare in connection with this study.
References
- [1].Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001;2:533–43. [DOI] [PubMed] [Google Scholar]
- [2].Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127:S5–16. [DOI] [PubMed] [Google Scholar]
- [3].El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol 2002;35:S72–8. [DOI] [PubMed] [Google Scholar]
- [4].Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421–30. [DOI] [PubMed] [Google Scholar]
- [5].European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- [6].Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014;63:844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kudo M. Local ablation therapy for hepatocellular carcinoma: current status and future perspectives. J Gastroenterol 2004;39:205–14. [DOI] [PubMed] [Google Scholar]
- [9].Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47:82–9. [DOI] [PubMed] [Google Scholar]
- [10].N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475–83. [DOI] [PubMed] [Google Scholar]
- [11].Lupo L, Panzera P, Giannelli G, et al. Single hepatocellular carcinoma ranging from 3 to 5cm: radiofrequency ablation or resection? HPB (Oxford) 2007;9:429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cho CM, Tak WY, Kweon YO, et al. [The comparative results of radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma]. Korean J Hepatol 2005;11:59–71. [PubMed] [Google Scholar]
- [13].Hong SN, Lee SY, Choi MS, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol 2005;39:247–52. [DOI] [PubMed] [Google Scholar]
- [14].Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vivarelli M, Guglielmi A, Ruzzenente A, et al. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg 2004;240:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Montorsi M, Santambrogio R, Bianchi P, et al. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg 2005;9:62–8. discussion 67–68. [DOI] [PubMed] [Google Scholar]
- [17].Daniel F. McCaffrey LFB, Beth Ann Griffin, Craig Martin Propensity Scores for Multiple Treatments, A Tutorial for the MNPS Macro in the TWANG SAS Macros; 2015. [Google Scholar]
- [18].Matthew Cefalu SL, Craig Martin, Toolkit for Weighting and Analysis of Nonequivalent Groups: A Tutorial on the TWANG Commands for Stata; 2015. [Google Scholar]
- [19].Lane Burgette BAGnaDM, RAND Corporation. Propensity scores for multiple treatments: A tutorial for the mnps function in the twang package; 2016. [Google Scholar]
- [20].Yin XY, Xie XY, Lu MD, et al. [Ultrasound-guided percutaneous composite thermal ablation technique in treatment of medium and large hepatocellular carcinoma]. Zhonghua Wai Ke Za Zhi 2004;42:1029–32. [PubMed] [Google Scholar]
- [21].Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201–9. [DOI] [PubMed] [Google Scholar]
- [22].Tateishi R, Shiina S, Ohki T, et al. Treatment strategy for hepatocellular carcinoma: expanding the indications for radiofrequency ablation. J Gastroenterol 2009;44(Suppl):142–6. [DOI] [PubMed] [Google Scholar]


