Abstract
Lymphatic vessel invasion (LVI) is promising in determining prognosis and treatment strategies, but the application of LVI as a histopathological criterion in breast cancer patients especially those of different subgroups is controversial. This research aims to evaluate the prognostic value of LVI assessed by D2-40 not only in patients with early invasive breast cancer but also in lymph node-negative, lymph node-positive, luminal A-like, luminal B-like, HER2-enriched, and triple-negative subgroups.
The study cohort included 255 patients with a median follow-up of 101 months. Immunohistochemical staining for D2-40 was performed to identify LVI.
LVI was present in 64 (25.1%), 15 (12.1%), 49 (37.4%), 19 (20.9%), 23 (27.7%), 13 (31.7%), and 9 (22.5%), respectively, in the whole cohort, lymph node-negative, lymph node-positive, luminal A-like, luminal B-like, HER2-enriched, and triple-negative patients. LVI was associated with large tumor size (P = .04), high histological grade (P = .004), involved lymph node (P < .001), and high expression of Ki-67 (P = .003). No significant difference was found among patients with different subtypes and LVI status. The presence of LVI was significantly associated with adverse disease-free survival in the whole cohort (P < .001), lymph node-negative (P < .001), lymph node-positive (P < .001), luminal A-like (P < .001), and luminal B-like patients (P < .001) in both of the univariate and multivariate survival analysis.
This study indicated that the presence of LVI stained by D2-40 provided independent prognostic information not only in the whole cohort but also in the subgroup of patients with lymph node-negative, lymph node-positive, luminal A-like, and luminal B-like diseases, which may make a case for routine clinical assessment of LVI using D2-40.
Keywords: breast carcinoma, D2-40, lymphatic vessel invasion, prognostic, recurrence
1. Introduction
Breast cancer is a common malignant disease in women and one of the main causes of cancer death in the female. In Chinese population, >268,000 women were diagnosed with breast cancer and about 70,000 cases died from it in 2015, accounting for 15% of all female new cancers and 7% of all female deaths due to cancers.[1] However, due to detection and systemic adjuvant therapy, the survival rate has improved over the last decade.
Prediction of breast cancer prognosis based on specific markers can provide useful information to guide early therapeutic decisions. Predict factors including tumor size, lymph node status, histological type and nuclear grade have been established as conventional clinical factors; estrogen receptor (ER), progesterone receptor (PR), and HER2 status are recognized as molecular biological factors.[2] Among these, lymph node metastasis, which initially occurs by migration of carcinoma cells into the lymphatic vessels at the primary site, is one of the most important prognostic factors for breast cancer. The presence of lymphovascular invasion in a primary tumor has been used as an indication for the ability of this tumor to metastasis outside the breast and was recognized as one of the factors that should determine a treatment plan of breast cancer according to the 2005 St. Gallen consensus meeting.[3] The term “lymphovascular invasion” refers to invasion of either blood vessels or lymphatic vessels.[4] Because the invasion of lymphatic vessels was found to be the major type of lymphovascular invasion in breast cancer, the present study assessed only the invasion of lymphatic vessels used lymphatic vessel specific marker (D2-40) and referred it as “lymphatic vessel invasion” (LVI).
Since the prognostic value of LVI in breast cancer was first reported in 1964,[5] numerous studies had confirmed the importance of LVI as a prognostic factor, but the application of LVI as a histopathological criterion remained controversial.[6–11] According to the expression status of ER, PR, HER2, and Ki-67, breast cancer can be categorized as 4 molecular subtypes: luminal A-like, luminal B-like, HER2-enriched, and triple-negative.[12] In a review of previous studies, we found that those studies often examined a combination of lymph node negative and positive breast cancers and patients of all subtypes (luminal A-like, luminal B-like, HER2-enriched, and triple-negative). Undoubtedly, these patients were heterogeneous both in behavior and therapy accepted. Therefore, the prognostic values of LVI in these subgroups are as yet uncertain. Additionally, the presence or absence of axillary lymph node involvement, which is defined as lymph node-positive or lymph node-positive negative, is associated with significantly different prognosis for breast cancer. Lymph node-negative breast cancer has a relatively good prognosis (10–20% mortality) and the improvement of survival with adjuvant chemotherapy in these patients is less than in lymph node-positive cases. Therefore, reliable prognostic markers are important in deciding whether to use adjuvant systemic therapy or not. In comparison, we assessed LVI not only in the whole cohort but also in each subgroup: lymph node-negative, lymph node-positive, luminal A-like, luminal B-like, HER2-enriched, and triple-negative patients. Additionally, the majority of former studies used hematoxylin and eosin (H&E) stain, by which blood vessel invasion could not be distinguished. D2-40 is a novel monoclonal antibody that reacts with a fixation-resistant epitope, which is a glycosylated or non-glycosylated epitope of gp36, on the lymphatic endothelium but did not react with the endothelium of capillaries, arteries, and veins in normal and neoplastic tissues on formalin-fixed paraffin-embedded tissues.[13] Its usefulness for detecting intratumoral lymph vessels has been reported in various carcinomas, including breast.[13–16] The sensitivity and specificity of using D2-40 as a method in detecting lymphatic invasion in breast cancer as well as other cancer types are 97.3% and 98.8%, respectively.[17] Since then, several studies have concluded that relying on D2-40 to detect LVI was a much responsible approach in predicting outcomes in patients with breast cancer.[2,18–20]
In this study, we examine the prognostic value of LVI using D2-40 stain in Chinese patients with early, and in particular lymph node-negative, lymph node-positive, luminal A-like, luminal B-like, HER2- enriched, and triple-negative invasion breast cancer.
2. Materials and methods
2.1. Patients
Primary tumors from patients who underwent surgery between the years 2005 to 2008 at Shandong Cancer Hospital Affiliated to Shandong University were formalin-fixed and paraffin-embedded for this study (n = 255). To limit the potential confounding effects of other tumor types on the analysis, only invasive ductal carcinomas of the breast were included in the present study. Patients that received no surgical treatment, diagnosed with invasive ductal carcinomas in situ or metastasis, and lack insufficient follow-up data, pathology slides, and tissue blocks were excluded. Age, tumor size, lymph node status, histological type, and grade were retrieved from routine reports. The median age of patients at time of diagnosis was 48 years (range, 26–72 years). Patients were treated with radiation, hormones, or chemotherapy according to their pathological reports. Disease-free survival (DFS) was defined as the period from the date of primary surgery until the date of the first recurrence of breast cancer. Written informed consent was obtained from each patient, and the protocol was approved by the ethics committee of Shandong Cancer Hospital Affiliated to Shandong University.
2.2. Immunohistochemistry
A single representative block from each of the 255 specimens was stained with D2-40 (Princeton, New Jersey, Covance, Monoclonal Antibody, SIG-3730) diluted 1:100. Tissue sections (4-μm thick) were dewaxed in xylene and rehydrated in a sequence of descending concentrations of ethanol. Block endogenous hydrogen peroxidase activity in 3% H2O2 for 15 minutes and block nonspecific binding by incubation in 10% horse serum for 30 minutes. Subsequently, sections were incubated with the primary antibody at room temperature for an hour. Detect sites of binding with 3, 30 diaminobenzidine (Vector, code SK 4001, Burlingame, CA) as the chromogenic substrate by the Envision technique (Dako, code K5007), according to the manufacturer's instruction. After counter-stained with hematoxylin, the sections were dehydrated and mounted with DPX.
ER and PR status was defined with the cutoff value of 1% positive tumor cells.[21] HER2-positive was defined as scored 3+ by immunohistochemistry (IHC); for scored 2+, FISH was performed to determine HER2 positivity; and 0 and 1+ are regarded as negative.[22] Ki-67 is frequently measured both as a static marker of proliferative activity and as a possible dynamic intermediate or surrogate marker of treatment efficacy.[23] Ki-67-positive tumor cells were identified by the method described by Bukholm et al.[24] In brief, a total of 10 fields of cell nuclei Ki-67 stained cells were randomly chosen and 500 cells were counted under each field. Then, we calculated the percentages of Ki-67 positive cells. Ki-67 ≤14% was defined as low expression and Ki-67 >14% as high expression.[25,26] LVI was identified by tumor cells within D2-40 positively stained vessels.[18] The cases were categorized as LVI-positive or LVI-negative. Typical histologic pictures of LVI-positive and LVI-negative by D2-40 staining are shown in Figure 1A and B, respectively. The molecular subtypes of breast cancer were categorized as follows: luminal A-like (ER and PR positive and HER2 negative and low Ki-67), luminal B-like (ER and/or PR positive and at least one of the following: HER2 negative and high Ki-67; HER2 positive), HER2-enriched (ER and PR negative, HER2 positive), and triple-negative (ER, PR and HER2 negative)).[12] Interpretation of IHC results was made by 2 investigators without knowledge of clinical characteristics and the status of other prognostic variables.
Figure 1.
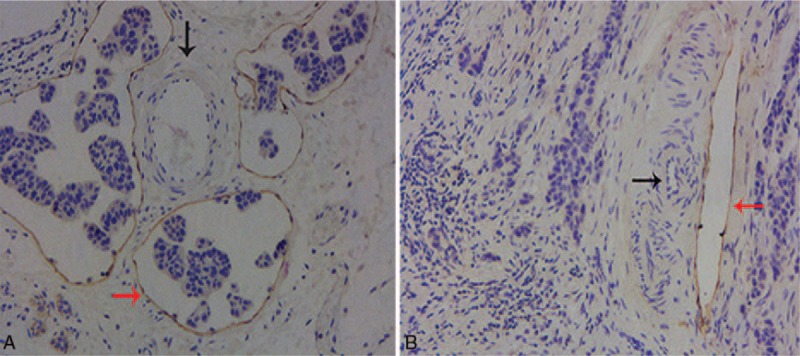
(A) LVI-positive by D2-40 staining. Positive staining of lymphatic endothelium with D2-40 shows the presence of tumor emboli in the lumen of the lymph vessels (red arrow). The endothelia of the adjacent blood vessels are negative for D2-40 (black arrow) (×100). (B) LVI-negative by D2-40 staining. No tumor emboli are noted within the lumen of the lymph vessels positive stained by D2-40 (red arrow). The endothelia of the adjacent blood vessels are negative for D2-40 (black arrow) (×100). LVI = lymphatic vessel invasion.
2.3. Statistical analysis
Statistical analysis was performed using SPSS statistics 19.0. Chi-square test was used to analyze the significance of relationships between the status of LVI and variables in the whole cohort (Table 1) and the subgroups including lymph node-negative, lymph node-positive, luminal A-like, luminal B-like, HER2-enriched, and triple-negative disease (Table 2). Survival curves for patients were calculated by the Kaplan-Meier method and analyzed by the log-rank test. Univariate and multivariate survival analysis were performed in the whole cohort and subgroups (Table 3) using Kaplan-Meier method and Cox proportional hazards model with a stepwise backward elimination to derive a final model of variables with a significant independent relationship with DFS. For each variable, the hazard ratio (HR) and the 95% confidence interval (CI) were calculated. All statistical analyses were 2-sided with significance defined as P < .05.
Table 1.
The inter-relationship between clinic-pathological characteristics and LVI in patients with invasive ductal breast cancer.
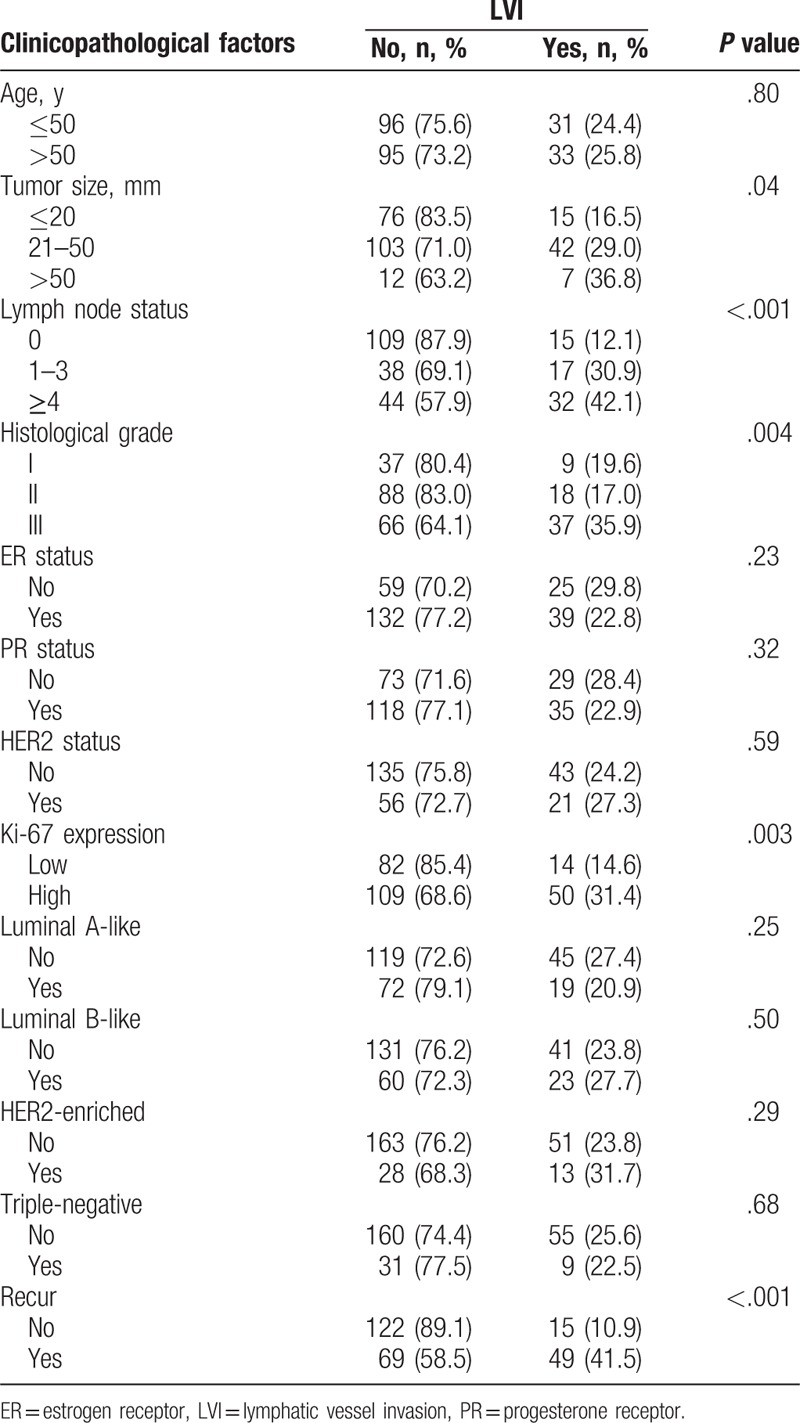
Table 2.
The inter-relationship between clinicopathological characteristics and LVI in patients with lymph node-negative, lymph node-positive, luminal A-like, luminal B-like, HER2-enriched, and triple-negative disease.
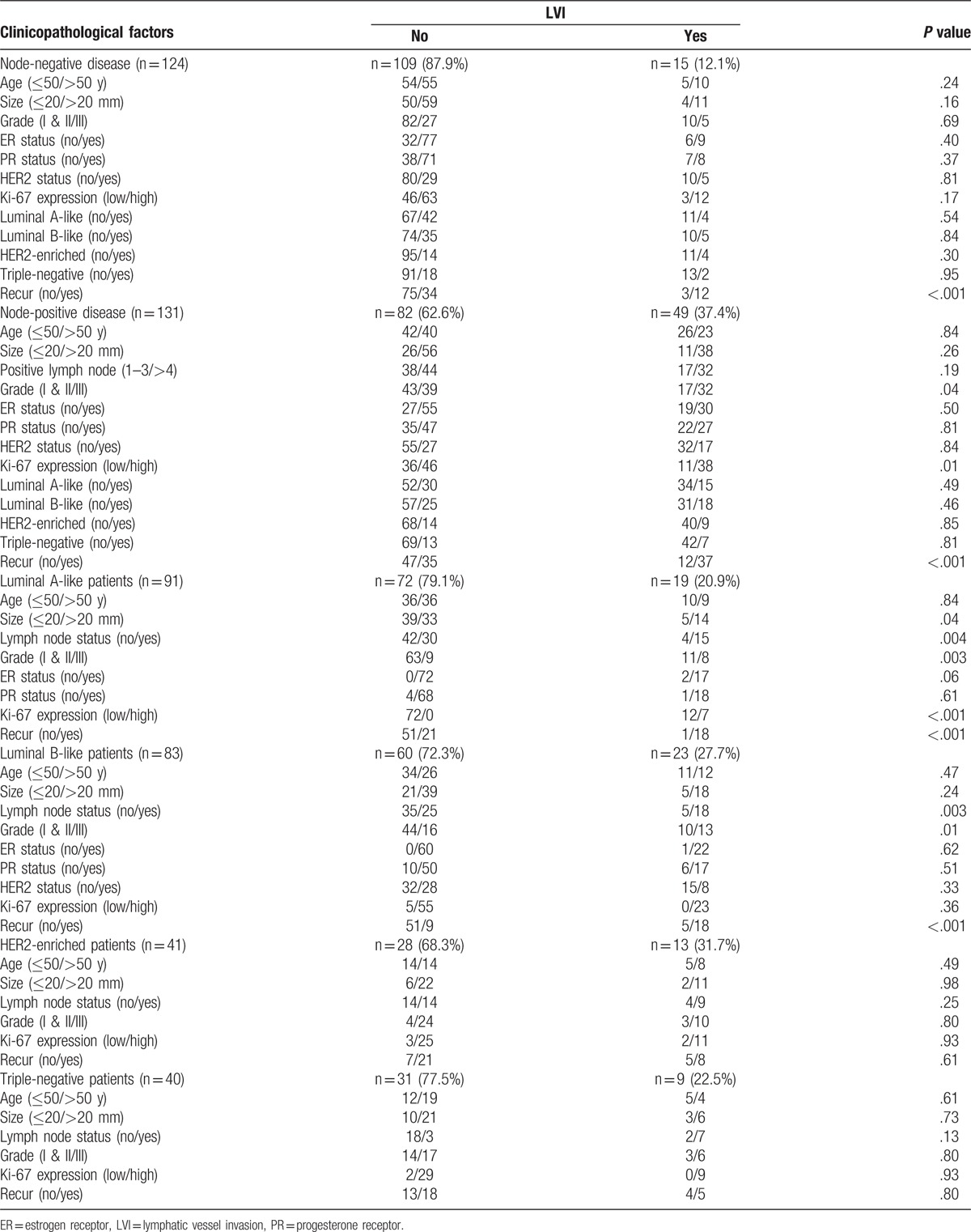
Table 3.
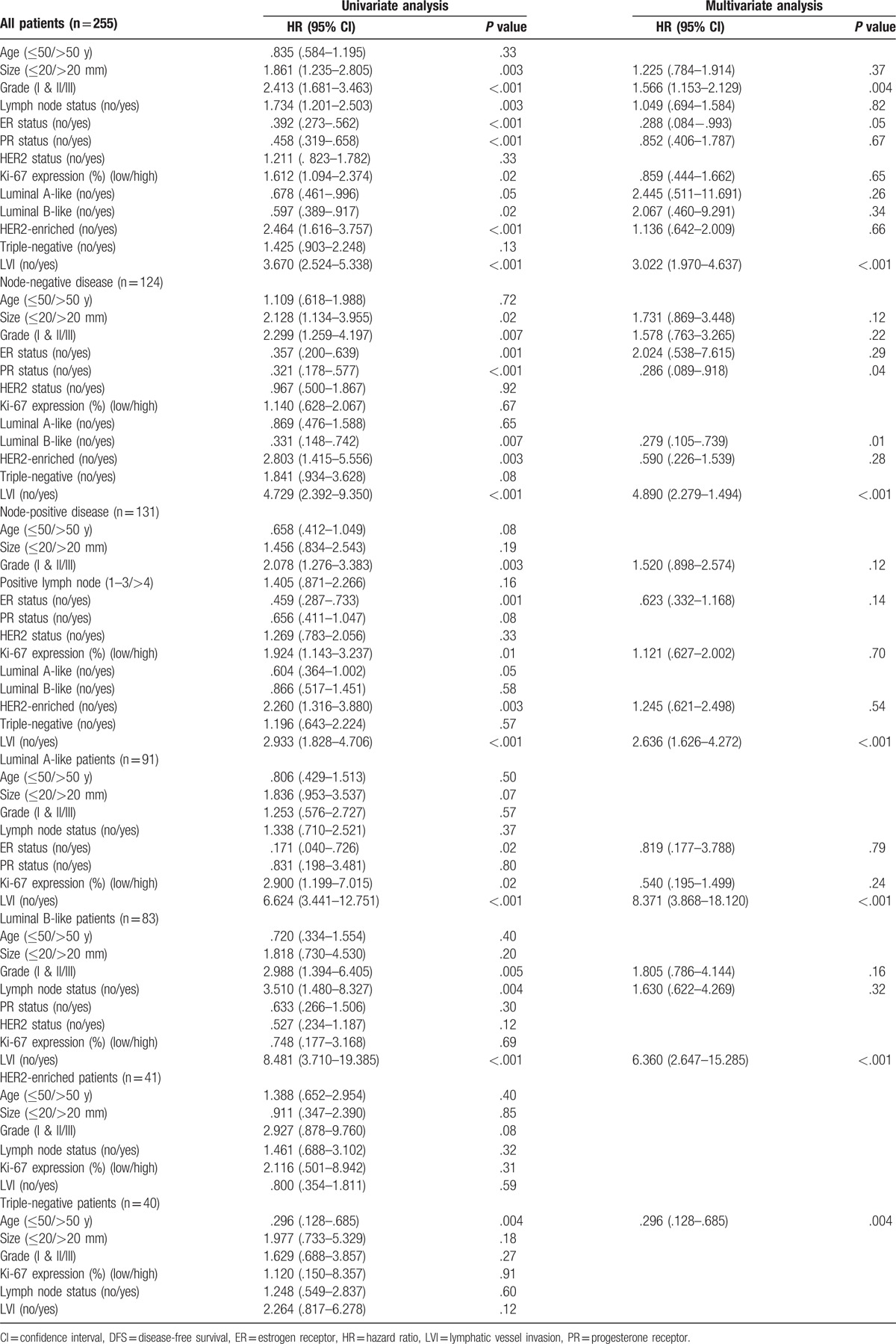
Correlation between DFS and clinicopathological variables in patients with primary operable invasive ductal breast cancer.
3. Results
3.1. Correlation between LVI and clinicopathological factors in the whole cohort, in lymph node-negative patients, in lymph node-positive patients and in breast cancer subtypes patients
As shown in Table 1, LVI was detected in 25.1% of the whole cohort. LVI was associated with large tumor size (P = .04), high histological grade (P = .004), involved lymph node (P < .001), high expression of Ki-67 (P = .003), and tumor recurrence (P < .001). No association was seen with patient age, ER status, PR status, HER2 status, and breast cancer subtypes. The proportion of LVI was highest in the HER2-enriched group (31.7%) and was lowest in the luminal A-like group (20.9%).
Table 2 shows that only tumor recurrence (P < .001) was significantly associated with LVI in lymph node-negative patients. In lymph node-positive patients, LVI was significantly more frequent in patients in with high tumor grade (P = .04), high Ki-67 expression (P = .01) and tumor recurrence (P < .001). In luminal A-like patients, the presence of LVI was associated with large tumor size (P = .04), involved lymph node (P = .004), high tumor grade (P = .003), high Ki-67 expression (P < .001), and tumor recurrence (P < .001). In luminal B-like patients, LVI was associated with high histological grade (P = .003), involved lymph node (P = .01), and tumor recurrence (P < .001). No parameter was found significantly associated with LVI in neither HER2-enriched nor triple-negative subtypes.
3.2. Survival analysis of LVI in the whole cohort, in lymph node-negative patients, in lymph node-positive patients, and in breast cancer subtypes patients
The mean follow-up period was 97 ± 28.219 months. The presence of LVI was analyzed with DFS data using the Kaplan-Meier analysis and Cox regression. Kaplan-Meier curves showed a significantly higher risk of recurrence in the whole cohort (Fig. 2), lymph node-negative cases (Fig. 3A), lymph node-positive cases (Fig. 3B), luminal A-like cases (Fig. 3C), and luminal B-like cases (Fig. 3D) (all P < .001). By contrast, no correlation was found in HER2-enriched patients (P = .59) (Fig. 3E) and triple-negative patients (P = .11) (Fig. 3F).
Figure 2.
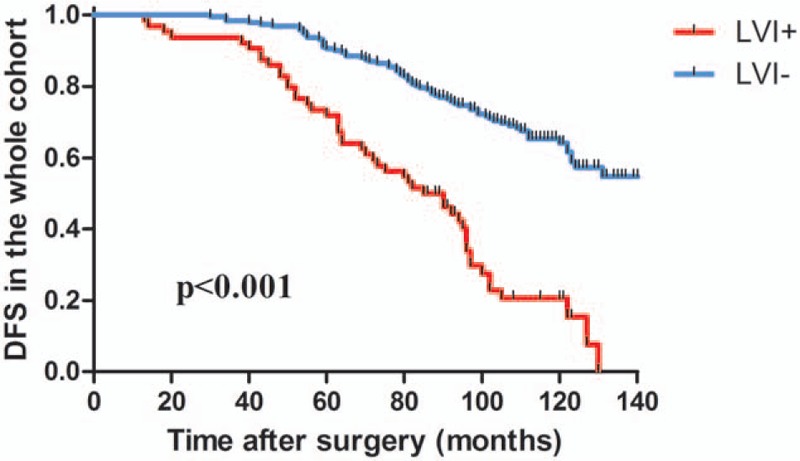
Kaplan-Meier survival analysis of DFS depending on LVI status in the whole cohort. LVI+ status exhibited significantly worse DFS compared with LVI− in the whole cohort (P < .001, log-rank test). DFS = disease-free survival, LVI = lymphatic vessel invasion.
Figure 3.
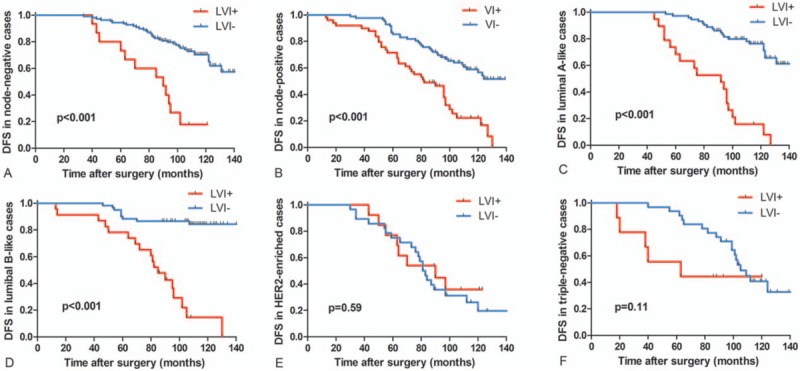
Kaplan-Meier survival analysis of DFS depending on LVI status in lymph node-negative, lymph node-positive, and breast cancer subtypes patients. LVI+ status exhibited significantly worse DFS compared with LVI− in lymph node-negative cases (A), lymph node-positive cases (B), luminal A-like cases (C), and luminal B-like cases (D) (all P < .001, log-rank test). LVI+ status exhibited no significantly worse DFS compared with LVI− in HER2-enriched cases (P = .589, log-rank test) (E) and triple-negative cases (P = .106, log-rank test) (F). DFS = disease-free survival, LVI = lymphatic vessel invasion.
Univariate analysis indicated that the present of LVI was significantly associated with DFS in the whole cohort, lymph node-negative, lymph node-positive, luminal A-like, and luminal B-like patients (all P < .001). Additionally, tumor size (P = .003), histological grade (P < .001), lymph node status (P = .003), ER status (P < .001), PR status (P < .001), Ki-67 expression (P = .02), luminal A-like (P = .05), luminal B-like (P = .02), and HER2-enriched (P < .001) were significantly associated with DFS in the whole cohort; tumor size (P = .02), histological grade (P = .007), ER status (P = .001), PR status (P < .001), luminal B-like (P = .007), HER2-enriched (P = .003), and LVI (P < .001) were significantly associated with DFS in lymph node-negative patients; histological grade (P = .003), ER status (P = .001), Ki-67 expression (P = .01), HER2-enriched (P = .003), and LVI (P < .001) were significantly associated with DFS in lymph node-positive patients; ER status (P = .02), Ki-67 expression (P = .02), and LVI (P < .001) were significantly associated with DFS in luminal A-like cases; histological grade (P = .005), lymph node status (P = .004), and LVI (P < .001) were significantly associated with DFS in luminal B-like cases; no variables were significantly associated with DFS in HER2-enriched disease; only patient age (P = .004) was significantly associated with DFS in triple-negative disease (Table 3).
In multivariate analysis for the whole cohort, histological grade (HR = 1.57; P = .004), ER status (HR = .29; P = .05) and LVI (HR = 3.02; P < .001) remained independently associated with DFS. In multivariate survival analysis for lymph node-negative patients, PR status (HR = .29; P = .04), luminal B-like subtype (HR = .28; P = .01), and LVI (HR = 4.89; P < .001) remained independent predictors of shorter DFS. In lymph node-positive, luminal A-like, and luminal B-like cases, only LVI was significantly related to a poorer outcome on multivariate analysis (all P < .001). No parameters were found significantly associated with DFS in HER2-enriched and triple-negative subtypes in multivariate analysis (Table 3).
4. Discussion
As a result of early detection and systemic adjuvant therapy, recurrence and distant metastasis, rather than primary tumors, are becoming the leading causes of breast cancer death.[27] It is well known that LVI of regional lymph nodes or distant sites occurs early in tumor metastasis, and the presence of LVI in earlier cancer has been used as an indicator for its ability to metastasize out of the breast.[28] Such tumors, therefore, receive more intense therapy than tumors with no LVI in the same disease stage.[29–31]
In the present study, the proportion of patients with LVI (25.1%) was consistent with most previous studies using a similar approach (21–42%),[32] but lower than that of former studies (12.1%) compared with (15–28%) in lymph node-negative patients and (22.5%) compared with (26–41%) in triple-negative cases.[32,33] Breast cancer is a heterogeneous disease, encompassing a number of distinct biological characters. The prognosis and treatment strategy vary among different subtypes (luminal A-like, luminal B-like, HER2-enriched, and triple-negative). However, few studies have investigated the prognostic value of LVI in different subtypes. Therefore, we conducted analysis not only in the whole cohort but also in each subgroup.
To standardize the use of LVI in patient management, the method of detection of LVI is the primary issue needed to be addressed. As mentioned earlier, LVI was detected in the past using H&E stain in samples of breast cancer patients in which blood vessel invasion could not be discerned. With advances in IHC technique, new markers such as D2-40 have been discovered. The straightforward staining technique needed for D2-40 and the excellent staining performance make it a robust marker for the detection of LVI lesions. Numerous studies have concluded that LVI detected by D2-40 was a more reliable approach in predicting outcomes in cases with breast cancer.[2,18–20] In a pilot study of 50 breast cancers, D2-40 increased the detection of LVI by 16% in lymph node-positive cases and 20% in lymph node-negative cases compared with that examined by H&E.[34] In the present study, we assessed LVI by IHC using D2-40, which could increase the accuracy of LVI detection relative to examined by H&E.[34]
The associations between LVI and other well established prognostic factors varied among different studies.[13,18–20,28,31,32,35–38] Similar to previous studies, tumor size, lymph node status, histological grade, and Ki-67 expression in our study showed a significant correlation with LVI in the whole cohort.[2,18–20,32] In terms of lymph node-negative patients, however, no parameter was significantly associated with LVI, whereas most former studies reported that LVI was independent of tumor size in lymph node-negative cases.[10,33,35,37,39,40] This inconsistency could be explained by variations in sample sizes, types of the clone of antibody, positive cells interpretations, and statistical analysis.
In line with the majority of studies reported,[2,14,33,35,37,41] a significant correlation between LVI and tumor recurrence was observed in our study. In multivariate analyses, a significant increase in the HR for tumor recurrence was observed in higher histological grade, ER-negative and the presence of LVI in early breast cancer patients. The results were similar with other studies that included both lymph node-negative and lymph node-positive disease. Furthermore, the presence of LVI provided independent prognostic information not only in the whole cohort but also in the subgroup of patients with lymph node-negative, lymph node-positive, luminal A-like, and luminal B-like disease in the present study.
In theory, LVI may be predictive of lymph node metastasis. Indeed, the presence of LVI has been correlated with presence of lymph node involvement, local recurrence, and poor survival in breast cancer, and 20% of patients with node-negative breast cancer will experience a recurrence and die of systemic disease.[32,37,42,43] There may be some kind of lymphovascular shunt in the primary tumor through which tumor cells can directly pass from the lymphatic circulation to the blood circulation,[44] which appears plausible to explain how tumor cells access to the blood circulation can be achieved without lymph node involvement. Therefore, identification of LVI, especially using D2-40, could objectively identify a higher-risk subgroup of node-negative patients who might benefit from adjuvant chemotherapy. The results from our study indicated that LVI is an independent poor prognostic factor for the development of recurrence in lymph node-negative breast cancer, which are consistent with previous studies that assessed LVI objectively using D2-40.[14,36,37,41] A large recent study on 1005 patients found that through the use of D2-40, identification of the presence of any LVI in the primary tumor, even single small lesions, is a powerful independent adverse prognostic factor in patients with lymph node-negative breast cancer.[37] But the results differ from those of the Ejlertsen et al[38] study, which identified LVI by conventional histological assessment in 16,172 breast cancer patients. They found that the presence of LVI should not be considered sufficient to reclassify breast cancer patients who are at a low risk (older than 35 years, with lymph node-negative disease, tumor size ≤2 cm, and positive hormonal status) of recurrence into a high-risk category. Another recent substantive study by Gudlaugsson et al[35] conducted on 240 lymph node-negative invasive breast cancer patients found that the presence of LVI, identified by D2-40/p63 (which stains myoepithelial cell nuclei), has strong prognostic value only in patients ≥55 years old. The reasonable explanation for such discrepancy are that the use of different methods in LVI detection and relatively short follow-up period in the aforementioned studies.
The data concerning the prognostic value of LVI in lymph node-positive disease are still limited and controversial in previous studies.[45,46] Some recent studies have found a significant prognostic impact for LVI in lymph node-positive disease; however, the methods for routine assessment of LVI and standardization of its use in management still need further assessment.[31,45,47] In the present study, the presence of LVI in lymph node-positive disease was significantly associated with poorer DFS using both univariate and multivariate analysis. In a study that examined LVI by D2-40 staining in 557 patients with lymph node-positive breast cancer, it was found to be an independent poor prognostic factor in lymph node-positive breast cancer and associated with increased number of positive lymph nodes.[31] However, the study by Ragage and colleagues[45] showed that the presence of lymphovascular invasion stained by H&E was not associated with the number of involved lymph nodes, which was consistent with the result in our study.
Luminal A-like patients, who comprise the majority of women diagnosed with breast cancer, are at lower risk relative to those with the HER2-enriched and triple-negative disease. However, not all such patients do well. In the 2009 St. Gallen meeting, the presence of LVI was reported to be one of the parameters that indicate the usage of chemo-endocrine therapy in early cases with ER-positive, HER2-negative breast cancer, and its absence was a relative indication for endocrine therapy alone.[37,47] Similarly, in the 2015 St. Gallen meeting, the majority (67.6%) of panelists regarded LVI as a sole indicator for adjuvant chemotherapy.[48] We suggest that the presence of LVI is a powerful prognostic factor that could potentially be used for clinical stratification of those patients through identification of a high-risk subgroup, an issue also identified by Mohammed et al.[37] These findings suggest that LVI detected by D2-40 might usefully be incorporated into the routine clinical pathological staging of patients with luminal A-like breast cancer.
However, the major limitation existed in the present study is the small number of samples. It is worth noting that the present cohort showed a large discrepancy about the prognostic value of LVI in triple-negative patients between the present study (P = .80) and that reported by Gujam and colleagues (P = .01).[18] We suppose that the small number of triple-negative cases (n = 40) in our study may account for the difference. Further work is required to confirm the prognostic value of LVI in such cases, which are frequently associated with worse prognosis. Nevertheless, the results are interesting and make a case for further prospective studies, with a larger population and longer follow-up, of routine clinical assessment of LVI by D2-40 stain.
In conclusion, the present study demonstrated that the presence of LVI predicted tumor recurrence in Chinese women with early invasive breast cancer. Furthermore, LVI provides independent prognostic information in the subgroup of patients with lymph node-negative, lymph node-positive, luminal A-like, and luminal B-like diseases. The results of this study suggest that the presence of LVI represents an important criterion for evaluating prognosis of invasive breast cancer patients with early, lymph node-negative, lymph node-positive, luminal A-like, and luminal B-like diseases, which make a case for routine clinical assessment of LVI using D2-40.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, ER = estrogen receptor, H&E = hematoxylin and eosin, HR = hazard ratio, IHC = immunohistochemistry, LVI = lymphatic vessel invasion, PR = progesterone receptor.
KWH and JJS contributed equally to this study.
This study was supported by grants from the Natural Science Foundation of Shandong Province (ZR2015HM055) and the Key Research and Development Plan of Shandong Province (2016GSF201185).
The authors report no conflicts of interest.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Tezuka K, Onoda N, Takashima T, et al. Prognostic significance of lymphovascular invasion diagnosed by lymphatic endothelium immunostaining in breast cancer patients. Oncol Rep 2007;17:997–1003. [PubMed] [Google Scholar]
- [3].Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005;16:1569–83. [DOI] [PubMed] [Google Scholar]
- [4].Gilchrist KW, Gould VE, Hirschl S, et al. Interobserver variation in the identification of breast carcinoma in intramammary lymphatics. Hum Pathol 1982;13:170–2. [DOI] [PubMed] [Google Scholar]
- [5].Teel P. Vascular invasion as a prognostic factor in breast carcinoma. Surg Gynecol Obstet 1964;118:1006–8. [PubMed] [Google Scholar]
- [6].Bettelheim R, Mitchell D, Gusterson BA. Immunocytochemistry in the identification of vascular invasion in breast cancer. J Clin Pathol 1984;37:364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bettelheim R, Penman HG, Thorntonjones H, et al. Prognostic significance of peritumoral vascular invasion in breast cancer. Br J Cancer 1984;50:771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pinder SE, Ellis IO, Galea M, et al. Pathological prognostic factors in breast cancer. III. Vascular invasion: relationship with recurrence and survival in a large study with long-term follow-up. Histopathology 1994;24:41–7. [DOI] [PubMed] [Google Scholar]
- [9].de Mascarel I, Bonichon F, Durand M, et al. Obvious peritumoral emboli: an elusive prognostic factor reappraised. Multivariate analysis of 1320 node-negative breast cancers. Eur J Cancer 1998;34:58–65. [DOI] [PubMed] [Google Scholar]
- [10].Lauria R, Perrone F, Carlomagno C, et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer 1995;76:1772–8. [DOI] [PubMed] [Google Scholar]
- [11].McCready DR, Chapman JA, Hanna WM, et al. Factors affecting distant disease-free survival for primary invasive breast cancer: use of a log-normal survival model. Ann Surg Oncol 2000;7:416–26. [DOI] [PubMed] [Google Scholar]
- [12].Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 2015;107:djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 2002;15:434–40. [DOI] [PubMed] [Google Scholar]
- [14].Yamauchi C, Hasebe T, Iwasaki M, et al. Accurate assessment of lymph vessel tumor emboli in invasive ductal carcinoma of the breast according to tumor areas, and their prognostic significance. Hum Pathol 2007;38:247–59. [DOI] [PubMed] [Google Scholar]
- [15].Giorgadze TA, Zhang PJ, Pasha T, et al. Lymphatic vessel density is significantly increased in melanoma. J Cutan Pathol 2004;31:672–7. [DOI] [PubMed] [Google Scholar]
- [16].Fogt F, Zimmerman RL, Ross HM, et al. Identification of lymphatic vessels in malignant, adenomatous and normal colonic mucosa using the novel immunostain D2-40. Oncol Rep 2004;11:47–50. [DOI] [PubMed] [Google Scholar]
- [17].Evangelou E, Kyzas PA, Trikalinos TA. Comparison of the diagnostic accuracy of lymphatic endothelium markers: Bayesian approach. Mod Pathol 2005;18:1490–7. [DOI] [PubMed] [Google Scholar]
- [18].Gujam FJ, Going JJ, Mohammed ZM, et al. Immunohistochemical detection improves the prognostic value of lymphatic and blood vessel invasion in primary ductal breast cancer. BMC Cancer 2014;14:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee JA, Bae JW, Woo SU, et al. D2-40, Podoplanin, and CD31 as a prognostic predictor in invasive ductal carcinomas of the breast. J Breast Cancer 2011;14:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Widodo I, Ferronika P, Harijadi A, et al. Clinicopathological significance of lymphangiogenesis and tumor lymphovascular invasion in Indonesian breast cancers. Asian Pac J Cancer Prev 2013;14:997–1001. [DOI] [PubMed] [Google Scholar]
- [21].Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 2007;134:907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mohammed ZM, Going JJ, McMillan DC, et al. Comparison of visual and automated assessment of HER2 status and their impact on outcome in primary operable invasive ductal breast cancer. Histopathology 2012;61:675–84. [DOI] [PubMed] [Google Scholar]
- [23].Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005;23:7212–20. [DOI] [PubMed] [Google Scholar]
- [24].Bukholm IR, Bukholm G, Holm R, et al. Association between histology grade, expression of HsMCM2, and cyclin A in human invasive breast carcinomas. J Clin Pathol 2003;56:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang J, Sang D, Xu B, et al. Value of breast cancer molecular subtypes and Ki67 expression for the prediction of efficacy and prognosis of neoadjuvant chemotherapy in a Chinese population. Medicine 2016;95:e3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005;5:591–602. [DOI] [PubMed] [Google Scholar]
- [28].Marinho VF, Metze K, Sanches FS, et al. Lymph vascular invasion in invasive mammary carcinomas identified by the endothelial lymphatic marker D2-40 is associated with other indicators of poor prognosis. BMC Cancer 2008;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mohammed RA, Ellis IO, Elsheikh S, et al. Lymphatic and angiogenic characteristics in breast cancer: morphometric analysis and prognostic implications. Breast Cancer Res Treat 2009;113:261–73. [DOI] [PubMed] [Google Scholar]
- [30].Mittendorf EA, Sahin AA, Tucker SL, et al. Lymphovascular invasion and lobular histology are associated with increased incidence of isolated tumor cells in sentinel lymph nodes from early-stage breast cancer patients. Ann Surg Oncol 2008;15:3369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mohammed RA, Menon S, Martin SG, et al. Prognostic significance of lymphatic invasion in lymph node-positive breast carcinoma: findings from a large case series with long-term follow-up using immunohistochemical endothelial marker. Mod Pathol 2014;27:1568–77. [DOI] [PubMed] [Google Scholar]
- [32].Gujam FJ, Going JJ, Edwards J, et al. The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hematol 2014;89:231–41. [DOI] [PubMed] [Google Scholar]
- [33].Arnaout-Alkarain A, Kahn HJ, Narod SA, et al. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol 2007;20:183–91. [DOI] [PubMed] [Google Scholar]
- [34].Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest 2002;82:1255–7. [DOI] [PubMed] [Google Scholar]
- [35].Gudlaugsson E, Skaland I, Undersrud E, et al. D2-40/p63 defined lymph vessel invasion has additional prognostic value in highly proliferating operable node negative breast cancer patients. Mod Pathol 2011;24:502–11. [DOI] [PubMed] [Google Scholar]
- [36].Mohammed RA, Martin SG, Gill MS, et al. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol 2007;31:1825–33. [DOI] [PubMed] [Google Scholar]
- [37].Mohammed RA, Martin SG, Mahmmod AM, et al. Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: Findings from a large case series with long-term follow-up. J Pathol 2011;223:358–65. [DOI] [PubMed] [Google Scholar]
- [38].Ejlertsen B, Jensen MB, Rank F, et al. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst 2009;101:729–35. [DOI] [PubMed] [Google Scholar]
- [39].Neville AM, Bettelheim R, Gelber RD, et al. Factors predicting treatment responsiveness and prognosis in node-negative breast cancer. The International (Ludwig) Breast Cancer Study Group. J Clin Oncol 1992;10:696–705. [DOI] [PubMed] [Google Scholar]
- [40].Clayton F. Pathologic correlates of survival in 378 lymph node-negative infiltrating ductal breast carcinomas. Mitotic count is the best single predictor. Cancer 1991;68:1309–17. [DOI] [PubMed] [Google Scholar]
- [41].Schoppmann SF, Bayer G, Aumayr K, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg 2004;240:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leitner SP, Swern AS, Weinberger D, et al. Predictors of recurrence for patients with small (one centimeter or less) localized breast cancer (T1a,b N0 M0). Cancer 1995;76:2266–74. [DOI] [PubMed] [Google Scholar]
- [43].Pinder SE, Ellis IO, Galea M, et al. Pathological prognostic factors in breast cancer. III. Vascular invasion: relationship with recurrence and survival in a large study with long-term follow-up. Histopathology 2010;24:41–7. [DOI] [PubMed] [Google Scholar]
- [44].Stacker SA, Achen MG, Jussila L, et al. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002;2:573–83. [DOI] [PubMed] [Google Scholar]
- [45].Ragage F, Debled M, Macgrogan G, et al. Is it useful to detect lymphovascular invasion in lymph node-positive patients with primary operable breast cancer? Cancer 2010;116:3093–101. [DOI] [PubMed] [Google Scholar]
- [46].Yildirim E, Berberoglu U. Lymph node ratio is more valuable than level III involvement for prediction of outcome in node-positive breast carcinoma patients. World J Surg 2007;31:276–89. [DOI] [PubMed] [Google Scholar]
- [47].Song YJ, Sun HS, Jin SC, et al. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer 2011;14:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jackisch C, Harbeck N, Huober J, et al. 14th St. Gallen International Breast Cancer Conference 2015: evidence, controversies, consensus—primary therapy of early breast cancer: opinions expressed by German experts. Breast Care 2015;10:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


