Abstract
Rationale:
We present a rare case of malignant pheochromocytoma with thoracic metastases during pregnancy that presented with symptoms of myelopathy and was treated with circumferential decompression, stabilization, and radiation. The management of this unique case is not well documented. The clinical manifestations, imaging results, pathological characteristics, treatment and prognosis of the case were analyzed.
Patient concerns:
A 26-year-old pregnant woman with a history of paroxysmal hypertension during the second trimester presented with lower extremity weakness, numbness, urinary incontinence, and back pain. Imaging studies revealed a right adrenal pheochromocytoma, multiple metastases at T8, T11, T12, and the pelvis girdle causing significant multilevel cord compression and significant osteolytic lesions at T11 and T12.
Diagnoses:
We believe this is the first reported case of metastatic pheochromocytoma of the thoracic spine presenting with symptoms of myelopathy during pregnancy.
Interventions:
A healthy neonate was delivered by emergency caesarean section at 34 weeks. Subsequently, the patient underwent a circumferential spinal cord decompression and a stabilization procedure.
Outcomes:
The patient's neurological deficits improved significantly after the surgery, and the postoperative period was uneventful at the 6-month follow-up visit.
Lessons:
This article emphasizes that metastatic pheochromocytoma of the spine, although rare, should be part of the differential when a patient presents with elevated blood pressure, weakness, and urinary incontinence.
Keywords: acute incomplete paralysis, metastatic spinal pheochromocytoma, pregnancy, radiation therapy, surgical treatment
1. Introduction
Pheochromocytoma is a metabolically-active tumor originating from the chromaffin cells of the adrenal medulla. Five-year survival rates range from 84% to 96% for benign pheochromocytoma, to about 40% for malignant pheochromocytoma.[1] Malignant forms account for nearly 10% of all cases with the lymph nodes, liver, and lungs being the most common site of metastasis.[2] Metastatic spread to the spine, however, is rare. Only 17 cases of metastatic pheochromocytoma of the spine have been reported in the literature.[3–5] Classically, pheochromocytoma manifests as paroxysmal attacks of the following 4 characteristics: headaches, diaphoresis, palpitations, and severe hypertensive episodes.[6] Long-term exposure to catecholamines leads to arrhythmias and cardiomyopathy. Initial laboratory evaluations are based on index of suspicion. High-risk patients should undergo 24-hour urinary fractionated metanephrines and catecholamines measurements (sensitivity = 98%, specificity = 98%).[7–9] Biochemical confirmation of the diagnosis should be followed by radiological evaluation of the tumor's location, using computed tomography (CT) and magnetic resonance imaging (MRI) (sensitivity 98%–100%, specificity 70%). Approximately 95% of the tumors are located within the abdomen and pelvis.[10] Additional imaging studies like iodine-123 metaiodobenzylguanidine (MIBG) scintigraphy are warranted. Genetic testing should be part of the diagnostic process. Von Hippel Lindau syndrome, Multiple Endocrine Neoplasia 2, neurofibromatosis type 1 are associated with pheochromocytoma. In pregnant women, the diagnosis is usually based on 24-hour fractionated metanephrines and catecholamines, as well as MRI. MIBG scintigraphy is not considered safe for this cohort.
To the best of our knowledge, this is the first reported case of metastatic pheochromocytoma to the spine in a pregnant woman presenting with acute myelopathy. Our patient experienced a history of paroxysmal hypertension, sudden onset numbness and decreased muscle strength of bilateral lower extremities, as well as urinary incontinence. The patient did not present with significant anal sphincter nor sexual function disturbances. To our experience, main causes of myelopathy in a pregnant woman include lumbar disc herniation, spinal canal stenosis, spondylolisthesis, nerve sheath tumors in the spinal canal such as schwannoma and neurofibroma, and rarely with spinal metastases. We performed surgical exploration, circumferential decompression, and stabilization surgery. In the short term, the patient's conditions improved significantly postoperatively.
2. Case presentation
In August of 2015, a 26-year-old G1P0 Chinese woman at 34-weeks gestation with no notable past medical history presented to a hospital in Edinburgh, Scotland with a 3-hour history of sudden onset numbness and decreased muscle strength of bilateral lower limbs, as well as urinary incontinence. Abdominal CT and MRI revealed a right adrenal mass (10 cm × 7.8 cm × 13 cm) with thoracic spinal metastases at T8, T11, and T12. An ultrasound-guided biopsy of the adrenal mass revealed features consistent with pheochromocytoma. Subsequently, a healthy female neonate was delivered by emergency caesarean section during week 34 of gestation. No major complications were documented. Because of the extent of the thoracic metastases, the physicians believed at the time that the vertebral fixation could not be safely completed after decompression surgery. The patient was placed on palliative combined radiotherapy (T7-T12, Dt 20Gy/5f, from August 29, 2015 to September 2, 2015) and chemotherapy (2 cycles of cisplatin/etoposide) to reduce the size of the tumor and to relieve the neurological deficits. After 2 cycles of chemotherapy, imaging studies were repeated showing the right adrenal mass measuring 9 cm × 7 cm × 14.5 cm, a 10% decrease in total volume compared to the previous image study. With extensive bone metastases and minimal decrease in tumor volume after 2 cycles of chemotherapy, the physicians decided to forgo the third cycle of chemotherapy. Because the patient wished to continue her treatment in China, she was discharged from the hospital with a prescription for 4 mg of doxazosin daily to control her blood pressure until she can be admitted again.
The patient presented to the Peking Union Medical College Hospital (PUMCH) in November 2015. The patient has been experiencing a gradual decrease in muscle strength in her bilateral lower extremities, and worsening numbness for 3 months after she first presented to the hospital in Edinburgh. Paroxysmal lower back and hip pain were present for approximately 3 months. The patient denied experiencing chest palpitations, diaphoresis, paresthesia, headaches, fatigue, and facial flushing, symptoms commonly associated with pheochromocytoma. Upon further questioning, the patient recalled a history of paroxysmal hypertension above 150/100 mm Hg during early second trimester of her pregnancy. However, the elevated blood pressure was inconsistent among each doctor's visits, and thus was thought to be nonpathological at the time. No pertinent family history was identified.
On physical examination, the patient showed decreased sensation to pin-prick below the T10 sensory level indicating possible cord compression at the T10 level and decreased sensation to fine-touch of bilateral lower extremities bilaterally, and exhibited a 2/5 strength in all major muscle groups of the lower extremities. Deep tendon reflexes revealed hyporeflexia, 1+, for both knee-jerk and Achilles tendon reflexes bilaterally. Babinski sign was negative. Due to decreased strength of the lower extremities, gait ataxia could not be performed. Cranial nerves and higher mental function examinations were normal, and the rest of the neurological examination showed no abnormalities. The syndromic diagnosis was suggestive of T10 myelopathy (paraparesis, urinary incontinence, T10 level hypoesthesia) and radiculopathy (back pain, decreased reflexes) respectively. Routine laboratory tests were ordered, including electrolytes, liver and kidney function tests, and complete blood count. Genetic evaluations for RET, VHL, SDHB, SDHC, SDHD and 24-hour urine fractionated catacholamines were ordered. Abdominal MRI, Fluorodeoxyglucose-Positron emission tomography/computed tomography, and MIBG were ordered to visualize the metastatic lesions, assess the stability of the vertebral column, and aid in the formulation of a surgical approach. Preoperative hemodynamic and cardiovascular assessments included electrocardiogram, echocardiogram, and chest x-ray. Twenty-four-hour urine fractionated catecholamine revealed a normal urinary epinephrine level of 4.04 μg/24 h, an elevated urinary norepinephrine level of 125.34 μg/24 h, and an elevated urinary dopamine level of 857.14 μg/24 h. Furthermore, NSE was significantly elevated at 32.5 ng/mL. Genetic investigation was negative. An MRI of the thoracolumbar spine revealed widespread abnormal signals involving multiple vertebral levels consistent with diffused metastatic infiltration. MRI-spine showed progressive spinal cord compression at T8 caused by the epidural component of the mass, with increased metastatic marrow infiltration of the vertebral body. Specifically, the tumor had infiltrated through the T8 vertebral body and into the right pedicle and posterior elements. Furthermore, there was extraosseous spread into the right lateral aspect of the epidural space extending posteriorly, and resulting in cord compression. Metastatic infiltration of the tumor also involved the vertebral body at T11 and T12 levels extending into the right pedicle and the right side of the epidural space. The cord was visibly displaced. Spinal stenosis was significant at these 2 levels (Fig. 1). A PET/CT scan revealed multiple osteolytic lesions of the spine, which were especially prominent at T11 and T12. PET/CT also demonstrated a right-side adrenal pheochromocytoma and multiple suspicious metastases of the spine, the right kidney, the pelvic girdle, and the left erector spinae muscle (Fig. 2). Furthermore, a MIBG scan revealed extra-adrenal hotspots in regions T8, T11, and the pelvic girdle.
Figure 1.
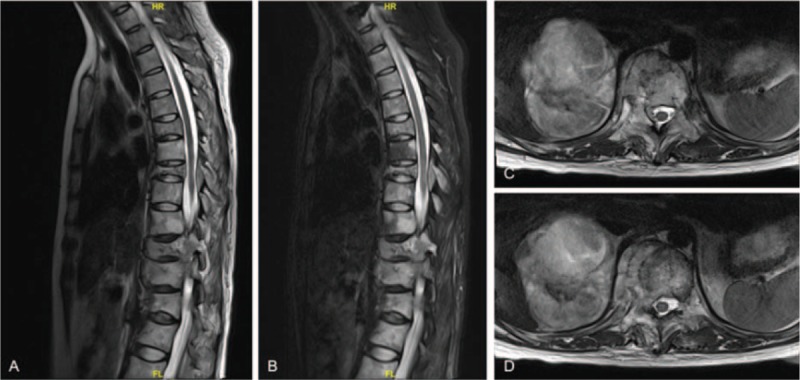
A, Preoperative sagittal T2-weighted MRI scan revealing several vertebral metastases, pathological vertebral fractures, and multilevel thoracic spinal cord compressions caused by metastatic malignant pheochromocytoma. B, Preoperative sagittal T2-weighted fat-saturated MRI scan revealing vertebral metastases, vertebral fractures, and thoracic spinal cord stenosis. C and D, Preoperative transverse T2-weighted MRI images showing vertebral metastasis and metastatic adrenal pheochromocytoma. MRI = magnetic resonance imaging.
Figure 2.
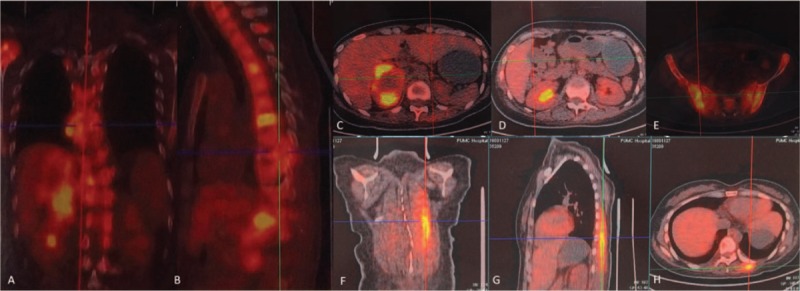
A-H, Positron emission tomography-computed tomography revealed adrenal pheochromocytoma of the right side and multiple metastases of the spine, right kidney, pelvis, and left erector spinae.
In consultation with the Department of Endocrinology, we decided to perform a circumferential decompression procedure of the spinal metastasis to alleviate the symptoms caused by the spinal cord compression and subsequently stabilize the vertebral spine. Because of the size of the primary adrenal pheochromocytoma and the extent of the metastases, the risk of surgical intervention and general anesthesia was high. Full blood pressure control, with a goal of 140/90 mm Hg using phenoxybenzamine 10 mg Three times a day (TID) for 4 weeks prior to the operation, was recommended by the endocrinologist. Furthermore, extensive monitoring of preoperative cardiovascular and hemodynamic functions was also recommended. Criteria included the absence of ST-segment elevations on electrocardiogram and less than 1 ventricular premature complex over 5 minutes on a Holter monitor within the 4-week period prior to the surgery.
In brief, posterior circumferential decompression and T7 to L2 internal fixation were performed (Fig. 3A and B). For the posterior approach, the paraspinal muscles were detached gently on each side after a midline longitudinal incision was made over the spinous processes while avoiding the possible metastatic lesion in the left erector spinae that was revealed on PET. The pedicle entry points were exposed via step-by-step bilateral dissection. At first, the pedicle screws were placed bilaterally at T9, T10, and L1, followed by pedicle screw insertion at T7 and L2. Because the patient did not exhibit hemodynamic instability to the placement of the pedicle screws, fixation using a Moss SI screw-rod system was employed. Likewise, neither dissection nor screw insertion resulted in significant alterations of the blood pressure or cardiac dysfunction parameters.
Figure 3.
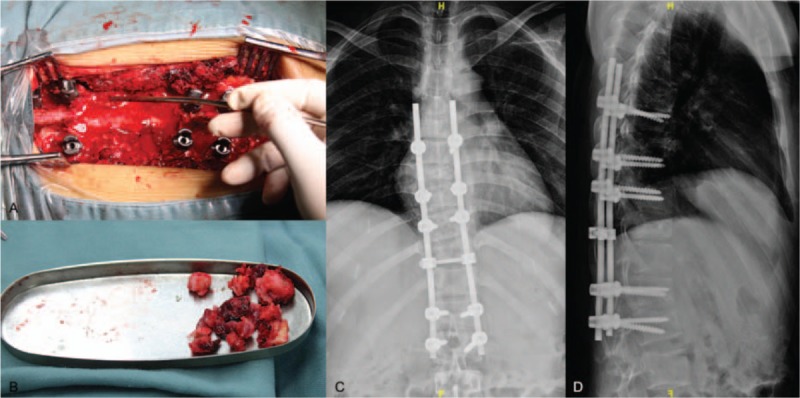
A, Intraoperative photography depicting the exposed spinal cord. B, Intraoperative photography depicting partially resected metastatic tumor. C, Posteroanterior (PA) x-ray image of the thoracic spine obtained postoperatively. D, Lateral x-ray image of the thoracic spine obtained postoperatively.
Visual inspection using the intraoperative fluoroscopy showed optimal position of all pedicle screws. Perioperative blood pressure was stable, and intraoperative blood loss was approximately 600 mL. The patient's course in the intensive care unit (ICU) was uneventful. An x-ray after the surgery confirmed the correct positioning of the implants and no signs of displacement of the screws and rods (Fig. 3C and D). The postoperative pathology report confirmed malignant pheochromocytoma (Fig. 4). Pathological result was positive for chromogranin A, synaptophysin, S-100, and P53 indicating pheochromocytoma from chromaffin cells of the adrenal medulla.
Figure 4.
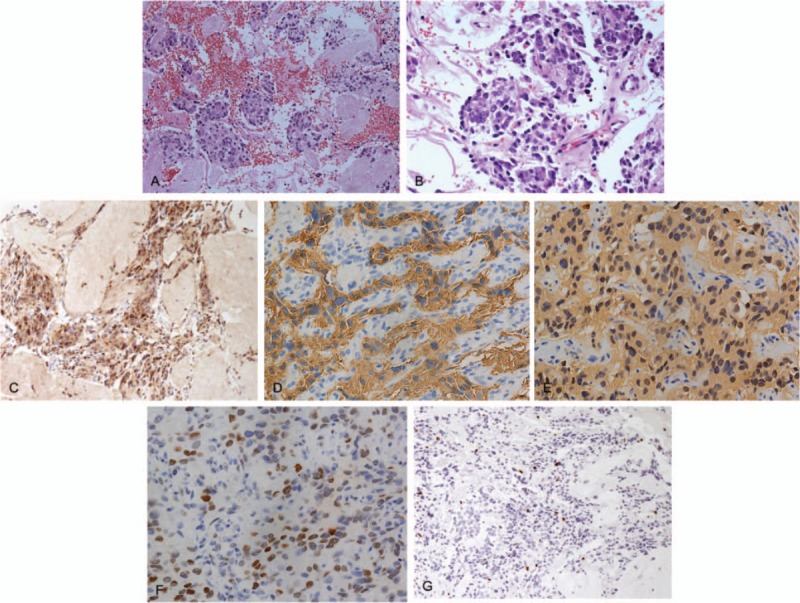
Pathologic histology of spinal metastases. A and B, Microphotography showing characteristic nests of tumor cells separated by vascular septa (Zellballen) with cells showing significant nuclear pleomorphism with prominent nucleoli (Hematoxylin and Eeosin, original magnification ×20 and ×40). C, Chromogranin A immunostaining is strongly positive in the chromaffin cells. Chromogranin A is present in the secretory granules. D, Synaptophysin immunostaining shows strong, diffuse cytoplasmic staining in the tumor cells. E, The sustentacular cells of the spinal metastases of pheochromocytoma showing characteristic staining of S100. F, P-53 immunostaining is sporadically positive. G, Ki-67 immunostaining shows 3% Ki-67 positive cells. Ki-67 staining is localized in the tumor nuclei.
One week after the operation, the patient's muscle strength in the bilateral lower extremities improved to grade IV and the tendon reflexes returned to baseline. Following wound healing, the patient underwent adjuvant radiation therapy (32Gy/8f, 400cGy/f, 3–5f/w) and was prescribed phenoxybenzamine 10 mg TID for blood pressure management. Three months after the operation, she received the second cycle of MIBG therapy. The postoperative 6-month follow-up visit showed no tumor progression and no new symptoms. The blood pressure remained slightly elevated but stable.
3. Discussion
The incidence of pheochromocytoma is 0.2 to 0.9 case per 100,000 individuals per year, and the malignant forms account for approximately 10% of all cases.[1,2] The percentage of pheochromocytoma in patients with adrenal incidentaloma is about 0.4%.[11] Pheochromocytoma is extraordinarily rare, with a frequency of 0.002% in all pregnancies; thus, it can be difficult to diagnose and may result in devastating consequences if mismanaged.[12,13] Paroxysmal hypertension during pregnancy can often mimic gestational hypertension, the most common cause of elevated blood pressure (affecting 6%–8% of all pregnancies), making timely diagnosis of pheochromocytoma difficult without a high level of suspicion.[14] Maternal and fetal mortality rates are high, particularly in those who are not diagnosed until delivery. A systematic review by Biggar and Lennard[15] of 77 pregnancies in women with pheochromocytoma showed the maternal and fetal mortality rates were 8% and 17%, respectively. Pheochromocytomas are often endocrinologically active; any stimulation bears a huge risk of unwanted release of catecholamine, thus hypertension secondary to pheochromocytoma tends to be paroxysmal. A thorough investigation of the patient's hypertension history can be extremely high yield. Hypertension resistant to blood pressure medications points toward possible pheochromocytoma, while the previous history of hypertension during pregnancy or family history of hypertension during pregnancy makes gestational hypertension more likely. Second, endocrine laboratory tests may give clinicians additional information to better separate hypertension secondary to pheochromocytoma or pregnancy. Last, abdominal ultrasonography and MRI are highly recommended in patients with a high-level suspicion for pheochromocytoma. When diagnosis of pheochromocytoma was made during the antenatal period, maternal and fetal survival rates are increased significantly.[15] However, many constitutional symptoms of metastatic pheochromocytoma of the spine are vague, like back pain, which is common during pregnancy and are often overlooked. Since metastatic lesions are endocrinologically active, any stimulation bears a huge risk of unwanted release of catecholamine, resulting in hemodynamic complications.[16] Accordingly, we believe that changes in the intra-abdominal pressure or direct compression of the tumor by the enlarged uterus could have caused catecholamines to be released from the tumor, leading to the elevated blood pressure observed in our patient.
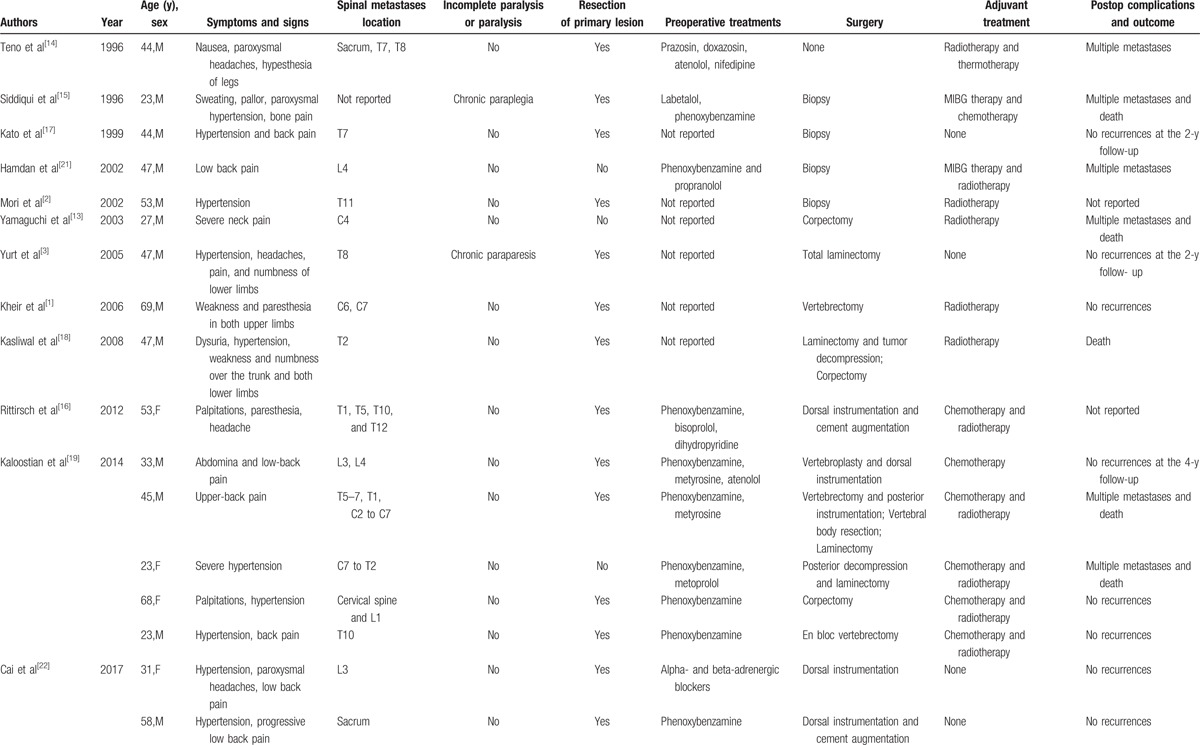
To our knowledge, this is the first reported case of a pregnant patient diagnosed with metastatic pheochromocytoma of the spine. Clinical studies looking at metastatic pheochromocytoma to the spine are lacking due to the extremely low incidence rate. Based on our review of the 17 case reports on PubMed (Table 1),[3–5,17–24] metastatic pheochromocytoma of the spine is slightly more common in the thoracic region (47%) and is more commonly diagnosed during the fourth and fifth decades of life for the sporadic form (mean age: 43.2 years; range: 23–69 years).[25] Familial forms tend to develop at a younger age and are usually bilateral, whereas sporadic tumors are more commonly unilateral.[6,26,27]
Table 1.
Clinical review of 17 previously published metastatic pheochromocytomas of spine.
Malignant pheochromocytoma has similar radiographic and histologic characteristics as its benign counterparts. Malignant pheochromocytoma is based on the invasion of adjacent structures or the presence of multifocal disease. The location of the spinal lesion determines the neurological deficits. Cord compression in the thoracic and lumbar regions usually shows symptoms of myelopathy, which include lower back pain, lower extremity paresthesia and weakness, and urinary incontinence. Imaging studies play a crucial role in the surgical intervention decision making. Imaging studies can demonstrate spinal cord compression, and pathological vertebral fractures. In previous case reports, MRI of metastatic spinal cord pheochromocytoma appears as inhomogeneous lesion of spine, isointense on T1WI and hyperintense on T2WI, and indistinguishable from other metastatic spinal lesions.[28,29] The “gold-standard” to diagnose pheochromocytoma relies on pathological findings. Histopathologically, metastatic spinal pheochromocytomas are characterized by an architecture of nests of tumor cells separated by vascular septa with the cells showing significant nuclear pleomorphism with prominent nucleoli. Generally, metastatic pheochromocytomas are commonly immunoreactive for chromogranin A and synaptophysin.[30]
The best treatment for metastatic spinal pheochromocytomas causing myelopathy is posterior decompression and tumor resection, followed by internal fixation.[31–33] This protocol accomplishes 2 objections: it alleviates the neurological deficits by decompressing the stenosis and at the same time provides histopathological specimens for diagnosis, which is valuable in cases where the patient presents with atypical clinical and radiological findings.[34] There are several considerations to keep in mind: preoperative hemodynamic instability and cardiac arrhythmia control, possible incomplete tumor resection, intraoperative blood loss and hemodynamic instability, and, lastly, postoperative adjuvant therapy. During the preoperative period, patients should receive alpha blockade, with or without metyrosine for at least 2 weeks prior to surgery. Cardiovascular functions should be monitored with periodic Electrocardiographs, a Holter monitor, and echocardiography. The highly vascular nature of the tumor and its potential for infiltration makes total resection difficult, thus recurrence is common. As the tumor is highly vascular, there may be significant intraoperative blood loss which may necessitate blood transfusion. Intraoperative blood loss can be minimized by routine use of preoperative alpha blockade and embolization.[35,36] A review of the literature revealed that the risk of hemodynamic instability is prominent during the resection of an active lesion. Fluctuations in heart rate and blood pressure can result from anesthesia induction and tumor manipulation.[28,37] However, intraoperative hemodynamic and cardiovascular complications resulting from the resection of metastatic spinal lesions of pheochromocytoma are infrequent.[1] Adjuvant treatment aiming at controlling residual tumor is recommended for most patients.[38] This includes MIBG therapy, radiotherapy, and chemotherapy. The survival benefit of resection of spinal metastases is still unproven. However, such a procedure does have the benefit of reducing the tumor burden, decompressing the spinal stenosis, and facilitating subsequent chemotherapy and radiation therapy. Due to the rarity of the disease, there is currently no consensus on the appropriate chemotherapy regiments for the management of pheochromocytoma with spinal cord metastases. However, the commonly used chemotherapy drugs include Cisplatin, Etoposide, Cytoxan, vincristine, and dacarbazine.[1]
Six months after the procedure, our patient's postoperative condition remained uneventful and her neurological functions returned to baseline. Nevertheless, abnormal coagulation and cardiovascular remodeling are common postoperative complications. They account for a significant percentage of morbidity following resection of metastatic pheochromocytoma in the spine.[1] We hope the readers of this manuscript can see how we managed a pregnant patient with pheochromocytoma and secondary spinal cord metastases presenting with symptoms of myelopathy. Limitations of this case report include that we did not perform a combined resection of the spinal metastasis and the primary adrenal pheochromocytoma as 1-stage operation, mainly limited by the high risks associated with the surgical procedure. We believed that changes in the intra-abdominal pressure or accidental compression of the tumor could cause significant fluctuations in heart rate and the blood pressure. Last, in our patient, dexamethasone was not initiated at admission. Despite her functional status returning to baseline after the procedure, high-dose dexamethasone should have been started upon the diagnosis of spinal cord compression to avoid potential spinal cord edema before surgical intervention.
4. Conclusion
Although rare, metastatic pheochromocytoma of the spine should be part of the differential when a patient presents with neurological deficits and labile blood pressure. Paroxysmal hypertension is often attributed to gestational hypertension in pregnant patients and thus overlooked without a high level of suspicion for pheochromocytoma. Clinical symptoms are generally the result of the tumor burden, and pathological findings remain the “gold standard” for diagnosing malignant pheochromocytoma. We recommend the posterior approach for spinal decompression of the metastatic tumor when the tumor has caused neurological deficits, especially myelopathy. With a multidisciplinary team approach, proper planning, and adequate perioperative medical management, metastatic pheochromocytoma in the spine can be managed effectively.
Ethic statement: Written informed consent was obtained from the patient for the publication of this article, a copy of which is available for review from the editors of Medicine. Because this article does not involve any human or animal trials, it did not require institutional ethical review and approval.
Footnotes
Abbreviations: BP = blood pressure, CT = computed tomography, ECG = Electrocardiograph, FDG-PET/CT = Fluorodeoxyglucose-Positron emission tomography/computed tomography, H&E = Hematoxylin and Eeosin, ICU = intensive care unit, MIBG = metaiodobenzylguanidine, MRI = magnetic resonance imaging, NSE = serum neuron-specific enolase, PUMCH = Peking Union Medical College Hospital, T1WI = T1-weighted image, T2WI = T2-weighted image, TID = Three times a day.
SL, AS, and XZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Kaloostian PE, Zadnik PL, Kim JE, et al. High incidence of morbidity following resection of metastatic pheochromocytoma in the spine. J Neurosurg Spine 2014;20:726–33. [DOI] [PubMed] [Google Scholar]
- [2].Kaloostian PE, Zadnik PL, Awad AJ, et al. En bloc resection of a pheochromocytoma metastatic to the spine for local tumor control and for treatment of chronic catecholamine release and related hypertension. J Neurosurg Spine 2013;18:611–6. [DOI] [PubMed] [Google Scholar]
- [3].Kheir E, Pal D, Mohanlal P, et al. Cervical spine metastasis from adrenal pheochromocytoma. Acta Neurochir (Wien) 2006;148:1219–20. [DOI] [PubMed] [Google Scholar]
- [4].Mori S, Okura T, Kitami Y, et al. A case of metastatic extra-adrenal pheochromocytoma 12 years after surgery. Hypertens Res 2002;25:141–4. [DOI] [PubMed] [Google Scholar]
- [5].Yurt A, Arda MN, Vardar E. Metastatic pheochromocytoma of the thoracic spinal extradural space. Case report and review of the literature. Kobe J Med Sci 2005;51:49–53. [PubMed] [Google Scholar]
- [6].Beard CM, Sheps SG, Kurland LT, et al. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc 1983;58:802–4. [PubMed] [Google Scholar]
- [7].Perry CG, Sawka AM, Singh R, et al. The diagnostic efficacy of urinary fractionated metanephrines measured by tandem mass spectrometry in detection of pheochromocytoma. Clin Endocrinol (Oxf) 2007;66:703–8. [DOI] [PubMed] [Google Scholar]
- [8].Kudva YC, Sawka AM, Young WF., Jr Clinical review 164: the laboratory diagnosis of adrenal pheochromocytoma: the Mayo Clinic experience. J Clin Endocrinol Metab 2003;88:4533–9. [DOI] [PubMed] [Google Scholar]
- [9].Sawka AM, Jaeschke R, Singh RJ, et al. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003;88:553–8. [DOI] [PubMed] [Google Scholar]
- [10].Bravo EL. Evolving concepts in the pathophysiology, diagnosis, and treatment of pheochromocytoma. Endocr Rev 1994;15:356–68. [DOI] [PubMed] [Google Scholar]
- [11].Loh HH, Yee A, Loh HS, et al. The natural progression and outcomes of adrenal incidentaloma: a systematic review and meta-analysis. Minerva Endocrinol 2017;42:77–87. [DOI] [PubMed] [Google Scholar]
- [12].Sarathi V, Lila AR, Bandgar TR, et al. Pheochromocytoma and pregnancy: a rare but dangerous combination. Endocr Pract 2010;16:300–9. [DOI] [PubMed] [Google Scholar]
- [13].Lenders JW. Pheochromocytoma and pregnancy: a deceptive connection. Eur J Endocrinol 2012;166:143–50. [DOI] [PubMed] [Google Scholar]
- [14].Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis 2013;20:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Biggar MA, Lennard TW. Systematic review of phaeochromocytoma in pregnancy. Br J Surg 2013;100:182–90. [DOI] [PubMed] [Google Scholar]
- [16].Goodwin CR, Clarke MJ, Gokaslan ZL, et al. En bloc resection of solitary functional secreting spinal metastasis. Global Spine J 2016;6:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yamaguchi S, Hida K, Nakamura N, et al. Multiple vertebral metastases from malignant cardiac pheochromocytoma—case report. Neurol Med Chir (Tokyo) 2003;43:352–5. [DOI] [PubMed] [Google Scholar]
- [18].Teno S, Tanabe A, Nomura K, et al. Acutely exacerbated hypertension and increased inflammatory signs due to radiation treatment for metastatic pheochromocytoma. Endocr J 1996;43:511–6. [DOI] [PubMed] [Google Scholar]
- [19].Siddiqui MZ, Von Eyben FE, Spanos G. High-voltage irradiation and combination chemotherapy for malignant pheochromocytoma. Cancer 1988;62:686–90. [DOI] [PubMed] [Google Scholar]
- [20].Rittirsch D, Battegay E, Zimmerli LU, et al. Cement-augmented dorsal instrumentation of the spine as a safe adjunct to the multimodal management of metastatic pheochromocytoma: a case report. Patient Saf Surg 2012;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kato H, Suzuki M, Mukai M, et al. Clinicopathological study of pheochromocytoma of the urinary bladder: immunohistochemical, flow cytometric and ultrastructural findings with review of the literature. Pathol Int 1999;49:1093–9. [DOI] [PubMed] [Google Scholar]
- [22].Kasliwal MK, Sharma MS, Vaishya S, et al. Metachronous pheochromocytoma metastasis to the upper dorsal spine-6-year survival. Spine J 2008;8:845–8. [DOI] [PubMed] [Google Scholar]
- [23].Hamdan A, Hirsch D, Green P, et al. Pheochromocytoma: unusual presentation of a rare disease. Isr Med Assoc J 2002;4:827–8. [PubMed] [Google Scholar]
- [24].Cai S, Kong X, Yan C, et al. Successful treatment of metastatic pheochromocytoma in the spine with cement augmentation. Medicine (Baltimore) 2017;96:e5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guerrero MA, Schreinemakers JM, Vriens MR, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg 2009;209:727–32. [DOI] [PubMed] [Google Scholar]
- [26].Stein PP, Black HR. A simplified diagnostic approach to pheochromocytoma. A review of the literature and report of one institution's experience. Medicine (Baltimore) 1991;70:46–66. [DOI] [PubMed] [Google Scholar]
- [27].Pacak K, Linehan WM, Eisenhofer G, et al. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med 2001;134:315–29. [DOI] [PubMed] [Google Scholar]
- [28].Homma K, Hayashi K, Wakino S, et al. Primary malignant hepatic pheochromocytoma with negative adrenal scintigraphy. Hypertens Res 2006;29:551–4. [DOI] [PubMed] [Google Scholar]
- [29].Sundgren P, Annertz M, Englund E, et al. Paragangliomas of the spinal canal. Neuroradiology 1999;41:788–94. [DOI] [PubMed] [Google Scholar]
- [30].Lau D, La Marca F, Camelo-Piragua S, et al. Metastatic paraganglioma of the spine: case report and review of the literature. Clin Neurol Neurosurg 2013;115:1571–4. [DOI] [PubMed] [Google Scholar]
- [31].Yang C, Li G, Fang J, et al. Clinical characteristics and surgical outcomes of primary spinal paragangliomas. J Neurooncol 2015;122:539–47. [DOI] [PubMed] [Google Scholar]
- [32].Lee JH, Stein M, Roychowdhury S. Percutaneous treatment of a sacral metastasis with combined embolization, cryoablation, alcohol ablation and sacroplasty for local tumor and pain control. Interv Neuroradiol 2013;19:250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zileli M, Kalayci M, Basdemir G. Paraganglioma of the thoracic spine. J Clin Neurosci 2008;15:823–7. [DOI] [PubMed] [Google Scholar]
- [34].Laufer I, Edgar MA, Hartl R. Primary intraosseous paraganglioma of the sacrum: a case report. Spine J 2007;7:733–8. [DOI] [PubMed] [Google Scholar]
- [35].Bruynzeel H, Feelders RA, Groenland TH, et al. Risk factors for hemodynamic instability during surgery for pheochromocytoma. J Clin Endocrinol Metab 2010;95:678–85. [DOI] [PubMed] [Google Scholar]
- [36].Ahlman H. Malignant pheochromocytoma: state of the field with future projections. Ann N Y Acad Sci 2006;1073:449–64. [DOI] [PubMed] [Google Scholar]
- [37].Eisenhofer G, Schott M, Bornstein S. Pheochromocytoma and paraganglioma: recent progress and new vistas for improved patient care. Horm Metab Res 2012;44:325–7. [DOI] [PubMed] [Google Scholar]
- [38].Adler JT, Meyer-Rochow GY, Chen H, et al. Pheochromocytoma: current approaches and future directions. Oncologist 2008;13:779–93. [DOI] [PubMed] [Google Scholar]


