Abstract
Objective
To examine an association between melanoma and Parkinson’s disease (PD).
Patients and Methods
(Phase I) Rochester Epidemiology Project (REP) records were used to identify PD patients in Olmsted County, MN (1/1/1976–12/31/2013), with three matched controls per case. Following review, JMP statistical software with logistic regression analysis was used to assess risk of prior history of melanoma in PD patients versus controls. (Phase II) All REP cases of melanoma were identified (1/1/1976–12/31/2014), with one control each. A Cox proportional hazards model was used to assess risk of developing PD post-index date in cases versus controls, and Kaplan-Meier analysis was performed to determine 35-year cumulative risk of PD. A Cox proportional hazards model was used to calculate risk of death from metastatic melanoma in melanoma patients without PD compared to those with PD.
Results
(Phase I) PD subjects had a 3.8-fold increased probability of having pre-existing melanoma compared to controls (CI 2.1–6.8, P<.001). (Phase II) Melanoma patients had a 4.2-fold increased risk of developing PD (CI 2.0–8.8, P<.001). Kaplan-Meier analysis revealed an increased 35-year cumulative risk of PD in melanoma patients (11.8%) compared to controls (2.6%), P<.001. Melanoma patients without PD had a 10.5-fold increased relative risk of dying from metastatic melanoma compared to melanoma patients with PD (CI 1.5–72.2, P=.02).
Conclusion
There appears to be an association between melanoma and PD. Further study is warranted; but based on these results, physicians may consider counseling melanoma patients regarding PD risk and implementing cutaneous and ocular melanoma surveillance in PD patients.
Keywords: Parkinson’s disease, melanoma, cutaneous, ocular, genetics, immunology
Introduction
There has been much speculation on the relationship between Parkinson’s disease (PD) and melanoma.1 Dating back to 1972, there have been numerous reports suggesting that levodopa therapy may be implicated in malignant melanoma.2–5 This association is plausible because levodopa is an intermediary product involved in melanin synthesis, and it has been shown to increase melanin and melanoma cell growth in plant and human cell studies, respectively.6–8 Moreover, levodopa has been speculated to accelerate the growth of pre-existing malignant melanomas in humans.9 However, randomized controlled trials and prospective studies have not substantiated these claims, begging the question of whether medical treatment of PD increases the risk for melanoma or if it is mere coincidence.10–13
Some have hypothesized that there is an association between melanoma and PD itself, regardless of PD treatment. Indeed, several publications indicate an increased risk of melanoma in patients with PD, ranging from a two-fold increased risk up to a reported seven-fold increased risk in a recent prospective North American study.10,14–19 In one study, there was actually an increase in the prodromal markers for PD in patients with a prior history of melanoma.20 Nevertheless, other work suggests that there is not a strong association between melanoma and PD prior to treatment with levodopa.21 Overall, this is a highly controversial and compelling subject that has given much consideration to the need for more rigorous melanoma screening measures in this patient population.
Most of the large studies performed in this area have been done in regard to cutaneous melanoma, with no large studies dedicated to uveal melanoma available on extensive chart review. With regard to the ophthalmologist, there have been a few case reports of choroidal melanoma in PD patients on levodopa as well as one case of an eyelid margin melanoma.4,22,23 Due to the curable nature of melanoma if detected early, this is an important topic about which ophthalmologists, dermatologists, neurologists, oncologists, and general practitioners should all be aware, and there is a need for further investigation in this area.
Furthermore, most prior studies have examined the risk of melanoma within PD cohorts rather than examining the risk of developing PD within melanoma cohorts. There have, however, been prior reports that there is an increased occurrence of melanoma prior to development of PD.5 If there is an increased risk of developing PD following a melanoma diagnosis, this could be important information with regard to counseling patients in the setting of a melanoma diagnosis and, therefore, also warrants additional study.
The Rochester Epidemiology Project (REP) medical records linkage system provides a large cohort of patients who are all residents of Olmsted County, which allows for further investigation of these under-addressed issues. To test our hypothesis that PD patients have an increased risk of developing cutaneous and choroidal melanoma following PD diagnosis, we reviewed the charts of all available PD cases in the REP database intending to compare the prevalence of melanoma to a group of age- and sex-matched controls. The results from our initial study led to a new hypothesis and review of all available melanoma cases to evaluate the risk of subsequently developing PD in these patients. Finally, we reviewed all Mayo Clinic records for additional cases of concomitant PD and conjunctival or uveal melanoma. The findings from review of both the PD and melanoma cohorts as well as additional relevant cases from review of Mayo Clinic records are reported here.
Methods
This study is in compliance with Health Insurance Portability and Accountability Act (HIPAA). This study received institutional IRB approval and adhered to the tenets of the Declaration of Helsinki.
This study was designed as a retrospective cohort study using the REP medical records linkage system. The database consists only of residents of Olmsted County, MN, and queries yield only subjects who have provided informed consent for research. Medical care of this population is provided primarily by Mayo Clinic and Olmsted Medical Center, but additional small independent clinics also participate in the REP, resulting in the capture of nearly all Olmsted County residents.24–26 Prior work has shown that this database is not biased toward patients with health conditions that require more frequent monitoring.27,28
Previous studies have shown that the REP medical records database has excellent agreement for patient status at last contact, date of last contact, and date of death when compared with manual record abstraction.29 The REP database was used when possible for these elements of the chart review.
Statistical analysis was carried out using JMP statistical discovery software from SAS (SAS Institute, Cary, NC). P-values≤.05 were considered statistically significant. When applicable, Shapiro-Wilks tests were used for normality and, when appropriate, means were compared using a two-tailed t-test.
Parkinson’s Disease Cohort
Cases
We hypothesized that there would be a significantly higher incidence of melanoma diagnosed in patients with PD compared to an age- and sex-matched cohort with similar environmental exposures. We suspected that most melanomas in patients with PD would be diagnosed after the PD diagnosis. To test this hypothesis, we found all potential cases of PD between January 1, 1976, and December 31, 2013, by searching the REP database for 33 unique H-ICDA codes and 3 unique ICD-9 codes. We then narrowed these to only those cases of PD that were diagnosed or confirmed by a neurologist. We included cases of Lewy body dementia (LBD) because it is considered to be a subdivision of the same underlying condition as PD with dementia.30 We excluded any cases that were not confirmed by a neurologist. We excluded patients with parkinsonism if such cases never met a neurologist’s criteria for PD or LBD.
Controls
Each case was individually matched by age (±1 year) and sex to three referent subjects residing in Olmsted County. Control subjects were free of PD, LBD, parkinsonism, tremor, or any of the H-ICDA and ICD-9 codes that were used to find potential cases at the index date (date of PD diagnosis in the matched PD case). The controls were generated from a list of all Olmsted County residents provided by the REP database who had recorded contact with a REP-participating provider at least once in the index year or three years thereafter.25
Controls were selected randomly from the complete list of REP subjects meeting criteria. Controls were excluded if they carried any of the 33 unique H-ICDA codes or 3 unique ICD-9 codes used to generate the case list at any time in their history of follow-up with REP-participating providers. Thus, patients who were free of PD at the index date but later developed PD that was recorded by a REP-participating provider were excluded from the control list. The presence of Alzheimer’s disease or vascular dementia was not an exclusion criterion. On manual review of the control list, one duplicate was discovered; the duplicate was removed and replaced with the next random control that met criteria.
Follow-Up and Ascertainment of Melanoma
The medical records of subjects were examined both pre- and post-index date for cases of melanoma with the aid of the REP system and manual record review. To ascertain cases of melanoma, we searched the REP database for H-ICDA and ICD-9 codes for melanoma and personal history of melanoma. All potential identified cases underwent manual chart review by a single physician to identify true melanoma cases. Cutaneous melanomas were considered true only if confirmatory pathology records were available, and cases of atypical cutaneous nevi without confirmed melanoma diagnosis by pathology were considered negative for melanoma. Uveal melanomas were considered true only if ophthalmology records confirmed the diagnosis. There were no cases of atypical choroidal nevi or conjunctival melanoma discovered.
Statistical Analysis
Logistic regression analysis was used to determine if PD patients were more likely to have a prior history of melanoma compared to controls. Patients who developed melanoma after the index date or after PD diagnosis were censored in this analysis. Average age at melanoma diagnosis and average years of pre- and post-index date follow-up were calculated as mean ± standard deviation for both PD cases and controls.
Melanoma Cohort
Cases
Results from the PD cohort study caused us to alter our hypothesis about the relationship between PD and melanoma. We hypothesized that patients with a history of melanoma would be more likely to develop PD following their melanoma diagnosis compared to age- and sex-matched controls with similar environmental exposures. To test this new hypothesis, we found all potential cases of melanoma between January 1, 1976, and December 31, 2014, by searching the REP database for 61 unique H-ICDA codes and 32 unique ICD-9 codes. All potential identified cases underwent manual chart review by a single physician to identify true melanoma cases. Cutaneous and conjunctival melanomas were confirmed by pathology records. Uveal melanomas required confirmatory ophthalmology records. No cases of atypical choroidal nevi were encountered on review, and cases of atypical cutaneous nevi without confirmed melanoma diagnosis by pathology were considered negative for melanoma.
Controls
Each case was individually matched by age (±1 year) and sex to one referent subject residing in Olmsted County selected randomly from the REP database in the same fashion as for the PD cohort. Control subjects were free of melanoma, atypical nevi, or any of the H-ICDA and ICD-9 codes that were used to find potential cases at the index date (date of melanoma diagnosis in the matched melanoma case) or at any time in their history of follow-up with REP-participating providers.
Follow-Up and Ascertainment of Parkinson’s Disease
The medical records of subjects were examined both pre- and post-index date for cases of PD with the aid of the REP system and manual record review. To ascertain cases of PD, we searched the REP database for H-ICDA and ICD-9 codes for PD and LBD. All potential identified cases underwent manual chart review by a single physician to identify only those PD and LBD cases with a neurologist-confirmed diagnosis. Finally, smoking status was recorded when available for patients in the melanoma cohort and their corresponding controls; the number of patients deceased in each group was recorded; and cause of death was recorded when applicable for patients in the melanoma cohort.
Statistical Analysis
Average age at PD diagnosis and average years of pre- and post-index date follow-up were calculated as mean ± standard deviation for both melanoma cases and controls. Of patients with available information, the percentage of patients who had ever smoked was determined for patients in the melanoma cohort and their corresponding controls. A two-tailed t-test was used to determine if any significant difference existed in the percentage of ever-smokers in the melanoma cohort compared with the controls.
A Cox proportional hazards model was used to determine the risk of post-index date PD in melanoma patients compared to controls. A Kaplan-Meier analysis with log-rank test was performed to determine if there was a difference in the 35-year cumulative risk of developing PD in the melanoma cohort when compared to controls. Patients who developed PD prior to melanoma diagnosis in the melanoma cohort or prior to the index date in the control group were censored in these analyses; and for the Kaplan-Meier analysis, the remaining patients were censored if and when their time of most recent follow-up was reached. A Cox proportional hazards model was used to calculate the risk of death from metastatic melanoma in melanoma patients with and without PD.
The results of this phase of the study were expected to be consistent with and complementary to the results of PD cohort analysis, as there was significant overlap with inclusion of patients of interest (those with both melanoma and PD).
Qualitative Case Review
A further qualitative case review was undertaken to specifically target patients relevant to the ophthalmologist. The Mayo Clinic electronic medical record system was reviewed from January 1, 1996, through August 31, 2014, for patients carrying diagnoses for both PD and uveal or conjunctival melanoma using ICD-9 codes. All cases found were manually reviewed for neurologist-confirmed PD and ophthalmologist-confirmed uveal melanoma or pathologist-confirmed conjunctival melanoma.
Results
Parkinson’s Disease Cohort
Patient Demographics
There were 974 patients diagnosed with PD between January 1976 and December 2013 in the REP database, all of whom were white. Of the 974, 58% (561) were male. The average age of PD diagnosis was 75 ± 10 and ranged from 42 to 98. All PD patients were treated with carbidopa-levodopa beginning in the index year, and treatment continued until most recent follow-up or death. A referent cohort of 2922 white patients ranged from ages 42 to 98 at the index date (date of matched PD diagnosis).
The average number of years that records were available prior to the index date was 42 ± 18 years for PD patients and 36 ± 18 years for referent subjects. The average follow-up post-index date was 5 ± 5 years for PD patients and 12 ± 8 years for referent subjects.
Risk of Melanoma
There were 32 total cases of confirmed melanoma in the PD cohort. Of these, 3 were choroidal melanomas and 29 were cutaneous melanomas. There were 63 total cases of confirmed melanoma in the group of referent subjects; one of these was choroidal melanoma, while the remainder were cutaneous. There were no cases of ciliary body, iris, or conjunctival melanoma in either group. There were no cases of melanoma involving the eyelid. All cases of choroidal melanoma for both PD and control groups were diagnosed pre-index date, and all of these cases were in male subjects.
In the PD group, only six melanomas (all cutaneous) were diagnosed after PD diagnosis. The remainder were diagnosed prior to patients receiving any PD treatment, leaving 26 total pre-index date melanomas (2.7% of the total PD cohort). In the control group, 21 (33%) of the total 63 melanomas were diagnosed pre-index date, corresponding to only 0.7% of the control group. Examining pre-index date melanomas alone, logistic regression analysis revealed a 3.8-fold increased likelihood of PD patients having a prior history of melanoma compared to controls (CI 2.1–6.8, P<.001). Because there were only six post-index date melanomas in the PD group, the data did not lend itself to Kaplan-Meier analysis with good reliability.
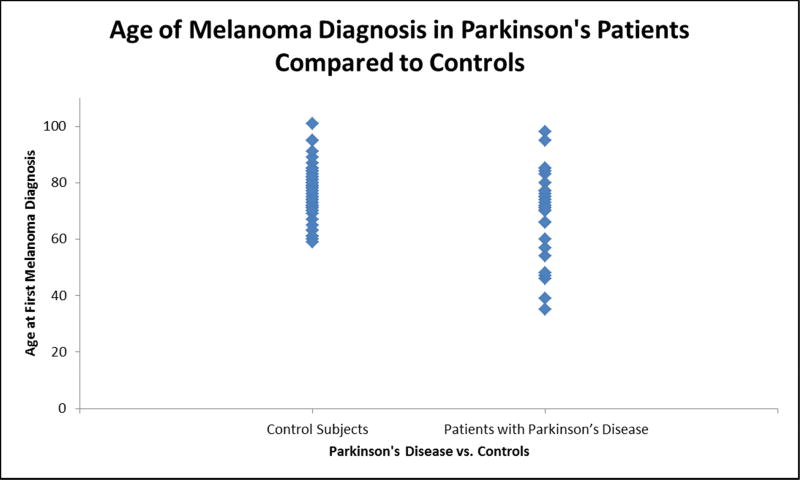
Prior to proceeding with t-test comparisons, Shapiro-Wilks tests were performed on subset data for age of melanoma diagnosis in both case and control groups to test normality, which showed P=.12 and P=.29, respectively. Patients with PD were found to develop melanoma an average of 7 years younger (70 ± 15) than patients in the control group (77 ± 8). This was statistically significant (P=.004, Figure 1).
Figure 1.
Age of melanoma diagnosis in Parkinson’s disease (PD) patients compared to controls. A scatterplot of the age at first melanoma diagnosis is shown for both controls and patients with PD. Patients with PD developed melanoma seven years younger (70 ± 15) than patients in the control group (77 ± 8). This reached statistical significance (P=.004).
Melanoma Cohort
Patient Demographics
There were 1544 patients diagnosed with melanoma between January 1976 and December 2014 in the REP database: 1540 whites, 2 Asians, 1 Pacific Islander, and 1 American Indian. Of the 1544, 52% (810) were male. The average age at melanoma diagnosis was 57 ± 18 and ranged from 10 to 101. Of the 1544 patients with melanoma, 1497 had cutaneous melanoma. Four of these cases involved the eyelid, and all but one patient who refused treatment underwent primary surgical excision. Of the remaining 47 patients, 40 had choroidal melanoma. Documentation of treatment was not found for all patients; but of the available information, 16 patients with choroidal melanoma underwent plaque brachytherapy, 10 had enucleation, 2 had transpupillary thermotherapy alone, 1 had cryotherapy alone, and 1 underwent exenteration for extrascleral extension. Finally, there were four patients with conjunctival melanoma who underwent surgical excision, two patients with ciliochoroidal melanoma for whom documentation of treatment was not found, and one patient with iris melanoma who underwent plaque brachytherapy.
A referent cohort of 1544 patients (of whom 1542 were white and 2 were Asian) ranged from ages 10 to 101 at the index date (date of matched melanoma diagnosis). The average number of years that records were available prior to the index date was 31 ± 19 years for melanoma patients and 44 ± 13 years for referent subjects. The average follow-up post-index date was 7 ± 8 years for melanoma patients and 8 ± 10 years for referent subjects.
Information regarding smoking status was available for 88% of patients in the melanoma cohort and 85% of the controls. Of patients with available data, 36% of the melanoma patients were prior or current smokers, and 40% of the controls were eversmokers. This difference was not statistically significant (P=.67).
Risk of Parkinson’s Disease
There were 43 total cases of confirmed PD in the melanoma cohort, all of whom were white. Of these, 3 were in patients with choroidal melanomas and 40 were in patients with cutaneous melanomas, none of which involved the eyelid. The 3 choroidal melanoma patients were male, and 28 of the cutaneous melanoma patients were male. There were 14 total cases of confirmed PD in the group of referent subjects, all of whom were white, and 9 of whom were male.
Before t-test comparisons, Shapiro-Wilks tests were performed on subset data for age of PD diagnosis in both case and control groups to test normality (P=.08 and P=.09, respectively). Patients with melanoma were found to develop PD at about the same age (77±11 years old) as patients without melanoma (78±11 years old), P=.78.
All three cases of choroidal melanoma developed PD post-index date, and these were the same three male cases found in the original PD cohort analysis. Melanoma treatment information was available for only one of the three cases, and he was reported to have had plaque brachytherapy. In the melanoma cohort, only 6 cases of PD were diagnosed prior to melanoma diagnosis (0.4%), and these were the same six cutaneous melanoma patients described in the PD cohort. The remaining cases of PD were diagnosed following initial diagnosis and treatment of melanoma; these patients, on average, developed PD 11±12 years following diagnosis of melanoma. However, PD was found to develop as late as 51 years following melanoma diagnosis in this study. In the control group, 5 of the total 14 cases of PD were diagnosed prior to the index date (0.3%), and the remaining 9 cases developed PD an average of 11±7 years following the index date. The percentage of pre-index date PD diagnoses was not statistically significant between the case and control groups (P=.76), and these patients were censored from further analysis.
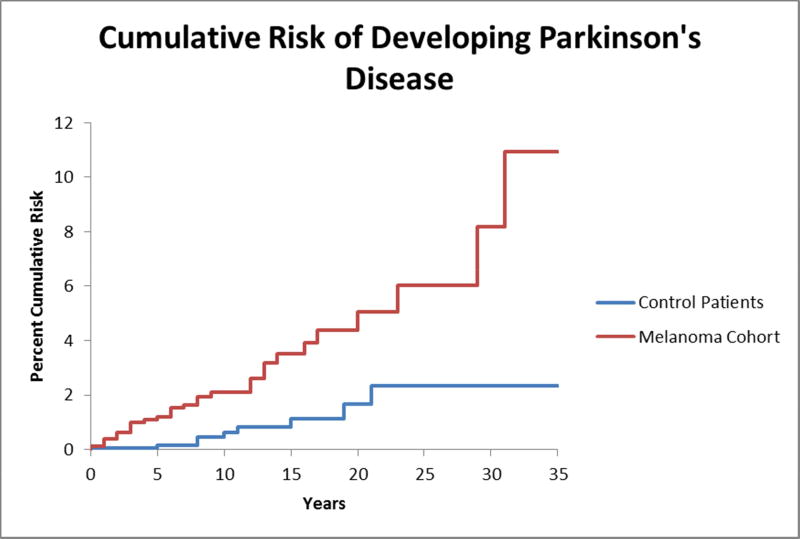
Using a Cox proportional hazards model, the melanoma cohort was found to have a 4.2-fold increased risk of developing PD post-index date compared to controls (CI 2.0–8.8, P<.001). Kaplan-Meier analysis and a log-rank test were performed to further examine the risk of developing PD following a melanoma diagnosis when compared to controls without melanoma. Patients in the melanoma cohort were found to have an 11.8% (CI 4.0–19.6%) 35-year cumulative risk of developing PD compared to a 2.6% (CI 0.4–4.8%) 35-year cumulative risk in controls, and this difference was statistically significant, P<.001 (Figure 2).
Figure 2.
Cumulative risk of developing Parkinson’s disease (PD) in patients with melanoma compared to age- and sex-matched controls. A failure plot derived from Kaplan-Meier analysis is shown for both patients with melanoma and their corresponding controls. Patients were censored from analysis if they carried a diagnosis of PD prior to melanoma diagnosis or prior to the index date. Patients were censored throughout the analysis if and when their time of last follow-up was reached. The 35-year cumulative risk of developing PD was approximately 11.8% in patients with a history of melanoma compared to only 2.6% in controls. This difference was statistically significant by log-rank test (P<.001).
Deceased Patients
At the time of record review, there were 345 patients (22%) deceased in the melanoma cohort, 316 without PD and 29 with PD; there were 322 patients (21%) deceased in the control group, 313 without PD and 9 with PD. Upon further examination of the 345 deceased patients in the melanoma cohort, the cause of death for 115 of the 345 (33%) was metastatic melanoma, and only 1 of those 115 had PD; that patient carried a diagnosis of cutaneous melanoma of the leg. Thus, within the melanoma cohort, patients without PD had a 10.5-fold increased risk of dying from metastatic melanoma compared to patients with PD (CI 1.5–72.2, P=.02).
Qualitative Case Review
An additional 10 patients (5 females) with PD were found to have choroidal melanoma (one ciliochoroidal) on review of the Mayo Clinic electronic records. There was also one case of ciliary body melanoma and one case of conjunctival melanoma found in male PD patients on chart review. Three of the patients with choroidal melanoma were diagnosed after PD diagnosis and after treatment with carbidopa-levodopa had begun. However, the remainder were diagnosed prior to PD diagnosis and prior to any PD treatment.
Discussion
This retrospective cohort study provides supportive evidence for an association between PD and melanoma, and the REP database provides an ideal platform for the study, ensuring that all subjects reside in the same geographic environment.
Parkinson’s Disease Cohort
Review of the PD cohort demonstrated an increased risk of prior melanoma in patients with PD. The 3.8-fold increased risk has important clinical implications. Melanoma is a curable disease if detected and treated early. Thus, a compelling argument can be made for more aggressive melanoma-screening protocols in PD patients.
Interestingly, the majority of melanomas diagnosed in the PD group were diagnosed prior to diagnosis or treatment for PD. Only six melanomas were diagnosed after PD treatment had begun, and those were all cutaneous melanomas. This is in contrast to the control group in which the majority (67%, 42 of 63) of melanomas was diagnosed after the index date. This makes a compelling case for a genetic association between melanoma and PD and argues against any role of levodopa in increasing melanoma risk, which is consistent with results previously found by Olsen et al.19 However, it is important to take into consideration that the control group had a longer duration of follow-up than the PD patients by an average of seven years. It is possible that this accounts for some of the disparity between the number of post-index date melanoma cases between the PD and control groups. If the PD patients had longer follow-up, it is likely that more cases of melanoma would have been found.
The PD patients in this study who developed melanoma were found to be seven years younger at first melanoma diagnosis than those in the control group. Combining this knowledge with the fact that most melanomas in PD patients were diagnosed prior to PD diagnosis in this study, it is clear that waiting for a PD diagnosis to begin aggressive screening measures for the majority of these patients would be too late. Thus, a combination of family history of PD and/or melanoma along with environmental risk factors may help guide screening. Additional risk factors associated with PD include: rural living, pesticide use (controversial), head trauma, depression, and family history of tremor.31,32 Certain genetic variations, including alpha-synuclein, parkin, and LRRK2 mutations, are also associated with development of PD and PD-associated dementia.33,34 It could be that some of the environmental or genetic risk factors for PD are also risk factors for melanoma; and as it stands, fair skin color and male sex are known risk factors for both diseases.35–38 Moreover, a recent study has documented an increase in the incidence of PD between 1976 and 2005, presumably related to a change in environmental risk factors,39 and there has been an increasing incidence of melanoma40,41 as well. Still, it remains unclear exactly how we might use these other risk factors to determine the necessity of melanoma screening for any given patient. Notably, smoking is not a risk factor for melanoma and was, therefore, not examined in this portion of the study.42,43
Melanoma Cohort
The melanoma cohort was collected and reviewed about a year following the original study of the PD cohort. This allowed time for an additional 11 patients with prior melanoma diagnoses to develop PD. These 11, who all had an existing diagnosis of cutaneous melanoma at the time of their PD diagnosis, were added to the 32 examined in the original PD cohort.
Because all but six patients developed PD after a pre-existing melanoma diagnosis, the melanoma cohort lent itself to Kaplan-Meier analysis, which showed a statistically significant difference in the 35-year cumulative risk of developing PD in melanoma patients (11.8%) compared to controls (2.6%). This was complementary to the 4.2-fold increased risk of post-index date PD found in the melanoma cohort using a Cox proportional hazards model. Given this information, it actually brings up an interesting thought that the physicians diagnosing patients with melanoma may need to consider counseling their patients about an increased risk of developing PD an average of 11 years down the road.
Smoking status was examined in the melanoma cohort and their corresponding controls because patients who have ever smoked have a known two-fold decreased risk of developing PD.44 There was no significant difference between the number of ever-smokers in the melanoma cohort when compared to controls, so we do not suspect that this was a confounding factor.
As a point of interest, the cause of death in the melanoma cohort patients was also examined. Of patients who were deceased at the time of record review, only 1 of the 29 patients with PD died of metastatic melanoma compared to 114 deaths from metastatic melanoma in the 316 patients without PD. This translated to a 10.5-fold increased risk of dying from metastatic melanoma in patients without PD in the setting of a known melanoma diagnosis. This suggests that somehow PD may be a protective factor when considering the risk of metastatic melanoma, but it is also possible that there is an underlying immune reaction that both suppresses development of metastatic melanoma and causes PD. It is known that patients with PD have inadequate innate immune responses, increased markers of adaptive immunity, and disruption of the blood brain barrier.45 This makes a common underlying immunologic association between PD and melanoma plausible.
Qualitative Case Review
The additional 11 cases of uveal melanoma and one case of conjunctival melanoma found in PD patients on qualitative review of the Mayo Clinic electronic records further compel the ophthalmologist to be on heightened alert both in terms of screening patients with PD for melanoma and potentially counseling patients diagnosed with uveal and conjunctival melanoma that they may be at increased risk for PD. Furthermore, because half (five of ten) of the choroidal melanoma cases were female in the qualitative case review; this suggests that a relationship between PD and uveal melanoma is not unique to males despite the fact that all three patients with concomitant PD and uveal melanoma diagnoses from the REP were male.
Limitations
As with all retrospective reviews, this study has its limitations. Detailed information was not available on a large enough proportion of subjects to adequately assess differences in environmental risk factors between the case and control groups. However, all patients were taken from the REP database, meaning that all were residents of Olmsted County. This allows for some degree of uniformity with regard to environmental exposures. Moreover, use of the REP database limits referral bias that can often be found with studies performed at large academic institutions, while ensuring good capture of all patients with PD and melanoma diagnoses residing in Olmsted County. However, one must still consider that patients diagnosed with cancer are more likely to have regular physician contact, which could possibly lead to an increased rate or earlier diagnosis of PD in patients with a cancer history. Finally, there were limitations on the number of subjects available for review in this study due to the relatively small geographic region that was chosen. Thus, it was not possible to perform reliable statistical analysis of sub-groups, such as cutaneous or choroidal melanomas alone, nor was it possible to reliably perform Kaplan-Meier analysis in the phase I study.
Recommendations and Future Directions
The results of this study provide supportive evidence for an association between PD and melanoma and discourage acceptance of prior suggestions that levodopa is the root cause of this link. It is more likely that there are common environmental, genetic, or immunologic abnormalities underlying both conditions in these patients, but future research is necessary to confirm and better characterize the underlying cause of this relationship. After the underlying cause of the relationship between melanoma and PD is better understood, more definitive recommendations can be made regarding the level of rigor and frequency of melanoma screening required in PD patients as well as the likelihood that a given patient with melanoma will develop PD in the future. For now, because of the apparent increase in incidence of both diseases and their reciprocal relationships, it is important for physicians to be vigilant for one disease in the context of the other.39–41
Conclusion
The association between PD and melanoma holds importance to a wide array of healthcare providers, including general practitioners, dermatologists, neurologists, ophthalmologists, and oncologists. Future research will provide further insight into the cause of this relationship as well as necessary screening or patient counseling measures that should be implemented.
Acknowledgments
Financial Support: Supported by a grant from the VitreoRetinal Surgery Foundation, Minneapolis, MN, and unrestricted grants from Research to Prevent Blindness Inc., New York, NY, the Paul Family and the Deshong Family. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CI
Confidence interval
- LBD
Lewy body dementia
- PD
Parkinson’s Disease
- REP
Rochester Epidemiology Project
- RR
Relative risk
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest to disclose.
For consideration of publication in Mayo Clinic Proceedings.
References
- 1.Ganguli M, Lotze MT. Parkinson Disease and Malignant Disease: Minding Cancer's Own Business. JAMA Oncol. 2015;1(5):641–642. doi: 10.1001/jamaoncol.2015.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skibba JL, Pinckley J, Gilbert EF, Johnson RO. Multiple primary melanoma following administration of levodopa. Arch Pathol. 1972;93(6):556–561. [PubMed] [Google Scholar]
- 3.Fiala KH, Whetteckey J, Manyam BV. Malignant melanoma and levodopa in Parkinson's disease: causality or coincidence? Parkinsonism Relat Disord. 2003;9(6):321–327. doi: 10.1016/s1353-8020(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 4.Abramson DH, Rubenfeld MR. Choroidal melanoma and levodopa. Jama. 1984;252(8):1011–1012. [PubMed] [Google Scholar]
- 5.Liu R, Gao X, Lu Y, Chen H. Meta-analysis of the relationship between Parkinson disease and melanoma. Neurology. 2011;76(23):2002–2009. doi: 10.1212/WNL.0b013e31821e554e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachinohe M, Matsumoto H. Involvement of reactive oxygen species generated from melanin synthesis pathway in phytotoxicty of L-DOPA. J Chem Ecol. 2005;31(2):237–246. doi: 10.1007/s10886-005-1338-9. [DOI] [PubMed] [Google Scholar]
- 7.Pawelek JM, Korner AM. The biosynthesis of mammalian melanin. Am Sci. 1982;70(2):136–145. [PubMed] [Google Scholar]
- 8.Osman AM, Amer TM. Effect of L-dopa on the growth of human melanoma cells in vitro. J Pharm Belg. 1987;42(5):323–326. [PubMed] [Google Scholar]
- 9.Sandyk R. Accelerated growth of malignant melanoma by levodopa in Parkinson's disease and role of the pineal gland. Int J Neurosci. 1992;63(1–2):137–140. doi: 10.3109/00207459208986663. [DOI] [PubMed] [Google Scholar]
- 10.Bertoni JM, Arlette JP, Fernandez HH, et al. Increased melanoma risk in Parkinson disease: a prospective clinicopathological study. Arch Neurol. 2010;67(3):347–352. doi: 10.1001/archneurol.2010.1. [DOI] [PubMed] [Google Scholar]
- 11.Weiner WJ, Singer C, Sanchez-Ramos JR, Goldenberg JN. Levodopa, melanoma, and Parkinson's disease. Neurology. 1993;43(4):674–677. doi: 10.1212/wnl.43.4.674. [DOI] [PubMed] [Google Scholar]
- 12.Gurney H, Coates A, Kefford R. The use of L-dopa and carbidopa in metastatic malignant melanoma. J Invest Dermatol. 1991;96(1):85–87. doi: 10.1111/1523-1747.ep12515896. [DOI] [PubMed] [Google Scholar]
- 13.Elbaz A, Peterson BJ, Bower JH, et al. Risk of cancer after the diagnosis of Parkinson's disease: a historical cohort study. Mov Disord. 2005;20(6):719–725. doi: 10.1002/mds.20401. [DOI] [PubMed] [Google Scholar]
- 14.Olsen JH, Friis S, Frederiksen K, McLaughlin JK, Mellemkjaer L, Moller H. Atypical cancer pattern in patients with Parkinson's disease. Br J Cancer. 2005;92(1):201–205. doi: 10.1038/sj.bjc.6602279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigel DS, Patel Z, Bolognia J, Eichler C, Ellis DL, Friedman RJ. Evaluation of Parkinson's disease (PD) prevalence in patients with malignant melanoma (Poster 22) Mov Disord. 2006;21(S13):S58. [Google Scholar]
- 16.Constantinescu R, Elm J, Auinger P, et al. Malignant melanoma in early-treated Parkinson's disease: the NET-PD trial. Mov Disord. 2014;29(2):263–265. doi: 10.1002/mds.25734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constantinescu R, Romer M, Kieburtz K. Malignant melanoma in early Parkinson's disease: the DATATOP trial. Mov Disord. 2007;22(5):720–722. doi: 10.1002/mds.21273. [DOI] [PubMed] [Google Scholar]
- 18.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Prospective case-control study of nonfatal cancer preceding the diagnosis of Parkinson's disease. Cancer Causes Control. 2007;18(7):705–711. doi: 10.1007/s10552-007-9005-9. [DOI] [PubMed] [Google Scholar]
- 19.Olsen JH, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology. 2006;17(5):582–587. doi: 10.1097/01.ede.0000229445.90471.5e. [DOI] [PubMed] [Google Scholar]
- 20.Walter U, Heilmann E, Voss J, et al. Frequency and profile of Parkinson's disease prodromi in patients with malignant melanoma. J Neurol Neurosurg Psychiatry. 2015 doi: 10.1136/jnnp-2014-310239. [DOI] [PubMed] [Google Scholar]
- 21.Elbaz A, Peterson BJ, Yang P, et al. Nonfatal cancer preceding Parkinson's disease: a case-control study. Epidemiology. 2002;13(2):157–164. doi: 10.1097/00001648-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Haider SA, Thaller VT. Lid melanoma and parkinsonism. Br J Ophthalmol. 1992;76(4):246–247. doi: 10.1136/bjo.76.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Rens GH, De Jong PT, Demols EE, Brihaye-Van Geertruyden MF. Uveal malignant melanoma and levodopa therapy in Parkinson's disease. Ophthalmology. 1982;89(12):1464–1466. doi: 10.1016/s0161-6420(82)34616-3. [DOI] [PubMed] [Google Scholar]
- 24.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 25.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 26.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campion ME, Naessens JM, Leibson CL, Shaller D, Ballard DJ. The Olmsted County Benchmark Project: primary study findings and potential implications for corporate America. Mayo Clin Proc. 1992;67(1):5–14. doi: 10.1016/s0025-6196(12)60270-5. [DOI] [PubMed] [Google Scholar]
- 28.Phillips SJ, Whisnant JP, O'Fallon WM, Hickman RD. A community blood pressure survey: Rochester, Minnesota, 1986. Mayo Clin Proc. 1988;63(7):691–699. doi: 10.1016/s0025-6196(12)65531-1. [DOI] [PubMed] [Google Scholar]
- 29.Elbaz A, Bower JH, Peterson BJ, et al. Survival study of Parkinson disease in Olmsted County, Minnesota. Arch Neurol. 2003;60(1):91–96. doi: 10.1001/archneur.60.1.91. [DOI] [PubMed] [Google Scholar]
- 30.Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson's disease with dementia. J Neurol. 2008;255(Suppl):539–47. doi: 10.1007/s00415-008-5007-0. [DOI] [PubMed] [Google Scholar]
- 31.Hubble JP, Cao T, Hassanein RE, Neuberger JS, Koller WC. Risk factors for Parkinson's disease. Neurology. 1993;43(9):1693–1697. doi: 10.1212/wnl.43.9.1693. [DOI] [PubMed] [Google Scholar]
- 32.Taylor CA, Saint-Hilaire MH, Cupples LA, et al. Environmental, medical, and family history risk factors for Parkinson's disease: a New England-based case control study. Am J Med Genet. 1999;88(6):742–749. [PubMed] [Google Scholar]
- 33.Nalls MA, Escott-Price V, Williams NM, et al. Genetic risk and age in Parkinson's disease: Continuum not stratum. Mov Disord. 2015;30(6):850–854. doi: 10.1002/mds.26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romo-Gutierrez D, Yescas P, Lopez-Lopez M, Boll MC. Genetic factors associated with dementia in Parkinson's disease (PD) Gac Med Mex. 2015;151(1):110–118. [PubMed] [Google Scholar]
- 35.Pan T, Li X, Jankovic J. The association between Parkinson's disease and melanoma. Int J Cancer. 2011;128(10):2251–2260. doi: 10.1002/ijc.25912. [DOI] [PubMed] [Google Scholar]
- 36.Cho E, Rosner BA, Feskanich D, Colditz GA. Risk factors and individual probabilities of melanoma for whites. J Clin Oncol. 2005;23(12):2669–2675. doi: 10.1200/JCO.2005.11.108. [DOI] [PubMed] [Google Scholar]
- 37.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. 2004;75(4):637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Family history of melanoma and Parkinson disease risk. Neurology. 2009;73(16):1286–1291. doi: 10.1212/WNL.0b013e3181bd13a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Time Trends in the Incidence of Parkinson Disease. JAMA Neurol. 2016;73(8):981–989. doi: 10.1001/jamaneurol.2016.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed KB, Brewer JD, Lohse CM, Bringe KE, Pruitt CN, Gibson LE. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87(4):328–334. doi: 10.1016/j.mayocp.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 42.Westerdahl J, Olsson H, Masback A, Ingvar C, Jonsson N. Risk of malignant melanoma in relation to drug intake, alcohol, smoking and hormonal factors. Br J Cancer. 1996;73(9):1126–1131. doi: 10.1038/bjc.1996.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman DM, Sigurdson A, Doody MM, Rao RS, Linet MS. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control. 2003;14(9):847–857. doi: 10.1023/b:caco.0000003839.56954.73. [DOI] [PubMed] [Google Scholar]
- 44.Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD. Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. American journal of epidemiology. 2002;155(8):732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, Halliday GM. Aspects of innate immunity and Parkinson's disease. Frontiers in pharmacology. 2012;333 doi: 10.3389/fphar.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]