Abstract
The cardiac transverse (T)-tubule membrane system is the safeguard for cardiac function and undergoes dramatic remodeling in response to cardiac stress. However, the mechanism by which cardiomyocytes repair damaged T-tubule network remains unclear. In the present study, we tested the hypothesis that MG53, a muscle-specific membrane repair protein, antagonizes T-tubule damage to protect against maladaptive remodeling and thereby loss of excitation-contraction coupling and cardiac function. Using MG53-knockout (MG53-KO) mice, we first established that deficiency of MG53 had no impact on maturation of the T-tubule network in developing hearts. Additionally, MG53 ablation did not influence T-tubule integrity in unstressed adult hearts as late as 10 months of age. Following left ventricular pressure overload-induced cardiac stress, MG53 protein levels were increased by approximately three-fold in wild-type mice, indicating that pathological stress induces a significant upregulation of MG53. MG53-deficient mice had worsened T-tubule disruption and pronounced dysregulation of Ca2+ handling properties, including decreased Ca2+ transient amplitude and prolonged time to peak and decay. Moreover, MG53 deficiency exacerbated cardiac hypertrophy and dysfunction and decreased survival following cardiac stress. Our data suggest MG53 is not required for T-tubule development and maintenance in normal physiology. However, MG53 is essential to preserve T-tubule integrity and thereby Ca2+ handling properties and cardiac function under pathological cardiac stress.
Keywords: Cardiomyocytes, MG53 (or TRIM72), T-tubules, Calcium, Sodium-calcium exchanger, Excitation-contraction coupling
1. Introduction
Repair of damage to the plasma membrane is an active and dynamic process that includes detection of acute membrane injury, recruitment of intracellular vesicles to the injury site, and vesicle fusion to enable formation of a membrane patch to reseal the damaged membrane. MG53, also known as tripartite motif 72 (TRIM72), a muscle-specific member of the tripartite motif/RING B-box Coiled Coil (TRIM/RBCC) family proteins, is a critical component of the sarcolemma repair machinery in striated muscle [1–5]. In response to acute membrane damage, MG53 facilitates vesicle translocation to the injury sites to reseal the damaged membrane within a matter of seconds [5,6]. Mice deficient in MG53 have defective membrane repair function after acute muscle injury and develop progressive myopathy with age [1].
While the majority of studies have analyzed the role of MG53 in striated muscle, genetic ablation of MG53 prohibits emergency sarcolemmal resealing after infrared laser–induced acute membrane damage in intact hearts and increases myocardial vulnerability to ischemia/reperfusion injury [2,3]. In line with a potential role for MG53 in cardiac function, gene delivery of MG53 enhances membrane repair, ameliorates pathology, and improves cardiac functions in hamsters with congestive heart failure [7]. However, the mechanisms by which MG53 provides cardiac protection following injury still remain elusive.
It is well-established that cardiac stress is associated with massive deterioration of the cardiomyocyte transverse (T)-tubule membrane system. The organized T-tubule system provides a structural basis for rapid electric excitation, initiation and synchronous triggering of SR Ca2+ release, and thus coordinated contraction of each contractile unit throughout the entire myocyte [8,9]. Compelling evidence from our group and others demonstrates that disruption of the T-tubule network is mechanistically involved in excitation-contraction (E-C) coupling dysfunction and the development and progression of heart failure [9–18].
While T-tubule damage is considered to be caused by pathological remodeling, it may also result from ineffective membrane repair in response to injury. Since others have reported that MG53 deletion abolishes membrane resealing after injury and thereby exaggerates ischemia/reperfusion-induced cardiac injury [2,3], our objective in this study was to examine the contribution of MG53 to maintenance of myocyte ultrastructure and cardiac function in developing hearts and following cardiac stress. We hypothesized that the MG53 is involved in T-tubule biogenesis and maintenance. Using MG53-deficient mice, we found that MG53 is dispensable for T-tubule development and cardiac function as late as 10 months of age at baseline. However, in response to LV pressure overload-induced hypertrophy and heart failure, MG53 is essential to maintain T-tubule integrity and thereby preserve Ca2+ handling properties and gross cardiac function.
2. Materials and methods
2.1. Animals and MG53 gene knockout mice
Animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85–23, revised 1996) and were approved by the Institutional Animal Care and Use Committee at the University of Iowa. MG53 knockout (MG53-KO) mice were generated, as described previously [1]. MG53-KO mice were backcrossed with C57BL/6J mice for 10 generations to generate MG53-KO mice on a C57BL/6J background. Conventional PCR was used for genotyping. Wild-type littermates were used as control mice.
2.2. Transaortic banding surgery
Male MG53-KO mice and WT littermates (9–10 weeks) were subjected to pressure overload by transaortic banding (TAB) surgery, as previously described [19]. Briefly, mice were anesthetized with keta-mine/xylazine (87.5 mg/kg/12.5 mg/kg, i.p.) and ventilated with a small rodent ventilator. A thoracotomy was created between the second and third intercostal space, and the aortic arch was visualized. Aortic constriction was performed by tying a 7–0 nylon suture ligature against a 27-gauge needle to yield a narrowing 0.4 mm in diameter when the needle was removed and a reproducible TAB of ~ 70%. In sham mice, the aortic arch was visualized but not banded. Mouse survival rate was recorded from 48 h after surgery; mice that died within 48 h of surgery were excluded from the analysis. Left ventricle (LV) function was examined by echocardiography 5 weeks after TAB or sham surgery as previously described [20]. Confocal imaging of T-tubule structure and intracellular Ca2+ in subepicardial myocytes from intact hearts was performed on the next day after echocardiography.
2.3. Mouse exercise training protocol
C57BL/6 mice were trained on treadmill at 20° incline every other day for 4 weeks. Each session consisted of 4 sets of 6 min intervals at 80 – 90% of maximum velocity (Vmax), with 4 min of active rest between intervals. The control (sedentary) group was handled like the training group and placed on non-moving treadmill for same time periods.
2.4. Confocal calcium and T-tubule imaging
Cytosolic Ca2+ from intact hearts was stained with Rhod-2 AM at 10 μmol/L (AAT BioQuest, CA) in 1.8 mmol/L [Ca2+]o Tyrode solution via Langendorff perfusion at room temperature for 30 min [21]. Calcium transients were acquired using confocal microscope (LSM 510, Carl Zeiss MicroImaging Inc., Germany) with 63× lens in line–scan mode along the transverse axis of myocytes under autonomous beating. After completing Ca2+ imaging, hearts were perfused with MM 4–64 (2.5 μmol/L, AAT BioQuest, CA) in [Ca2+]o free Tyrode solution at room temperature for 30 min to stain T-tubule membranes. The structure of T-tubules was visualized with the same confocal microscope in frame-scan mode. Quantitative analysis of T-tubule integrity was processed with IDL image analysis program as previously reported. The power value (TTpower) reflects the strength of the regularity of T-tubule organization [15].
2.5. Protein preparation and western blotting
Heart lysates were prepared as previously described [22]. Briefly, the whole hearts were homogenized and sonicated in lysis buffer (in mmol/L: Tris-HCl 50 [pH 7.4], NaCl 150, NaF 10, Na3VO4 1, EGTA 5, EDTA 5, 0.5% Triton X-100, 0.5% Na deoxycholate, 0.1% SDS, and protease inhibitors (#P8340, Sigma-Aldrich)). After standing on ice for 1 h allowing lysis, the homogenates were centrifuged at 13,000g 4 °C for 15 min. The supernatants were kept as whole cell proteins. Protein concentration was measured by BCA assay.
Proteins were separated on 4–12% Bis-Tris gels and transferred to PVDF membrane. The membrane was probed with primary antibodies against MG53 (provided by Dr. Jianjie Ma), NCX1 (1:1000, #R3F1, Swant, Switzerland), RyR2 (#MA3-916, Thermo Scientific), SERCA2a (#MA3-919, Thermo Scientific), Junctophilin-2 (JP2, #SC51313, Santa Cruz Biotechnology), Amphiphysin 2 (Bin1, #SC23918, Santa Cruz Biotechnology) and GAPDH (1:10,000, #G8975, Sigma-Aldrich) overnight at 4 °C, followed by incubation with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000–1:10,000 dilution in PBS solution). The immunoreactions were visualized using an enhanced-chemiluminescent detection kit and the protein bands were quantified with Quantity One 1-D Analysis Software (Bio-Rad, USA).
2.6. Statistical analysis
Data are expressed as mean±SE. One-way ANOVA followed by Bonferroni procedure was applied to multiple group comparisons. Statistical analyses were carried out using SPSS V15.0 software. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. MG53 protein is not required for maturation of the T-tubule system during cardiac development
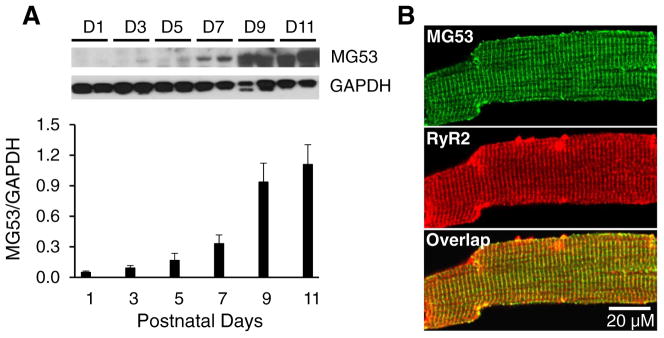
We first established the expression pattern of MG53 in whole heart lysates from C57BL/6 mice at postnatal days 1, 3, 5, 7, 9, and 11. MG53 was detected as early as postnatal Day 7, increased substantially at Day 9 and continued to increase at Day 11 (Fig. 1A). We previously reported that some sporadic T-tubules are present at postnatal Day 8, with an obvious T-tubule network apparent at Day 10 [23]. In adult cardiomyocytes, MG53 co-localized with RyR2 in the T-tubule/SR junctional region (Fig. 1B).
Fig. 1.
Expression pattern of MG53 in developing hearts and its localization in cardiomyocytes. A, Representative immunoblot and summary data of MG53 expression in C57Bl/6J murine hearts. GAPDH: loading control. Data shown are mean ±SE. B, Representative Confocal images of immunofluorescent staining of MG53 (green) and RyR2 (red) in 2 months old of adult cardiomyocytes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
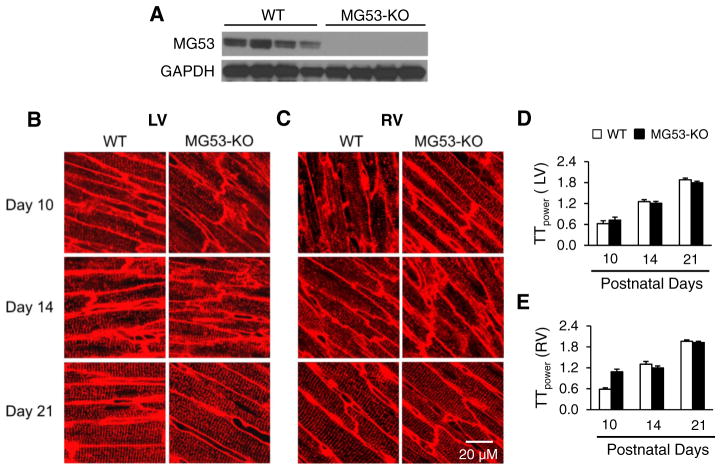
Since the pattern of MG53 expression occurred earlier than T-tubule development, we hypothesized that MG53 may participate in T-tubule maturation. Therefore, we examined T-tubule structure in intact hearts from WT and MG53-KO mice using in situ confocal imaging. We confirmed that MG53-KO mice have no detectable MG53 protein in heart lysates (Fig. 2A). Consistent with our previous study [23], an immature T-tubule network was observed in both left and right ventricles in WT mice at postnatal Day 10, with a fully mature T-tubule network at Day 21 (Fig. 2B–E). Unexpectedly, deficiency of MG53 did not alter the time course of T-tubule maturation. Quantification of T-tubule structural integrity using the T-tubule power (TTpower) index revealed no significant difference in TTpower in LV or RV myocytes from WT and MG53-KO hearts at Days 10, 14, and 21 (Fig. 2D, E). These data indicate that MG53, unlike JP2 [23], is not necessary for T-tubule maturation during cardiac development.
Fig. 2.
Deficiency of MG53 does not alter T-tubule maturation. A, Immunoblots of MG53 protein expression in heart lysates from WT and MG53-KO mice. GAPDH: loading control. B & C, Representative confocal of left ventricular (LV, B) or right ventricular (RV, C) T-tubules from WT or MG53-KO mice at postnatal days 10, 14, and 21. T-tubules were stained with a lipophilic marker MM 4–64. D & E, Mean values of TTpower in the LV (D) and RV (E) of WT and MG53-KO mice at different ages. N=4 mice in each group. Data shown are mean ± SE.
3.2. MG53 is not required for maintaining T-tubule integrity under physiological conditions
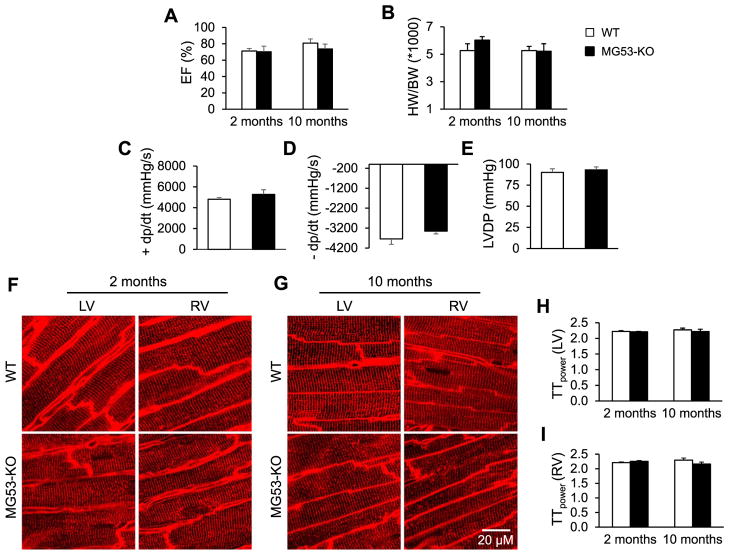
We next examined the impact of MG53 deletion on cardiac function and T-tubule integrity in adult mice under physiological conditions. Echocardiographic data showed no impairment in gross cardiac function in 2- and 10-month-old MG53-KO mice as compared to age-matched WT mice (Fig. 3A). There was also no difference in heart weight to body weight ratio (Fig. 3B). In vivo hemodynamic measurement of LV development pressure indicated no change in cardiac contractile function in 10-month-old MG53-KO mice (Fig. 3C–E). Confocal imaging of T-tubules in intact hearts revealed that MG53 deletion did not affect T-tubule integrity in either the left and right ventricles at 2 months and 10 months of age (Fig. 3F–I). Thus, MG53 expression is not necessary to maintain cardiac function or T-tubule integrity in myocardium under physiological conditions.
Fig. 3.
Cardiac function and T-tubule integrity is normal in MG53-KO mice at 2 and 10 months of age under normal physiological conditions. A, Ejection fraction (EF). B, Ratio of heart weight to body weight (HW/BW). C–E, In vivo hemodynamic measurement of LV development pressure in mice of 10 months old. F–G, Representative confocal T-tubule images in the LV and the RV of hearts from WT and MG53-KO mice at 2 months (F) and 10 months of age (G). H & I, Mean values of TTpower in LV (H) and RV (I) of WT and MG53-KO mice at different ages. N =5 mice in each group. Data shown are mean ±SE.
3.3. MG53 preserves T-tubule ultrastructure and Ca2+ handling properties following cardiac stress
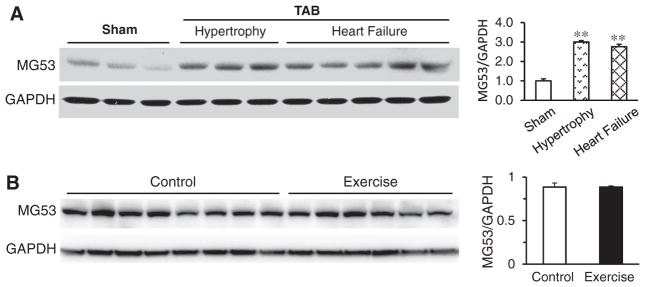
Given that MG53 is necessary for membrane repair in response to injury, and T-tubules are an extension of the plasma membrane, we tested the hypothesis that MG53 is required to maintain T-tubule integrity following cardiac stress. Within 5 weeks of left ventricular pressure overload (TAB), MG53 levels were increased by approximately three-fold in WT mice as compared to sham-operated controls (Fig. 4A). On the contrary, MG53 expression levels were not altered under physiological stress such as exercise training (Fig. 4B). These data indicate that pathological but not physiological stress promotes increased MG53 expression.
Fig. 4.
Pathological but not physiological stress promotes MG53 expression. A, MG53 expression levels were significantly increased by 3-fold and 2.76-fold, respectively, in compensated hypertrophy and heart failure following 5-week pressure overload cardiac stress. **, p< 0.01 vs sham group. B, MG53 expression levels were not altered in mice following 4 weeks exercise training.
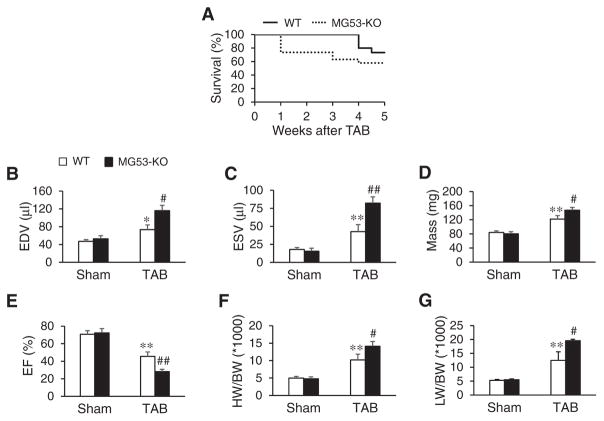
Following TAB, MG53-KO mice displayed a significantly reduced survival rate as compared to WT littermate controls (Fig. 5A), which corresponded with a marked increase in end diastolic volume (Fig. 5B), end systolic volume (Fig. 5C), and LV mass (Fig. 5D) and a decrease in EF (Fig. 5E). As compared to WT littermates, deficiency of MG53 further increased the heart weight to body weight ratio and the lung weight to body weight ratio after TAB surgery (Fig. 5F, G).
Fig. 5.
Deficiency of MG53 significantly decreases survival and impairs cardiac function in response to pressure overload-induced cardiac stress. A, Kaplan-Meier survival curve of WT and MG53-KO mice after TAB surgery. N= 15 and 19 for WT and MG53-KO mice, respectively. B–E, Echocardiographic data in WT and MG53-KO mice 5 weeks after TAB or sham surgery. End diastolic volume (EDV, B), end systolic volume (ESV, C), mass (D) and ejection fraction (EF, E). F, HW/BW: the ratio of heart weight to body weight. G, LW/BW: the ratio of lung weight to body weight. N= 5, 5, 6, 11 mice per group, respectively. *p< 0.05, **p< 0.01 vs sham group; #p< 0.05, ##p< 0.01 vs WT TAB group (one-way ANOVA). Data shown are mean±SE.
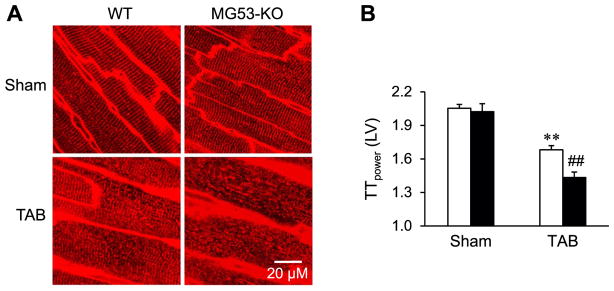
Next, we compared LV T-tubule ultrastructure in Langendorff-perfused intact WT and MG53-KO hearts after TAB. Consistent with results in 2- and 10-month-old mice, MG53 deletion did not affect T-tubule integrity in sham-operated mice (Fig. 6A). By contrast, MG53-KO mice had severely disrupted T-tubule structure following LV pressure overload (Fig. 6A). TTpower analysis revealed that, following TAB, MG53 deficiency aggravated T-tubule remodeling in the LV as compared to WT littermates (Fig. 6B).
Fig. 6.
MG53-KO mice have severely disrupted T-tubule ultrastructure following pressure overload. A, Representative LV T-tubule images from intact WT or MG53-KO hearts stained with a lipophilic marker MM 4–64, 5 weeks after sham or TAB surgery. B, Mean values of LV TTpower. N=5, 5, 9, 9 mice per group, respectively. **p <0.01 vs sham group; ##p< 0.01 vs WT TAB group (One-way ANOVA). Data shown are mean ±SE.
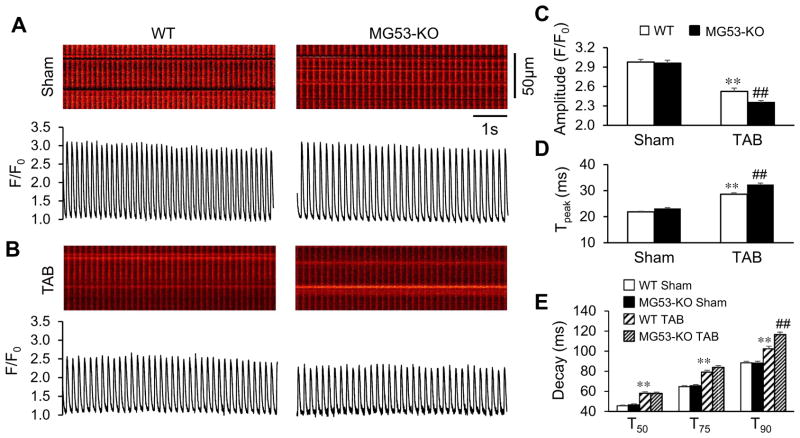
Since T-tubule integrity is a determinant of Ca2+ handling properties in cardiomyocytes, we next examined the effect of MG53 in E-C coupling following TAB. Shown in Fig. 7A are representative Ca2+ images recorded from intact hearts under simultaneous beating. There was no difference in Ca2+ handling properties in sham-operated WT and MG53-KO hearts (Fig. 7A, C, D), suggesting that MG53 is not required for baseline E-C coupling function. As anticipated, the amplitude of Ca2+ transients was decreased and the time to peak increased in WT hearts following TAB surgery as compared to sham-operated hearts (Fig. 7B, C, D). In line with the exacerbated T-tubule disruption under MG53 deficiency, MG53-KO hearts had a significantly lower Ca2+ transient amplitude and longer time to peak as compared to WT TAB hearts (Fig. 7C, D).
Fig. 7.
MG53 is required to maintain E-C coupling function following pressure overload-induced cardiac stress. A, Representative cytosolic Ca2+ images (upper) and traces (lower) under autonomous beating in LV of intact hearts from WT mice and MG53-KO mice 5 weeks after sham or TAB surgery. B, Average data of the amplitude, time to peak, and decay (T50, T75, T90) of Ca2+ transients. n = 5, 5, 5, 7 mice per genotype for sham and TAB, respectively. **p <0.01 vs sham group; ##p< 0.01 vs WT TAB group (One-way ANOVA). Data shown are mean±SE.
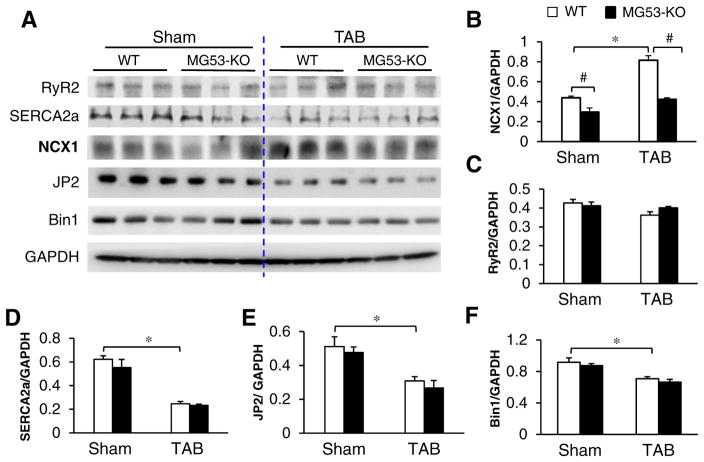
We also measured the decay time constant at 50% (T50), 75% (T75), and 90% (T90) and found that, in sham-operated hearts, MG53 deficiency had no impact on baseline decay constants (Fig. 7E). TAB induced an anticipated increase in T50, T75, and T90 in WT heart, and MG53 deficiency only further increased T90 (Fig. 7E). The prolongation in T90 is indicative of Na+/Ca2+ exchanger (NCX) downregulation [24–26]. Analysis of cardiac NCX1 expression by Western blotting demonstrated a 35% reduction in NCX1 protein levels in MG53-KO myocytes at baseline and a 48% reduction 5 weeks after TAB (Fig. 8A & B). Although a dramatic reduction in levels of SERCA2a and JP2, and to a lesser extent in Bin1, were induced by TAB, MG53 deficiency had no additional impact on their expressions (Fig. 8A, D–F). Taken with the data in Figs. 5 and 6, these results identify a critical role for MG53 in preserving cardiac function, T-tubule integrity, and E-C coupling following cardiac stress.
Fig. 8.
Downregulation of NCX1 in MG53-KO hearts. Representative immunoblots (A) and summary data of NCX1 (B), RyR2 (C) SERCA2a (D), JP2 (E) and Bin1 (F) in heart lysates from WT and MG53-KO mice of 3 months old (sham-operated or TAB). GAPDH: loading control. *p< 0.05 vs sham group; #p< 0.05 vs MG53-KO.
4. Discussion
T-tubule dysregulation is characteristic feature of human hearts with end-stage dilated or ischemic cardiomyopathy [14,27] and a key underlying mechanism for cardiac dysfunction in failing hearts [9–18]. Loss of T-tubule integrity alters the spatial distribution of L-type calcium channels and increases ‘orphaned’ RyRs [11], which leads to a reduced amplitude and slower rise of Ca2+ release. Using in situ confocal imaging of Langendorff-perfused intact hearts, we discovered that T-tubule remodeling is not just a manifestation of end-stage heart failure [15]. Instead, the remodeling of the T-tubules starts early in hypertrophy, before the onset of left ventricle (LV) systolic dysfunction. The T-tubule system in the LV undergoes progressive deterioration from compensated hypertrophy through early heart failure to advanced heart failure [15]. T-tubule integrity is highly correlated with cardiac ejection fraction of diseased hearts, suggesting T-tubule integrity is an important determinant of cardiac function [15]. To date, however, no studies have address the concept of membrane repair as it relates to the maintenance of the T-tubule network following cardiac stress.
Following ischemia/reperfusion injury to the heart, MG53 rapidly translocates to the site of membrane damage [3]. Genetic ablation of MG53 exacerbates the consequences of ischemia/reperfusion injury, including substantial cardiomyocyte death [2,3,28]. Conversely, infusion of recombinant human MG53 restores cardiac function in murine model of heart failure [29]. These studies provide clear evidence that MG53 is critical to repair damaged membrane and thus protect against cardiac dysfunction, though the mechanisms remain incompletely defined. Herein we examined the impact of MG53 deficiency in a chronic cardiac stress model. Survival following pressure overload-induced cardiac stress was significantly decreased in MG53-KO mice, with enhanced hypertrophy and loss of cardiac function as compared to WT littermates. Our data identify disruption of the organized T-tubule network and resultant E-C coupling dysfunction as a potential mechanism for the exacerbated response to cardiac stress in MG53-KO mice. By contrast, MG53 was dispensable for normal cardiac and T-tubule development and maintenance of the T-tubule ultrastructure in adult murine hearts at baseline. These data suggest that MG53 plays a surveillance role in the heart, primed to respond to membrane damage.
4.1. Mechanisms of action for MG53
Several studies have provided insight into the precise mechanisms by which MG53 mediates membrane repair. First, the cytoskeletal motor protein nonmuscle myosin type IIA (NM-IIA) interacts with MG53 and recruits MG53-containing intracellular vesicles to the injury site [30]. Second, polymerase I and transcript release factor (PTRF) senses exposed membrane cholesterol at the injury site and acts as a docking protein for MG53 [31]. Third, once at the membrane, MG53 binds to phosphatidylserine, oligomerizes, and serves as a protein scaffold for membrane repair components such as dysferlin [1,32–35]. These MG53-containing vesicles then form a membrane patch to repair the damaged sarcolemma. Evidence also suggests that caveolin-3 is involved in MG53-mediated membrane repair [36]. Caveolin-3 co-localizes and interacts with MG53 and dysferlin, thereby regulating MG53 activity such as MG53-mediated membrane fusion events [32]. Mutations in caveolin-3 that cause retention of caveolin-3 in Golgi apparatus result in aberrant localization of MG53 and dysferlin, leading to defective membrane repair [37]. In addition to facilitating vesicle trafficking, MG53 also possesses E3 ligase activity [38,39]. While defective vesicle trafficking and subsequent loss of membrane repair function likely mediates the disruption of the T-tubule structure in MG53-KO mice following cardiac stress, it remains possible that loss of MG53 E3 ligase activity may also mechanistically contribute to the maladaptive T-tubule remodeling. As another potential mechanism, we found that deficiency of MG53 resulted in a decrease in NCX1 expression in both baseline and stressed hearts. Following TAB, the T90 decay constant was significantly increased in MG53-KO hearts as compared to WT littermates, which is consistent with the lower NCX1 expression. NCX1 is a crucial player in Ca2+ homeostasis in cardiomyocytes [24]. Recent evidence from atrial-specific NCX1 knockout model [40] and cardiac-specific inducible NCX1 overexpression mice [41] suggest that NCX1 may mechanistically contribute to the proper spatial localization of JP2 and maintenance of T-tubule organization. This may represent an additional mechanism for the dysregulated E-C coupling and T-tubule remodeling in MG53-KO mice under stress conditions. Future studies are necessary to delineate the mechanism by which MG53 deficiency results in decreased NCX1 expression.
4.2. Impact of MG53 deficiency on cardiac development
Our data in developing hearts suggest that MG53 is not necessary for normal cardiac development, presumably because no membrane damage occurs during normal physiological growth that would require MG53-mediated repair. Moreover, cardiac function is normal in 2- and 10-month-old MG53-KO mice, indicating MG53 does not play a crucial role in maintaining baseline cardiac function. Similarly, skeletal muscle in MG53-KO mice has normal morphology and contractility under unstressed conditions until at least 11 months of age [1]. However, genetic deletion of MG53 causes a 35% downregulation of NCX expression in adult hearts under non-stressed conditions. The lack of a phenotype in MG53-KO mice is not surprising given that NCX-deficient mice also have no baseline cardiac dysfunction [42]. In addition, induced overexpression of NCX1 does not alter T50 [43], in line with our findings that T90 but not T50 or T75 was significantly greater in MG53-KO mice as compared to WT after TAB.
4.3. Impact of MG53 deficiency on T-tubule development
Expression of MG53 in developing murine hearts followed a similar time course as T-tubule maturation [23]. However, analysis of the cardiac T-tubule ultrastructure demonstrated that MG53 is not required for T-tubule maturation during cardiac development or for maintenance of T-tubule integrity in adult hearts. The lack of a role for MG53 in T-tubule development was unexpected given the contemporary theories for T-tubule morphogenesis: T-tubules form either from invagination of sarcolemma driven by caveolae and/or addition of new membrane to early T-tubule via a process involving exocytosis vesicle trafficking [44,45]. Since membrane vesicle trafficking is potentially important for T-tubule formation, and MG53 plays a key role in vesicle trafficking in response to membrane injury, [1,4] we anticipated that MG53 would be required for T-tubule maturation. Indeed, many proteins, such as caveolin-3, [46] JP2 [23,47,48] and BIN1 [49–52] are required for T-tubule morphogenesis and maintenance, and the recruitment of these proteins to T-tubules depends on protein-coated vesicle trafficking [53]. These data support our hypothesis that MG53 acts in a surveillance capacity to guard against membrane damage in the heart. Indeed, the present study showed MG53 ablation worsens T-tubule deterioration and cardiac dysfunction in response to chronic cardiac stress. These data suggest that MG53 is required to repair damaged T-tubule network and to preserve E-C coupling and cardiac function in stressed hearts.
4.4. Alterations in MG53 expression under physiological and pathological (acute vs chronic) cardiac stress
In the present study, we found that MG53 is unchanged in response to physiological stress (i.e., exercise training), but upregulated upon chronic pathological cardiac stress, such as in pressure overload-induced hypertrophy and heart failure. On the contrary, two previous studies have shown decreased MG53 levels under acute cardiac stress, for example, treatment with H2O2 and ischemia/reperfusion injury [2,54]. Note that both of these processes result in a highly oxidative state. Accordingly, Murphy and Kohr’s group demonstrated that the mechanism of MG53 downregulation in the setting of acute stress is through oxidation-mediated S-nitrosylation of MG53 [54]. Thus, the reduction of MG53 under acute stress compromises its protective membrane repair function. Although the data presented herein demonstrate that MG53 is required for repairing damaged T-tubule membrane under chronic stress, the long-term impact of MG53 upregulation in the setting of chronic stress remains to be determined.
In summary, our data provide strong insight into the mechanism by which MG53 protects against cardiac stress through preservation of the T-tubule network. Whereas previous studies have focused on the role of MG53-mediated membrane repair following acute injury, our findings demonstrate a specific role for MG53 in a chronic stress model of heart failure through actions on T-tubules.
Acknowledgments
This work was supported by NIH (R01 HL090905 and HL130346, L.S.S.; AR070752 and AR061385, J.M.), the United States Department of Veterans Affairs Biomedical Laboratory Research (Merit Review Award I01BX002334, L.S.S.; 2I01BX000776, C.J.B.), and China National Natural Science Foundation (NSFC 31371159, J.L.; 57201701 and 81570293, J.H.).
Footnotes
Disclosures
No conflict of interest to be disclosed.
References
- 1.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11(1):56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Zhang Y, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao RP. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121(23):2565–2574. doi: 10.1161/CIRCULATIONAHA.110.954628. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao RP, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res. 2010;107(1):76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- 4.McNeil P. Membrane repair redux: redox of MG53. Nat Cell Biol. 2009;11(1):7–9. doi: 10.1038/ncb0109-7. [DOI] [PubMed] [Google Scholar]
- 5.Weisleder N, Takeshima H, Ma J. Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol. 2009;2(3):225–226. doi: 10.4161/cib.2.3.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisleder N, Lin P, Zhao X, Orange M, Zhu H, Ma J. Visualization of MG53-mediated cell membrane repair using in vivo and in vitro systems. J Vis Exp. 2011;52 doi: 10.3791/2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He B, Tang RH, Weisleder N, Xiao B, Yuan Z, Cai C, Zhu H, Lin P, Qiao C, Li J, Mayer C, Li J, Ma J, Xiao X. Enhancing muscle membrane repair by gene delivery of MG53 ameliorates muscular dystrophy and heart failure in delta-Sarcoglycan-deficient hamsters. Mol Ther. 2012;20(4):727–735. doi: 10.1038/mt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo A, Zhang C, Wei S, Chen B, Song LS. Emerging mechanisms of T-tubule remodelling in heart failure. Cardiovasc Res. 2013;98(2):204–215. doi: 10.1093/cvr/cvt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92(11):1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 10.Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res. 2003;59(1):67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 11.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103(11):4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol. 2006;574(Pt 2):519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, Claus P, Dymarkowski S, Maes F, Bogaert J, Rademakers F, D’Hooge J, Sipido K. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102(3):338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 14.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci U S A. 2009;106(16):6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107(4):520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crossman DJ, Ruygrok PN, Soeller C, Cannell MB. Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS One. 2011;6(3):e17901. doi: 10.1371/journal.pone.0017901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacconi L, Ferrantini C, Lotti J, Coppini R, Yan P, Loew LM, Tesi C, Cerbai E, Poggesi C, Pavone FS. Action potential propagation in transverse-axial tubular system is impaired in heart failure. Proc Natl Acad Sci U S A. 2012;109(15):5815–5819. doi: 10.1073/pnas.1120188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, Luther S, Hasenfuss G, Parlitz U, Jafri MS, Hell SW, Lederer WJ, Lehnart SE. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res. 2012;111(4):402–414. doi: 10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo A, Zhang X, Iyer VR, Chen B, Zhang C, Kutschke WJ, Weiss RM, Franzini-Armstrong C, Song LS. Overexpression of junctophilin-2 does not enhance baseline function but attenuates heart failure development after cardiac stress. Proc Natl Acad Sci U S A. 2014;111(33):12240–12245. doi: 10.1073/pnas.1412729111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen B, Li Y, Jiang S, Xie YP, Guo A, Kutschke W, Zimmerman K, Weiss RM, Miller FJ, Anderson ME, Song LS. beta-Adrenergic receptor antagonists ameliorate myocyte T-tubule remodeling following myocardial infarction. FASEB J. 2012;26(6):2531–2537. doi: 10.1096/fj.11-199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B, Guo A, Gao Z, Wei S, Xie YP, Chen SR, Anderson ME, Song LS. In situ confocal imaging in intact heart reveals stress-induced Ca(2+) release variability in a murine catecholaminergic polymorphic ventricular tachycardia model of type 2 ryanodine receptor(R4496C+/−) mutation. Circ Arrhythm Electrophysiol. 2012;5(4):841–849. doi: 10.1161/CIRCEP.111.969733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Chen B, Guo A, Zhu Y, Miller JD, Gao S, Yuan C, Kutschke W, Zimmerman K, Weiss RM, Wehrens XH, Hong J, Johnson FL, Santana LF, Anderson ME, Song LS. Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation. 2014;129(17):1742–1750. doi: 10.1161/CIRCULATIONAHA.113.008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen B, Guo A, Zhang C, Chen R, Zhu Y, Hong J, Kutschke W, Zimmerman K, Weiss RM, Zingman L, Anderson ME, Wehrens XH, Song LS. Critical roles of junctophilin-2 in T-tubule and excitation-contraction coupling maturation during postnatal development. Cardiovasc Res. 2013;100(1):54–62. doi: 10.1093/cvr/cvt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottolia M, Torres N, Bridge JH, Philipson KD, Goldhaber JI. Na/Ca exchange and contraction of the heart. J Mol Cell Cardiol. 2013;61:28–33. doi: 10.1016/j.yjmcc.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neco P, Rose B, Huynh N, Zhang R, Bridge JH, Philipson KD, Goldhaber JI. Sodium-calcium exchange is essential for effective triggering of calcium release in mouse heart. Biophys J. 2010;99(3):755–764. doi: 10.1016/j.bpj.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song LS, Guia A, Muth JN, Rubio M, Wang SQ, Xiao RP, Josephson IR, Lakatta EG, Schwartz A, Cheng H. Ca2+ signaling in cardiac myocytes over-expressing the alpha(1) subunit of L-type Ca2+ channel. Circ Res. 2002;90(2):174–181. doi: 10.1161/hh0202.103230. [DOI] [PubMed] [Google Scholar]
- 27.Kaprielian RR, Stevenson S, Rothery SM, Cullen MJ, Severs NJ. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation. 2000;101(22):2586–2594. doi: 10.1161/01.cir.101.22.2586. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Lv F, Jin L, Peng W, Song R, Ma J, Cao CM, Xiao RP. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res. 2011;91(1):108–115. doi: 10.1093/cvr/cvr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Duann P, Lin PH, Zhao L, Fan Z, Tan T, Zhou X, Sun M, Fu M, Orange M, Sermersheim M, Ma H, He D, Steinberg SM, Higgins R, Zhu H, John E, Zeng C, Guan J, Ma J. Modulation of wound healing and scar formation by MG53 protein-mediated cell membrane repair. J Biol Chem. 2015;290(40):24592–24603. doi: 10.1074/jbc.M115.680074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin P, Zhu H, Cai C, Wang X, Cao C, Xiao R, Pan Z, Weisleder N, Takeshima H, Ma J. Nonmuscle myosin IIA facilitates vesicle trafficking for MG53-mediated cell membrane repair. FASEB J. 2012;26(5):1875–1883. doi: 10.1096/fj.11-188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N, Ma J. Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J Biol Chem. 2011;286(15):12820–12824. doi: 10.1074/jbc.C111.221440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, Komazaki S, Takeshima H, Ma J. MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem. 2009;284(5):3314–3322. doi: 10.1074/jbc.M808866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lek A, Evesson FJ, Lemckert FA, Redpath GM, Lueders AK, Turnbull L, Whitchurch CB, North KN, Cooper ST. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. J Neurosci. 2013;33(12):5085–5094. doi: 10.1523/JNEUROSCI.3560-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuda C, Miyake K, Kameyama K, Keduka E, Takeshima H, Imamura T, Araki N, Nishino I, Hayashi Y. The C2A domain in dysferlin is important for association with MG53 (TRIM72) PLoS Curr. 2012;4 doi: 10.1371/5035add8caff4. (e5035add8caff4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang M, Ko JK, Weisleder N, Takeshima H, Ma J. Redox-dependent oligo-merization through a leucine zipper motif is essential for MG53-mediated cell membrane repair. Am J Phys Cell Phys. 2011;301(1):C106–14. doi: 10.1152/ajpcell.00382.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blazek AD, Paleo BJ, Weisleder N. Plasma membrane repair: a central process for maintaining cellular homeostasis. Physiology (Bethesda) 2015;30(6):438–448. doi: 10.1152/physiol.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284(23):15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song R, Peng W, Zhang Y, Lv F, Wu HK, Guo J, Cao Y, Pi Y, Zhang X, Jin L, Zhang M, Jiang P, Liu F, Meng S, Zhang X, Jiang P, Cao CM, Xiao RP. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature. 2013;494(7437):375–379. doi: 10.1038/nature11834. [DOI] [PubMed] [Google Scholar]
- 39.Yi JS, Park JS, Ham YM, Nguyen N, Lee NR, Hong J, Kim BW, Lee H, Lee CS, Jeong BC, Song HK, Cho H, Kim YK, Lee JS, Park KS, Shin H, Choi I, Lee SH, Park WJ, Park SY, Choi CS, Lin P, Karunasiri M, Tan T, Duann P, Zhu H, Ma J, Ko YG. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat Commun. 2013;4:2354. doi: 10.1038/ncomms3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue X, Zhang R, Kim B, Ma A, Philipson KD, Goldhaber JI. Heterogeneity of transverse-axial tubule system in mouse atria: remodeling in atrial-specific Na+–Ca2+ exchanger knockout mice. J Mol Cell Cardiol. 2017;108:50–60. doi: 10.1016/j.yjmcc.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ujihara Y, Mohri S, Katanosaka Y. Effects of induced Na+/Ca2+ exchanger overexpression on the spatial distribution of L-type Ca2+ channels and junctophilin-2 in pressure-overloaded hearts. Biochem Biophys Res Commun. 2016;480(4):564–569. doi: 10.1016/j.bbrc.2016.10.090. [DOI] [PubMed] [Google Scholar]
- 42.Komuro I, Ohtsuka M. Forefront of Na+/Ca2+ exchanger studies: role of Na+/Ca2+ exchanger—lessons from knockout mice. J Pharmacol Sci. 2004;96(1):23–26. doi: 10.1254/jphs.fmj04002x5. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Chan TO, Zhang XQ, Gao E, Song J, Koch WJ, Feldman AM, Cheung JY. Induced overexpression of Na+/Ca2+ exchanger transgene: altered myocyte contractility, [Ca2+]i transients, SR Ca2+ contents, and action potential duration. Am J Physiol Heart Circ Physiol. 2009;297(2):H590–601. doi: 10.1152/ajpheart.00190.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang B, Zelhof AC. Amphiphysins: raising the BAR for synaptic vesicle recycling and membrane dynamics, Bin-Amphiphysin-Rvsp. Traffic. 2002;3(7):452–460. doi: 10.1034/j.1600-0854.2002.30702.x. [DOI] [PubMed] [Google Scholar]
- 45.Di Maio A, Karko K, Snopko RM, Mejia-Alvarez R, Franzini-Armstrong C. T-tubule formation in cardiacmyocytes: two possible mechanisms? J Muscle Res Cell Motil. 2007;28(4–5):231–241. doi: 10.1007/s10974-007-9121-x. [DOI] [PubMed] [Google Scholar]
- 46.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276(24):21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Wu H, Wang Q, Wang S. Morphogenesis of T-tubules in heart cells: the role of junctophilin-2. Sci China Life Sci. 2013;56(7):647–652. doi: 10.1007/s11427-013-4490-4. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds JO, Chiang DY, Wang W, Beavers DL, Dixit SS, Skapura DG, Landstrom AP, Song LS, Ackerman MJ, Wehrens XH. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc Res. 2013;100(1):44–53. doi: 10.1093/cvr/cvt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297(5584):1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 50.Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marban E, Jan LY, Shaw RM. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. 2014;20(6):624–632. doi: 10.1038/nm.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tjondrokoesoemo A, Park KH, Ferrante C, Komazaki S, Lesniak S, Brotto M, Ko JK, Zhou J, Weisleder N, Ma J. Disrupted membrane structure and intracellular Ca2+ signaling in adult skeletal muscle with acute knockdown of Bin1. PLoS One. 2011;6(9):e25740. doi: 10.1371/journal.pone.0025740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caldwell JL, Smith CE, Taylor RF, Kitmitto A, Eisner DA, Dibb KM, Trafford AW. Dependence of cardiac transverse tubules on the BAR domain protein amphiphysin II (BIN-1) Circ Res. 2014;115(12):986–996. doi: 10.1161/CIRCRESAHA.116.303448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chadda R, Mayor S. PTRF triggers a cave in. Cell. 2008;132(1):23–24. doi: 10.1016/j.cell.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 54.Kohr MJ, Evangelista AM, Ferlito M, Steenbergen C, Murphy E. S-nitrosylation of TRIM72 at cysteine 144 is critical for protection against oxidation-induced protein degradation and cell death. J Mol Cell Cardiol. 2014;69:67–74. doi: 10.1016/j.yjmcc.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]