Abstract
Objective
To study the long-term risk of cerebrovascular events, seizures, and cognitive impairment in patients with transient global amnesia (TGA).
Patients and Methods
Data for all patients diagnosed with possible TGA in Olmsted County, Minnesota, between January 1, 1985, through December 31, 2010, were retrieved from the Rochester Epidemiology Project database. Transient global amnesia was defined clinically. End points were cerebrovascular event (stroke or transient ischemic attack), seizure, or cognitive impairment (mild cognitive impairment or dementia) during follow-up. End points were studied using Kaplan-Meier survival plots and log-rank test.
Results
A total of 221 patients with TGA were identified and 221 age- and sex-matched controls were included in the analysis. The mean duration of follow-up was 12 years in both groups (range, 0.07-29.93). Prevalence of vascular risk factors and history of seizures were similar between both groups. Previous migraine was more common in the TGA group (42 patients [19.1%] vs 12 patients [5.4%]; P<.001). There was no statistically significant difference between survival curves for the TGA group and the control group using time to any type of cerebrovascular event (log-rank P=.30), time to seizures event (log-rank P=.55), and time to cognitive impair event (log-rank P=.88) as end points. The TGA recurrence occurred in 5.4% of patients after a median interval of 4.21 years (interquartile range, 2.82-8.44). Modified Rankin scale and death rates at last follow-up were also similar between both groups.
Conclusion
Our findings indicate that having an episode of TGA does not increase the risk of subsequent cerebrovascular events, seizures, or cognitive impairment.
Transient global amnesia (TGA) is characterized by the sudden onset of anterograde amnesia, generally lasting up to 24 hours.1-4 It has an annual incidence of 3.4 to 10.4 per 100,000 people.5-7
The leading hypothesis on the pathogenesis of TGA is abnormal venous drainage of the temporal lobes caused by increased intrathoracic pressure resulting in jugular hypertension.8-11 Others include arterial ischemia,12 migraine-,5,13,14 and epilepsy-related.15
The recurrence rate of TGA has been estimated to be between 2.9% and 23.8%.13,16-19 Available studies about the long-term outcome are few and limited in scope. Acquiring information is necessary to enhance clinical practice.
Available data suggest that TGA does not put patients at a higher risk of cerebrovascular events, myocardial infarction, or peripheral artery disease.13,16,19,20 Data on the risk of seizures after TGA are inconsistent. Studies that have suggested a higher risk of seizures concluded that at least some of the patients could have had a seizure mimicking TGA, as suggested by abnormal interictal electroencephalograms (EEGs).5,11
The long-term risk of cognitive decline has not been sufficiently evaluated. Complete recovery of cognitive function has been reported 5 days to 6 months after the episode.21-24 Other investigators have found memory and visuoperceptual dysfunction.25-30 Long-term risk was assessed in one study with an average follow-up of 82.2 months, showing no difference in the incidence of dementia (2.9%) compared with general population.16
We conducted a population-based, matched cohort study on patients with TGA to establish the long-term risk of cerebrovascular events, seizures, and cognitive impairment.
Patients and Methods
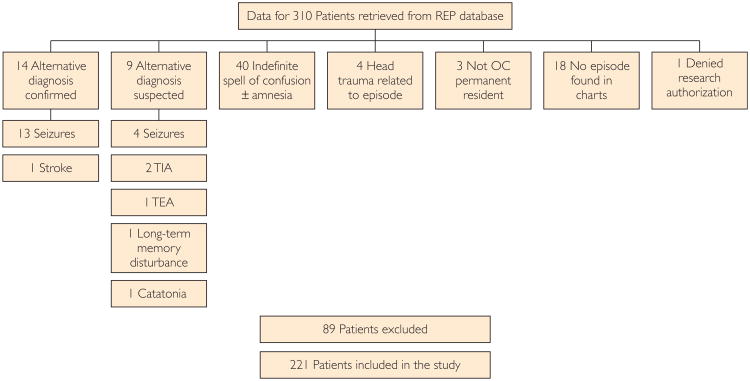
A list of all patients with a potential diagnosis of TGA among residents of Olmsted County, Minnesota, occurring from January 1, 1985, through December 31, 2010, was obtained using the resources of the Rochester Epidemiology Project31 database. The list included patients diagnosed in both the outpatient and inpatient settings, including cases seen at Mayo Clinic, and those seen at other medical providers in Olmsted County. We reviewed electronic and paper medical records for all patients. TGA was defined clinically, and all cases were confirmed using previously proposed criteria.1,32,33 Cases in which an alternative diagnosis was suspected or could not be ruled out were excluded from the study. Fourteen patients were excluded because of a confirmed diagnosis of seizures or stroke. The other 75 patients were excluded for suspicion of alternative diagnosis, including the classical differential diagnosis of TGA such as transient ischemic attack [TIA], seizures, and transient epileptic amnesia, with the latter consisting of focal seizures with similar clinical features, shorter duration and higher recurrence, and sometimes accompanied by other manifestations such as oral automatisms. One age- and sex-matched control for each case was selected from the same database; controls were also matched for date of assessment.
Data collected in both cases and controls included demographic characteristics, relevant cerebrovascular risk factors, and conditions reported to be associated with TGA (hypertension, dyslipidemia, diabetes mellitus, smoking history, coronary artery disease, atrial fibrillation, peripheral vascular disease, alcohol abuse, personal or familial history of stroke, personal or familial history of migraine, personal or familial history of seizures or epilepsy).
The primary end points for our analysis were cerebrovascular events (TIA or stroke), seizures, and cognitive impairment (diagnosis of mild cognitive impairment and dementia). The diagnosis of stroke or TIA was made on the basis of comprehensive data from the medical record, and using the definition on current guidelines.34,35 The presence of seizures was recorded according to the clinical diagnosis by the treating neurologist, sometimes aided by an abnormal ictal or interictal EEG. The presence of cognitive impairment was considered to be the first time the patient was given a clinical diagnosis of mild cognitive impairment or dementia by a neurologist.36,37 Secondary end points were death, functional outcome at last follow-up as assessed by the modified Rankin score (mRS)38, and TGA recurrence.
Approval from the Ethics Standards Committee was obtained to conduct this retrospective study.
Statistical Analyses
Descriptive summaries were reported as mean ± SD or median and interquartile range (IQR) as appropriate. Comparisons between cases and controls for continuous variables such as body mass index (calculated as the weight in kilograms divided by the height in meters squared) and duration of follow-up were done using Wilcoxon rank sum test. Categorical variables such as relevant medical history, family history, and smoking history were performed using Fisher exact test. To account for differential follow-up and censoring, events of interest at follow-up such as death, first cerebrovascular event, seizures, or diagnosis of cognitive impairment were analyzed using Kaplan-Meier survival curves. Comparisons of curves between the cases and controls were performed using the log-rank test. All tests were 2-sided, and P values of less than .05 were considered statistically significant. All analyses were performed using SAS 9.3.
Results
Two hundred twenty-one cases and the same number of matched controls were included in the study (Figure 1). Baseline data for cases and controls are presented in the Table.
Figure 1.
Patient selection flowchart. OC = Olmsted County; REP = Rochester Epidemiology Project; TEA = transient epileptic amnesia; TIA = transient ischemic attack.
Table. Baseline Characteristics of Patients With TGA Compared With Controlsa,b.
| Characteristic | TGA | Controls | P |
|---|---|---|---|
| Number of subjects | 221 | 221 | |
| Age (y), mean ± SD | 65.6± 12.2 | 63.3±16.5 | .10 |
| Sex: Female | 111 (50.2) | 111 (50.2) | 1.00 |
| Hypertension | 92 (41.6) | 85 (38.4) | .56 |
| BMI, mean ± SD | 28.5±4.5 | 29.9±5.9 | .25 |
| Dyslipidemia | 59 (26.7) | 57 (25.8) | .91 |
| Diabetes mellitus | 10 (4.5) | 23 (10.4) | .03 |
| Smoking history | 39 (17.6) | 40 (18.1) | 1.00 |
| CHD | 11 (5.0) | 19 (8.6) | .19 |
| PVD | 0 (0.0) | 3 (1.4) | .25 |
| Atrial fibrillation | 10 (4.5) | 5 (2.3) | .30 |
| Previous stroke | 16 (7.2) | 14 (6.3) | .71 |
| Alcohol abuse history | 10 (4.5) | 11 (5.0) | 1.00 |
| History of migraine | 42 (19.10) | 12 (5.4) | <.001 |
| Family history of migraine | 5 (2.3) | 1 (0.5) | .22 |
| History of seizures/epilepsy | 4 (1.8) | 4 (1.8) | 1.00 |
BMI = body mass index; CHD = congestive heart disease; PVD = peripheral vascular disease; TGA=transient global amnesia.
Values represent n (%) unless indicated otherwise.
Among the patients with TGA, 20 (9.0%) had experienced 1 or more previous episodes of TGA. Twelve (5.4%) patients had recurrences after the index episode; 10 had only 1 recurrence and 2 had 4 recurrences each. The median interval to the first recurrence was 4.2 years (IQR, 2.8-8.4 years). The resulting recurrence rate from the index event was 67 per 100 person-years of follow-up (95% CI, 52.3-84.5).
The mean ± SD age at onset of TGA was 65.6±12.2 years (range, 0.07-29.93 years). A total of 111 (50.2%) patients were women. Data on TGA attack duration were available in 107 patients. They lasted from 0.16 to 36 hours, with a mean ± SD of 5.5±5.6 hours.
Potential precipitating factors for the episode were identified in 62 (28.0%) patients; 53 (24.0%) of them involved exercise, sexual intercourse, or a potential Valsalva-like maneuver such as coughing/choking or vomiting; 6 (2.7%) episodes occurred after exposure to heat while showering, and 3 (1.4%) after emotional stress.
A total of 172 (77.8%) patients underwent a computed tomography scan of the head as part of their workup. None of them was suggestive of an alternative diagnosis. Magnetic resonance imaging was performed in 47 of the 221 (21.27%) patients included in the study. Only 2 (4.26%) of them showed the classically described punctate hyperintensity on diffusion weighted imaging in the hippocampal region. No other abnormalities were found on magnetic resonance imaging. The EEG was done in 72 (32.6%) patients, including 11 on the same day of the episode; the rest of the patients had the EEG within a median of 4 days (IQR, 2-13 days) after the TGA episode had resolved. Of 11 EEGs performed on the day of the episode, only 1 was abnormal, showing temporal lobe slowing. Among the other 61 EEGs, 6 (9.8%) showed temporal slowing. No epileptiform discharges or seizures were noted.
The prevalence of cerebrovascular risk factors and conditions associated with TGA was compared between patients with TGA and controls (Table). No significant differences were found in the frequency of hypertension, dyslipidemia, history of coronary artery disease, atrial fibrillation, peripheral vascular disease, smoking history, or personal or familial history of stroke or seizures. Body mass index was not different between the 2 groups. Diabetes mellitus had a higher prevalence among controls (P=.033). History of migraine was the only baseline factor that was more frequent among TGA cases (42 [19.1%] vs 12 [5.4%]; P<.001). However, family history of migraine was not different between the 2 groups.
The mean follow-up period in subjects with TGA was 12±7 years, with a median mRS of 0 (IQR, 0-0; range, 0-6) at last follow-up. In controls, the length of follow-up was 12.3 ± 7 years and median mRS at last follow-up was 0 (IQR, 0-0; range, 0-5). During follow-up, there were 142 deaths in the TGA group and 127 among controls (P=.34). Median time to death was similar between the TGA group and the control group (22.5 vs 17.1 years; log-rank P=.34).
There were no statistically significant differences between survival curves for the TGA group and the control group using time to any type of cerebrovascular event (log-rank P=.30), time to seizures event (log-rank P=.55), and time to cognitive impair event (log-rank P=.88) as end points (Figure 2). No particular dementia etiology (degenerative or vascular) predominated in one group over the other. In the TGA group, there was also no evidence of an association between TGA recurrence and cognitive impairment (P=77), cerebrovascular event (P=71), or seizures (P=46). Median time to death was similar between the TGA group and the control group (21.7 vs 17.1; log-rank P=34).
Figure 2.
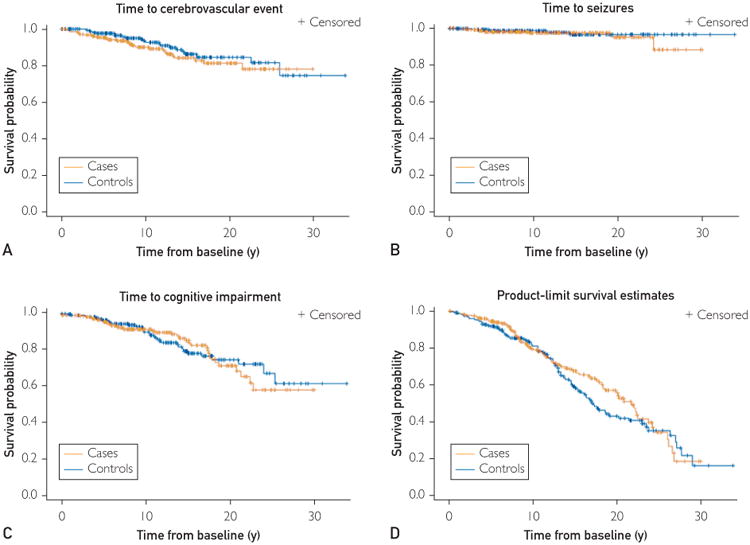
Kaplan-Meyer plots for events of interest at follow-up. Plots for the time to occurrence of first vascular event (A), seizure (B), diagnosis of cognitive impairment (C), and death (D).
Discussion
In this case cohort, population-based study, we examined the risk of cerebrovascular events, seizures, and cognitive impairment after a single episode or multiple episodes of TGA. Our results demonstrate that patients are not at a higher risk of having a cerebrovascular event, seizures, or developing cognitive impairment over the long-term after an episode of TGA. The population-based, casematched design and long length of follow-up of our study strongly support the validity of our results. Previous reports suggested that the incidence of cerebrovascular events after an episode of TGA does not differ from that in the general population, though these previous studies typically had shorter follow-up and therefore could not be as conclusive.13,16,19,20 In addition, our results provide further argument against the hypothesis that arterial ischemia could be the pathophysiological mechanism of TGA, not only because of the lack of association with subsequent stroke/TIA but also because there were no differences in baseline cardiovascular risk factors between cases of patients with TGA and controls. Instead, the finding that exercise or a Valsalva maneuver was the most common precipitating factor suggests that venous hypertension might be responsible for the symptoms.8-11
Our study found that the risk of seizures after an episode of TGA is not increased and previous seizures are not a risk factor for TGA. These findings argue against an epileptic mechanism for TGA. Of note, focal seizures can present with manifestations resembling TGA. These patients diagnosed with transient amnesia associated with epilepsy have a different disease (ie, epilepsy) and were consequently not included in our cohort. Epilepsy initially masquerading as TGA may explain the cases of epilepsy diagnosed after an episode of TGA in previous smaller studies.5,19
Our study found that having 1 or more episodes of TGA does not impact the risk of developing subsequent cognitive impairment. This conclusion is reassuring and clinically important because previous studies evaluating the long-term risk of cognitive impairment in patients with TGA had produced variable results.21-30 However, only 1 of those studies had a relatively long follow-up (∼6 years).16 We did not find a higher incidence of cognitive impairment in the TGA group over a mean follow-up of 12 years, even among those patients with recurrent TGA. We cannot confirm nor refute whether preexisting structural abnormalities in the limbic system might precipitate an episode of TGA as has been previously postulated,39 but our results suggest that recurrent episodes of TGA would not represent a structural disruption in connectivity of memory pathways given that the long-term incidence of cognitive impairment is not increased. This is also supported by previous imaging studies that have shown no evidence of structural brain injury after TGA.40
We found a rate of recurrence of 5.4% in our patients with TGA. This rate is lower than in most previous studies.13,16-20 This difference may be related to the strict criteria we used for the diagnosis of TGA (ie, careful exclusion of patients with possible alternative diagnoses).
The association between migraine and TGA has been previously reported5,13,14 and was confirmed in our analysis. This association between the 2 conditions and their occasional familial aggregation may suggest a possible genetic predisposition, which needs to be investigated in future research. It is difficult to rule out, however, that a higher prevalence of migraine could be consistently found among patients with TGA due to the bias introduced by treating clinicians specifically enquiring about it, knowing their proposed association.
The population-based design and length of follow-up longer than a decade make our study unique. Although the possibility of selection bias had been a consistent limitation in most previous literature on TGA, this bias is much less likely in our population-based cohort. Meanwhile, the length of follow-up in our study was markedly longer than in any previous study on TGA, thus supporting the reliability of our findings.
We acknowledge that missing information is always a possibility with any retrospective data collection; yet, this is unlikely given the comprehensive nature of our medical records and the fact that we had full access to all the medical information from the 2 centers where our patients could have received local medical care. The workup of patients varied according to the preference of the treating physician, but we do not think that such variability compromises the validity of our main results.
Conclusion
We conclude that having 1 or more episodes of TGA does not increase the long-term risk of cerebrovascular events, seizures, or cognitive impairment, compared with general population. This is very valuable reassuring information for the counseling of patients with TGA and their families. The previously reported association between TGA and migraine was confirmed in our study. Further research is warranted to determine the origin of such association.
Acknowledgments
Grant Support: This study was made possible by the Rochester Epidemiology Project (grant number R01-AG034676; Principal Investigator: Walter A. Rocca, MD, MPH, and Jennifer L. St Sauver, PhD).
Abbreviations and Acronyms
- EEG
electroencephalogram
- IQR
interquartile range
- mRS
modified Rankin score
- TGA
transient global amnesia
- TIA
transient ischemic attack
References
- 1.Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264–272. doi: 10.1016/j.mayocp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Bender MB. Syndrome of isolated episode of confusion with amnesia. J Hillside Hospital. 1956;5:212–215. [Google Scholar]
- 3.Courjon J, Guyotat J. Amnestic strokes. J Med Lyon. 1956;37(882):697–701. [PubMed] [Google Scholar]
- 4.Fisher CM, Adams RD. Transient global amnesia. Trans Am Neurol Assoc. 1958;83:143–146. [Google Scholar]
- 5.Hodges JR, Warlow CP. The aetiology of transient global amnesia: a case-control study of 114 cases with prospective follow-up. Brain. 1990;113(Pt 3):639–657. doi: 10.1093/brain/113.3.639. [DOI] [PubMed] [Google Scholar]
- 6.Koski KJ, Marttila RJ. Transient global amnesia: incidence in an urban population. Acta Neurol Scand. 1990;81(4):358–360. doi: 10.1111/j.1600-0404.1990.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 7.Lauria G, Gentile M, Fassetta G, Casetta I, Caneve G. Incidence of transient global amnesia in the Belluno province, Italy: 1985 through 1995. Results of a community-based study. Acta Neurol Scand. 1997;95(5):303–310. doi: 10.1111/j.1600-0404.1997.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 8.Agosti C, Borroni B, Akkawi NM, Padovani A. Cerebrovascular risk factors and triggers in transient global amnesia patients with and without jugular valve incompetence: results from a sample of 243 patients. Eur Neurol. 2010;63(5):291–294. doi: 10.1159/000292502. [DOI] [PubMed] [Google Scholar]
- 9.Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397–399. doi: 10.1016/S0140-6736(98)01442-1. [DOI] [PubMed] [Google Scholar]
- 10.Sander D, Winbeck K, Etgen T, Knapp R, Klingelhöfer J, Conrad B. Disturbance of venous flow patterns in patients with transient global amnesia. Lancet. 2000;356(9246):1982–1984. doi: 10.1016/S0140-6736(00)03313-4. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber SJ, Doepp F, Klingebiel R, Valdueza JM. Internal jugular vein valve incompetence and intracranial venous anatomy in transient global amnesia. J Neurol Neurosurg Psychiatry. 2005;76(4):509–513. doi: 10.1136/jnnp.2004.043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Filippo M, Calabresi P. Ischemic bilateral hippocampal dysfunction during transient global amnesia. Neurology. 2007;69(5):493. doi: 10.1212/01.wnl.0000271085.87627.72. [DOI] [PubMed] [Google Scholar]
- 13.Zorzon M, Antonutti L, Masè G, Biasutti E, Vitrani B, Cazzato G. Transient global amnesia and transient ischemic attack: natural history, vascular risk factors, and associated conditions. Stroke. 1995;26(9):1536–1542. doi: 10.1161/01.str.26.9.1536. [DOI] [PubMed] [Google Scholar]
- 14.Lin KH, Chen YT, Fuh JL, et al. Migraine is associated with a higher risk of transient global amnesia: a nationwide cohort study. Eur J Neurol. 2014;21(5):718–724. doi: 10.1111/ene.12346. [DOI] [PubMed] [Google Scholar]
- 15.Jacome DE. EEG features in transient global amnesia. Clin Electroencephalogr. 1989;20(3):183–192. doi: 10.1177/155005948902000312. [DOI] [PubMed] [Google Scholar]
- 16.Gandolfo C, Caponnetto C, Conti M, Dagnino N, Del Sette M, Primavera A. Prognosis of transient global amnesia: a long-term follow-up study. Eur Neurol. 1992;32(1):52–57. doi: 10.1159/000116787. [DOI] [PubMed] [Google Scholar]
- 17.Klotzsch C, Sliwka U, Berlit P, Noth J. An increased frequency of patent foramen ovale in patients with transient global amnesia: analysis of 53 consecutive patients. Arch Neurol. 1996;53(6):504–508. doi: 10.1001/archneur.1996.00550060046014. [DOI] [PubMed] [Google Scholar]
- 18.Melo TP, Ferro JM, Ferro H. Transient global amnesia: a case control study. Brain. 1992;115(Pt 1):261–270. doi: 10.1093/brain/115.1.261. [DOI] [PubMed] [Google Scholar]
- 19.Pantoni L, Bertini E, Lamassa M, Pracucci G, Inzitari D. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350–356. doi: 10.1111/j.1468-1331.2004.00982.x. [DOI] [PubMed] [Google Scholar]
- 20.Mangla A, Navi BB, Layton K, Kamel H. Transient global amnesia and the risk of ischemic stroke. Stroke. 2014;45(2):389–393. doi: 10.1161/STROKEAHA.113.003916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartsch T, Alfke K, Stingele R, et al. Selective affection of hip-pocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain. 2006;129(Pt 11):2874–2884. doi: 10.1093/brain/awl248. [DOI] [PubMed] [Google Scholar]
- 22.Jäger T, Bäzner H, Kliegel M, Szabo K, Hennerici MG. The transience and nature of cognitive impairments in transient global amnesia: a meta-analysis. J Clin Exp Neuropsychol. 2009;31(1):8–19. doi: 10.1080/13803390801955193. [DOI] [PubMed] [Google Scholar]
- 23.Kritchevsky M, Squire LR. Transient global amnesia: evidence for extensive, temporally graded retrograde amnesia. Neurology. 1989;39(2 Pt 1):213–218. doi: 10.1212/wnl.39.2.213. [DOI] [PubMed] [Google Scholar]
- 24.Quinette P, Guillery B, Desgranges B, de la Sayette V, Viader F, Eustache F. Working memory and executive functions in transient global amnesia. Brain. 2003;126(Pt 9):1917–1934. doi: 10.1093/brain/awg201. [DOI] [PubMed] [Google Scholar]
- 25.Caffarra P, Moretti G, Mazzucchi A, Parma M. Neuropsychological testing during a transient global amnesia episode and its follow-up. Acta Neurol Scand. 1981;63(1):44–50. doi: 10.1111/j.1600-0404.1981.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 26.Hodges JR. Semantic memory and frontal executive function during transient global amnesia. J Neurol Neurosurg Psychiatry. 1994;57(5):605–608. doi: 10.1136/jnnp.57.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodges JR, Oxbury SM. Persistent memory impairment following transient global amnesia. J Clin Exp Neuropsychol. 1990;12(6):904–920. doi: 10.1080/01688639008401030. [DOI] [PubMed] [Google Scholar]
- 28.Le Pira F, Giuffrida S, Maci T, Reggio E, Zappalà G, Perciavalle V. Cognitive findings after transient global amnesia: role of pre-frontal cortex. Appl Neuropsychol. 2005;12(4):212–217. doi: 10.1207/s15324826an1204_5. [DOI] [PubMed] [Google Scholar]
- 29.Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125–1127. doi: 10.1007/s00415-004-0497-x. [DOI] [PubMed] [Google Scholar]
- 30.Gallassi R, Stracciari A, Morreale A, Lorusso S, Rebucci GG, Lugaresi E. Transient global amnesia: neuropsychological findings after single and multiple attacks. Eur Neurol. 1993;33(4):294–298. doi: 10.1159/000116957. [DOI] [PubMed] [Google Scholar]
- 31.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., III History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caplan L, editor. Transient global amnesia. Amsterdam: Elsevier; 1985. [Google Scholar]
- 33.Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834–843. doi: 10.1136/jnnp.53.10.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Easton JD, Saver JL, Albers GW, et al. American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 35.Sacco RL, Kasner SE, Broderick JP, et al. American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonita R, Beaglehole R. Modification of Rankin Scale: recovery of motor function after stroke. Stroke. 1988;19(12):1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 39.Park KM, Han YH, Kim TH, et al. Pre-existing structural abnormalities of the limbic system in transient global amnesia. J Clin Neurosci. 2015;22(5):843–847. doi: 10.1016/j.jocn.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Moon Y, Moon WJ, Han SH. The structural connectivity of the recurrent transient global amnesia. Acta Neurol Scand. 2016;134(2):160–164. doi: 10.1111/ane.12518. [DOI] [PubMed] [Google Scholar]