Abstract
Background
Psoriasis is a chronic inflammatory disorder of skin and joints for which conventional treatments that are effective in clearing the moderate-to-severe disease are limited due to long-term safety issues. This necessitates exploring the usefulness of botanical agents for treating psoriasis. We previously showed that delphinidin, a diet-derived anthocyanidin endowed with antioxidant and anti-inflammatory properties, induces normal epidermal keratinocyte differentiation and suggested its possible usefulness for the treatment of psoriasis [1]
Objectives
To investigate the effect of delphinidin (0–20 µM ; 2–5 days) on psoriatic epidermal keratinocyte differentiation, proliferation and inflammation using a three-dimensional reconstructed human psoriatic skin equivalent (PSE) model.
Methods
PSEs and normal skin equivalents (NSEs) established on fibroblast-contracted collagen gels with respective psoriatic and normal keratinocytes and treated with/without delphinidin were analyzed for histology, expression of markers of differentiation, proliferation and inflammation using histomorphometry, immunoblotting, immunochemistry, qPCR and cultured supernatants for cytokine with a Multi-Analyte ELISArray Kit.
Results
Our data show that treatment of PSE with delphinidin induced (1) cornification without affecting apoptosis and (2) the mRNA and protein expression of markers of differentiation (caspase-14, filaggrin, loricrin, involucrin). It also decreased the expression of markers of proliferation (Ki67 and proliferating cell nuclear antigen) and inflammation (inducible nitric oxide synthase and antimicrobial peptides S100A7-psoriasin and S100A15-koebnerisin, which are often induced in psoriatic skin). ELISArray showed increased release of psoriasis-associated keratinocyte-derived proinflammatory cytokines in supernatants of the PSE cultures, and this increase was significantly suppressed by delphinidin.
Conclusions
These observations provide a rationale for developing delphinidin for the management of psoriasis.
Keywords: Delphinidin; Differentiation; Caspase14; Cell proliferation; Filaggrin; Inflammation; Inducible nitric oxide synthase; Ki67; Loricrin; Proliferating cell nuclear antigen procaspase-3,-7,-9; Psoriasis; Normal human and psoriatic skin equivalent model; Psoriasin; Koebnerisin
Introduction
Psoriasis is an autoimmune-like chronic and currently incurable inflammatory skin and joint disease that affects 2–3% of the world population [2, 3]. Histologic characteristics reveal marked epidermal hyperproliferation and thickening as well as increased elongated rete ridges, and the clinical features include skin inflammation with demarcated erythematous papules and scaly dermatological plaques [2]. The etiology of psoriasis is not fully understood, but there is evidence showing a complex interaction of genetic, environmental, innate and adaptive immune-mediated factors as well as perturbed keratinocyte function in its pathogenesis [4].
In normal suprabasal epidermis, caspase-14 proteolyzes profilaggrin (pro-FLG) into multiple units of FLG monomers and further cleaves FLG to free amino acids and other products responsible for proper skin barrier function [5–8]. These critically important skin barrier components, caspase-14 and FLG, are downregulated in psoriasiform lesions mostly involving impaired skin barrier [9].
The expression of antimicrobial peptides such as psoriasin (S100A7) and koebnerisin (S100A15), two evolutionary and highly homologous S100 calcium-binding proteins, are strongly increased in inflamed psoriatic skin [10–12]. Although both proteins are highly homologous, they have distinct expression pattern, function and mechanism of action, but synergize as endogenous chemoattractants as well as pro-inflammatory cytokines ‘alarmins’ to promote the inflammatory response in psoriasis [13].
Although psoriasis is sensitive to physiological derivatives of vitamins A and D, all-trans retinoic acid (RA) and 1α,25-dihydroxyvitamin D3 (Vit-D3), and their analogues, these options are limited clinically by issues of efficacy and toxicity, thus imposing the need to develop novel treatment strategies [14, 15].
Our recent observation that delphinidin, a dietary anthocyanidin, possesses prodifferentiation properties in normal human epidermal keratinocytes and in a three-dimensional (3D) normal epidermal equivalent model [1] led us to hypothesize that in addition to its known anti-inflammatory properties, it may be useful for treating psoriasis [1]. To test this hypothesis we examined the ability of delphinidin to induce differentiation and inhibit the expression of proliferation and inflammation markers in a full-thickness 3D reconstituted human skin model of psoriasis [psoriatic skin equivalent; (PSE)] in relation to normal 3D human skin equivalent (NSE). Here, we provide evidence that delphinidin treatment induces differentiation and inhibits proliferation and inflammation in PSE that allows complete skin regeneration. Further, when comparing the effect of delphinidin with RA and Vit-D3, our results suggest that delphinidin is at least as effective as Vit-D3 and is superior to RA.
Materials and Methods
Antibodies, Reagents, Cell Cultures and Drug Treatment
Antibodies (Abs), reagents, normal human epidermal keratinocyte cultures and preparation of drug solutions has been described elsewhere [1], unless otherwise stated. NSEs and PSEs were treated with delphinidin 4 days and 24 h after exposure to air-liquid interfaces, respectively. DMSO at 0.01–0.05% (v/v) served as a vehicle-control and tissues were incubated with/without the drugs for 48, 72 and 120 h until harvest.
Generation of 3D Full-Thickness NSE and PSE
Full-thickness 3D NSEs were generated as described previously [16] with slight modifications and cultured at an air-liquid interface for 12 days to reconstitute a 3D multilayered skin equivalent consisting of a dermal substratum and an outermost differentiating epidermis. Primary normal human epidermal keratinocytes established in low calcium Epi-Life growth medium supplemented with HKGS (Invitrogen), were seeded at 20% confluence and synchronically switched and maintained in progenitor cell-targeted cell culture media (CnT-02–07 CELLnTEC, ZenBio, Research Triangle Park, N.C., USA) prior to establishment of 3D cultures. The dermal and epidermal components were prepared as previously described [17]. The full-thickness human PSE model (SOR-300-FT) consisted of human psoriatic keratinocytes and collagen-contracted fibroblasts cultured to form a multilayered, highly differentiated epidermis (MatTek Corporation, Ashland, Mass., USA). Metabolically active PSE tissues were shipped at 4 ° C, sealed with agarose gel on medium-supplemented and supplied in inserts each per well of 24 well-tissue culture plates. Upon receipt, the psoriatic equivalent was equilibrated at 37 ° C, 5% CO2 for 24 h and maintained in SOR-300-FT-MM media. Throughout the experiment, the tissue cultures were maintained at 37 ° C in a humidified atmosphere containing 5% CO2. After every alternate day, fresh prewarmed media supplemented or not with delphinidin was replenished for 4–12 days. At harvest, punch biopsies (diameter: 3–4 mm) from each insert were obtained and processed for hematoxylin and eosin staining (HE), immunostaining and morphometry, and the rest of the tissues used for Western blot [1], qPCR and supernatants were collected for cytokine and chemokine analysis using Multi-Analyte ELISAray as described below.
RNA Isolation, cDNA Synthesis and qRT-PCR
Isolation of mRNA from NSEs and PSEs and RT-PCR was performed as described earlier [18]. RT-PCR was performed using the iScript cDNA Synthesis Kit (Bio-Rad; Hercules, Calif., USA) according to the manufacturer’s instructions and a standardization kit (Epicenter Biotechnologies; Madison, Wisc., USA). qRT-PCR measurements of transcripts of test markers were done using SYBR Green-based PCR core reagents and detected on a 7500 Fast Cycler (Applied Biosystems). Table 1 lists the primers and sequences used, and the ΔΔCt method was used to normalize relative transcript expression of the target genes to GAPDH. All analyses were performed in triplicate from three independent experiments.
Table 1.
Primer sequences for qPCR analysis
| Gene | Forward | Reverse |
|---|---|---|
| FLG | 5′-AAGGTTCACATTTATTGCCAAA-3′ | 5′-GGATTTGCCGAAATTCCTTT-3′ |
| β-Actin | 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ | 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′ |
| Caspase-14 | 5′-AAATGAGCAATCCGCGGTCTTTGG-3′ | 5′-CCGTGGAATAAACGTGCAAGGCAT-3′ |
| Involucrin | 5′-CTCCACCAAAGCCTCTGC-3′ | 5′-CTGCTTAAGCTGCT GCTC-3′ |
| TGase-1 | 5′-TGAATAGTGACAAGGTGTACTGGCA-3′ | 5′-GTGGCCTGAGACATTGAGCAGCAT-3′ |
| Psoriasin-hS100A7 | 5′-AGACGTGATGACAAGATTGAC-3′ | 5′-TGTCCTTTTTCTCAAAGACGTC-3′ |
| Koeberisin 15S-hS100A15S | 5′-CAAGTTCCTTCTGCTCCATCTTAG-3′ | 5′-AGCCTTCAGGAAATAAAGACAATC-3′ |
| Koebneris 15L-hS100A15L | 5′-ACGTCACTCCTGTCTCTCTTTGCT-3′ | 5′-TGATGAATCAACCCATTTCCTGGG-3′ |
Preparation of Tissue Lysates, SDS-PAGE and Immunoblotting
The reconstituted tissues were harvested at the indicated time points, and whole tissue lysates were prepared and immunoblotting was performed as previously described [1]. In brief, PSE tissue lysates (25–30 µg) prepared as described and proteins were separated using a 12% SDS-polyacrylamide gel, transferred to nitrocellulose membranes and incubated with blocking buffer (Tris-buffered saline, pH 7.4, with 5% milk, 0.1% Tween 20) for 30 min, primary Ab (anti-hS100A15, 1 µg ml−1 ; anti-hS100A7 Ab, 1 µg ml−1; anti-β-actin, 1: 20,000 sigma) overnight, and secondary Ab for 1 h with several washes (TBS, pH 7.4, 0.1% Tween 20) between incubations and were visualized using ECL as described [1].
Immunohistochemistry, Immunofluorescence and Morphometric Analyses
For histology, morphometry and immunohistochemical staining, deparaffinized 5-µm tissue sections were processed and immunostained, and the dehydrated stained slides were mounted in xylene-based mounting medium (Fisher Diagnostics, Middletown, Va., USA) and analyzed as previously described [1]. The sections were processed and analyzed for thickness, morphology and HE as described earlier [1]. For morphometric analysis, HE sections were systematically sampled and analyzed as described previously [1]. Immunofluorescence staining was performed on serial 5-µm frozen sections of PSE fixed in a cold 1: 1 acetone-methanol mixture. The sections were blocked in 10% normal goat serum, and incubated overnight with anti-S100A15 (5 µg ml−1), anti-S100A7 (10 µg ml−1 ; Abcam, Cambridge, UK). The sections were then incubated with Alexa Fluor 488 goat anti-rabbit IgG (H+L) highly cross-adsorbed Ab (Invitrogen) and with Texas Red conjugated goat anti-mouse Ab (Invitrogen) diluted in 10% normal goat serum and incubated for 1 h at room temperature in a dark humidified chamber. Staining with secondary Abs only was performed as a negative control. Sections were overlaid with 4′-6-diamidino-2-phenylindole or DAPI containing ProLong Gold antifade reagent (Invitrogen). Images of fluorescent-stained tissues were recorded using a CCD digital camera on a fluorescent microscope Zeiss ImagerZ1 (Zeiss, Jena, Germany). Expression of psoriasin and koebnerisin was separately quantified in the PSE sections using Image J 1.44 (National Institutes of Health, Bethesda, Md., USA).
Multi-Analyte ELISArray Detection of Released Cytokines and Chemokines
Following harvest of the PSE tissue cultures treated for 5 days with or without delphinidin, culture media suspensions were centrifuged at 1,000 g for 10 min before the supernatants were collected and stored at −86° C until used. During analysis, the supernatant samples were thawed on ice and added to the ELISArray plates according to the manufacturer’s recommendation. Detection of the levels of various cytokines and chemokines was carried out using the human Multi-Analyte ELISArray Kit (SABiosciences, Frederick, Md., USA) as described by the manufacturer’s user manual. The kit analyzes the concentrations of IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17A, IFN-γ, TNF-α, and GM-CSF. Negative and positive controls supplied by the kits were also included, and for low levels of detection, samples were spiked.
Statistical Analysis
Data are presented as means ± SD and were analyzed using GraphPad Prism v4.03 by one-way ANOVA, Bonferroni correction, a Tukey multiple comparisons post hoc test, or an unpaired Student’s post hoc t test. p ≤ 0.05 was considered significant.
Results
Delphinidin Treatment Enhances Cornification Associated with Epidermal Thinning, and Increases in the mRNA and Protein Expression of Markers of Differentiation but Not Apoptosis in a 3D PSE Model
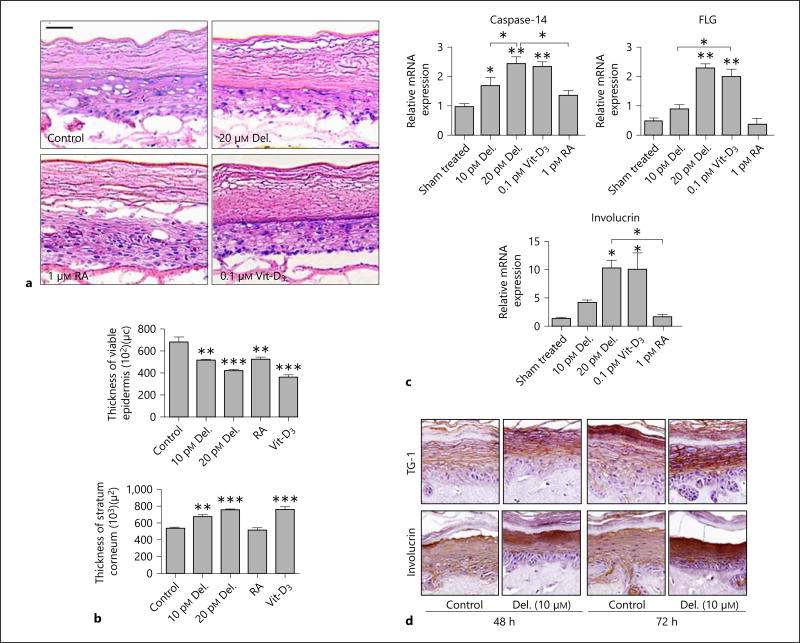
We first determined the effect of delphinidin in an optimized full-thickness 3D PSE (SOR-300-FT) model, and compared it with an NSE model, both of which mimic their respective in vivo skin phenotypes. In NSEs, control tissues showed lesser development of the intermediate and cornified layers and thicker epidermis compared with delphinidin-treated tissues, with a much prominently thicker stratum corneum and thinner viable epidermis similar to previous epidermal equivalent work [1] (data not shown). Interestingly, delphinidin treatment (0– 20 µM) of the PSE (48–72 h) profoundly induced cornification. The effect on morphology and thickness were examined and compared upon prolonged treatment for up to 5 days with delphinidin, RA or Vit-D3. Delphinidin was found to further enhance differentiation associated with decreases in viable epidermal thickness, which was comparable to the effects exhibited by Vit-D3, but superior to the effects by RA (fig. 1 a,b).
Fig. 1.
Delphinidin treatment enhances cornification associated with epidermal thinning, and increases expression of markers of differentiation, but not apoptosis, in a 3D PSE model. Full-thickness psoriatic (PSE) and normal (NSE) reconstructed tissues were treated with or without delphinidin for 48, 72 and 120 h (PSE) and 4 and 8 days (NSE) at an air-liquid interface after which they were harvested, analyzed and evaluated for their morphology, thickness and differentiation prospective as described in Materials and Methods. a Representative photomicrographs of HE-stained PSE tissue sections treated with or without delphinidin, RA or Vit-D3 (controls were treated with medium containing the vehicle). b Histograms represent the quantification of the thicknesses of the viable-epidermis (left panel) and the stratum corneum (right panel) of PSE treated for 5 days with or without delphinidin, RA or Vit-D3 and analyzed as described in Materials and Methods. c Histograms showing an dose-dependent increase in the mRNA expression of differentiation markers caspase-14, FLG and involucrin in control vs. drug (delphinidin, Vit-D3 or RA)-treated PSE. d Representative photomicrographs of immunohistochemical staining showing the expression of differentiation markers in control vs. delphinidin-treated PSE tissue sections. Data represent means ± SD of three independent experiments each performed in triplicate. * p < 0.05, * p < 0.01, ** p < 0.0001; one-way analysis of variance. Bar = 20 µm. Del. = Delphinidin; TG-1 = transglutaminase-1.
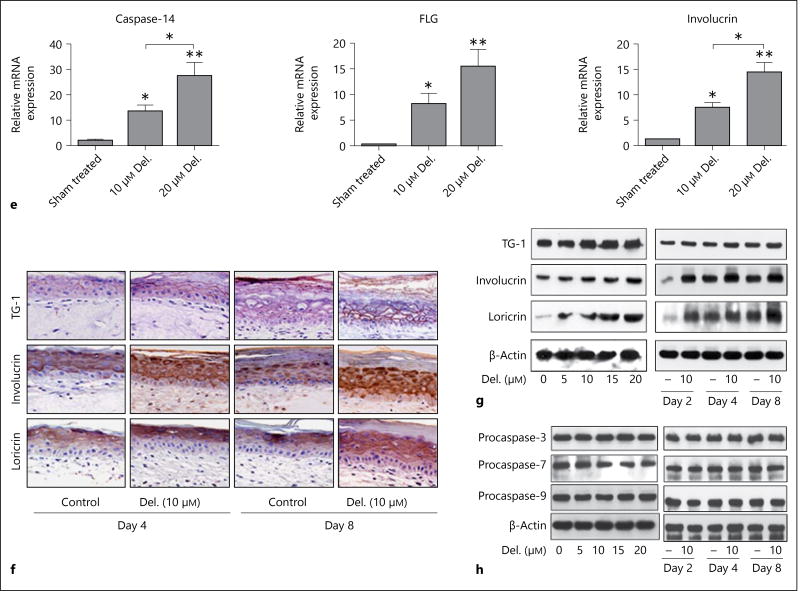
Delphinidin treatment enhances cornification associated with epidermal thinning, and increases expression of markers of differentiation, but not apoptosis, in a 3D PSE model. Full-thickness psoriatic (PSE) and normal (NSE) reconstructed tissues were treated with or without delphinidin for 48, 72 and 120 h (PSE) and 4 and 8 days (NSE) at an air-liquid interface after which they were harvested, analyzed and evaluated for their morphology, thickness and differentiation prospective as described in Materials and Methods. e Histograms showing the equivalent dose-dependent increase in the mRNA expression of differentiation markers, caspase-14, FLG and involucrin in NSE treated with delphinidin. f Representative photomicrographs of immunostaining showing the expression of loricrin, involucrin and transglutaminase-1 in control vs. delphinidin-treated NSE tissue sections. Immunoblots showing control vs. delphinidin-treated NSE tissue section expressions of differentiation markers (g) and markers of apoptosis (h). Data represent means ± SD of three independent experiments each performed in triplicate. * p < 0.05, ** p < 0.01, *** p < 0.0001; one-way analysis of variance. Bar = 20 µm. Del. = Delphinidin; TG-1 = transglutaminase-1.
Based on our recent finding showing a prodifferentiation effect of delphinidin in an in vitro normal epidermal equivalent model [1], we next assessed its effects on the expression of early and late markers of differentiation in the PSE model. Again in this study, upon delphinidin treatment we observed a marked induction of the mRNA and protein expression of the early and late markers of epidermal differentiation in both PSEs (fig. 1c,d) and NSEs (fig. 1e–g). This protein induction in PSEs was further increased by prolonged treatment (up to 5 days) in culture (data not shown). Next, to better understand the role of delphinidin-induced differentiation, we examined its effect on markers of apoptosis, caspase-3, −7 and −9, and observed in both models that delphinidin did not induce apoptosis at the time points investigated. This was evident by the consistent absence of activated caspases or apoptotic cells (caspase-3 positive) as assessed by immunoblotting and immunostaining analyses (fig. 1h).
Delphinidin Treatment Enhances the Expression and Processing of Critical Epidermal Differentiation Markers, Caspase-14 and FLG in a PSE Model
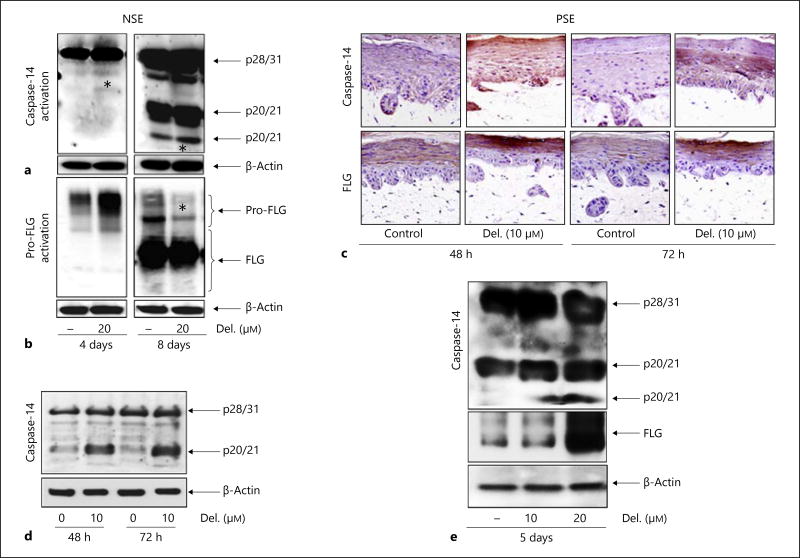
Critically important skin barrier components such as the nonapoptotic protease caspase-14 (which proteolyzes pro-FLG into multiple units of FLG monomers, free amino acids and other products) and the structural filament aggregating protein FLG are downregulated in psoriasiform lesions [8]. By employing immunohistochemistry and immunoblotting analyses, we examined the expression of these cornification-specific markers, caspase-14 and FLG, in both models. The kinetics of procaspase-14 and pro-FLG activation during cornification was established in NSEs by monitoring the appearance of their cleaved products. As early as day 4, we observed an increase in procaspase-14 expression (fig. 2a, left panel) as well as a slight induction of the processed form (*), evident as the p20 subfragment of cleaved caspase-14 upon delphinidin treatments (fig. 2a, panel). Under these conditions, complete cornification was established after 8 days, as evidenced by the appearance of both the cleaved fragments (p10 and p20) in control NSE (fig. 2a, right panel). Interestingly, by day 8 delphinidin (20 µM) accelerated cornification associated with increases in the cleaved p10 and p20 fragments compared to control NSE (*), which is indicative of increased differentiation (fig. 2a, right panel). Analogous to caspase-14, pro-FLG expression appeared earlier and was significantly induced by treatment with delphinidin (20 µM) compared to control NSE (fig. 2b, left panel). By day 8, pro-FLG was almost completely processed upon delphinidin treatment (*) (fig. 2b, right panel). In the PSE model, caspase-14 and FLG expressions were increased after 48–72 h of treatment with delphinidin (10 µM) compared to untreated PSE. Nonetheless, immunoblotting revealed that caspase-14 was not completely processed (fig. 2c,d).
Fig. 2.
Delphinidin (Del.) treatment promotes procaspase-14 and pro-FLG protein expression and activation in 3D reconstituted human NSE and PSE. Reconstituted tissues were treated with or without delphinidin for 4–8 days (NSE), and 48, 72 and 120 h (PSE). Immunoblot analysis of control vs. delphinidin-treated tissue lysates was analyzed for expression and processing of procaspase-14 (a) and pro-FLG (b). c Representative photomicrographs of PSE tissue sections treated with or without delphinidin (10 µM) and immunostained for caspase-14 and FLG. Immunoblot analysis of PSE lysates probed with human caspase-14 Abs showing incomplete activation of procaspase-14 after 72 h (d) and complete processing of caspase-14 and FLG upon prolonged (5 days) treatment with delphinidin (e). Equal loading was confirmed by stripping and reprobing the blots for β-actin. Figures are representative blots of three independent experiments for each treatment group with similar results. * See explanation in text.
Next, we explored the effect of delphinidin (10–20 µM; 5 days) in the PSE model and observed significant increases in the expression and processing of procaspase-14 and pro-FLG (fig. 2e).
Delphinidin Treatment Inhibits the Expression of Markers of Proliferation and Inflammation in a 3D PSE Model
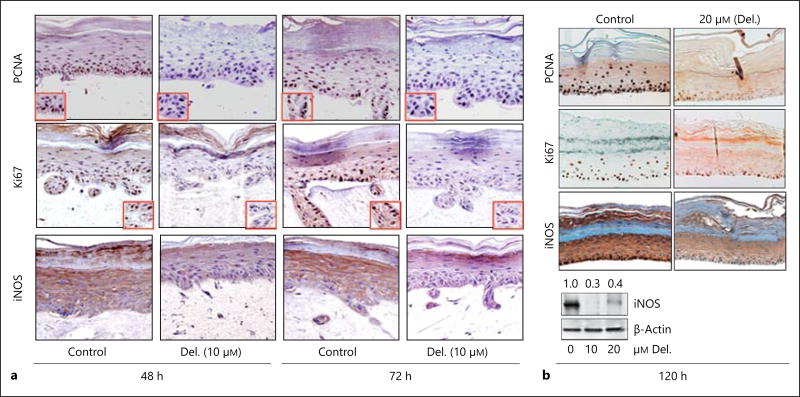
Histologically, epidermal hyperproliferation and inflammation in psoriatic lesions is characterized by an overrepresentation of basaloid keratinocytes, acanthosis, parakeratosis and about a 10-fold increase in epidermal keratinocyte transit time with perturbed differentiation and increased expression of proinflammatory markers [19–21] Here, assessment of the expression of these markers showed a strong expression of markers of proliferation [Ki67 and proliferating cell nuclear antigen (PCNA)] and inflammation [inducible nitric oxide synthase (iNOS)] in control PSE which was found to be significantly reduced upon short treatment with delphinidin (10 µM, 48–72 h; fig. 3a). Furthermore, a prolonged treatment of the PSE model with delphinidin (20 µM) for up to 5 days significantly suppressed the expression of Ki67, PCNA and iNOS (fig. 3b).
Fig. 3.
Delphinidin (Del.) treatment reduces the protein expression of proliferation (PCNA and Ki67) and inflammation (iNOS) markers in 3D reconstituted human PSE. Representative photomicrographs of immunohistochemical staining showing that proliferation and inflammation markers are strongly and predominantly expressed in the basal and granular layers of control PSE. a More intense staining of PCNA, Ki67 and iNOS protein expression was observed in control PSE tissue sections, which was markedly depressed but not completely absent in 48- and 72-hour delphinidin-treated tissues (10 µM) compared to untreated controls. b Protein expression of PCNA, Ki67 and iNOS were markedly and almost completely inhibited in delphinidin-treated constructs (20 µM) after prolonged treatment of 120 h. Immunoblots of iNOS (lower panel) expression in control vs. delphinidin-treated PSEs. Bars = 20 µm.
Delphinidin Treatment Dose-Dependently Suppresses the Expression of Proinflammatory ‘Alarmins’, Psoriasin and Koebnerisin in the PSE Model
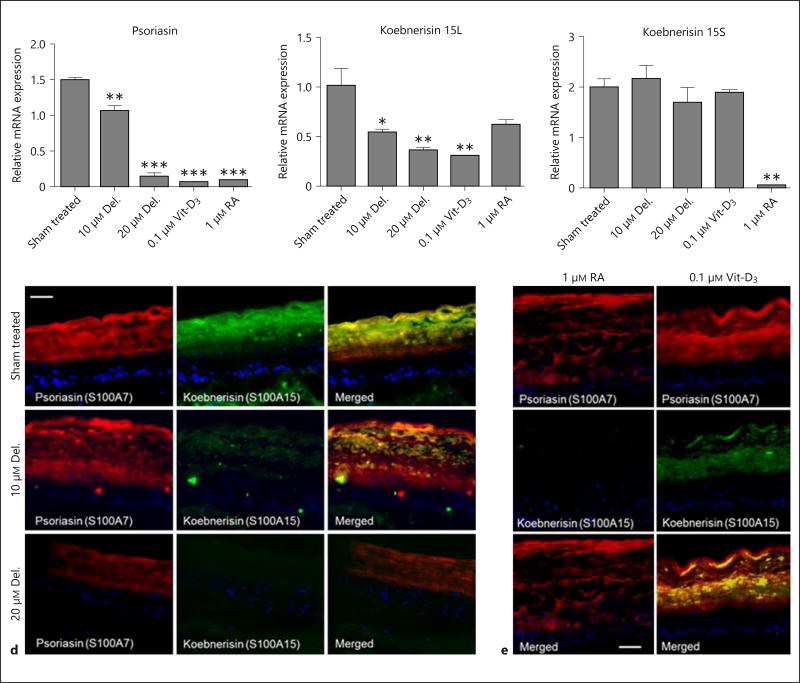
The expression of both evolutionary and highly homologous epidermal S100 calcium-binding antimicrobial peptides, psoriasin (S100A7) and koebnerisin (S100A15), are associated with cutaneous inflammation in psoriasis [4, 13, 22]. Both proteins possess distinct roles, expression patterns and regulatory mechanisms as proinflammatory pathobiological factors whose secretion is increased in hyperplastic psoriatic skin [23] ; they serve as alarmins and chemoattractants. In this PSE model, we observed a significant increase in mRNA expression of psoriasin and koebnerisin, suggesting an induction in psoriatic keratinocytes. Here, we observed that treatment with delphinidin or single doses of RA and Vit-D3 significantly suppressed the increases in mRNA expression of psoriasin (fig. 4a), and differentially regulated the mRNA expressions of both alternate spliced isoforms of koebnerisin (S100A15L and S100A15S). To a similar extent to that of psoriasin, delphinidin, Vit-D3 and RA suppressed the mRNA expression of the S100A15L isoform (fig. 4b). However, apart from RA alone which decreased it, both delphinidin and Vit-D3 did not seem to play an important role in the suppression of the S100A15S isoform in the PSE model (fig. 4c). Next, immunofluorescence data of the PSE tissues showed a strong staining of psoriasin and koebnerisin which was dose-dependently diminished upon treatment with delphinidin (fig. 4d), and similar decreases were also observed in the Vit-D3 and RA-treated PSEs (fig. 4e).
Fig. 4.
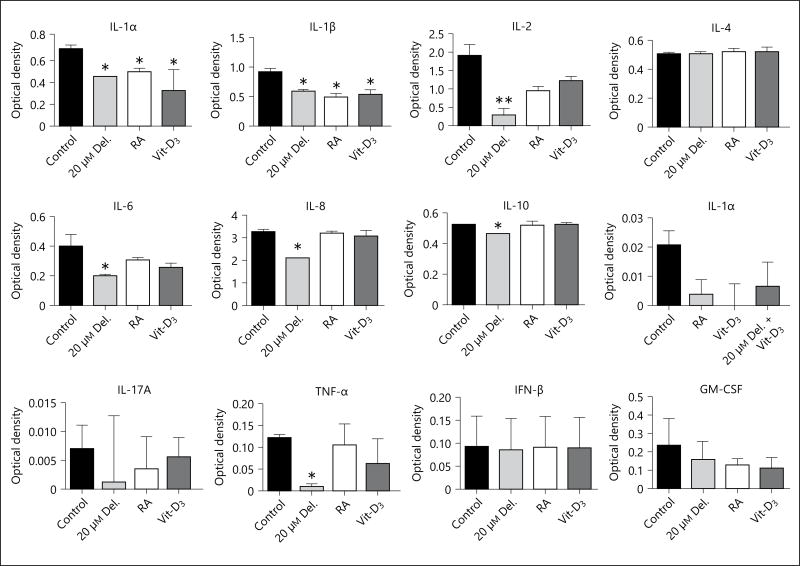
Delphinidin (Del.) treatment suppresses the expression of proinflammatory ‘alarmins’ psoriasin (S100A7) and koebnerisin (S100A15), and inhibits the increased release of proinflammatory cytokines in PSE and its culture supernatant. Psoriasin (S100A7) and koebnerisin (S100A15) are differentially induced and secreted in hyperplastic psoriatic skin. The mRNA and protein expression of these antimicrobial peptides in PSE treated with or without delphinidin, RA or Vit-D3. a–c The mRNA expression of psoriasin (S100A7) and koebnerisin (S100A15L and S100A15S) alternate isoforms analyzed after 5 days by qRT-PCR. Immunofluorescence staining of frozen sections showing control vs. delphinidin-treated (d) and RA and Vit-D3 treated (e) PSE stained for psoriasin (S100A7, red), koebnerisin (S100A15, green). The levels of colocalization (merged yellow) for each treatment are shown on the extreme-right panels and nuclei were stained with 4′-6-diamidino-2-phenylindole (DAPI; blue). Bar = 150 µm. Multi-Analyte ELISArray of PSE culture supernatants using a panel of specific Ab against human proinflammatory cytokines and chemokines. e Histograms summarizing the average optical densities of the different cytokines/chemokines under different treatment conditions. Delphinidin treatment decreased the increased production and secretion of IL-1α, IL-1β, IL-6, IL-8, IL-10 and TNF-α. Data represent means ± SD of three independent experiments each performed in triplicate; * p < 0.05, ** p < 0.01, determined by one-way ANOVA followed by Tukey’s post hoc test or Student’s t test.
Delphinidin Treatment Inhibits the Increased Release of Keratinocyte-Associated Proinflammatory Cytokines in Cultured Supernatants of a 3D PSE Model
Using the human proinflammatory cytokine and chemokine Multi-Analyte ELISArray Kit for analysis of the release in PSE cultures treated with or without delphinidin, RA and Vit-D3, we found increased release of cytokines, IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12, TNF-α, and low IL-4, but undetectable levels of release of immune cell-derived cytokines IL-17A and IFN-γ and GM-CSF in control untreated PSE-cultured supernatants (fig. 4e). Our observation demonstrated that delphinidin treatment significantly suppressed the increased release of proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, IL-10 and TNF-α) in the PSE culture supernatants (fig. 4e). Furthermore, Vit-D3 and RA treatment only decreased the release of IL-1α and IL-1β (p < 0.05 when compared to untreated controls).
Discussion
Delphinidin is a dietary agent that plays functionally important anticancer, antioxidant and antiproliferative roles, and recently we have shown that it also exhibits prodifferentiation effects [1, 24]. Thus, its identification and development may be a welcome addition to the armamentarium of prodifferentiating agents, and we provide evidence that it may be useful for the treatment of psoriasis. Disease phenotype can be mimicked in vitro in 3D reconstituted skin equivalent models by culturing disaggregated patient-derived cells in a spatially organized setting to display in vivo-like architectural features [16], which is disengaged and lost in 2D culture systems. The therapeutic effects of dietary agents such as delphinidin can then be studied optimally in such systems. The current study employed such a model as PSEs consisting of psoriatic keratinocytes and fibroblasts, and demonstrated that delphinidin treatment significantly suppressed the expression of proliferation and inflammation as well as concomitantly induced epidermal differentiation markers. In addition to the antiproliferative, anti-inflammatory and prodifferentiation effects, delphinidin significantly decreased the thickness of the viable normal and hyperplastic epidermis, and consolidated the differentiating and cornified layers of the skin equivalent.
Furthermore, caspase-14 and FLG are tightly regulated during cornification [6, 25], where caspase-14 proteolyzes pro-FLG into multiple units of FLG monomers [5, 6] and further breaks down FLG to amino acids and other moisturizing factors, resulting in an enhanced skin barrier [7] The current study provides evidence that delphinidin-induced differentiation is associated with increases in expression and processing of both caspase-14 and pro-FLG. Time-dependent studies have revealed an early induction and further enhancement of procaspase-14 and pro-FLG expression and activation over time (8 days). These increases may be related to the increase in caspase-14 activity, which in concert might have further enhanced the proteolysis of FLG. This suggestion concurs with recent data demonstrating that caspase-14 further controls the proteolysis of FLG monomers to enhance skin barrier function [7]. Additionally, reports have shown increased sensitivity to UVB-induced apoptosis in caspase-14-deficient murine skin, a potential UVB scavenging effect of caspase-14 [26]. Therefore, it is also possible that compounds such as delphinidin that possess the ability to modulate the turnover of caspase-14 and FLG might be useful for treating psoriasis. Analogous to previous reports on Vit-D3 [15, 27, 28], treatment with delphinidin also displayed increased caspase-14 and FLG expression and processing, whereas RA treatment diminished these effects. It is noteworthy here that compared to RA, delphinidin, similar to Vit-D3, strongly enhanced differentiation in both the PSE and NSE models.
The expression of antimicrobial peptides including the two highly homologous S100 calcium-binding proteins, psoriasin (S100A7) and koebnerisin (S100A15), which have distinct mode of actions and expression, are strongly increased in inflamed psoriastic skin where they synergize as endogenous chemoattractants and proinflammatory cytokines [13]. Here, we observed that delphinidin treatment of the PSE model significantly reduced the expressions of psoriasin and koebnerisin, which was analogous to suppression induced by physiologic Vit-D3 [23] on these genes [29].
Finally, high levels of expressions and release of proinflammatory cytokines and chemokines are critical facets in the pathogenesis of psoriasis [30]. Importantly, delphinidin inhibits the increased release of keratinocyte-associated inflammatory cytokines without affecting the immunocyte-mediated components (IL-17A, GM-CSF and IFN-γ). This absence of immune cells in the current model possibly supports the lack of a triggering feedback loop often induced by specific immune cells that are not present in the PSE model employed. It is important to mention here that the PSE model, apart from keratinocytes, lacks other immune cells that are critical in psoriasis pathogenesis. As such, only keratinocyte-driven cytokines were induced or suppressed. Although the model is encouraging, further studies using well-suited animal, in vitro 3D and human psoriatic skin transplanted mice models that incorporate the immune axis are warranted to fully delineate its detailed molecular mechanism and validate the usefulness of delphinidin for psoriasis management. In summary, considering the complexity of psoriasis etiology, it is apparent that an agent like delphinidin that is capable of targeting several crucial biological endpoints in psoriasis disease is a potential therapeutic agent for psoriasis. Taken together, this study further provides an in vitro rationale for further preclinical and clinical studies to evaluate delphinidin for the management of psoriasis.
Acknowledgments
Work in H.M.’s laboratory was supported by Grants RO1 AR059742; T32AR055893; MRSG-11-019-01-CNE, and F.A. was supported by grants R21 AT004966 and P30AR50948. The authors wish to acknowledge Dr. Ronald Wolf for providing the koebnerisin Ab and Dr. Kimberly A. Toops for assistance with epifluorescence microscopy.
Footnotes
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Chamcheu JC, Afaq F, Syed DN, Siddiqui IA, Adhami VM, Khan N, Singh S, Boylan BT, Wood GS, Mukhtar H. Delphinidin, a dietary antioxidant induces human epidermal keratinocyte differentiation but not apoptosis studies in submerged and three-dimensional epidermal equivalent models. Exp Dermatol. 2013;22:342–348. doi: 10.1111/exd.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 3.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 4.Sabat R, Philipp S, Hoflich C, Kreutzer S, Wallace E, Asadullah K, Volk HD, Sterry W, Wolk K. Immunopathogenesis of psoriasis. Exp Dermatol. 2007;16:779–798. doi: 10.1111/j.1600-0625.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 5.Denecker G, Ovaere P, Vandenabeele P, Declercq W. Caspase-14 reveals its secrets. J Cell Biol. 2008;180:451–458. doi: 10.1083/jcb.200709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippens S, Denecker G, Ovaere P, Vandenabeele P, Declercq W. Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ. 2005;12(suppl 2):1497–1508. doi: 10.1038/sj.cdd.4401722. [DOI] [PubMed] [Google Scholar]
- 7.Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, Gilbert B, Lippens S, De Groote P, Roelandt R, Van Damme P, Gevaert K, Presland RB, Takahara H, Puppels G, Caspers P, Vandenabeele P, Declercq W. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233–2241. doi: 10.1038/jid.2011.153. [DOI] [PubMed] [Google Scholar]
- 8.Hoste E, Denecker G, Gilbert B, Van Nieuwerburgh F, van der Fits L, Asselbergh B, De Rycke R, Hachem JP, Deforce D, Prens EP, Vandenabeele P, Declercq W. Caspase-14-deficient mice are more prone to the development of parakeratosis. J Invest Dermatol. 2013;133:742–750. doi: 10.1038/jid.2012.350. [DOI] [PubMed] [Google Scholar]
- 9.Walsh DS, Borke JL, Singh BB, Do NN, Hsu SD, Balagon MV, Abalos RM. Psoriasis is characterized by altered epidermal expression of caspase 14, a novel regulator of keratinocyte terminal differentiation and barrier formation. J Dermatol Sci. 2005;37:61–63. doi: 10.1016/j.jdermsci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Anderson KS, Wong J, Polyak K, Aronzon D, Enerback C. Detection of psoriasin/S100A7 in the sera of patients with psoriasis. Br J Dermatol. 2009;160:325–332. doi: 10.1111/j.1365-2133.2008.08904.x. [DOI] [PubMed] [Google Scholar]
- 11.Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem. 2003;51:675–685. doi: 10.1177/002215540305100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruse M, Lambert A, Robinson N, Ryan D, Shon KJ, Eckert RL. S100A7, S100A10, and S100A11 are transglutaminase substrates. Biochemistry. 2001;40:3167–3173. doi: 10.1021/bi0019747. [DOI] [PubMed] [Google Scholar]
- 13.Wolf R, Howard OM, Dong HF, Voscopoulos C, Boeshans K, Winston J, Divi R, Gunsior M, Goldsmith P, Ahvazi B, Chavakis T, Oppenheim JJ, Yuspa SH. Chemotactic activity of S100A7 (psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol. 2008;181:1499–1506. doi: 10.4049/jimmunol.181.2.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Kerkhof PC. Update on retinoid therapy of psoriasis in an update on the use of retinoids in dermatology. Dermatol Ther. 2006;19:252–263. doi: 10.1111/j.1529-8019.2006.00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Lippens S, Kockx M, Denecker G, Knaapen M, Verheyen A, Christiaen R, Tschachler E, Vandenabeele P, Declercq W. Vitamin D3 induces caspase-14 expression in psoriatic lesions and enhances caspase-14 processing in organotypic skin cultures. Am J Pathol. 2004;165:833–841. doi: 10.1016/S0002-9440(10)63346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamcheu JC, PihlLundin I, Mouyobo CE, Gester T, Virtanen M, Moustakas A, Navsaria H, Vahlquist A, Torma H. Immortalized keratinocytes derived from patients with epidermolytic ichthyosis reproduce the disease phenotype a useful in vitro model for testing new treatments. Br J Dermatol. 2011;164:263–272. doi: 10.1111/j.1365-2133.2010.10092.x. [DOI] [PubMed] [Google Scholar]
- 17.Calvo-Castro L, Syed DN, Chamcheu JC, Vilela FM, Perez AM, Vaillant F, Rojas M, Mukhtar H. Protective effect of tropical highland blackberry juice (Rubus adenotrichos Schltdl.) against UVB-mediated damage in human epidermal keratinocytes and in a reconstituted skin equivalent model. Photochem Photobiol. 2013;89:1199–1207. doi: 10.1111/php.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamcheu JC, Navsaria H, Pihl-Lundin I, Liovic M, Vahlquist A, Torma H. Chemical chaperones protect epidermolysis bullosa simplex keratinocytes from heat stress-induced keratin aggregation involvement of heat shock proteins and MAP kinases. J Invest Dermatol. 2011;131:1684–1691. doi: 10.1038/jid.2011.93. [DOI] [PubMed] [Google Scholar]
- 19.Leigh IM, Pulford KA, Ramaekers FC, Lane EB. Psoriasis maintenance of an intact monolayer basal cell differentiation compartment in spite of hyperproliferation. Br J Dermatol. 1985;113:53–64. doi: 10.1111/j.1365-2133.1985.tb02044.x. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein GD, McCullough JL, Ross P. Cell proliferation in normal epidermis. J Invest Dermatol. 1984;82:623–628. doi: 10.1111/1523-1747.ep12261462. [DOI] [PubMed] [Google Scholar]
- 21.Vanscott EJ, Ekel TM. Kinetics of hyperplasia in psoriasis. Arch Dermatol. 1963;88:373–381. [PubMed] [Google Scholar]
- 22.Hattinger E, Zwicker S, Ruzicka T, Yuspa SH, Wolf R. Opposing functions of psoriasin (S100A7) and koebnerisin (S100A15) in epithelial carcinogenesis. Curr Opin Pharmacol. 2013;13:588–594. doi: 10.1016/j.coph.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegyi Z, Zwicker S, Bureik D, Peric M, Koglin S, Batycka-Baran A, Prinz JC, Ruzicka T, Schauber J, Wolf R. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 ‘alarmins’ psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J Invest Dermatol. 2012;132:1416–1424. doi: 10.1038/jid.2011.486. [DOI] [PubMed] [Google Scholar]
- 24.Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, Khan N, Zaid MA, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007;127:222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 25.Ballaun C, Karner S, Mrass P, Mildner M, Buchberger M, Bach J, Ban J, Harant H, Tschachler E, Eckhart L. Transcription of the caspase-14 gene in human epidermal keratinocytes requires AP-1 and NFkappaB. Biochem Biophys Res Commun. 2008;371:261–266. doi: 10.1016/j.bbrc.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 26.Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, Van den Broecke C, Van Damme P, D’Herde K, Hachem JP, Borgonie G, Presland RB, Schoonjans L, Libert C, Vandekerckhove J, Gevaert K, Vandenabeele P, Declercq W. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- 27.Lippens S, Kockx M, Knaapen M, Mortier L, Polakowska R, Verheyen A, Garmyn M, Zwijsen A, Formstecher P, Huylebroeck D, Vandenabeele P, Declercq W. Epidermal differentiation does not involve the proapoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 2000;7:1218–1224. doi: 10.1038/sj.cdd.4400785. [DOI] [PubMed] [Google Scholar]
- 28.Eckhart L, Declercq W, Ban J, Rendl M, Lengauer B, Mayer C, Lippens S, Vandenabeele P, Tschachler E. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol. 2000;115:1148–1151. doi: 10.1046/j.1523-1747.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 29.Datta Mitra A, Raychaudhuri SP, Abria CJ, Mitra A, Wright R, Ray R, Kundu-Raychaudhuri S. 1α,25-Dihydroxyvitamin-D3-3-bromoacetate regulates AKT/mTOR signaling cascades a therapeutic agent for psoriasis. J Invest Dermatol. 2013;133:1556–1564. doi: 10.1038/jid.2013.3. [DOI] [PubMed] [Google Scholar]
- 30.Trepicchio WL, Ozawa M, Walters IB, Kikuchi T, Gilleaudeau P, Bliss JL, Schwertschlag U, Dorner AJ, Krueger JG. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J Clin Invest. 1999;104:1527–1537. doi: 10.1172/JCI6910. [DOI] [PMC free article] [PubMed] [Google Scholar]