Abstract
The T4 replisome has provided a unique opportunity to investigate the intricacies of DNA replication. We present a comprehensive review of this system focusing on the following: its 8-protein composition, their individual and synergistic activities, and assembly in vitro and in vivo into a replisome capable of coordinated leading/lagging strand DNA synthesis. We conclude with a brief comparison with other replisomes with emphasis on how coordinated DNA replication is achieved.
Keywords: bacteriophage, DNA-binding protein, DNA helicase, DNA polymerase, DNA primase, DNA replication
Introduction
Enterobacteria phage T4 infects Escherichia coli bacteria. Its genome is 170 kb (1) long and encodes 289 proteins. The DNA genome within an icosahedral head of a virus whose tail is hollow passes into the bacterial cell for infection. The rate of DNA replication in the cell is 400–700 nucleotides s−1 (2) with a mutation rate per base pair of only 7 × 10−8 (3). This efficient and highly accurate replication system is the subject of this review.
In vitro reconstitution of a T4 replication system capable of leading- and lagging-strand synthesis on a duplex DNA substrate requires a minimum of seven proteins: the DNA polymerase (gp43); the ssDNA2-binding protein (gp32); the clamp loader (gp44/62); the clamp (gp45); the helicase (gp41); and the primase (gp61). The helicase loader (gp59) accelerates the reconstitution of the replication system but is not essential once assembled. The numbers in parentheses are the T4 gene designations. The brilliant biochemistry by the Alberts group and in parallel with the Nossal laboratory established the basis for functional and structural characterization of the system (4–7). An attractive reason for study of the T4 system is the strong similarities between it and the less accessible eukaryotic replication complexes (8).
Polymerase (gp43)
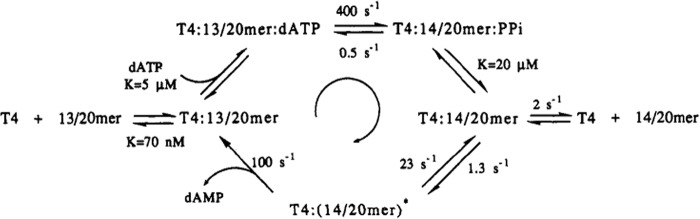
As a first step in understanding the contributions of individual proteins to the functional properties of the complex is the elucidation of their properties. Kinetic schemes for the 5′–3′ polymerase and 3′–5′ exonuclease activities of gp43 were determined by pre-steady-state kinetic methods and fit by computer simulation (9). The minimal kinetic scheme for the action of gp43 on a model duplex is depicted in Fig. 1.
Figure 1.
Kinetic scheme for the T4 polymerase. The minimal kinetic scheme for the polymerase and exonuclease activities of the T4 gp43 enzyme as integrated in an idling process, i.e. the nucleotide is inserted and then excised, is shown. Reprinted with permission from ACS Publications, obtained via RightsLink Copyright Clearance Center (Capson, T. L., Peliska, J. A., Kaboord, B. F., Frey, M. W., Lively, C., Dahlberg, M., and Benkovic, S. J. (1992) Kinetic characterization of the polymerase and exonuclease activities of the gene 43 protein of bacteriophage T4. Biochemistry 31, 10984–10994).
Incorporation of a single correct base follows a minimal five-step kinetic sequence with an observed rate constant for single nucleotide incorporation of >400 s−1 assigned to the chemical step and close to that observed in the cell. Thus, the accessory proteins do not increase the rate of the polymerization reaction per se. The dissociation rate of gp43 from the duplex sets the observed steady-state velocity. The polymerization process is further distinguished by a high degree of dNTP-binding discrimination—up to 300-fold—in the ternary reactive complex.
gp43 exhibits an active 3′–5′-exonuclease cleavage rate of 100 s−1. Note there is a kinetic barrier that protects a properly base-paired 3′ terminus from excision as illustrated by a partitioning between the polymerase site and the exonuclease site biased 23:1 in favor of the polymerase site for correct base pairing. In the case of a mismatched 3′ terminus, this partitioning drops to 5:1 markedly favoring excision. The distance between the two active sites has been estimated as 2–3 nucleotides (10). The 2.8-Å resolution structure of the bacteriophage RB69 gp43 (63% identical in amino acid sequence to its homolog from T4 phage) as well as a 2.6-Å structure with primer/template and nucleotide bound are central for examining protein-protein interactions that maintain the replisome (11). A ribbon representation of the enzyme showing gp43 and its active sites can be found in supplemental Fig. S1. The revealed palm domain contains the three conserved carboxylates (Asp-411, Asp-621, and Asp-623) implicated in catalyzing the nucleotidyl transfer reaction. The conversion of Asp-219 in the exonuclease domain to Ala-219 generates an enzyme that is devoid of exonuclease activity but retains unchanged polymerase activity (12). The distance between the polymerase and exonuclease sites in the crystal structure can be spanned by a 4-base oligonucleotide or a three-nucleotide duplex DNA (13).
Holoenzyme (HE)
In the absence of accessory proteins, gp43 has limited ability to extend DNA templates (2). Numerous physical studies and processivity assays found that gp44/62, gp45, and gp43 in the presence of ATP formed an active replication complex capable of extending large primed circular ssDNA templates (M13 or ΦX174) or polyoligonucleotides (14–18). Experiments on end-blocked linear primer/template substrates established that the core HE consisted of a complex of gp45 and gp43 with the gp44/62 acting catalytically to load gp45 but not acting in its unloading (19–21). The molecular basis for the increased processivity of the HE was revealed by the structure of gp45 that, like other processivity factors for the bacterial and eukaryotic DNA polymerases, is a highly symmetrical, three-subunit, ring-shaped structure through which duplex DNA can be threaded (supplemental Fig. S2) (22).
Investigations of the solution structure of gp45 by various physical methods found a cooperative assembly of the monomers into an open complex composed of two closed subunit interfaces with a third subunit interface separated by a distance of 35–38 Å (23). This intriguing finding was further scrutinized by time-resolved fluorescent spectroscopy of gp45 labeled across its three-subunit interfaces with a pair of dyes capable of FRET. gp45 was found to exist in two forms in solution with 67% in a closed state and 33% with a gap between two subunits of 42 Å (24). The gap is sufficiently large to permit clamp loading to form a functional replication complex without the gp44/62 (25).
Interactions between gp45 and gp43 were first uncovered by deleting the last six C-terminal amino acids of gp43. This mutant, which retained all the kinetic parameters of the parent, does not form an HE (26). A solution model of the HE bound to DNA (supplemental Fig. S3) (27) was created through studies with the following: (i) a fluorescently labeled peptide based on the gp43 C-terminal residues as well as an analogous peptide cross-linker; (ii) FRET-based stopped-flow measurements (gp45 was labeled in multiple positions on opposite sides of the subunit interface) that tracked the kinetics of HE formation (28, 29); and (iii) a crystal structure of the C-terminal peptide bound to gp45 (13). The model shows the C terminus of gp43 inserted into the open subunit interface of gp45.
Assembly/disassembly of the HE
Before discussing how gp44/62 solves the topological problem of opening and closing gp45 on DNA, it is instructive to view the structure of gp44/62. The general organization of the clamp loader consists of one copy of gp62 and four copies of gp44. The architecture of a gp44/62·gp45·DNA complex with gp44/62 bound to an open gp45 has been solved by X-ray crystallography (supplemental Fig. S4) (30).
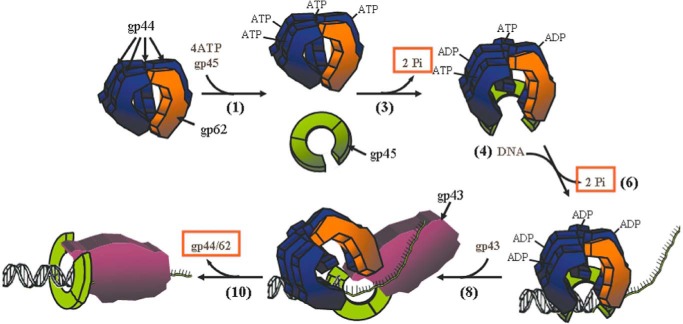
Germane to integrating this structure with function is the proposed reaction cycle for loading gp45 by gp44/62 followed by the latter's dissociation from the DNA. In the presence of ATP bound to the four subunits of gp44, gp44/62 binds, opens gp45, and loads gp45 onto the duplex (Fig. 2, steps 1–4). ATP hydrolysis is associated with both loading and departure of gp44/62 but not for gp43 binding (31). Hydrolysis of ATP promotes closure of gp45 and ejection of gp44/62 (Fig. 2, steps 4–10). All of these steps are associated with large conformational changes in the four gp44 subunits (supplemental Fig. S5) (30, 32). The stoichiometry of ATP hydrolysis by gp44/62 has been measured by pulse-chase kinetics; however, the numbers vary from 2 to 4 ATPs per turnover cycle apparently arising from differences in the quenching procedures (33–35).
Figure 2.
Representation of the bacteriophage T4 DNA polymerase holoenzyme assembly process derived from pre- and steady-state kinetics. A proposed structural representation of the key steps 1, 3, 4, 6, 8, and 10 is depicted. Orange boxes highlight the important conclusions, namely the hydrolysis of ATP in step 3 to open the clamp, the hydrolysis of ATP in step 6 to close the clamp around DNA, and the dissociation of gp44/62 from the holoenzyme in step 10. Figure has been adapted with permission from Elsevier, obtained via RightsLink by Copyright Clearance Center (Trakselis, M. A., Berdis, A. J., and Benkovic, S. J. (2003) Examination of the role of the clamp-loader and ATP hydrolysis in the formation of the bacteriophage T4 polymerase holoenzyme. J. Mol. Biol. 326, 435–451).
Besides loading gp45 onto duplex DNA, gp44/62 acts as a chaperone to escort gp43 to its binding site on the DNA duplex, consistent with the finding that gp43 binds to the same face of gp45 as gp44/62 (36). Moreover, the formation of the HE can occur through one of four pathways as illustrated in supplemental Fig. S6 (37, 38).
The dissociation of the HE from the DNA duplex is governed by the dissociation of gp45 subunits into monomers at a rate of (3.3 ± 0.6) × 10−3 s−1 as measured using a FRET signal engineered across the subunit interface (39). Unexpectedly, gp43 in the HE was found to exchange with an unbound gp43 in solution (40). Neither ATP hydrolysis nor the presence of gp44/62 was required for the exchange. Two possible models for the exchange process are shown in supplemental Fig. S7 (40).
gp32 is often considered part of the HE because it stimulates replisome processivity and the rate at which gp43 traverses helical regions of the DNA by melting out adventitious secondary structure (41). It is essential for leading- and lagging-strand synthesis in vitro (42). Its crystal structure revealed an ssDNA-binding cleft with a positively charged surface parallel to a series of hydrophobic pockets conferring sequence independence and high discrimination against duplex DNA (43). Consequently, the protein may slide along the ssDNA, although its cooperative binding favors complete coverage of the ssDNA.
The primosome (helicase gp41/primase gp61)
The gp41·gp61 complex exhibits both helicase and primase activities (44, 45). The preferred substrate for gp41 is a replication fork with single-stranded extensions of >29 nucleotides on both strands of the fork duplex consistent with gp41 interacting with both the leading and lagging strands (46). Unwinding requires ATP or GTP hydrolysis and proceeds at a rate of 30 bp/s (47). gp61 stimulates gp41 unwinding less than 2-fold by facilitating its binding to the ssDNA (46). At physiological concentrations, gp41 exists primarily as a dimer, but the binding of ATP/GTP or ATPγS/GTPγS drives the assembly of the dimers into an asymmetric hexametric ring complex (supplemental Fig. S8) (48). Electron microscopy images of gp41 support open and closed forms of the ring (49). gp41 is highly processive in the presence of the six other replication proteins (excluding gp59) with an association half-life of ∼11 min (50), sufficiently long to accomplish the replication of the entire T4 genome implying that gp41 also has an accelerated rate in the presence of the other replication proteins.
gp61 generates the pentameric ribonucleotide primers required to initiate Okazaki fragment synthesis (51, 52). The biological relevant primers with the sequence pppApC(pN)3 are the products of the gp41·gp61 complex; in the absence of gp41, gp61 can generate dimers as well as products greater than five nucleotides (52). The primase activity is greatly stimulated by complexation with gp41. In fact, gp41·gp61 complexes on templates coated with gp32 exhibit a physiologically relevant priming rate of about 1 primer s−1 to provide sufficient primers for lagging-strand synthesis given the rate of replication (53). The stoichiometry of gp61 binding to a gp41 hexamer has been reported as 1:1 (54), 6:1 (53, 55), or 3:1.3 The last stoichiometry measurement was done with single-molecule photobleaching and is more definitive and reflective of physiological conditions. Moreover, the variability of this stoichiometry probably arises from the dissociative rather than processive nature of gp61 (56), and it is likely that only one gp61 is scanning the ssDNA to synthesize a primer at a given time. The primosome synthesizes far more primers than needed with only ∼25% being utilized for Okazaki fragment synthesis (57).
Assembling the replisome
Replication initiates from R- and D-loops for origin-dependent and recombinant-dependent replication, respectively (58). Origins of replication facilitate the formation of RNA primers within the R-loop to start leading-strand DNA synthesis implying that it is primed by the gp41·gp61 complex or coupled with gp61-dependent lagging-strand synthesis. Recombinant-dependent replication begins with the strand-invasion reaction that creates D-loops with the invading 3′-end of the DNA being used to prime leading-strand synthesis following loading of a gp41·gp61 complex and gp59 on the displaced strand of the D-loop.
gp41 and gp59 form a 1:1 hexameric complex with the lagging strand of the replication fork passing through the center of the ring-shaped helicase (47). Loading of gp41 onto gp32 that coats ssDNA exposed during replication initiation is facilitated by gp59 that destabilizes the interaction between gp32 and ssDNA through a direct contact with gp32 (59). At least two to three gp32 proteins (binding-site size of 8 nucleotides each) must be released for loading of gp41 (binding-site size of 12–20 nucleotides per hexamer) (60, 61), and indeed, gp32 promotes gp59 oligomerization (62). In turn, direct interactions between the C-terminal peptides of gp59 and those of gp41 promote the latter's assembly (63). A plausible mechanism revealed by cross-linking experiments is depicted in supplemental Fig. S9. The hexameric nature of gp59 when bound to forked DNA was definitively confirmed by single-molecule photobleaching (64). The stepwise assembly of the primosome was then traced by single-molecule FRET microscopy leading to the sequence illustrated in Fig. 3 (65).
Figure 3.
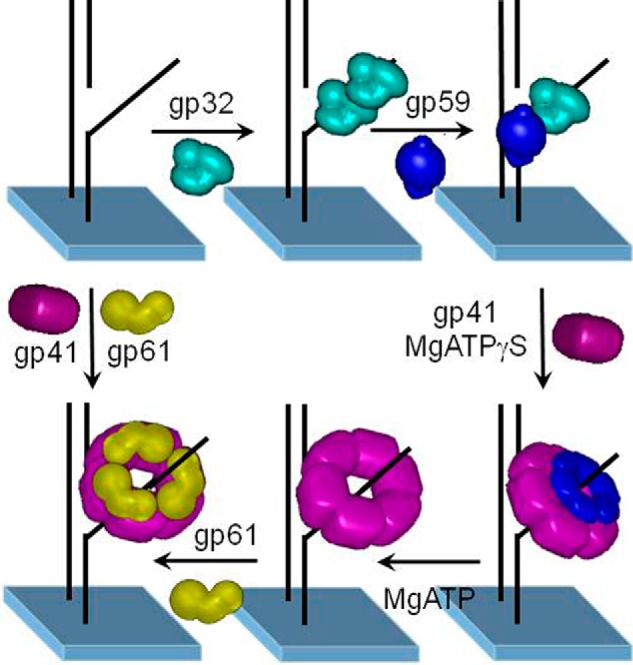
Assembly mechanism of the T4 lagging-strand primosome on forked DNA. The gp32 protein binds to forked DNA with either subsequent or concurrent binding of gp59. Subsequently, gp41 binds to gp59 and is loaded onto the forked DNA in the presence of nucleotide. ATP hydrolysis is required for gp41 to displace gp32 and gp59, either directly or by translocation. The gp61 protein then binds and interacts closely with gp41 on forked DNA. In the absence of gp32 and gp59, both gp41 and gp61 bind to forked DNA. Figure has been adapted to reflect the current opinion on primase stoichiometry with permission from the National Academy of Sciences (© (2005) National Academy of Sciences. Zhang, Z., Spiering, M. M., Trakselis, M. A., Ishmael, F. T., Xi, J., Benkovic, S. J., and Hammes, G. G. (2005) Assembly of the bacteriophage T4 primosome: single-molecule and ensemble studies. Proc. Natl. Acad. Sci. U.S.A. 102, 3254–3259).
The leading-strand HE can readily assemble on the DNA fork as the primer strand becomes available. What's to prevent premature synthesis before the primosome is in place? Mutations in gp59, which have a deleterious effect on origin-dependent DNA replication in vivo, suggest a gatekeeper role for it (66). In vitro studies found a complex formed between gp59 and the leading-strand gp43, whose structure was modeled (supplemental Fig. S10) (67). Both the synthesis and exonuclease activities of gp43 are inhibited. Single-molecule FRET microscopy showed this complex was “unlocked” by the addition of gp41 followed by gp61 to form a functional primosome and subsequently a fully active replisome (supplemental Fig. S11) (68). The “unlocking” stems from the loss of gp59 contacts with the replication fork (69) leading to its loss from the active replisome (68). We have summarized the known interactions between the proteins within the replisome in supplemental Fig. S12.
The in vitro gp41 unwinding rate is ∼10-fold less than the replication rates observed in vivo and in vitro. Likewise, an independent HE is very inefficient at strand displacement synthesis at a rate of about 1 nt/s (70). However, no physical interaction was found between the two to account for their rate of replication when both are present at a replication fork, leading to the postulate of a functional coupling that depended on interactions modulated by the DNA replication fork (71). In this model, the trailing HE traps the ssDNA product of the gp41 unwinding activity preventing the separated strands from reannealing and causing back slippage of gp41 and thereby increasing the unwinding rate. From investigations with magnetic tweezers, a collaborative model was postulated where gp41 prevents the HE from stalling and in turn HE blocks gp41 slippage so both activities are stimulated through the DNA fork (key data and instrumentation are shown in supplemental Fig. S13) (70, 72). Consequently the coupled replication of gp41 and gp43 manifests a rate of 300–400 bp/s in accord with the rates for replication fork movement noted above.
Coordinated leading- and lagging-strand replication
DNA synthesis in vivo is tightly coordinated with the synthesis of both strands completed simultaneously despite the continuous replication of the leading strand and the discontinuous replication of the lagging strand. How is this achieved?
As first steps in understanding leading- and lagging-strand DNA synthesis, reconstitution in vitro of an active replisome was achieved on a model replication fork or a minicircle substrate (42, 73). The latter allows for the quantitation of synthesis of each strand (supplemental Fig. S14). The synthesis was initiated with all eight proteins and was tightly coordinated. With a two-hybrid system based on the λ phage C1 repressor, a homodimerization domain was established in the 400–600-amino acid region of gp43 (42) and then narrowed by cross-linking to Cys-507 specifically (74). The physical tethering of the two gp43s in a replisome necessitates the formation of a replication loop (5) visually observed in electron micrographs (75).
Experiments showed that the activity of coordinated synthesis by a reconstituted replisome was highly resistant to dilution provided the buffer was supplemented with gp45, gp44/62, and gp32 (76). More sensitive trapping experiments additionally revealed gp61 dissociated as well (56). As noted earlier, in the presence of excess gp43 in solution, the gp43s in the replisome will exchange but not impede the processivity of the replisome (40). Thus, only gp41 and the gp43s do not dissociate from the replisome within lifetimes sufficiently long to permit processive duplication of the entire T4 genome.
Central to the synthesis of Okazaki fragments on the lagging strand is the need to accommodate gp41 and gp61 translocating in opposite directions (5′ to 3′ unwinding versus 3′ to 5′ primer synthesis). Three possible scenarios can be visualized (supplemental Fig. S15): pausing both gp41 and gp61; disassembly of the primosome to synthesize a primer forming a pppRNA·gp61 complex while gp41 continues unwinding; and having the primosome remain intact forming a priming loop with the unwound DNA. With magnetic tweezers experiments, two mechanisms were observed: disassembly of the primosome to form pppRNA·gp61 complexes and priming loop formation with an intact primosome. No pausing was found (77). Primosome disassembly during primer synthesis has important ramifications for the discontinuous lagging-strand synthesis.
Lagging-strand synthesis requires transient release of gp43 from the DNA template upon Okazaki fragment completion, yet it remains associated with the replisome during recycling to initiate a new fragment (76, 78). Recycling can be triggered by the lagging-strand gp43 colliding with the end of the previous Okazaki fragment that accelerates the transient dissociation, i.e. the collision mechanism (5, 79, 80). However, the size and number of the Okazaki fragments can be manipulated by varying the activity of gp61, the gp45 and gp44/62 levels, and the rate of synthesis by the lagging-strand gp43 to create a pattern of gapped Okazaki fragments, i.e. the signaling mechanism (57, 81). The cumulative events of primer synthesis and gp43 recycling to initiate another Okazaki fragment compete with the advance of the leading-strand HE to increase the separation of the two HEs and potentially disrupt coordinated replication by the replisome. How then is replication coordination maintained?
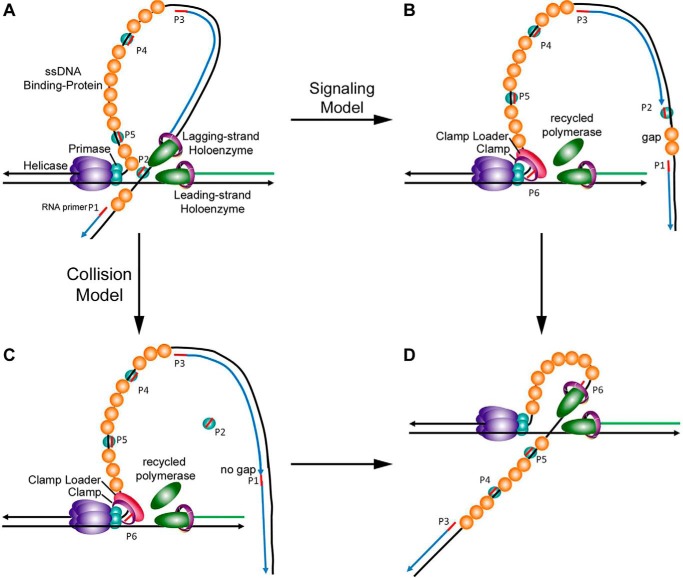
Recognizing that only 20–30% of the primers produced by gp61 are actually utilized for Okazaki fragment synthesis, many unused primers in the form of pppRNA·gp61 complexes could build up ahead of the lagging-strand HE. Indeed, the collision with such complexes was found to trigger early termination of Okazaki fragment synthesis (82). Consequently, the mechanism for dissociation of gp43 to recycle is always collision either with the previous Okazaki fragment or an unused pppRNA·gp61 complex. These signaling and collision mechanisms are illustrated in Fig. 4 (82) and accommodate the above factors that affect the signaling pathway.
Figure 4.
Proposed model for pppRNA·primase complex as the signal for lagging-strand initiation. A, during the replication phase of Okazaki fragment synthesis, primase subunits within a replisome stochastically synthesize 5-mer RNA primers at priming sites along the lagging-strand DNA; these primers are stabilized on the DNA by primase subunits dissociated from the primosome. B, pppRNA·primase complex forms a block, triggering recycling of the lagging-strand polymerase in the signaling model, and results in a gap between Okazaki fragments. C, collision with the previous Okazaki fragment triggers recycling of the lagging-strand polymerase in the collision model and results in no gap between Okazaki fragments. D, holoenzyme is assembled on the new RNA primer to initiate the next Okazaki fragment. Reprinted with permission from National Academy of Sciences (© (2005) National Academy of Sciences. Spiering, M. M., Hanoian, P., Gannavaram, S., and Benkovic, S. J. (2017) RNA primer–primase complexes serve as the signal for polymerase recycling and Okazaki fragment initiation in T4 phage DNA replication. Proc. Natl. Acad. Sci. U.S.A. 114, 5635–5640).
A model derived from all the available kinetic data (82) reproduces the observed distribution of Okazaki fragments (83). Several important conclusions may be drawn from the modeling. First, all Okazaki fragments originate from primers synthesized by the looping mechanism. Second, the interplay between the recycling gp43 binding to an already available pppRNA·gp45·gp44/62 complex (<1 s) and a median time of ∼6 s for the formation of a pppRNA·gp45·gp44/62 complex provides a mechanism for a semi-random distribution of Okazaki fragment lengths in our simulation. Finally, the lagging-strand gp43 will recycle to bind newly synthesized primers a mean distance of 400 nt from gp41 as a result of the brief lifetime of a naked primer and the slightly longer lifetime of a pppRNA·gp45·gp44/62 complex. Thus, signaling alone is sufficient to keep the leading- and lagging-strand synthesis coordinated even though the two gp43s are moving at the same mean velocity.
Comparison with three DNA replication systems
We conclude with a comparison of three DNA replication systems focusing on how they coordinate leading- and lagging-strand synthesis.
The bacteriophage T7 replication system has been the subject of a very recent comprehensive review (84). The replisome and its constituent proteins are depicted in supplemental Fig. S16. The slowest event in coordinated replication stems from the collection of steps involved with primer synthesis (a short tetraribonucleotide) requiring some 6–12 s. In contrast, the polymerase (gp5) with bound processivity factor, thioredoxin, extends primers at a rate of 114–122 bp/s. Importantly, this is also the rate of duplex unwinding by the helicase (gp4) in this gp4·gp5 complex that is accelerated some 13-fold over that of gp4 alone. Recall a similar phenomenon was noted for the T4 helicase in a gp41·HE complex (72).
This large difference between the rate of priming versus the rate of the gp4·gp5 complex advance would result in the loss of coordination between the syntheses of the two strands (85). This problem can be resolved by pausing duplex unwinding and leading-strand synthesis, by having the lagging-strand gp5 advance at a faster rate than the leading-strand gp5, or by having more frequent gp5 recycling and Okazaki fragment initiation via the signaling mechanism. An initial report found pausing of the gp4 and leading-strand synthesis (86); a later report found no pausing but an ∼30% faster rate for lagging-strand synthesis versus leading-strand synthesis (87). Given the observations of concomitant primer synthesis and efficient inter-protein transfer of the primer to gp5, the faster copying of the lagging-strand template ensures synthesis of both strands remains coordinated.
The T7 system, however, also exhibits both signaling and collision mechanisms in the synthesis of Okazaki fragments. The signal for premature release of gp5 appears to be primer synthesis followed by replication loop release before completion of the previous Okazaki fragment (88) rather than polymerase release upon collision with unused primer·primase complexes because the helicase and primase activities are properties of a single polypeptide.
An unusual characteristic is the binding of additional units of gp5 to gp4 (up to six gp5s theoretically can bind to the six C termini of hexameric gp4), which offers a switching mechanism enabling exchange of the synthesizing lagging-strand polymerase and the non-synthesizing polymerase reservoir (89). This exchange could also contribute to the early termination of Okazaki fragments as a signal along with primer synthesis. Both the faster synthesis rate of the lagging-strand gp5 and the added benefit of the premature recycling of gp5 via the switching mechanism act together to retain coordination of DNA replication by the T7 replisome.
The Escherichia coli replisome has also been the subject of a recent review (90). The replisome with its more complex constituent proteins is exhibited in supplemental Fig. S17. Moreover, the E. coli system features the helicase (DnaB) and primase (DnaG) activities residing on separate proteins like the T4 replisome. The processive coupling of DNA synthesis on both template strands faces the same timing issues noted above. The slowest event surrounds priming of the lagging-strand polymerase. Like T4, the primer synthesis activity of DnaG is accelerated ∼5,000-fold by the presence of DnaB reducing the time for this part of the process to ∼0.2 s. Deposition of primers then occurs at the physiological 1–2-s interval similar to the T4 replisome (91).
The leading-strand polymerase complexed to β-clamp and in the presence of DnaB in single-molecule experiments shows bursts of synthesis at 500–600 nt/s and mean rates of about 350 nt/s independent of DnaG activity (92, 93). Again, the activity of DnaB is stimulated by the presence of the leading-strand HE; by itself, the DnaB unwinds duplex DNA at a rate of only 84–86 bp/s (93). Consequently, a displacement of about 350–700 bp between the two polymerases can be imagined as a consequence of each Okazaki fragment synthesized (90).
The question again is how the lagging-strand polymerase is recycled after Okazaki fragment synthesis. Testing for the collision mechanism found it might act 40% of the time (94). However, recent reports contend the polymerase recycling time for the collision mechanism is greater than 2 min and would lead to lengthening of successive Okazaki fragments contrary to observation (95). An additional hypothesis is that the torsional strain generated by a replisome with two interconnected polymerases is responsible for polymerase recycling (96), but a direct measure of the force required to break the connection would bolster this proposal. Consequently, the literature appears to favor a more conventional signaling mechanism (93, 97).
The signal that triggers lagging-strand polymerase recycling has been ascribed to the synthesis of a primer (97), consistent with an earlier observation that the frequency of primer synthesis and the efficiency of primer utilization control Okazaki fragment size (98). Pertinent to our discussion, however, is that primer utilization varies from 70 to 95% and that DnaG acts distributively (99) like gp61. This raises the untested possibility that the recycling of the E. coli lagging-strand polymerase might also be triggered by collision with an unused primer or pppRNA·DnaG complex similar to the T4 system.
Recent single-molecule experiments found the replication velocity of the leading- and lagging-strand polymerases to be equivalent, but punctuated with stops/starts to change replication rates (93). Consequently, their behavior would not be coordinated but stochastic. Because there is no difference in velocity to compensate for the time required for various events related to Okazaki fragment synthesis, one can question whether the stochastic rate fluctuations can be coordinated to achieve the DNA replication necessary for a long genome. A further complicating factor is the observation that both in vivo and in vitro studies indicate that two polymerases may function on the lagging strand, which is not taken into account in the single-molecule experiments. So the issue of replisome coordination or lack thereof is somewhat unsettled.
Much remains to be done on eukaryotic replisomes whose constituent proteins are more numerous and complex. For example, there are two distinct, multisubunit polymerases: one for leading- (ϵ) and one for lagging-strand replication (δ). The fact that the primase is both an RNA and DNA polymerase is another prominent difference. An understanding of the present status of the eukaryotic replication machine and a brief comparison with bacterial replisomes have appeared recently (100). Many questions that have been answered for T4, T7, and E. coli are only now being explored for eukaryotic replisomes. One wonders to what extent similarities will be universal and whether the differences between phage, bacteria, and eukaryote replisomes are merely evolutionary nuances with the key features of the mechanistic solution to DNA replication already solved by primitive organisms.
Supplementary Material
This work was supported by National Institutes of Health Grant GM013306 (to S. J. B.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Figs. S1–S17.
M. M. Spiering, manuscript in preparation.
- ssDNA
- single-stranded DNA
- HE
- holoenzyme
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- nt
- nucleotide.
References
- 1. Miller E. S., Kutter E., Mosig G., Arisaka F., Kunisawa T., and Rüger W. (2003) Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 67, 86–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mace D. C., and Alberts B. M. (1984) T4 DNA polymerase. Rates and processivity on single-stranded DNA templates. J. Mol. Biol. 177, 295–311 [DOI] [PubMed] [Google Scholar]
- 3. Drake J. W. (1970) The Molecular Basis of Mutation, Holden-Day, San Francisco, CA [Google Scholar]
- 4. Liu C. C., Burke R. L., Hibner U., Barry J., and Alberts B. (1979) Probing DNA replication mechanisms with the T4 bacteriophage in vitro system. Cold Spring Harb. Symp. Quant. Biol. 43, 469–487 [DOI] [PubMed] [Google Scholar]
- 5. Alberts B. M., Barry J., Bedinger P., Formosa T., Jongeneel C. V., and Kreuzer K. N. (1983) Studies on DNA replication in the bacteriophage T4 in vitro system. Cold Spring Harb. Symp. Quant. Biol. 47, 655–668 [DOI] [PubMed] [Google Scholar]
- 6. Nossal N. G., and Peterlin B. M. (1979) DNA replication by bacteriophage T4 proteins. The T4 43, 32, 44–62, and 45 proteins are required for strand displacement synthesis at nicks in duplex DNA. J. Biol. Chem. 254, 6032–6037 [PubMed] [Google Scholar]
- 7. Nossal N. G. (1980) RNA priming of DNA replication by bacteriophage T4 proteins. J. Biol. Chem. 255, 2176–2182 [PubMed] [Google Scholar]
- 8. Tsurimoto T., and Stillman B. (1990) Functions of replication factor C and proliferating-cell nuclear antigen: functional similarity of DNA polymerase accessory proteins from human cells and bacteriophage T4. Proc. Natl. Acad. Sci. U.S.A. 87, 1023–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capson T. L., Peliska J. A., Kaboord B. F., Frey M. W., Lively C., Dahlberg M., and Benkovic S. J. (1992) Kinetic characterization of the polymerase and exonuclease activities of the gene 43 protein of bacteriophage T4. Biochemistry 31, 10984–10994 [DOI] [PubMed] [Google Scholar]
- 10. Gopalakrishnan V., and Benkovic S. J. (1994) Spatial relationship between polymerase and exonuclease active sites of phage T4 DNA polymerase enzyme. J. Biol. Chem. 269, 21123–21126 [PubMed] [Google Scholar]
- 11. Wang J., Sattar A. K., Wang C. C., Karam J. D., Konigsberg W. H., and Steitz T. A. (1997) Crystal structure of a pol α family replication DNA polymerase from bacteriophage RB69. Cell 89, 1087–1099 [DOI] [PubMed] [Google Scholar]
- 12. Frey M. W., Nossal N. G., Capson T. L., and Benkovic S. J. (1993) Construction and characterization of a bacteriophage T4 DNA polymerase deficient in 3′–>5′ exonuclease activity. Proc. Natl. Acad. Sci. U.S.A. 90, 2579–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shamoo Y., and Steitz T. A. (1999) Building a replisome from interacting pieces: sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex. Cell 99, 155–166 [DOI] [PubMed] [Google Scholar]
- 14. Jarvis T. C., Paul L. S., Hockensmith J. W., and von Hippel P. H. (1989) Structural and enzymatic studies of the T4 DNA replication system. II. ATPase properties of the polymerase accessory protein complex. J. Biol. Chem. 264, 12717–12729 [PubMed] [Google Scholar]
- 15. Jarvis T. C., Newport J. W., and von Hippel P. H. (1991) Stimulation of the processivity of the DNA polymerase of bacteriophage T4 by the polymerase accessory proteins. The role of ATP hydrolysis. J. Biol. Chem. 266, 1830–1840 [PubMed] [Google Scholar]
- 16. Capson T. L., Benkovic S. J., and Nossal N. G. (1991) Protein-DNA cross-linking demonstrates stepwise ATP-dependent assembly of T4 DNA polymerase and its accessory proteins on the primer-template. Cell 65, 249–258 [DOI] [PubMed] [Google Scholar]
- 17. Munn M. M., and Alberts B. M. (1991) DNA footprinting studies of the complex formed by the T4 DNA polymerase holoenzyme at a primer-template junction. J. Biol. Chem. 266, 20034–20044 [PubMed] [Google Scholar]
- 18. Gogol E. P., Young M. C., Kubasek W. L., Jarvis T. C., and von Hippel P. H. (1992) Cryoelectron microscopic visualization of functional subassemblies of the bacteriophage T4 DNA replication complex. J. Mol. Biol. 224, 395–412 [DOI] [PubMed] [Google Scholar]
- 19. Kaboord B. F., and Benkovic S. J. (1993) Rapid assembly of the bacteriophage T4 core replication complex on a linear primer/template construct. Proc. Natl. Acad. Sci. U.S.A. 90, 10881–10885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaboord B. F., and Benkovic S. J. (1995) Accessory proteins function as matchmakers in the assembly of the T4 DNA polymerase holoenzyme. Curr. Biol. 5, 149–157 [DOI] [PubMed] [Google Scholar]
- 21. Kaboord B. F., and Benkovic S. J. (1996) Dual role of the 44/62 protein as a matchmaker protein and DNA polymerase chaperone during assembly of the bacteriophage T4 holoenzyme complex. Biochemistry 35, 1084–1092 [DOI] [PubMed] [Google Scholar]
- 22. Moarefi I., Jeruzalmi D., Turner J., O'Donnell M., and Kuriyan J. (2000) Crystal structure of the DNA polymerase processivity factor of T4 bacteriophage. J. Mol. Biol. 296, 1215–1223 [DOI] [PubMed] [Google Scholar]
- 23. Alley S. C., Shier V. K., Abel-Santos E., Sexton D. J., Soumillion P., and Benkovic S. J. (1999) Sliding clamp of the bacteriophage T4 polymerase has open and closed subunit interfaces in solution. Biochemistry 38, 7696–7709 [DOI] [PubMed] [Google Scholar]
- 24. Millar D., Trakselis M. A., and Benkovic S. J. (2004) On the solution structure of the T4 sliding clamp (gp45). Biochemistry 43, 12723–12727 [DOI] [PubMed] [Google Scholar]
- 25. Reddy M. K., Weitzel S. E., and von Hippel P. H. (1993) Assembly of a functional replication complex without ATP hydrolysis: a direct interaction of bacteriophage T4 gp45 with T4 DNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 90, 3211–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berdis A. J., Soumillion P., and Benkovic S. J. (1996) The carboxyl terminus of the bacteriophage T4 DNA polymerase is required for holoenzyme complex formation. Proc. Natl. Acad. Sci. U.S.A. 93, 12822–12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alley S. C., Trakselis M. A., Mayer M. U., Ishmael F. T., Jones A. D., and Benkovic S. J. (2001) Building a replisome solution structure by elucidation of protein-protein interactions in the bacteriophage T4 DNA polymerase holoenzyme. J. Biol. Chem. 276, 39340–39349 [DOI] [PubMed] [Google Scholar]
- 28. Alley S. C., Abel-Santos E., and Benkovic S. J. (2000) Tracking sliding clamp opening and closing during bacteriophage T4 DNA polymerase holoenzyme assembly. Biochemistry 39, 3076–3090 [DOI] [PubMed] [Google Scholar]
- 29. Trakselis M. A., Alley S. C., Abel-Santos E., and Benkovic S. J. (2001) Creating a dynamic picture of the sliding clamp during T4 DNA polymerase holoenzyme assembly by using fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. U.S.A. 98, 8368–8375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelch B. A., Makino D. L., O'Donnell M., and Kuriyan J. (2011) How a DNA polymerase clamp loader opens a sliding clamp. Science 334, 1675–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berdis A. J., and Benkovic S. J. (1996) Role of adenosine 5′-triphosphate hydrolysis in the assembly of the bacteriophage T4 DNA replication holoenzyme complex. Biochemistry 35, 9253–9265 [DOI] [PubMed] [Google Scholar]
- 32. Pietroni P., Young M. C., Latham G. J., and von Hippel P. H. (2001) Dissection of the ATP-driven reaction cycle of the bacteriophage T4 DNA replication processivity clamp loading system. J. Mol. Biol. 309, 869–891 [DOI] [PubMed] [Google Scholar]
- 33. Trakselis M. A., Berdis A. J., and Benkovic S. J. (2003) Examination of the role of the clamp-loader and ATP hydrolysis in the formation of the bacteriophage T4 polymerase holoenzyme. J. Mol. Biol. 326, 435–451 [DOI] [PubMed] [Google Scholar]
- 34. Sexton D. J., Kaboord B. F., Berdis A. J., Carver T. E., and Benkovic S. J. (1998) Dissecting the order of bacteriophage T4 DNA polymerase holoenzyme assembly. Biochemistry 37, 7749–7756 [DOI] [PubMed] [Google Scholar]
- 35. Pietroni P., Young M. C., Latham G. J., and von Hippel P. H. (1997) Structural analyses of gp45 sliding clamp interactions during assembly of the bacteriophage T4 DNA polymerase holoenzyme. I. Conformational changes within the gp44/62-gp45-ATP complex during clamp loading. J. Biol. Chem. 272, 31666–31676 [DOI] [PubMed] [Google Scholar]
- 36. Latham G. J., Bacheller D. J., Pietroni P., and von Hippel P. H. (1997) Structural analyses of gp45 sliding clamp interactions during assembly of the bacteriophage T4 DNA polymerase holoenzyme. III. The Gp43 DNA polymerase binds to the same face of the sliding clamp as the clamp loader. J. Biol. Chem. 272, 31685–31692 [DOI] [PubMed] [Google Scholar]
- 37. Zhuang Z., Berdis A. J., and Benkovic S. J. (2006) An alternative clamp loading pathway via the T4 clamp loader gp44/62-DNA complex. Biochemistry 45, 7976–7989 [DOI] [PubMed] [Google Scholar]
- 38. Smiley R. D., Zhuang Z., Benkovic S. J., and Hammes G. G. (2006) Single-molecule investigation of the T4 bacteriophage DNA polymerase holoenzyme: multiple pathways of holoenzyme formation. Biochemistry 45, 7990–7997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soumillion P., Sexton D. J., and Benkovic S. J. (1998) Clamp subunit dissociation dictates bacteriophage T4 DNA polymerase holoenzyme disassembly. Biochemistry 37, 1819–1827 [DOI] [PubMed] [Google Scholar]
- 40. Yang J., Zhuang Z., Roccasecca R. M., Trakselis M. A., and Benkovic S. J. (2004) The dynamic processivity of the T4 DNA polymerase during replication. Proc. Natl. Acad. Sci. U.S.A. 101, 8289–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang C. C., Hearst J. E., and Alberts B. M. (1981) Two types of replication proteins increase the rate at which T4 DNA polymerase traverses the helical regions in a single-stranded DNA template. J. Biol. Chem. 256, 4087–4094 [PubMed] [Google Scholar]
- 42. Salinas F., and Benkovic S. J. (2000) Characterization of bacteriophage T4-coordinated leading- and lagging-strand synthesis on a minicircle substrate. Proc. Natl. Acad. Sci. U.S.A. 97, 7196–7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shamoo Y., Friedman A. M., Parsons M. R., Konigsberg W. H., and Steitz T. A. (1995) Crystal structure of a replication fork single-stranded DNA binding protein (T4 gp32) complexed to DNA. Nature 376, 362–366 [DOI] [PubMed] [Google Scholar]
- 44. Liu C. C., and Alberts B. M. (1981) Characterization of the DNA-dependent GTPase activity of T4 gene 41 protein, an essential component of the T4 bacteriophage DNA replication apparatus. J. Biol. Chem. 256, 2813–2820 [PubMed] [Google Scholar]
- 45. Venkatesan M., Silver L. L., and Nossal N. G. (1982) Bacteriophage T4 gene 41 protein, required for the synthesis of RNA primers, is also a DNA helicase. J. Biol. Chem. 257, 12426–12434 [PubMed] [Google Scholar]
- 46. Richardson R. W., and Nossal N. G. (1989) Characterization of the bacteriophage T4 gene 41 DNA helicase. J. Biol. Chem. 264, 4725–4731 [PubMed] [Google Scholar]
- 47. Raney K. D., Carver T. E., and Benkovic S. J. (1996) Stoichiometry and DNA unwinding by the bacteriophage T4 41:59 helicase. J. Biol. Chem. 271, 14074–14081 [DOI] [PubMed] [Google Scholar]
- 48. Dong F., Gogol E. P., and von Hippel P. H. (1995) The phage T4-coded DNA replication helicase (gp41) forms a hexamer upon activation by nucleoside triphosphate. J. Biol. Chem. 270, 7462–7473 [DOI] [PubMed] [Google Scholar]
- 49. Norcum M. T., Warrington J. A., Spiering M. M., Ishmael F. T., Trakselis M. A., and Benkovic S. J. (2005) Architecture of the bacteriophage T4 primosome: electron microscopy studies of helicase (gp41) and primase (gp61). Proc. Natl. Acad. Sci. U.S.A. 102, 3623–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schrock R. D., and Alberts B. (1996) Processivity of the gene 41 DNA helicase at the bacteriophage T4 DNA replication fork. J. Biol. Chem. 271, 16678–16682 [DOI] [PubMed] [Google Scholar]
- 51. Cha T. A., and Alberts B. M. (1986) Studies of the DNA helicase-RNA primase unit from bacteriophage T4. A trinucleotide sequence on the DNA template starts RNA primer synthesis. J. Biol. Chem. 261, 7001–7010 [PubMed] [Google Scholar]
- 52. Hinton D. M., and Nossal N. G. (1987) Bacteriophage T4 DNA primase-helicase. Characterization of oligomer synthesis by T4 61 protein alone and in conjunction with T4 41 protein. J. Biol. Chem. 262, 10873–10878 [PubMed] [Google Scholar]
- 53. Valentine A. M., Ishmael F. T., Shier V. K., and Benkovic S. J. (2001) A zinc ribbon protein in DNA replication: primer synthesis and macromolecular interactions by the bacteriophage T4 primase. Biochemistry 40, 15074–15085 [DOI] [PubMed] [Google Scholar]
- 54. Jing D. H., Dong F., Latham G. J., and von Hippel P. H. (1999) Interactions of bacteriophage T4-coded primase (gp61) with the T4 replication helicase (gp41) and DNA in primosome formation. J. Biol. Chem. 274, 27287–27298 [DOI] [PubMed] [Google Scholar]
- 55. Yang J., Xi J., Zhuang Z., and Benkovic S. J. (2005) The oligomeric T4 primase is the functional form during replication. J. Biol. Chem. 280, 25416–25423 [DOI] [PubMed] [Google Scholar]
- 56. Trakselis M. A., Roccasecca R. M., Yang J., Valentine A. M., and Benkovic S. J. (2003) Dissociative properties of the proteins within the bacteriophage T4 replisome. J. Biol. Chem. 278, 49839–49849 [DOI] [PubMed] [Google Scholar]
- 57. Nelson S. W., Kumar R., and Benkovic S. J. (2008) RNA primer handoff in bacteriophage T4 DNA replication: The role of single-stranded DNA binding protein and polymerase accessory proteins. J. Biol. Chem. 283, 22838–22846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kreuzer K. N., and Brister J. R. (2010) Initiation of bacteriophage T4 DNA replication and replication fork dynamics: a review in the Virology Journal series on bacteriophage T4 and its relatives. Virol. J. 7, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lefebvre S. D., Wong M. L., and Morrical S. W. (1999) Simultaneous interactions of bacteriophage T4 DNA replication proteins gp59 and gp32 with single-stranded (ss) DNA. Co-modulation of ssDNA binding activities in a DNA helicase assembly intermediate. J. Biol. Chem. 274, 22830–22838 [DOI] [PubMed] [Google Scholar]
- 60. Ishmael F. T., Alley S. C., and Benkovic S. J. (2001) Identification and mapping of protein-protein interactions between gp32 and gp59 by cross-linking. J. Biol. Chem. 276, 25236–25242 [DOI] [PubMed] [Google Scholar]
- 61. Young M. C., Schultz D. E., Ring D., and von Hippel P. H. (1994) Kinetic parameters of the translocation of bacteriophage T4 gene 41 protein helicase on single-stranded DNA. J. Mol. Biol. 235, 1447–1458 [DOI] [PubMed] [Google Scholar]
- 62. Ishmael F. T., Alley S. C., and Benkovic S. J. (2002) Assembly of the bacteriophage T4 helicase: architecture and stoichiometry of the gp41-gp59 complex. J. Biol. Chem. 277, 20555–20562 [DOI] [PubMed] [Google Scholar]
- 63. Delagoutte E., and von Hippel P. H. (2005) Mechanistic studies of the T4 DNA (gp41) replication helicase: functional interactions of the C-terminal tails of the helicase subunits with the T4 (gp59) helicase loader protein. J. Mol. Biol. 347, 257–275 [DOI] [PubMed] [Google Scholar]
- 64. Arumugam S. R., Lee T. H., and Benkovic S. J. (2009) Investigation of stoichiometry of T4 bacteriophage helicase loader protein (gp59). J. Biol. Chem. 284, 29283–29289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang Z., Spiering M. M., Trakselis M. A., Ishmael F. T., Xi J., Benkovic S. J., and Hammes G. G. (2005) Assembly of the bacteriophage T4 primosome: single-molecule and ensemble studies. Proc. Natl. Acad. Sci. U.S.A. 102, 3254–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dudas K. C., and Kreuzer K. N. (2005) Bacteriophage T4 helicase loader protein gp59 functions as gatekeeper in origin-dependent replication in vivo. J. Biol. Chem. 280, 21561–21569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xi J., Zhuang Z., Zhang Z., Selzer T., Spiering M. M., Hammes G. G., and Benkovic S. J. (2005) Interaction between the T4 helicase-loading protein (gp59) and the DNA polymerase (gp43): a locking mechanism to delay replication during replisome assembly. Biochemistry 44, 2305–2318 [DOI] [PubMed] [Google Scholar]
- 68. Xi J., Zhang Z., Zhuang Z., Yang J., Spiering M. M., Hammes G. G., and Benkovic S. J. (2005) Interaction between the T4 helicase loading protein (gp59) and the DNA polymerase (gp43): unlocking of the gp59-gp43-DNA complex to initiate assembly of a fully functional replisome. Biochemistry 44, 7747–7756 [DOI] [PubMed] [Google Scholar]
- 69. Nelson S. W., Yang J., and Benkovic S. J. (2006) Site-directed mutations of T4 helicase loading protein (gp59) reveal multiple modes of DNA polymerase inhibition and the mechanism of unlocking by gp41 helicase. J. Biol. Chem. 281, 8697–8706 [DOI] [PubMed] [Google Scholar]
- 70. Manosas M., Spiering M. M., Ding F., Bensimon D., Allemand J. F., Benkovic S. J., and Croquette V. (2012) Mechanism of strand displacement synthesis by DNA replicative polymerases. Nucleic Acids Res. 40, 6174–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Delagoutte E., and von Hippel P. H. (2001) Molecular mechanisms of the functional coupling of the helicase (gp41) and polymerase (gp43) of bacteriophage T4 within the DNA replication fork. Biochemistry 40, 4459–4477 [DOI] [PubMed] [Google Scholar]
- 72. Manosas M., Spiering M. M., Ding F., Croquette V., and Benkovic S. J. (2012) Collaborative coupling between polymerase and helicase for leading-strand synthesis. Nucleic Acids Res. 40, 6187–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang J., Trakselis M. A., Roccasecca R. M., and Benkovic S. J. (2003) The application of a minicircle substrate in the study of the coordinated T4 DNA replication. J. Biol. Chem. 278, 49828–49838 [DOI] [PubMed] [Google Scholar]
- 74. Ishmael F. T., Trakselis M. A., and Benkovic S. J. (2003) Protein-protein interactions in the bacteriophage T4 replisome. The leading strand holoenzyme is physically linked to the lagging strand holoenzyme and the primosome. J. Biol. Chem. 278, 3145–3152 [DOI] [PubMed] [Google Scholar]
- 75. Chastain P. D. 2nd, Makhov A. M., Nossal N. G., and Griffith J. (2003) Architecture of the replication complex and DNA loops at the fork generated by the bacteriophage t4 proteins. J. Biol. Chem. 278, 21276–21285 [DOI] [PubMed] [Google Scholar]
- 76. Kadyrov F. A., and Drake J. W. (2001) Conditional coupling of leading-strand and lagging-strand DNA synthesis at bacteriophage T4 replication forks. J. Biol. Chem. 276, 29559–29566 [DOI] [PubMed] [Google Scholar]
- 77. Manosas M., Spiering M. M., Zhuang Z., Benkovic S. J., and Croquette V. (2009) Coupling DNA unwinding activity with primer synthesis in the bacteriophage T4 primosome. Nat. Chem. Biol. 5, 904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen D., Yue H., Spiering M. M., and Benkovic S. J. (2013) Insights into Okazaki fragment synthesis by the T4 replisome: the fate of lagging-strand holoenzyme components and their influence on Okazaki fragment size. J. Biol. Chem. 288, 20807–20816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hacker K. J., and Alberts B. M. (1994) The rapid dissociation of the T4 DNA polymerase holoenzyme when stopped by a DNA hairpin helix. A model for polymerase release following the termination of each Okazaki fragment. J. Biol. Chem. 269, 24221–24228 [PubMed] [Google Scholar]
- 80. Carver T. E. Jr, Sexton D. J., and Benkovic S. J. (1997) Dissociation of bacteriophage T4 DNA polymerase and its processivity clamp after completion of Okazaki fragment synthesis. Biochemistry 36, 14409–14417 [DOI] [PubMed] [Google Scholar]
- 81. Yang J., Nelson S. W., and Benkovic S. J. (2006) The control mechanism for lagging strand polymerase recycling during bacteriophage T4 DNA replication. Mol. Cell 21, 153–164 [DOI] [PubMed] [Google Scholar]
- 82. Spiering M. M., Hanoian P., Gannavaram S., and Benkovic S. J. (2017) RNA primer–primase complexes serve as the signal for polymerase recycling and Okazaki fragment initiation in T4 phage DNA replication. Proc. Natl. Acad. Sci. U.S.A. 114, 5635–5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chastain P. D. 2nd, Makhov A. M., Nossal N. G., and Griffith J. D. (2000) Analysis of the Okazaki fragment distributions along single long DNAs replicated by the bacteriophage T4 proteins. Mol. Cell 6, 803–814 [DOI] [PubMed] [Google Scholar]
- 84. Kulczyk A. W., and Richardson C. C. (2016) The replication system of bacteriophage T7. Enzymes 39, 89–136 [DOI] [PubMed] [Google Scholar]
- 85. Stano N. M., Jeong Y. J., Donmez I., Tummalapalli P., Levin M. K., and Patel S. S. (2005) DNA synthesis provides the driving force to accelerate DNA unwinding by a helicase. Nature 435, 370–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee J. B., Hite R. K., Hamdan S. M., Xie X. S., Richardson C. C., and van Oijen A. M. (2006) DNA primase acts as a molecular brake in DNA replication. Nature 439, 621–624 [DOI] [PubMed] [Google Scholar]
- 87. Pandey M., Syed S., Donmez I., Patel G., Ha T., and Patel S. S. (2009) Coordinating DNA replication by means of priming loop and differential synthesis rate. Nature 462, 940–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hamdan S. M., Loparo J. J., Takahashi M., Richardson C. C., and van Oijen A. M. (2009) Dynamics of DNA replication loops reveal temporal control of lagging-strand synthesis. Nature 457, 336–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Geertsema H. J., Kulczyk A. W., Richardson C. C., and van Oijen A. M. (2014) Single-molecule studies of polymerase dynamics and stoichiometry at the bacteriophage T7 replication machinery. Proc. Natl. Acad. Sci. U.S.A. 111, 4073–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lewis J. S., Jergic S., and Dixon N. E. (2016) The E. coli DNA replication fork. Enzymes 39, 31–88 [DOI] [PubMed] [Google Scholar]
- 91. Johnson S. K., Bhattacharyya S., and Griep M. A. (2000) DnaB helicase stimulates primer synthesis activity on short oligonucleotide templates. Biochemistry 39, 736–744 [DOI] [PubMed] [Google Scholar]
- 92. Tanner N. A., Hamdan S. M., Jergic S., Loscha K. V., Schaeffer P. M., Dixon N. E., and van Oijen A. M. (2008) Single-molecule studies of fork dynamics in Escherichia coli DNA replication. Nat. Struct. Mol. Biol. 15, 170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Graham J. E., Marians K. J., and Kowalczykowski S. C. (2017) Independent and stochastic action of DNA polymerases in the replisome. Cell 169, 1201–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Georgescu R. E., Kurth I., Yao N. Y., Stewart J., Yurieva O., and O'Donnell M. (2009) Mechanism of polymerase collision release from sliding clamps on the lagging strand. EMBO J. 28, 2981–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dohrmann P. R., Manhart C. M., Downey C. D., and McHenry C. S. (2011) The rate of polymerase release upon filling the gap between Okazaki fragments is inadequate to support cycling during lagging strand synthesis. J. Mol. Biol. 414, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kurth I., Georgescu R. E., and O'Donnell M. E. (2013) A solution to release twisted DNA during chromosome replication by coupled DNA polymerases. Nature 496, 119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yuan Q., and McHenry C. S. (2014) Cycling of the E. coli lagging strand polymerase is triggered exclusively by the availability of a new primer at the replication fork. Nucleic Acids Res. 42, 1747–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zechner E. L., Wu C. A., and Marians K. J. (1992) Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. II. Frequency of primer synthesis and efficiency of primer utilization control Okazaki fragment size. J. Biol. Chem. 267, 4045–4053 [PubMed] [Google Scholar]
- 99. Wu C. A., Zechner E. L., and Marians K. J. (1992) Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. I. Multiple effectors act to modulate Okazaki fragment size. J. Biol. Chem. 267, 4030–4044 [PubMed] [Google Scholar]
- 100. Zhang D., and O'Donnell M. (2016) The eukaryotic replication machine. Enzymes 39, 191–229 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.